Abstract
Background
Growth-differentiation factor-15 (GDF-15) is emerging as a prognostic marker in patients with cardiovascular disease (CVD), but its prognostic value in community-dwelling adults has not been reported. We hypothesized that GDF-15 would add incremental power for prediction of mortality in a population of community-dwelling older adults without known heart disease.
Methods and Results
We measured plasma GDF-15, NT-proBNP, and CRP levels in 1391 Rancho Bernardo Study participants, mean age 70, with no history of CVD, and followed them for a mean of 11 years. In models adjusted for traditional CVD risk factors, GDF-15 was a robust predictor of all-cause, cardiovascular, and non-cardiovascular mortality. GDF-15 was a stronger predictor of all-cause mortality than either NT-proBNP or C-reactive protein (hazard ratio [HR] [95% confidence interval] per standard deviation log10 units 1.5 [1.3–1.8], p<0.0001 for GDF-15, versus 1.3 [1.2–1.5], p<0.0001 for NT-proBNP; CRP was not a significant predictor). Among biomarkers considered, only GDF-15 predicted non-cardiovascular death (HR 1.6 [1.4–2.0], p<0.0001). Growth differentiation factor-15 improved discrimination and modestly but significantly improved reclassification for all-cause, and noncardiovascular mortality with borderline improvement for cardiovascular mortality; NT-proBNP significantly improved reclassification for all-cause and for cardiovascular mortality; C-reactive protein did not improve reclassification for any end point tested. Participants in the highest quartile of both GDF-15 and NT-proBNP had an increased risk of death compared to participants with only NT-proBNP elevated (HR 1.5 [1.1–2.0], p=0.01).
Conclusions
GDF-15 is a strong predictor of all-cause, cardiovascular, and non-cardiovascular mortality in community-dwelling older individuals, adding incremental value to traditional risk factors and to NT-proBNP and CRP levels.
Keywords: Biomarker, elderly, GDF-15, cardiovascular disease, natriuretic peptides
INTRODUCTION
Growth-differentiation factor-15 (GDF-15) is a divergent member of the transforming growth factor-β cytokine superfamily that was previously called macrophage-inhibitory cytokine-1 and is expressed by activated macrophages.1, 2 At baseline, GDF-15 is expressed in most parenchymal tissues only at very low levels;3, 4 the only human organ that expresses high levels of GDF-15 in healthy conditions is the placenta.5 However, in the presence of ischemic injury6 or pressure overload,3 mouse models have demonstrated markedly increased myocardial expression of GDF-15. In humans, GDF-15 is greatly upregulated in the myocardium in the setting of massive myocardial infarction6; it is also expressed by atherosclerotic plaques.7 GDF-15 is also overexpressed and prognostic in the setting of a number of human malignancies,8, 9 where it may enhance tumorigenic activity.10
GDF-15 has previously been shown to add incremental prognostic information to standard cardiovascular biomarkers and risk factors in patients with acute coronary syndromes and with chronic heart failure.11–13 A community-based cross-sectional study of 70-year-old Swedish adults found that GDF-15 levels are associated with endothelial dysfunction, carotid plaque burden, left ventricular hypertrophy, left ventricular systolic dysfunction, and prevalent cardiovascular disease (CVD), independent of traditional cardiovascular risk factors.14 In addition, a nested case-control study of healthy elderly women from the Women’s Health Study documented an increased 4-year risk of cardiovascular events associated with higher levels of GDF-15.15 To our knowledge, the long-term prognostic value of GDF-15 levels in the community has not been reported. We hypothesized that GDF-15 is an independent marker of increased mortality risk among relatively healthy community-dwelling older adults. We also sought to define the correlates of GDF-15 levels and to evaluate the potential usefulness of GDF-15 levels to improve risk stratification.
METHODS
Study Population
The Rancho Bernardo Study is a prospective, population-based study of the epidemiology of cardiovascular and other chronic diseases in older adults. Between 1972 and 1974, all adult residents ages 30 to 79 of Rancho Bernardo, a community in Southern California, were invited to participate in a study of heart disease risk factors; 82% (n=5,052) enrolled. Nearly all were Caucasian, middle to upper class, and relatively well-educated. In 1992–1996, 1781 of the surviving, locally resident participants attended a follow-up study visit. Of the 1740 (98%) who had sufficient blood banked for measurement of GDF-15, 1391 participants (80%) had no history of CVD at the time of this study visit, and are the focus of the present analyses. Prevalent CVD at baseline was defined as a history of physician-diagnosed myocardial infarction, coronary revascularization, stroke, transient ischemic attack, or peripheral arterial disease. As echocardiograms were not available, individuals with heart failure but no other prevalent CVD (e.g. dilated cardiomyopathy) were included in analyses. Since the primary focus was on CVD, we also did not exclude participants with a history of malignancy. Four participants had no follow-up and were not included in outcomes analyses. All participants provided written informed consent; the study protocol was approved by the human research protection program at the University of California at San Diego.
Data Collection
Baseline data for these analyses were collected at the 1992–1996 research clinic visit and included demographics, medical history (including history of cardiovascular events and revascularization procedures), and lifestyle information. Medical histories and information on physical activity (exercise 3+ times per week, yes/no), alcohol consumption (1+drinks per day versus less or none), and current smoking (yes/no) were obtained using standard questionnaires developed by the Rancho Bernardo Research Group. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. Blood pressure was measured in seated, resting participants using the Hypertension Detection and Follow-up Program protocol;16 the mean of two readings was used in analyses. Height and weight were measured in the clinic with participants wearing light clothing and no shoes, and body mass index (BMI; kg/m2) was calculated. Diabetes mellitus was defined as a fasting morning plasma glucose level ≥126 mg/dl, reported physician diagnosis, or use of diabetes-specific medication. Hypertension was defined as reported physician diagnosis, use of anti-hypertensive medication, or resting blood pressure ≥140 mmHg systolic or 90 mmHg diastolic. Estimated creatinine clearance (CrCl) was calculated using the Cockroft-Gault formula [CrCl (ml/min) = weight(kg)*(140-age)/(creatinine (mg/dl)*72)*0.85(if female)].17, 18 Participants were followed with periodic clinic visits and annual mailed questionnaires through July 30, 2009.
Definition of Endpoints
The primary outcome, all-cause mortality, was selected based on the well-known uncertainty of cause of death in the elderly. Pre-specified secondary endpoints were fatal CVD and non-cardiovascular death. Post-hoc exploratory analyses included neoplastic death, and the combined endpoint of coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery), myocardial infarction, or CVD death. Death certificates were obtained for decedents and coded by a certified nosologist using the International Classification of Disease–9th Revision criteria. CVD death included deaths assigned codes 390–459. Neoplastic death included deaths assigned codes 140–239.
Laboratory Methods
Serum and plasma were separated from fasting blood samples, and stored frozen at −70°C. Total cholesterol and triglyceride levels were measured using an ABA-200 Biochromatic Analyzer (Abbott Laboratories, Irving, Texas). High-density lipoprotein (HDL) was measured after precipitation of other lipoproteins with heparin and manganese chloride. Low-density lipoprotein (LDL) was estimated using the Friedewald formula.19 NT-proBNP was measured in 2010 using the Elecsys® proBNP sandwich immunoassay (measurable range 5–35,000 pg/ml; Roche Diagnostics, Indianapolis, Indiana) in EDTA plasma that had been stored at −70°C. Three of the 1391 participants did not have sufficient plasma for NT-proBNP measurement. Also in 2010, GDF-15 was measured using a Luminex platform with a sandwich immunoassay (measureable range 2–10,000 ng/L, limit of detection 2 ng/L, limit of quantification 11 ng/L, Alere Inc., Waltham, MA) in EDTA plasma. Intra-assay CV was 7%, and inter-assay CV was 10% at a GDF-15 level of 1,950 ng/L. CRP was also measured on the Luminex platform with a competitive immunoassay (measureable range 0.004–10.000 mg/dL; intra-assay CV 7%, inter-assay CV 10%).
Statistical Analysis
Continuous variables are presented as means±standard deviation; most laboratory values were not normally distributed, and are presented as medians (quartile 1–quartile 3). Dichotomous variables are presented as percentages. For prospective analyses, the 1391 participants without a history of CVD were divided into quartiles of GDF-15 levels. Trends in differences in baseline levels of risk factors and clinical characteristics by GDF-15 quartile were analyzed with ANOVA with linear trend for normally distributed variables, with Jonckheere-Terpstra tests for skewed variables, and with logistic regression for nominal variables.
Single-predictor associations between the clinical variables listed in Table 1 and logGDF-15 levels were determined by linear regression analysis. Backward multivariable regression analysis including variables with significant individual associations was used to determine which covariates were independently associated with logGDF-15 levels; repeating the analysis with forward regression analysis yielded identical results.
Table 1.
Baseline characteristics of the study population.
| Quartile of GDF15 | |||||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||
| Overall | No Prior CVD | (<962 ng/L) | (962–1268 ng/L) | (1269–1780 ng/L) | (>1780 ng/L) | ||
| (n=1740) | (n=1391) | (n=348) | (n=348) | (n=347) | (n=348) | p, trend | |
| Age | 71 ± 11 | 70 ± 11 | 60 ± 10 | 67 ± 9 | 73 ± 9 | 79 ± 8 | <0.0001 |
| % Male | 39 | 36 | 32 | 34 | 32 | 45 | 0.002 |
| Vital Signs | |||||||
| Heart rate, bpm | 65 ± 9 | 65 ± 11 | 64 ± 9 | 65 ± 12 | 64 ± 11 | 66 ± 12 | 0.19 |
| Systolic BP, mm Hg | 136 ± 22 | 135 ± 22 | 125 ± 20 | 131 ± 20 | 139 ± 20 | 144 ± 23 | <0.0001 |
| Diastolic BP, mm Hg | 76 ± 9 | 76 ± 9 | 77 ± 9 | 76 ± 9 | 76 ± 9 | 76 ± 10 | 0.04 |
| Cardiovascular Risk Factors | |||||||
| Hypertension, % | 53 | 49 | 32 | 41 | 57 | 66 | <0.0001 |
| Current smoking, % | 7 | 8 | 5 | 7 | 10 | 9 | 0.03 |
| Ever smoked, % | 57 | 55 | 52 | 54 | 54 | 62 | 0.02 |
| Diabetes, % | 14 | 12 | 7 | 9 | 14 | 18 | <0.0001 |
| Medication Use | |||||||
| Aspirin, % | 33 | 26 | 22 | 24 | 28 | 30 | 0.004 |
| Lipid-Lowering, % | 12 | 10 | 11 | 9 | 12 | 7 | 0.11 |
| Nutrition and Activity | |||||||
| Body mass index, kg/m2 | 25.4 ± 4.0 | 25.5 ± 4.0 | 25.5 ± 3.8 | 25.9 ± 4.2 | 25.2 ± 4.1 | 25.2 ± 4.0 | 0.11 |
| Waist-hip ratio, cm/cm | 0.84 ± 0.10 | 0.83 ± 0.09 | 0.82 ± 0.09 | 0.82 ± 0.10 | 0.83 ± 0.09 | 0.86 ± 0.09 | <0.0001 |
| Exercise ≥3x/wk, % | 71 | 71 | 70 | 76 | 70 | 69 | 0.38 |
| Alcohol ≥3x/wk, % | 45 | 46 | 44 | 50 | 46 | 42 | 0.42 |
| Laboratory Values | |||||||
| NT-proBNP†, pg/mL | 128 (62–257) | 112 (56–211) | 68 (36–114) | 91 (50–163) | 131 (71–207) | 204 (112–414) | <0.0001 |
| CRP†, mg/dL | 0.82 (0.39–1.77) | 0.78 (0.37–1.71) | 0.66 (0.30–1.80) | 0.73 (0.36–1.49) | 0.76 (0.37–1.58) | 0.91 (0.47–1.90) | 0.001 |
| Fasting glucose†, mg/dL | 94 (88–101) | 94 (88–100) | 93 (88–99) | 93 (87–100) | 94 (88–101) | 94 (88–101) | 0.02 |
| BUN†, mg/dL | 16 (13–19) | 15 (13–18) | 14 (12–17) | 14 (12–17) | 16 (13–19) | 18 (15–21) | <0.0001 |
| CrCl†, ml/min | 60 (46–76) | 62 (48–79) | 75 (63–88) | 67 (54–83) | 57 (46–70) | 48 (39–62) | <0.0001 |
| Total cholesterol†, mg/dL | 208 (185–232) | 209 (187–234) | 214 (191–240) | 211 (191–234) | 209 (185–236) | 203 (178–226) | <0.0001 |
| Triglycerides†, mg/dL | 104 (74–148) | 103 (74–146) | 106 (76–152) | 99 (71–138) | 104 (75–149) | 102 (69–145) | 0.29 |
| HDL†, mg/dL | 56 (45–69) | 57 (46–70) | 57 (46–71) | 59 (49–69) | 57 (46–68) | 54 (42–71) | 0.02 |
| LDL†, mg/dL | 125 (105–148) | 127 (106–148) | 129 (109–153) | 127 (109–149) | 125 (105–146) | 124 (100–144) | 0.0004 |
Median (Quartile 1 – Quartile 3)
BP=blood pressure; BPM=beats per minute; BUN=blood urea nitrogen; CrCl=creatinine clearance; CRP = C-reactive protein; CVD=cardiovascular disease; HDL=high-density lipoprotein; LDL=low-density lipoprotein; mm Hg = millimeters of mercury; NT-proBNP=N-terminal pro B-type natriuretic peptide.
Kaplan Meier cumulative incidence plots were constructed to compare risk of death by quartile of GDF-15, using the methods of Prentice et al to account for the presence of competing risks;20 the log-rank test was used to compare survival across groups. Cox proportional hazards regression models were used to determine the association of GDF-15 quartiles with each endpoint. Missing datapoints (<0.01% of data) were mean substituted. Model 1 adjusted for age and sex. Model 2 additionally adjusted for traditional cardiovascular risk factors, including categorically defined diabetes, hypertension, and current smoking, plus continuously defined systolic blood pressure, total cholesterol, and HDL. Model 3, the fully adjusted model, additionally adjusted for CrCl and BMI. Receiver-operator characteristic (ROC) curves were constructed and areas under the ROC curves (C-statistic) were calculated using a method adapted for survival models, to evaluate the incremental benefit of logGDF-15, when combined with the fully adjusted model, for predicting all-cause mortality.21
Model calibration was assessed using a Hosmer-Lemeshow test modified for use with Cox proportional hazards models.22 Likelihood ratio tests were used to assess whether global model fit improved with the addition of logGDF-15 to the fully adjusted models. Integrated discrimination improvement (IDI) and net reclassification improvement (NRI) for the addition of logGDF-15 to the fully adjusted models were calculated according to the methods of Pencina et al.23 Cause-specific cut-points of <10%, 10–30%, and >30% for all-cause mortality, and by tertiles of risk for both CVD mortality (<2%, 2–9%, >9%) and non-CVD mortality (<5%, 5–16%, >16%), were chosen to define risk categories for the purposes of NRI calculations. In addition, because NRI calculations are highly sensitive to chosen cut-points the categoryless NRI was calculated.24 For reclassification analyses, we estimated risk at 10 years. Finally, the relative utility of selected biomarkers was assessed by including logGDF-15, logNT-proBNP, and logCRP together in the fully adjusted Cox model, with results displayed in Forest plots. Participants were also divided into 4 groups based on whether their GDF-15 and/or NT-proBNP levels were in the highest quartile, and new Kaplan Meier cumulative incidence plots were constructed, again taking into consideration competing endpoints.
An interaction of GDF-15 with sex was tested for in all models; none were significant, so sex-specific analyses were not done. A two-tailed p<0.05 was considered statistically significant. Data were analyzed using SPSS 12.0 (Chicago, IL).
RESULTS
Baseline Characteristics
Baseline characteristics of all 1740 participants with measured GDF-15, and of the 1391 without prior CVD are shown in Table 1. The median GDF-15 level overall was 1370 ng/L (1008–1897); among the 349 participants with prevalent CVD, the median was 1740 ng/L (1313–2415). All Subsequent analyses were performed only on the 1391 free of known CVD at baseline, whose mean age at baseline was 70 ± 11 years; 36% were men. The median GDF-15 level in this group was 1268 ng/L (962–1781) and was higher in men than in women (1349 ng/L vs. 1229 ng/L, p=0.001). The 95% range of GDF-15 concentrations was from 634 to 2928 ng/L. Participants in the higher quartiles of GDF-15 levels were older and more likely to be men, to use aspirin, and to be hypertensive and diabetic. They also had lower CrCl and HDL levels and higher systolic blood pressure, waist-hip ratio, NT-proBNP, CRP, and fasting plasma glucose; but lower total cholesterol and LDL levels.
Correlates of GDF-15 Levels
Variables with significant individual associations with log GDF-15 levels are shown in Table 2. Older age, lower CrCl, higher systolic blood pressure, and higher levels of NT-proBNP and blood urea nitrogen showed the strongest single-predictor associations with GDF-15 levels. In multivariable analysis, variables independently associated with higher log GDF-15 levels were older age, lower CrCl, higher NT-proBNP level, current smoking, male sex, lower HDL and LDL levels, higher waist-hip ratio, diabetes, and higher blood urea nitrogen and CRP levels. The adjusted R2 value of this model was 0.38.
Table 2.
Significant Individual and Multivariable Covariates of logGDF-15 Levels
| Individual | Multivariable* | |||
|---|---|---|---|---|
| Variable | r | p | β | p |
| Demographics | ||||
| Age | 0.54 | <0.0001 | 0.33 | <0.0001 |
| Male Sex | 0.10 | 0.0001 | 0.09 | 0.011 |
| Vital Signs | ||||
| SBP | 0.26 | <0.0001 | ||
| DBP | −0.06 | 0.03 | ||
| Cardiovascular Risk Factors | ||||
| Hypertension | 0.21 | <0.0001 | ||
| Current Smoking | 0.06 | 0.033 | 0.10 | <0.0001 |
| Diabetes | 0.13 | <0.0001 | 0.07 | 0.001 |
| Nutrition and Activity | ||||
| Waist-Hip Ratio | 0.16 | <0.0001 | 0.07 | 0.041 |
| Laboratory Values | ||||
| log NT-proBNP | 0.39 | <0.0001 | 0.14 | <0.0001 |
| log CRP | 0.14 | 0.0001 | 0.06 | 0.007 |
| log Fasting Glucose | 0.10 | 0.0001 | ||
| log BUN | 0.31 | <0.0001 | 0.07 | 0.004 |
| log CrCl | −0.42 | <0.0001 | −0.16 | <0.0001 |
| log Total Cholesterol | −0.16 | <0.0001 | ||
| log HDL | −0.08 | 0.002 | −0.09 | 0.0006 |
| log LDL | −0.14 | <0.0001 | −0.09 | <0.0001 |
Abbreviations as in Table 1.
R2 = 0.38, β = standardized regression coefficient.
GDF-15 Levels and Outcomes
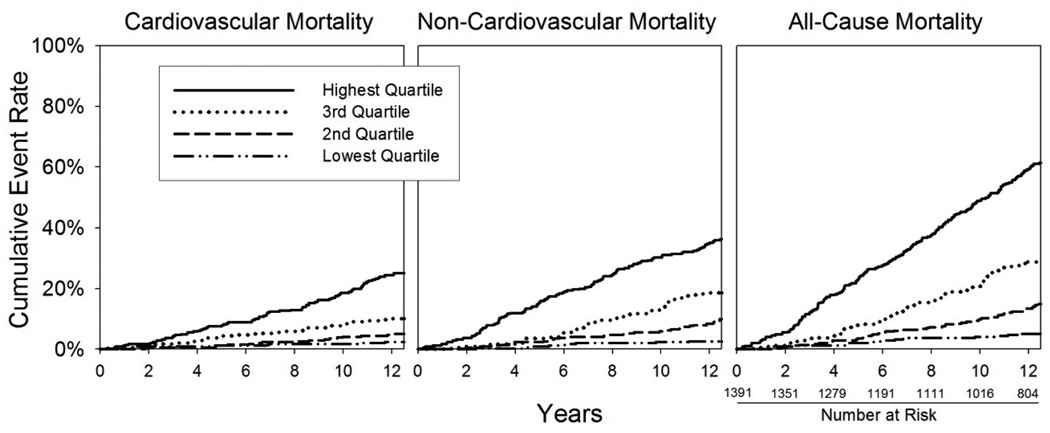
During a mean follow-up of 11.0 ± 3.7 years (maximum 16.2 years), there were 436 deaths (31%) of which 169 (39%) were cardiovascular and 108 (25%) were neoplastic. Overall, 101 participants suffered a fatal or non-fatal myocardial infarction during follow-up, and 75 underwent coronary revascularization (25 of whom also had a myocardial infarction). Figure 1 depicts Kaplan-Meier plots of cumulative cardiovascular, non-cardiovascular, and all-cause mortality by quartile of GDF-15. In each case, the time to death decreased with increasing quartile of GDF-15 (log-rank test p<0.0001 for each).
Figure 1.
Kaplan Meier curves adjusted for competing types of mortality, by GDF-15 quartile. Cut-points for GDF-15 quartiles are <962, 962–1268, 1269–1780, and >1780 ng/L. Log rank p-value <0.001 for each.
Multivariable Cox proportional hazards models were used to quantify the adjusted risk of death for each quartile of GDF-15 levels (Table 3). After adjusting for age and sex (Model 1), participants in the highest two quartiles of GDF-15 were at significantly increased risk of all-cause and of non-cardiovascular death compared with participants in the lowest quartile. Participants in the highest quartile were also at increased risk of cardiovascular death. After further adjusting for traditional CVD risk factors (Model 2), participants in the highest quartile had at least a 2.4 times increased risk of all-cause, cardiovascular, and non-cardiovascular death. Further adjusting for CrCl and BMI did not materially change the results (Model 3).
Table 3.
Multivariable Cox Proportional Hazard Models for Predicting CVD Events and Mortality by Quartile of GDF-15.
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
|---|---|---|---|---|
| (<962 ng/L) | (962–1268 ng/L) | (1268–1780 ng/L) | (>1780 ng/L) | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| MI, Coronary Revascularization, or CVD Death | ||||
| # of Events | 25 | 51 | 68 | 110 |
| Unadjusted | Reference | 1.87 (1.15–3.01) | 2.77 (1.75–4.39)** | 5.08 (3.28–7.86)** |
| Model 1 | Reference | 1.25 (0.76–2.03) | 1.39 (0.85–2.27) | 1.86 (1.12–3.06) |
| Model 2 | Reference | 1.17 (0.72–1.92) | 1.20 (0.73–1.97) | 1.61 (0.97–2.66) |
| Model 3 | Reference | 1.15 (0.70–1.88) | 1.20 (0.73–1.97) | 1.59 (0.96–2.64) |
| CVD Death | ||||
| # of Deaths | 9 | 23 | 43 | 94 |
| Unadjusted | Reference | 2.50 (1.16–5.41) | 5.24 (2.55–10.76)** | 16.92 (8.53–33.58)** |
| Model 1 | Reference | 1.31 (0.60–2.84) | 1.56 (0.74–3.27) | 3.00 (1.44–6.27)* |
| Model 2 | Reference | 1.16 (0.53–2.54) | 1.29 (0.61–2.72) | 2.42 (1.15–5.07) |
| Model 3 | Reference | 1.17 (0.53–2.55) | 1.29 (0.61–2.72) | 2.46 (1.17–5.18) |
| Non–CVD Death | ||||
| # of Deaths | 20 | 50 | 72 | 125 |
| Unadjusted | Reference | 2.42 (1.44–4.07)** | 3.84 (2.33–6.31)** | 9.91 (6.17–15.90)** |
| Model 1 | Reference | 1.52 (0.90–2.58) | 1.71 (1.02–2.88) | 2.93 (1.73–4.96)** |
| Model 2 | Reference | 1.44 (0.85–2.44) | 1.56 (0.92–2.64) | 2.61 (1.53–4.44)** |
| Model 3 | Reference | 1.46 (0.86–2.47) | 1.57 (0.93–2.65) | 2.62 (1.53–4.47)** |
| Cancer Death | ||||
| # of Deaths | 15 | 16 | 22 | 55 |
| Unadjusted | Reference | 1.05 (0.52–2.12) | 1.52 (0.78–2.95) | 5.52 (3.11–9.78)** |
| Model 1 | Reference | 0.82 (0.40–1.68) | 1.02 (0.50–2.08) | 2.86 (1.43–5.73)* |
| Model 2 | Reference | 0.79 (0.38–1.63) | 0.97 (0.47–2.00) | 2.68 (1.32–5.42)* |
| Model 3 | Reference | 0.78 (0.38–1.60) | 0.96 (0.47–1.98) | 2.55 (1.26–5.18) |
| All-Cause Death | ||||
| # of Deaths | 29 | 73 | 115 | 219 |
| Unadjusted | Reference | 2.45 (1.59–3.76)** | 4.27 (2.84–6.43)** | 12.07 (8.19–17.79)** |
| Model 1 | Reference | 1.43 (0.93–2.21) | 1.64 (1.07–2.50) | 2.94 (1.92–4.50)** |
| Model 2 | Reference | 1.32 (0.85–2.04) | 1.44 (0.94–2.21) | 2.53 (1.65–3.89)** |
| Model 3 | Reference | 1.33 (0.86–2.07) | 1.45 (0.94–2.22) | 2.56 (1.66–3.94)** |
Model 1 - Adjusted for age and sex.
Model 2 - Adjusted for Model 1 + diabetes, hypertension, and current smoking (dichotomous variables) and systolic blood pressure, total cholesterol, and HDL.
Model 3 - Adjusted for Model 2 + creatinine clearance and body mass index.
Bold values are significant at p<0.05.
values are significant at p<0.01.
values are significant at p<0.001.
We performed an additional post-hoc analysis to determine the predictive value of higher GDF-15 quartile for cancer death. GDF-15 levels were significantly associated with cancer mortality, with a HR of 1.52 per increasing quartile in the fully adjusted Model 3 (95% CI 1.20–1.93, p<0.001). As shown in Table 3, there appears to be a threshold effect for prediction of cancer death, with most of the increased risk appearing among patients with GDF-15 levels in the highest quartile.
In addition, we evaluated the combined post-hoc endpoint of coronary revascularization, myocardial infarction, or CVD death and found a significant linear trend, with an age- and sex-adjusted HR of 1.23 per increasing GDF-15 quartile (Model 1, 95% CI 1.06–1.42, p=0.007) and a fully adjusted HR of 1.17 per increasing quartile (Model 3, 95% CI 1.01–1.36, p=0.03).
Discrimination and Reclassification, and Comparison With NT-proBNP and C-Reactive Protein
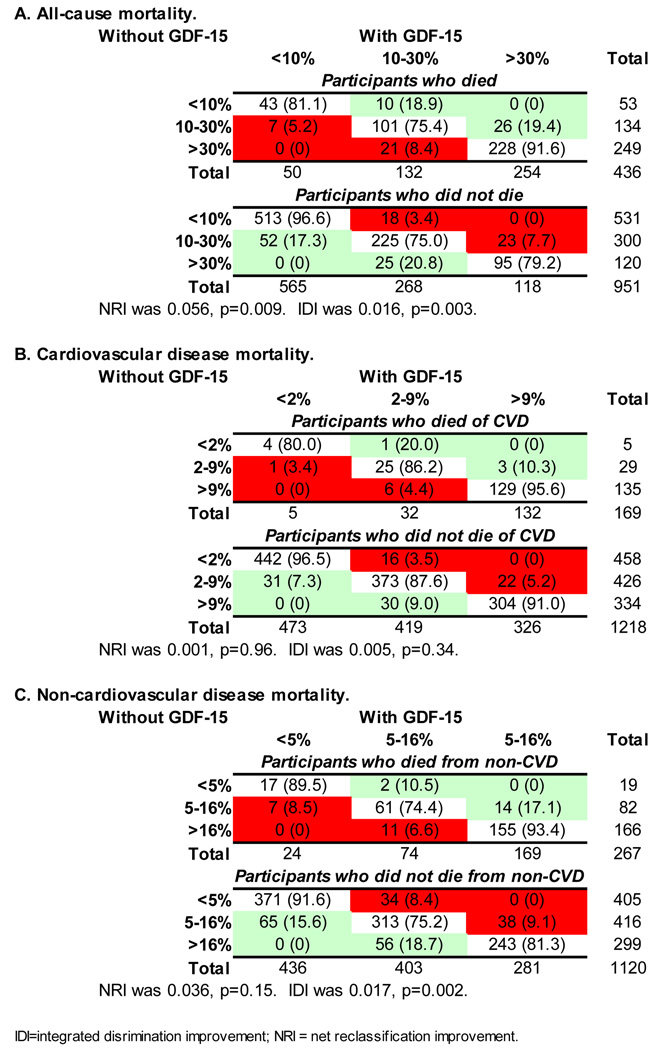
The addition of log GDF-15 to the fully adjusted model improved the area under the ROC curve (c-statistic) for prediction of all-cause mortality from 0.801 to 0.811, with a highly significant increment test in the Cox model (p<0.001). Adding log GDF-15 to a model that included log NT-proBNP and log CRP in addition to the risk factors also improved the c-statistic, from 0.806 to 0.815 (p<0.001). Reclassification as assessed by both the IDI and NRI was modestly but significantly improved with the addition of log GDF-15 to the fully adjusted model for all-cause and non-cardiovascular mortality, and improvement was of borderline significance for cardiovascular mortality based upon the categoryless NRI, but not the IDI or the NRI at the chosen cut-points (Figure 2). In the categoryless NRI, improvement in reclassification was due to both correct upward reclassification of events (net gain in reclassification of 0.13) and correct downward reclassification of non-events (net gain in reclassification of 0.17) for all-cause mortality; for cardiovascular mortality, the net gain in reclassification for events was 0.06 and for non-events was 0.11; for non-cardiovascular mortality the corresponding numbers were 0.18 and 0.16, respectively. In contrast, the addition of log NT-proBNP to the fully adjusted model improved reclassification for all-cause and for cardiovascular mortality, but not for non-cardiovascular mortality (except based on the categoryless NRI), whereas the addition of log CRP did not significantly improve the NRI nor IDI for any of the endpoints (Table 4).
Figure 2.
Reclassification based on GDF-15 levels. Individuals in the unshaded diagonal boxes did not change classification with the addition of GDF-15. Green shading indicates the number (percent) of individuals who were reclassified in a desirable direction when GDF-15 was added to the baseline model; red shading indicates individuals who were reclassified in an undesirable direction.
Table 4.
Comparison of NRI and IDI for GDF-15, NT-proBNP and CRP
| IDI | p | NRI* | p | Categoryless NRI | p | |
|---|---|---|---|---|---|---|
| All-Cause Death | ||||||
| GDF-15 | 0.016 | 0.003 | 0.056 | 0.009 | 0.296 | <0.0001 |
| NT-proBNP | 0.012 | 0.003 | 0.041 | 0.03 | 0.220 | <0.0001 |
| CRP | 0.002 | 0.12 | 0.000 | 0.99 | 0.105 | 0.07 |
| CVD Death | ||||||
| GDF-15 | 0.005 | 0.34 | 0.001 | 0.96 | 0.166 | 0.05 |
| NT-proBNP | 0.024 | 0.01 | 0.039 | 0.10 | 0.323 | <0.0001 |
| CRP | 0.005 | 0.08 | −0.016 | 0.24 | 0.056 | 0.53 |
| Non-CVD Death | ||||||
| GDF-15 | 0.017 | 0.002 | 0.036 | 0.15 | 0.333 | <0.0001 |
| NT-proBNP | 0.004 | 0.13 | 0.023 | 0.19 | 0.238 | 0.0006 |
| CRP | 0.001 | 0.48 | −0.009 | 0.50 | 0.136 | 0.05 |
Cut-points for low/high risk were <10%/>30% for all-cause death, and by tertiles of risk for CVD death (<2%/>9%) and non-CVD death (<5%/>16%).
Comparisons are to a baseline model adjusted for: age, sex, diabetes, hypertension, current smoking, systolic blood pressure, total cholesterol, HDL, CrCl and BMI.
CVD = cardiovascular disease; IDI = integrated discrimination improvement; NRI = net reclassification improvement.
Combinations of Markers
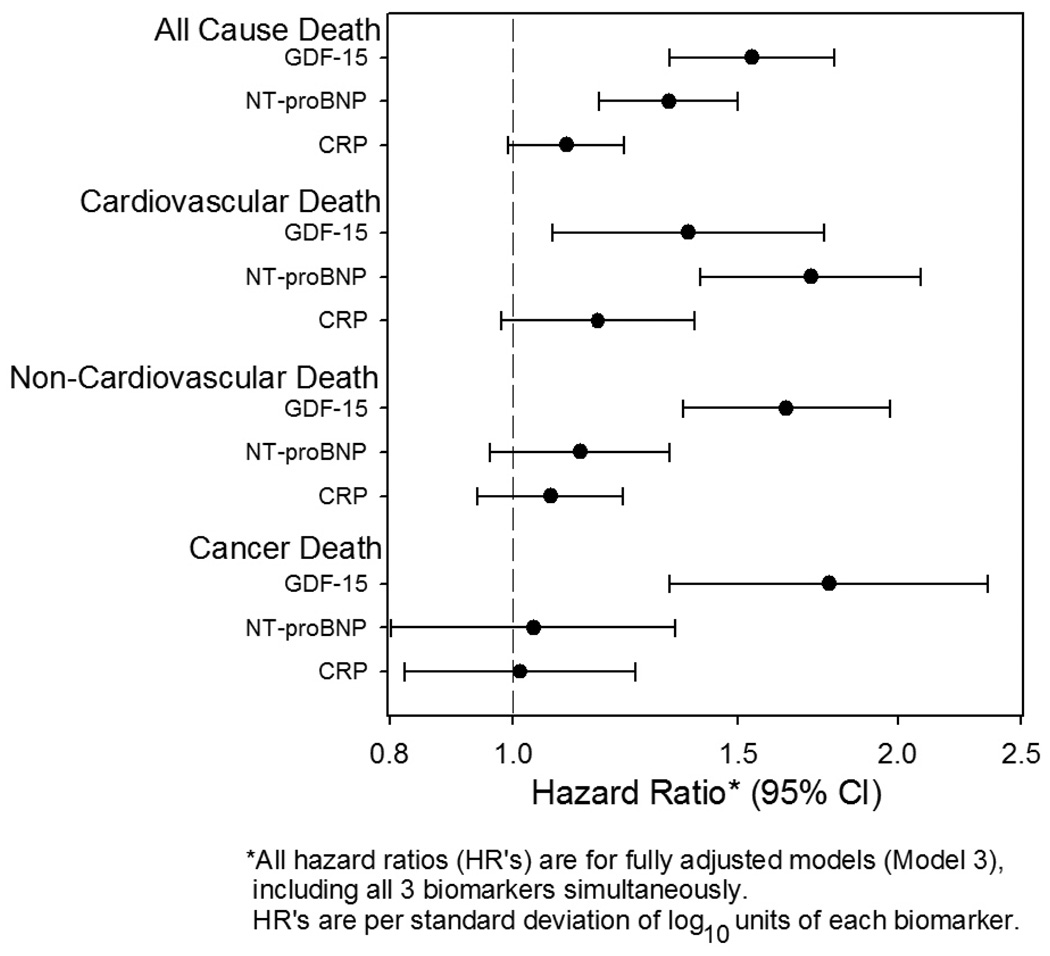
To assess the predictive value of GDF-15 in conjunction with NT-proBNP and CRP, two commonly used cardiovascular biomarkers, all three markers were included together in a multivariable Cox proportional hazards model that also included the fully adjusted Model 3 risk factors. As shown in Figure 3, both GDF-15 and NT-proBNP, but not CRP, added incremental value for prediction of all-cause and cardiovascular mortality, while only GDF-15 was independently associated with non-cardiovascular death (HR per standard deviation log10 unit [95% CI]: 1.6 [1.4–2.0], p<0.0001) and with cancer death (1.8 [1.3–2.4], p<0.0001). Based on point estimates, GDF-15 was the strongest predictor of all-cause mortality (HR 1.5 [1.3–1.8], p<0.0001 for GDF-15, versus 1.3 [1.2–1.5], p<0.0001 for NT-proBNP), while NT-proBNP was a stronger predictor of cardiovascular mortality (HR 1.7 [1.4–2.1] for NT-proBNP, vs. 1.4 [1.1–1.8] for GDF-15), though CI’s showed considerable overlap.
Figure 3.
Forest plot of adjusted hazard ratio and 95% confidence interval (CI) for risk of death per 1-standard deviation increase in log10GDF-15, log10NT-proBNP, and log10CRP level.
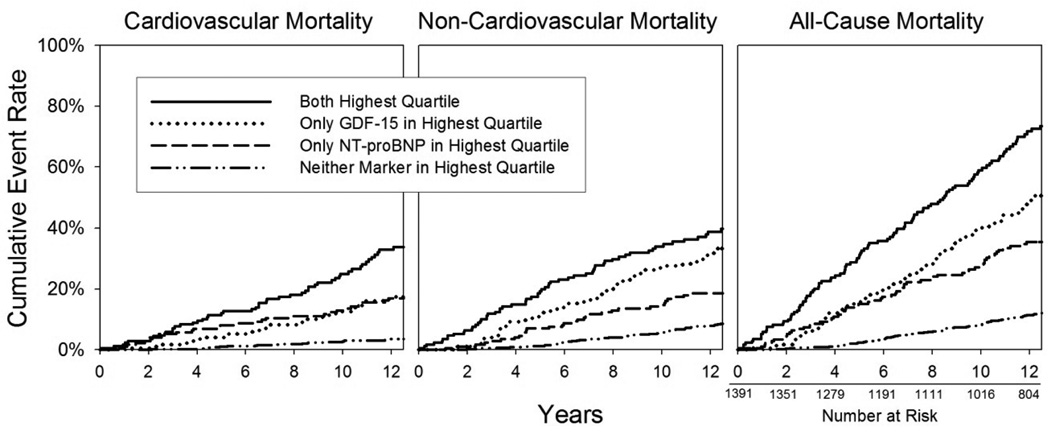
Next, participants were stratified into those in the highest quartile of both GDF-15 and NT-proBNP (n=171), those with elevated GDF-15 alone (n=176), those with elevated NT-proBNP alone (n=174), and those with neither marker in the top quartile (n=863). In fully adjusted models, participants with both GDF-15 and NT-proBNP levels in the highest quartile had a significantly increased risk of all-cause mortality compared to those with only elevated NT-proBNP (HR 1.5 [1.1–2.0], p=0.01), and compared to those with neither marker in the top quartile (HR 2.6 [2.0–3.5], p<0.0001). Participants with only one marker elevated had an intermediate risk of mortality which was still significantly higher than that observed in participants with neither marker in the top quartile (adjusted HR 1.8 [1.3–2.4], p<0.0001 for elevated NT-proBNP, and 2.0 [1.5–2.7], p<0.0001 for elevated GDF-15). A Kaplan Meier plot was also constructed, and showed that participants with neither an elevated GDF-15 nor NT-proBNP level were highly unlikely to die from CVD during the following 11 years (Figure 4). The pattern was similar for all-cause mortality and for non-cardiovascular death.
Figure 4.
Kaplan Meier curves adjusted for competing type of mortality, based on GDF-15 and NT-proBNP levels. Four groups are compared: those with both GDF-15 and NT-proBNP in the highest quartile (n=171; 56 cardiovascular, 64 non-cardiovascular deaths), those with only GDF-15 in the highest quartile (n=176; 38 cardiovascular, 61 non-cardiovascular deaths), those with only NT-proBNP in the highest quartile (n=174; 35 cardiovascular, 41 non-cardiovascular deaths), and those with neither marker elevated (n=863; 39 cardiovascular, 101 non-cardiovascular deaths). Three participants did not have NT-proBNP measured. Log rank p-value <0.001 for each.
DISCUSSION
Our study demonstrates that higher levels of GDF-15 are independently associated with an increased risk of all-cause mortality, and of both cardiovascular and non-cardiovascular death, in a cohort of older community-dwelling adults with no antecedent clinical CVD. We also provide novel evidence that GDF-15 adds significantly to the predictive value of NT-proBNP and CRP. To our knowledge, this is the largest study of community-dwelling individuals to report the clinical factors associated with GDF-15, and the first longitudinal community-based study to report the prognostic value of GDF-15.
Despite the covariance of GDF-15 with multiple traditional CVD risk factors and biomarkers (which accounted for 38% of the variability in GDF-15 levels, based on the model R2 value), GDF-15 remained a predictor of mortality even after adjusting for traditional risk factors, CRP, and NT-proBNP. In addition, GDF-15 was a stronger predictor of all-cause mortality than either NT-proBNP or CRP, and was the only one of the three markers to predict non-cardiovascular mortality.
We found that GDF-15 levels were independently and positively associated with age, male sex, reduced kidney function, current smoking, diabetes, and waist-hip ratio; and inversely associated with HDL and LDL levels. These results are remarkably similar to the associations found in the PIVUS study of 1004 community-based 70-year-olds from Uppsala, Sweden,14 and in a study of patients from GUSTO-IV with non-ST elevation myocardial infarction. 12 Like many CVD risk factors, GDF-15 had a relatively strong association with age, which suggests that GDF-15 is upregulated by physiologic processes (either normal or disease-related) associated with aging; nonetheless, levels remained predictive even in age-adjusted analyses. Despite the positive association with traditional risk factors in our study, GDF-15 associations with mortality were independent of these risk factors. The association of GDF-15 levels with reduced renal function could reflect a combination of altered renal clearance of GDF-15 along with increased expression in the setting of renal dysfunction, as has been shown in animal models of kidney injury.25 GDF-15 levels are also correlated with NT-proBNP and CRP levels, yet remain predictive of mortality independent of these two markers. The association with NT-proBNP levels indicates that GDF-15 expression may be induced by myocardial strain, and is consistent with animal models demonstrating an upregulation of GDF-15 in the setting of myocardial ischemia and pressure overload states.3, 6 GDF-15 was only weakly associated with CRP in our participants (adjusted rho 0.06), suggesting that while GDF-15 may be associated with inflammation, other stronger influences are likely.
Previously, a nested case-control analysis from the Women’s Health Study found that GDF-15 levels were higher at baseline in 257 apparently healthy older women who subsequently had cardiovascular events, compared with 257 matched controls.15 They also found the association was at least additive to CRP, although limitations of the case-control design may weaken some of these findings. In the PIVUS cross-sectional study, investigators found that GDF-15 levels were independently associated with clinical manifestations of coronary artery disease and heart failure.14 However, because PIVUS assessed GDF-15 levels and disease status concurrently, the temporal relationship could not be assessed. The fact that we demonstrated that elevated levels of GDF-15 in individuals without known CVD are predictive of mortality, and that the predictive value persists even a decade later, supports the hypothesis that GDF-15 is not merely a consequence of CVD nor a passive biomarker of the disease process, but may in fact play an active role in the pathophysiology of CVD.
In addition, since the prognostic value of GDF-15 was independent of both NT-proBNP and CRP and not specific to cardiovascular death, our results suggest that GDF-15 may reflect more than just cardiomyocyte strain and inflammation. Mouse models demonstrate that GDF-15 is expressed in the setting of cardiac ischemia, where it exerts a protective effect, limiting infarct size, myocyte apoptosis, and hypertrophy.3, 6 In the present study of community-dwelling, relatively healthy participants, it seems unlikely that a large number had silent ischemia, and it is unclear exactly what the elevated GDF-15 levels are reflecting in these individuals. Previous studies have shown that elevated GDF-15 levels are associated with reduced endothelium-dependent vasodilation in the microcirculation.14 In addition, GDF-15 levels may in part reflect atherosclerotic burden,14 as GDF-15 is expressed in human atherosclerotic plaque activated macrophages.7 It is noteworthy, however, that GDF-15 levels were more strongly associated with mortal events than with outcomes encompassing non-fatal coronary events. This is consistent with previous studies of GDF-15 levels in the setting of acute coronary syndromes, which also found a stronger association with mortality than with recurrent myocardial infarction.12, 26, 27 Elevations of GDF-15 may therefore reflect triggers from abnormalities in novel pathophysiologic pathways.
The strong relation of GDF-15 with non-cardiovascular death, an association not seen with NT-proBNP and CRP, suggests additional pathophysiologic mechanisms for GDF-15 expression and action. In post hoc analyses undertaken to evaluate this relation, we found a significant association between GDF-15 levels and increased risk of death from cancer, especially among those with levels in the highest quartile. GDF-15 is expressed in a number of aggressive malignancies including pancreatic,28 breast, and ovarian cancers,29 and it has been associated with tumorigenicity and worse prognosis in a variety of cancers including prostate,8 colorectal,30 and gastric cancers,31 melanoma,9 and glioblastoma.32 Although generally considered to be antitumorigenic as an inducer of apoptosis via both p53-dependent and p53-independent pathways,33 GDF-15 plays a complicated pathophysiologic role, and also may modulate tumor progression and invasiveness.10 GDF-15 expression is also increased in the settings of inflammation and tissue injury.10 The strong association between GDF-15 levels and non-cardiovascular mortality seen in the present study may reflect some of these triggers. Misclassification of cause of death is high in the elderly, which could partly explain the non-cardiovascular association.
Many cardiovascular risk prediction models that are based on traditional risk factors, including the Framingham Risk Score, show decreased predictive value in older individuals,34 yet identification of risk and preventive treatment in the elderly is still important.35 As a robust predictor of risk in older adults, GDF-15 has the possibility of improving prevention strategies for this growing population if confirmed in other cohorts.
The ultimate aim of any method of risk stratification is to identify individuals at high (and low) risk so that appropriate interventions may be undertaken to modify this risk. As described in recent guidelines for evaluation of novel markers of cardiovascular risk,36 risk stratification and the subsequent development of therapeutic interventions or methods of prevention are intricately related, both comprising integral parts of the evaluation of a novel risk marker. As GDF-15 is a relatively novel marker with little prospective clinical data (especially in the community-based setting), the aims of the present manuscript were to define the determinants of GDF-15 levels as well as their prognostic value in this population, and to assess the potential usefulness of GDF-15 for risk stratification. GDF-15 may ultimately be a worthy target for therapeutic interventions to prevent cardiovascular events, when the mechanism of action is clarified and predictive associations are confirmed in other cohorts.
Study Strengths and Limitations
Significant strengths of this study include the well-characterized, population-based sample of older adults, and the high rate of long-term follow-up. There are also limitations. The Rancho Bernardo Study population is largely white and middle to upper-middle class, therefore, these results may not be generalizable to other populations; however, this limitation is a strength to the extent that it limits confounding by socioeconomic status and access to health care.
Another limitation is the possible misclassification of prevalent CVD. Echocardiograms were not available, and participants with unreported angina and/or undiagnosed heart failure may be included in the study cohort. Prevalent CVD was based on self-report of a revascularization procedure or a physician diagnosis of CVD. However, the long-term participation in the Rancho Bernardo Study plus the relatively high education level among study participants, tend to improve health literacy and the reliability of self-reports. We confirmed 85% of reported cardiovascular events by medical record reviews of a 30% subset at an earlier Rancho Bernardo Study visit. In addition, the incidence plots continued to separate even a decade later, suggesting that the results are less likely to reflect occult disease.
Blood samples were stored for 14–18 years prior to measurement of GDF-15 levels, which raises questions about stability of the analyte. Although the long-term stability is difficult to assess directly, the fact GDF-15 levels were prognostic of outcomes argues that there is sufficient stability to preserve a clinical signal. It seems unlikely that there would be differential degradation of antigen associated with different participant outcomes, thereby creating a clinical signal where there was not one originally. However, the question of stability could still raise concerns of whether the particular cut-points identified would be the same in fresher samples. If some decay had occurred, this would lower the specific values identified.
Finally, while absolute values of improvement in model discrimination and reclassification were modest, they were nonetheless significant. Beyond showing improved risk prediction, another novel aspect of this study is its potential to provide insight into new pathophysiologic pathways.
Conclusion
GDF-15 levels are associated with increased all-cause, cardiovascular, and non-cardiovascular mortality among community-dwelling older individuals free from prior known CVD, adding incremental information to traditional risk factors and to NT-proBNP and CRP. This emerging biomarker may be a useful addition to current tools for risk stratification, if results are confirmed in other cohorts. The appropriate intervention for individuals with elevated levels of a marker of both CVD and cancer mortality is uncertain, but elevated GDF-15 levels could provide individuals with an incentive to make healthier lifestyle choices, which have beneficial effects for both.
Clinical Summary.
The goal of risk stratification for primary prevention of cardiovascular disease (CVD) is to identify individuals who may be candidates for interventions that could improve outcomes. Current risk stratification tools remain imperfect, and biomarkers that reflect novel pathophysiologic pathways could improve risk assessment as well as provide insight into potential therapeutic targets. Growth differentiation factor-15 (GDF-15) is a divergent member of the transforming growth factor-β cytokine superfamily that is upregulated in the myocardium after ischemic injury. Previous community-based studies have shown that higher levels of GDF-15 are associated with prevalent CVD. The present study of older community-dwelling adults free of known CVD from the Rancho Bernardo Study evaluated the association of GDF-15 levels with cardiovascular outcomes and mortality, and found that GDF-15 was a robust predictor of all-cause, cardiovascular, and non-cardiovascular mortality, even after adjusting for traditional CVD risk factors, renal function, and body size. In models containing all 3 markers, both GDF-15 and NT-proBNP, but not CRP, added incremental value for prediction of cardiovascular and all-cause mortality. When associations are confirmed in other cohorts, and when further studies clarify the mechanism of action, GDF-15 may ultimately be a worthy target for therapeutic interventions to prevent cardiovascular and all-cause death.
Acknowledgments
FUNDING SOURCES:
The Rancho Bernardo Study was funded by research grants AG07181 and AG028507 from the National Institute on Aging, and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also supported by grants from the American Heart Association (LBD & GAL) and the Sandra Daugherty Foundation (GAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
ASM and LBD have received research grants from Alere Inc. and Roche Diagnostics; there are no other conflicts to report.
REFERENCES
- 1.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 1999;65:2–5. doi: 10.1002/jlb.65.1.2. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 4.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, de Jesus GM, Wellington S, Knowles JA, Warburton D, Brown S, Soares MB. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203:17–26. doi: 10.1016/s0378-1119(97)00485-x. [DOI] [PubMed] [Google Scholar]
- 6.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 7.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Gronberg H, Breit SN, Wiklund FE. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin. Cancer Res. 2009;15:6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle GM, Pedley J, Martyn AC, Banducci KJ, Strutton GM, Brown DA, Breit SN, Parsons PG. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J. Invest. Dermatol. 2009;129:383–391. doi: 10.1038/jid.2008.270. [DOI] [PubMed] [Google Scholar]
- 10.Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, Breit SN. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 11.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 12.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 13.Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur. Heart J. 2007;28:2858–2865. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 14.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur. Heart J. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 15.Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 16.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev. Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.Vervoort G, Willems HL, Wetzels JF. Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation. Nephrol. Dial. Transplant. 2002;17:1909–1913. doi: 10.1093/ndt/17.11.1909. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 21.Kremers WK. Concordance for survival time data: fixed and time-dependent covariates and possible ties in predictor and time. Technical Report Series #80. 2007 Retrieved at http://mayoresearch.mayo.edu/biostat/upload/80.pdf.
- 22.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock. 2005;23:543–548. [PubMed] [Google Scholar]
- 26.Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur. Heart J. 2009;30:1057–1065. doi: 10.1093/eurheartj/ehn600. [DOI] [PubMed] [Google Scholar]
- 27.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L, Wollert KC. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler. Thromb. Vasc. Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 28.Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban RH, Breit SN, Kinzler KW, Vogelstein B, Goggins M. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin. Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 29.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson HF, Jr., Hampton GM. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin. Cancer Res. 2003;9:2642–2650. [PubMed] [Google Scholar]
- 31.Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH, Shin SM, Song KS, Lee YH, Kim YJ, Lee JJ, Choi I, Lee JH. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 2003;63:4648–4655. [PubMed] [Google Scholar]
- 32.Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, Schluep M, Ostermann S, Hamou MF, Stupp R, Breit SN, de Tribolet N, Hegi ME. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int. J. Cancer. 2009;125:2624–2630. doi: 10.1002/ijc.24639. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari N, Pfeffer U, Dell'Eva R, Ambrosini C, Noonan DM, Albini A. The transforming growth factor-beta family members bone morphogenetic protein-2 and macrophage inhibitory cytokine-1 as mediators of the antiangiogenic activity of N-(4-hydroxyphenyl)retinamide. Clin. Cancer Res. 2005;11:4610–4619. doi: 10.1158/1078-0432.CCR-04-2210. [DOI] [PubMed] [Google Scholar]
- 34.de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannel WB, D'Agostino RB. The Importance of Cardiovascular Risk Factors in the Elderly. The American journal of geriatric cardiology. 1995;4:10–23. [PubMed] [Google Scholar]
- 36.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr., Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC, Jr., Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]