Abstract
Noncovalent interactions play an essential role in biological and chemical processes. In the main chain of common protein secondary structures, the lone pair (n) of a carbonyl oxygen is delocalized into the antibonding orbital (π*) of the subsequent carbonyl group. Herein, experimental and computational data reveal that this n→π* interaction can be attenuated by the inductive electron withdrawal of one or two α-fluoro groups in the donor. The steric effect of three α-fluoro groups, however, overcomes the inductive withdrawal. These data evoke a means to modulate the n→π* interaction in peptides, proteins, and other systems.
Keywords: Inductive effect, non-covalent interaction, n→π* interaction, prolyl peptide bond, stereoelectronic effect
Introduction
Non-covalent interactions play an essential role in many biological processes.1 Yet, the precise nature of many non-covalent interactions is debated actively. For example, the nature of the hydrogen bond has been a subject of debate between proponents of “electrostatic”2 and “partial covalency”3 models. Only recently was the partial covalency of the hydrogen bond established by a direct X-ray measurement4 and NMR J-couplings.5
A second non-covalent interaction encountered in many protein secondary structures6 and protein–ligand interactions7 is the carbonyl–carbonyl interaction (C=O…·C=O). Analogous to a hydrogen bond, this interaction was postulated initially to have an electrostatic origin.7,8 We recently presented experimental and theoretical evidence for its partial covalency6b,c and showed that this interaction arises primarily from the delocalization of the carbonyl oxygen lone pair (n) into the antibonding orbital (π*) of the carbonyl group. Such n→π* electronic delocalization is reminiscent of the Bürgi–Dunitz trajectory for nucleophilic additions to carbonyl groups,9 and is accompanied by pyramidalization of the acceptor carbonyl group.6b
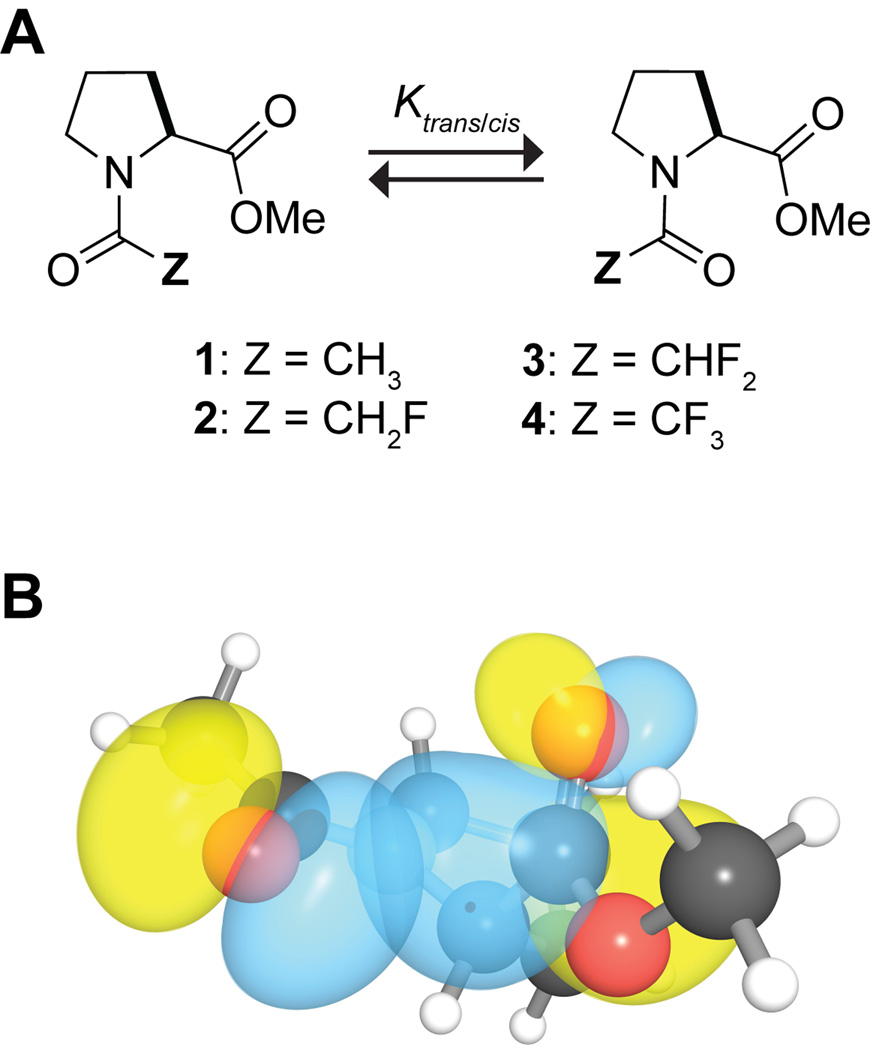
We have continued to explore the nature of the n→π* interaction by using an Ac-Pro-OMe model system. In this system, the trans amide-bond isomer is stabilized differentially over the cis isomer by the n→π* interaction (Figure 1). This differential stabilization is reflected in the value of Ktrans/cis, which can be measured experimentally with NMR spectroscopy. We and others have shown that the n→π* interaction alters the value of Ktrans/cis.6,9,10 For example, amplifying the n→π* interaction by replacing the donor oxygen with sulfur increases the population of the trans conformation.6b We speculated that attenuating the n→π* interaction should reduce the population of the trans conformation. Such attenuation can be achieved by reducing the electron-donating ability of the donor oxygen. We anticipated that replacing an acetyl hydrogen in the Ac-Pro-OMe system with the more electronegative fluorine would attenuate the n→π* interaction.
Figure 1.
Z-C(O)-Pro-OMe model system: A. Cis–trans equilibrium in Z-C(O)-Pro-OMe. B. Overlap of the n and π* orbitals in Ac-Pro-OMe generated with NBOView 1.1.20
The inductive electron withdrawal of fluorine can, however, be contravened by two other factors. First, electron donation from its lone pair into the antibonding orbital (π*) of the adjacent carbonyl group is an important conformational determinant in many α-halocarbonyl compounds.11 Second, the large size of fluorine relative to hydrogen (rF = 1.47 Å, rH = 1.20 Å) makes the monofluoromethyl group more demanding sterically than a methyl group. Indeed, the Taft steric parameters indicate that the monofluoromethyl group (H2FC–; Es = 1.48) is larger than an ethyl group (Es = 1.31), though smaller than an n-propyl group (Es = 1.60).12 As the cis conformation in the Ac-Pro-OMe model system is more sensitive than the trans-conformation to steric interactions, the larger size of the monofluoromethyl group will increase the value of Ktrans/cis. The steric requirements of the difluoromethyl (HF2C–) and the trifluoromethyl groups (F3C–) will be even greater than that of a monofluoromethyl group.
Here, we seek to modulate the n→π* interaction by inductive electron withdrawal of α-fluoro groups. We report on the synthesis and Ktrans/cis values o f Z-C(O)-Pro-OMe compounds with Z = H3C– (1), H2FC– (2), HF2C– (3), and F3C– (4) (Figure 1A). Additionally, we employ hybrid density functional theory and Natural Bond Orbital (NBO) analysis13 to reveal the origins of the conformational preferences. We conclude that α-fluoro groups alter the n→π* interaction by the mingling of other electronic effects, as well as steric effects.
Results and Discussion
Geometry optimization and frequency calculations were carried out on various conformations for each model compound (1–4) at the B3LYP/6–311+G(2d,p) level of theory using Gaussian ’03.14 The frequency calculations indicated that these conformations were true stationary points on the potential energy surface. Each of these optimized geometries was then analyzed with NBO 5.0 at B3LYP/6–311+G(2d,p) level of theory to determine the contribution of specific donor–acceptor orbital interactions to the conformational stability.
Conformational analyses of compound 2
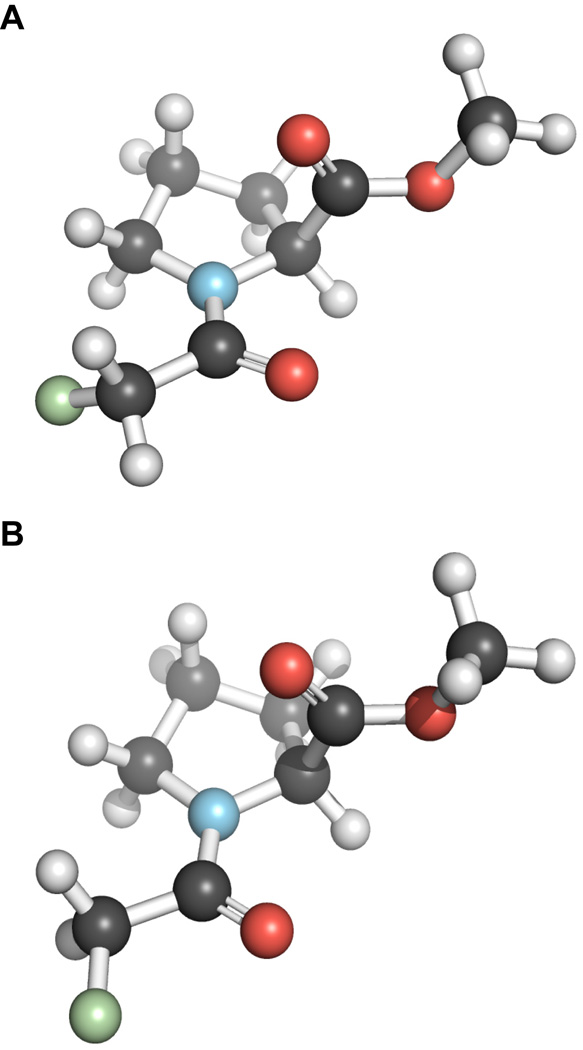
Our ab initio calculations indicate that in the low energy conformations of compound 2, the C–F bond can be oriented either antiperiplanar (ap) or synperiplanar (sp) to the amide carbonyl group (Ci=Oi) (Figure 2). The strength of the n→π* interaction is greater in the ap conformation than in the sp conformation (Table 1). It is known that the fluorine lone pairs can donate electron density into the antibonding orbital (π*) of the adjacent carbonyl group (Ci=Oi) in the ap conformation, but not in the sp conformation.11 Our NBO analysis suggests that this electron donation is worth 0.51 kcal/mol in the trans–ap conformation. Such electron donation enhances the ability of the carbonyl group to partake in the n→π* interaction.
Figure 2.
Conformations of 2: A. antiperiplanar conformation of 2. B. synperiplanar conformation of 2.
Table 1.
Values of Ktrans/cis and En→π* for compounds 1–4
| Compound | Z | Ktrans/cisa | Conformation | En→π* (kcal/mol) |
|---|---|---|---|---|
| 1 | H3C– | 4.0b | — | 0.40 |
| 2 | H2FC– | 2.7 | anti-periplanar | 0.76 |
| syn-periplanar | 0.29 | |||
| anti-periplanar | 0.30 | |||
| 3 | HF2C– | 3.4 | anti-clinical | 0.38 |
| syn-periplanar | 0.38 | |||
| 4 | F3C– | 4.6 | — | 0.37 |
Measured in CDCl3 at 25 °C.
from Ref 6a.
It is noteworthy that our calculations predict that the enhanced n→π* interaction in the trans–ap conformation will not be reflected in the value of Ktrans/cis. Close inspection reveals that the cis–ap conformation is stabilized by an n→π* interaction wherein the fluoro group rather than the carbonyl group acts as the electron-pair donor. Thus, although the energy difference between the trans– and cis–sp conformations is −1.21 kcal/mol in the gas phase, the energy difference for the ap conformations is only −0.36 kcal/mol. Such interactions of fluoro groups with carbonyl groups have been reported previously.7,15
The lower value of Ktrans/cis for compound 2 than 1 indicates that steric effects do not play an important role. Instead, Ktrans/cis is diminished, at least in part, because of the attenuation of the n→π* interaction in the trans–sp conformation.
Conformational analyses of compound 3
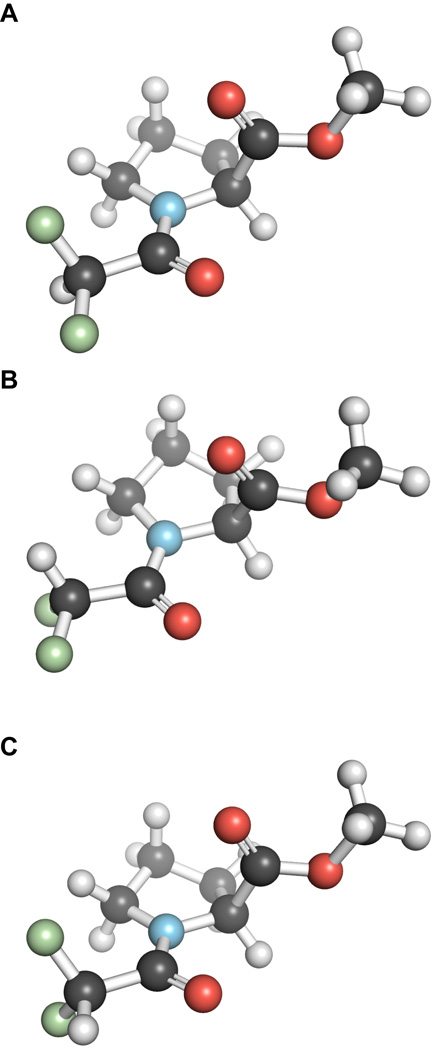
In the low energy conformations of compound 3, the C–H bond can be sp, ap, or anticlinal/synclinal (ac/sc) to the amide carbonyl group (Figure 3). Interestingly, the inductive effect of a difluoromethyl group does not attenuate significantly the strength of the n→π* interaction in the trans conformations of 3 (Table 1), as expected from the aforementioned delocalization of the fluorine lone pair into the antibonding orbital (π*) of the carbonyl group (Ci=Oi). Additionally, our NBO analysis indicates that all the trans conformations of 3 have higher amidic resonances than do those of 1. The inductive effect exerted by the difluoromethyl group tends to polarize the electron density away from the donor carbonyl oxygen and the amidic nitrogen. The former polarization attenuates the n→π* interaction, whereas the latter polarization strengthens the n→π* interaction via amidic resonance. The electron donation by the fluorine lone pair and the amidic resonance attenuate the effect of inductive electron withdrawal on the strength of the n→π* interaction.
Figure 3.
Conformations of 3: A. antiperiplanar conformation of 3. B. anticlinal conformation of 3. C. synperiplanar conformation of 3.
A comparison of the value of Ktrans/cis for compound 3 with those for 1 and 2 indicates that both inductive and steric effects are important. Apparently, the inductive effect is strong enough to lower slightly the value of Ktrans/cisfor 3 with respect to that of 1, but not strong enough to overcome the steric effect imposed by the difluoromethyl group. Thus, the value of Ktrans/cis for 3 is slightly less than that for 1 but greater than that for 2.
It is interesting to note that in some cis conformations of compounds 2 and 3, a weak inverse n→π* interaction is observed. In the inverse n→π* interaction, the roles of the donor and the acceptor carbonyl groups are reversed—the acceptor carbonyl acts as a donor while the donor carbonyl acts as an acceptor. This inverse n→π* interaction could stabilize the cis conformation of the prolyl peptide bond, which is the basis for a polyproline I helix,16 and certain conformations of peptoids.17
Conformational analyses of compound 4
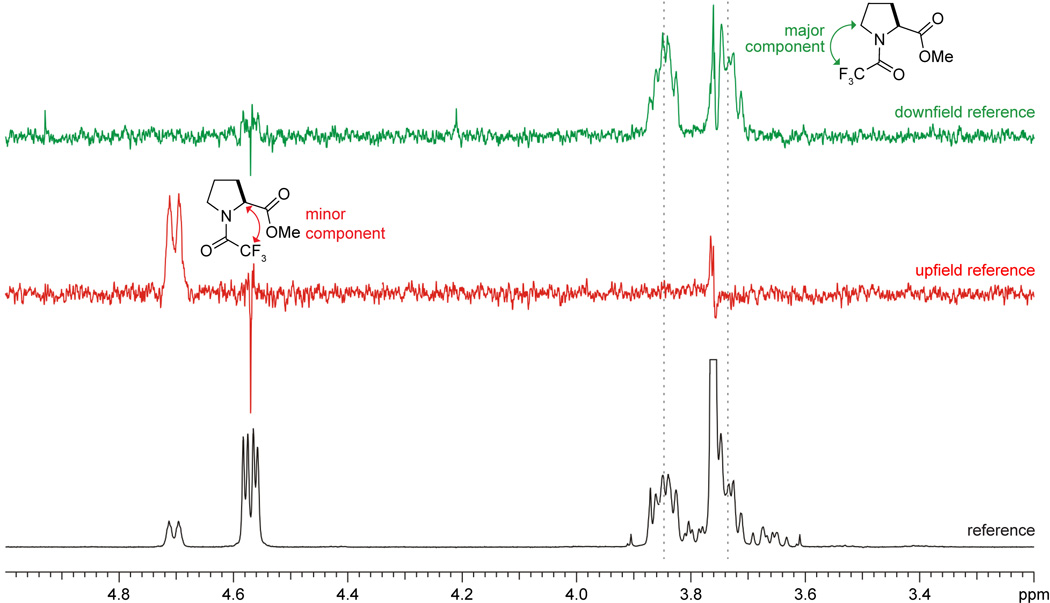
The strength of the n→π* interaction in compound 4 (Table 1) is nearly identical to that of 1 and the trans–sp and trans–ac conformations of 3. It follows that in the trans conformation the inductive electron withdrawal by the –CF3 group is compensated by the electron donation of the fluorine lone pair into the antibonding orbital (π*) of the carbonyl group (Ci=Oi) and enhanced amidic resonance. Based on the strength of the n→π* interaction alone, one would expect the Ktrans/cis of 4 to be identical to that of 1. Yet, the steric requirement of a trifluoromethyl group (−CF3) is greater than that of a methyl group. A strain values indicate that the CF3– (2.10 kcal/mol) is at least as large as an isopropyl group (2.15 kcal/mol), whereas Taft parameters indicate it to be as large as an isobutyl group.10 As the value of Ktrans/cis for 4 (Figure 4) is greater than that for 1, it follows that the large size of the trifluoromethyl group destabilizes the cis conformation significantly with respect to the trans conformation.
Figure 4.
NOE difference spectra with (top) CF3 of the trans conformation saturated; (middle) CF3 of the cis conformation saturated; and (bottom) baseline region in 19F spectrum saturated. Parameters for the experiments are provided in the text.
Conclusions
We have explored the conformational effects of attenuation of the n→π* interaction in model compounds 1−4. Our data suggest a dichotomous relationship between the inductive and the steric effects: the inductive effect tends to lower the value of Ktrans/cis by attenuating the n→π* interaction, but the steric effect tends to increase the value of Ktrans/cis. The cis–trans conformational equilibrium is dominated by the inductive effect in 2, and by the steric effect in 4. Our data also suggests that the attenuation of the n→π* interaction by the inductive effects of fluorine in α-fluorocarbonyl compounds can be compensated by the electronic delocalization of the fluorine lone pair into the antibonding orbital (π*) of the carbonyl group and by enhanced amidic resonance.
Experimental Section
General
Commercial chemicals were of reagent grade or better, and were used without further purification. Anhydrous THF, DMF, and CH2Cl2 were obtained from CYCLE-TAINER ® solvent delivery systems (J. T. Baker, Phillipsburg, NJ). Other anhydrous solvents were obtained in septum-sealed bottles. Reactions were monitored by thin-layer chromatography with visualization by UV light or staining with KMnO4 or I2. In all reactions involving anhydrous solvents, glassware was either oven- or flame-dried. Flash chromatography was performed with columns of silica gel 60, 230–400 mesh (Silicycle, Québec City, Canada). The removal of solvents and other volatile materials “under reduced pressure” refers to the use of a rotary evaporator at water-aspirator pressure (<20 torr) and a water bath of <45 °C. All reported yields are unoptimized.
N-Trifluoroacetyl–(2S)-proline methyl ester (4)
To an ice cooled solution of Boc-ProOH (1.0 g, 4.7 mmol) in anhydrous MeOH (15 mL) was added AcCl (15 mL) dropwise. The mixture was stirred for 6 h, concentrated under reduced pressure, and dried overnight. The residue was then dissolved in dichloromethane and cooled to 0 °C. To this solution was added dropwise Hünig’s base (1.2 g, 9.30 mmol), which was followed by trifluoroacetic anhydride (1.5 g, 7.10 mmol). After stirring for 0.5 h, the reaction mixture was quenched with MeOH, concentrated under reduced pressure, dissolved in 10% w/v citric acid solution, extracted with dichloromethane (4 × 100 mL), and dried over anhydrous MgSO4(s). Column chromatography using hexane/EtOAc afforded 4 as a pale yellow liquid (0.85 g, 81%). 1H NMR (CDCl3, 400 MHz, 4.6:1 mixture of two rotamers): δ 4.75-4.67 (m, 0.18H), 4.62-4.52 (m, 0.82H), 3.91-3.57 (m, 5H), 2.43-1.82 (m, 4H). 13C NMR (CDCl3, 100.6 MHz, 4.6:1 mixture of two rotamers): δ 171.5, 171.1, 156.1, 155.7, 117.6, 114.7, 105.0, 60.1, 59.3, 52.8, 52.6, 48.0, 47.2, 31.7, 28.5, 24.9, 21.2. ESI–MS: [M + Na]+ calcd. 248.0510; found 248.0512 (<1 ppm).
N-Difluoroacetyl–(2S)-proline methyl ester (3)
To an ice cooled solution of Boc-ProOH (0.75 g, 3.5 mmol) in anhydrous MeOH (15 mL) was added AcCl (15 mL) dropwise. The mixture was stirred for 6 h, concentrated under reduced pressure, and dried overnight. The residue was then dissolved in dichloromethane and cooled to 0 °C. To this solution were added difluoroacetic acid (0.4 g, 4.1 mmol), EDC–HCl (0.83 g, 4.4 mmol), HOBt (0.58 g, 4.4 mmol), and triethylamine (1.0 g, 10 mmol). The reaction mixture was stirred overnight, quenched with water, extracted with dichloromethane (4 × 100 mL), and dried over anhydrous MgSO4(s). Column chromatography using hexane/EtOAc afforded 3 as a colorless liquid (0.58 g, 80%). 1H NMR (CDCl3, 400 MHz, 3.2:1 mixture of two rotamers): δ 6.72-5.91 (t, J = 53.2 Hz, 0.76H), 6.12-5.86 (t, J = 53.6 Hz, 0.24H), 4.80-4.73 (m, 0.24H), 4.59-4.49 (m, 0.76H), 3.92-3.58 (m, 5H), 2.36-1.84 (m, 4H). 13C NMR (CDCl3, 100.6 MHz, 3.2:1 mixture of two rotamers): δ 172.0, 171.7, 161.4, 161.1, 112.5, 112.3, 109.9, 109.8, 107.4, 107.3, 59.5, 58.5, 52.7, 52.5, 47.5, 46.1, 31.5, 28.5, 24.9, 21.3. ESI–MS: [M + Na]+ calcd. 230.0605; found 230.0602 (1.3 ppm).
N-Monofluoroacetyl–(2S)-proline methyl ester (2)
To an ice-cooled solution of Boc-ProOH (0.75 g, 3.5 mmol) in anhydrous MeOH (15 mL) was added dropwise AcCl (15 mL). The mixture was stirred for 6 h, concentrated under reduced pressure, and dried overnight. To this solution were added sodium fluoroacetate (0.4 g, 4.2 mmol), EDC–HCl (0.82 g, 4.4 mmol), HOBt (0.59 g, 4.4 mmol), and triethylamine (1.0 g, 10 mmol). The reaction mixture was stirred overnight, quenched with water, extracted with dichloromethane (4 × 100 mL), and dried over anhydrous MgSO4(s). Column chromatography using hexane/EtOAc afforded 2 as a colorless liquid (0.4 g, 59%). 1H NMR (CDCl3, 400 MHz, 2.6:1 mixture of two rotamers): δ 5.04-4.81 (m, 2H), 4.65-4.60 (m, 0.28H), 4.59-4.53 (dd, J = 8.7, 3.6 Hz, 0.73H), 3.80-3.49 (m, 5H), 2.35-1.79 (m, 4H). 13C NMR (CDCl3, 100.6 MHz, 2.6:1 mixture of two rotamers): δ 172.4, 172.2, 165.9, 165.8, 81.9, 80.8, 80.1, 79.0, 59.1, 58.5, 58.4, 52.6, 52.4, 47.5, 45.9, 45.8, 31.6, 28.5, 24.9, 22.6. ESI–MS: [M + Na]+ calcd. 212.0699; found 212.0691 (3.8 ppm).
Measurement of Ktrans/cis values for compounds 2–4 with NMR spectroscopy
Each compound (5–10 mg) was dissolved in CDCl3. 1H spectra were acquired and worked up using the software package NUTS (Acorn NMR, Livermore, CA). Values of Ktrans/cis were determined from the relative areas of the trans and cis peaks. NOEDIFF experiments were carried out to confirm the proton assignments for 2 and 3. 19F–1H NOE experiments were carried out for 4. TOCSY1D experiments were used for assignments of overlapped 1H resonances from the two conformations. The TOCSY1D pulse sequence used was from the standard ChemPack library available for Varian spectrometers with a 5-mm inverse triple PFG-equipped probe. The sequence uses DPFGSE and SEDUCE-shaped RF pulses for multiplet selection, and an MLEV-17 spin lock for mixing. Zero-quantum filtering was implemented. Selection of the 4.705 or 4.570 ppm multiplets were clean, as demonstrated by mix = 0 spectra. The spin lock power equaled 9.4 kHz. Spectra at mix times = 0, 0.015, 0.035, 0.055, 0.080 and 0.150 s were obtained. Other parameters used were: d1 = 4 s, at = 2 s, sspul = ‘y’ (GZ–90°–GZ), ss = −4, and nt = 16. For processing, fn = 4 ×np, and lb = 0.5. TOCSY1D spectra were referenced to TMS, and obtained by using a Varian INOVA spectrometer with 1H resonating at 599.76 MHz. T1 values measured by standard inversion-recovery methods ranged from 1.5 to 3.2 s. The TOCSY1D spectra showed clean polarization of the two respective conformational spin systems, minor coupled through the 4.705 ppm and major through the 4.570 ppm multiplet.
All experiments involving 19F were performed on a Varian INOVA spectrometer, having resonance frequencies of 470.64 MHz for 19F and 500.23 MHz for 1H. All 19F spectra are referenced by the unified scale,18 with the 19F Ξ value referenced to CFCl3. The unified scale utilizes the 1H spectrum acquired from the same sample, experimentally referenced with TMS set to 0 ppm. A 5-mm dual-coil 1H–19F Varian probe was used.
Standard 19F 1D spectra were obtained without 1H decoupling using d1 = 4, at = 1.5, nt = 8, ss = 2, pw = 30°. The sweep width was reduced from an initial 400 ppm to 181 ppm to improve digital resolution. No peaks were observed except for the three singlets: CF3 peaks for the minor and major isomers at −71.712 and −72.758 ppm, respectively, and a small peak (<2%) at −75.754 ppm. The 1H–19F dual probe used for these studies did not have PFG capability. Hence, 1H–19F NOEs were obtained by using NOE difference methods as follows. Our INOVA-500 spectrometer has a single high-band AMT transmitter, so re-cabling to allow RF splitting and recombining while retaining high sensitivity for both nuclei was necessary.19
With NOE difference methods, a saturation pulse is applied to one multiplet; in our experiments, we chose the CF3 singlets. A saturation power between –10 and 0 dB was found to work well (that is, the pulse did not affect the nearby multiplet). Saturation equalizes the ±½ spin states of the 19F nuclei, which will then produce NOEs at nearby protons. Acquiring 1H spectra with the 19F saturation pulse applied at the reference position and subtracting that spectrum from another having the saturation pulse at the CF3 position produces the NOEdiff spectra, as shown in Figure 4. Parameters for these spectra were: nt = 1200, ss = 16, dpwr (=satpwr) = −4, d1 = 10, at = 4, bs = 16, il = ‘y’.
Acknowledgements
We are grateful to C. N. Bradford, B. R. Caes, and Dr. M. D. Shoulders for contributive discussions. This work was supported by Grants R01 AR044276 (NIH), S10 RR13866 (NIH), and CHE-9629688 (NSF).
Footnotes
This paper celebrates the distinguished careers of Drs. Cynthia A. and Bruce E. Maryanoff.
References
- 1.(a) Anfinsen CB. Science. 1973;181:223. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]; (b) Dill KA. Biochemistry. 1990;29:7133. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 2.(a) Morokuma K. Acc. Chem. Res. 1977;10:294. [Google Scholar]; (b) Kollman P. J. Am. Chem. Soc. 1977;99:4875. [Google Scholar]; (c) Buckingham AD, Fowler PW, Hutson JM. Chem. Rev. 1988;88:963. [Google Scholar]
- 3.(a) Sidgwick NV. The Electronic Theory of Valency. Clarendon, Oxford: 1927. [Google Scholar]; (b) Pimentel GC, McClellan AL. The Hydrogen Bond. San Francisco, CA: W. H. Freeman; 1960. [Google Scholar]; (c) Reed AE, Curtiss LA, Weinhold F. Chem. Rev. 1988;88:899. [Google Scholar]; (d) Weinhold F. Advances in Protein Chemistry: Peptide Solvation and H-Bonds. Vol. 72. Elsevier Academic Press; 2005. p. 121. [Google Scholar]
- 4.Isaacs ED, Shukla A, Platzman PM, Barbiellini DR, Tulk CA. Phys. Rev. Lett. 1999;82:600. [Google Scholar]
- 5.(a) Blake PR, Park JB, Adams MWW, Summers MF. J. Am. Chem. Soc. 1992;114:4931. [Google Scholar]; (b) Pervushin K, Ono A, Fernandez C, Szyperski T, Kainosho M, Wüthrich K. Proc. Natl. Acad. Sci. USA. 1998;95:14147. doi: 10.1073/pnas.95.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cornilescu G, Hu J-S, Bax A. J. Am. Chem. Soc. 1999;121:2949. [Google Scholar]
- 6.Hinderaker MP, Raines RT. Protein Sci. 2003;12:1188. doi: 10.1110/ps.0241903. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choudhary A, Gandla D, Krow GR, Raines RT. J. Am. Chem. Soc. 2009;131:7244. doi: 10.1021/ja901188y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jakobsche CE, Choudhary A, Miller SJ, Raines RT. J. Am. Chem. Soc. 2010;132:6651. doi: 10.1021/ja100931y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulini R, Müller K, Diederich F. Angew. Chem. Int. Ed. 2005;44:1788. doi: 10.1002/anie.200462213. [DOI] [PubMed] [Google Scholar]
- 8.(a) Maccallum PH, Poet R, Milner-White EJ. J. Mol. Biol. 1995;248:361. doi: 10.1016/s0022-2836(95)80056-5. [DOI] [PubMed] [Google Scholar]; (b) Maccallum PH, Poet R, Milner-White EJ. J. Mol. Biol. 1995;248:374. doi: 10.1016/s0022-2836(95)80057-3. [DOI] [PubMed] [Google Scholar]; (c) Allen FH, Baalham CA, Lommerse JPA, Raithby PR. Acta Crystallogr. B. 1998;B54:320. [Google Scholar]; (d) Fischer FR, Wood PA, Allen FH, Diederich F. Proc. Natl. Acad. Sci. USA. 2008;105:17290. doi: 10.1073/pnas.0806129105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. J. Am. Chem. Soc. 2002;124:2497. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]; (b) Hodges JA, Raines RT. Org. Lett. 2006;8:4695. doi: 10.1021/ol061569t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J. Am. Chem. Soc. 2001;123:777. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]; (b) Jenkins CL, Lin G, Duo J, Rapolu D, Guzei IA, Raines RT, Krow GR. J. Org. Chem. 2004;69:8565. doi: 10.1021/jo049242y. [DOI] [PubMed] [Google Scholar]; (c) Horng J-C, Raines RT. Protein Sci. 2006;15:74. doi: 10.1110/ps.051779806. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sonntag L-S, Schweizer S, Ochsenfeld C, Wennemers H. J. Am. Chem. Soc. 2006;128:14697. doi: 10.1021/ja0654938. [DOI] [PubMed] [Google Scholar]; (e) Shoulders MD, Guzei IA, Raines RT. Biopolymers. 2007;89:443. doi: 10.1002/bip.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kotch FW, Guzei IA, Raines RT. J. Am. Chem. Soc. 2008;130:2952. doi: 10.1021/ja800225k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Shoulders MD, Satyshur KA, Forest KT, Raines RT. Proc. Natl. Acad. Sci. USA. 2010;107:559. doi: 10.1073/pnas.0909592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Eisenstein O, Nguyen TA, Devaquet JA, Cantacuzene DJ, Salem L. Tetrahedron. 1974;30:1717. [Google Scholar]; (b) Basso EA, Kaiser C, Rittner R, Lambert JB. J. Org. Chem. 1993;58:7865. [Google Scholar]; (c) Eliel EL, Wilen SH. Stereochemistry of Organic Compounds. New York: Wiley Interscience Publication; 1996. [Google Scholar]; (d) Olivato PR, Guerrero SA, Yreijo MH, Rittner R, Tormena CF. J. Mol. Struct. 2002;607:87. [Google Scholar]; (e) Yoshinaga F, Tormena CF, Freitas MP, Rittner R, Abraham RJ. J. Chem. Soc., Perkin Trans. 2002;2:1494. [Google Scholar]
- 12.Kitazume T, Yamazaki T. Introduction in Experimental Methods in Organic Fluorine Chemistry. Tokyo: Gordon and Breach; 1998. [Google Scholar]
- 13.(a) Weinhold F. In: Encyclopedia of Computational Chemistry. Schleyer PvR, Allinger NL, Clark T, Gasteiger J, Kollman PA, Shaefer HF, III, Schreiner PR., editors. Vol. 3. Chichester, UK: John Wiley & Sons; 1998. p. 1792. [Google Scholar]; (b) Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. NBO 5.0. 2001. [Google Scholar]
- 14.C R, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03. Wallingford CT: Gaussian, Inc; 2004. [Google Scholar]
- 15.(a) Hof F, Scofield DM, Schweizer WB, Diederich F. Angew. Chem. Int. Ed. 2004;43:5056. doi: 10.1002/anie.200460781. [DOI] [PubMed] [Google Scholar]; (b) Fischer FR, Schweizer WB, Diederich F. Angew. Chem. Int. Ed. Engl. 2007;46:8270. doi: 10.1002/anie.200702497. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg IZ, Harrington WF, Berger A, Sela M, Katchalski EJ. J. Am. Chem. Soc. 1960;82:5263. [Google Scholar]
- 17.(a) Gorske BC, Bastian BL, Geske GD, Blackwell HE. J. Am. Chem. Soc. 2007;129:8928. doi: 10.1021/ja071310l. [DOI] [PubMed] [Google Scholar]; (b) Gorske BC, Stringer JR, Bastian BL, Fowler SA, Blackwell HE. J. Am. Chem. Soc. 2009;131:16555. doi: 10.1021/ja907184g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RK, Becker ED, Cabral de Menezes SM, Goodfellow R, Granger P. Pure Appl. Chem. 2001;73:1795. [Google Scholar]
- 19.Pomerantz WC, Hadley EB, Fry CG, Gellman SH. ChemBioChem. 2009;10:2177. doi: 10.1002/cbic.200900380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendt M, Weinhold F. NBOView 1.1. Madison, Wisconsin: Theoretical Chemistry Institute, University of Wisconsin-Madison; 2001. [Google Scholar]