Abstract
Background.
Recently, subclinical aspiration has been identified in approximately 30% of community-dwelling older adults. Given that the tongue is a key component of the safe swallow, we hypothesized healthy older adults who aspirate will generate less tongue strength than adults who do not aspirate. Furthermore, as muscle weakness may reflect a global effect of aging, we further investigated whether tongue strength is correlated with handgrip strength.
Methods.
We assessed 78 healthy community-dwelling older adults (M = 77.3 years, SD = 7.26) for aspiration status (37% aspirators) via flexible endoscopic evaluation of swallowing. Maximal isometric anterior and posterior tongue strength, anterior and posterior swallowing tongue strength, and maximum handgrip strength were measured.
Results.
Isometric tongue strength was significantly lower in aspirators versus nonaspirators (p = .03) at both the anterior (463 vs 548 mmHg, respectively) and posterior lingual locations (285 vs 370 mmHg, respectively). Likewise, swallowing tongue strength was significantly lower in aspirators versus nonaspirators at both the anterior (270 vs 317 mmHg, respectively) and posterior lingual locations (220 vs 267 mmHg, respectively). There was no difference between aspirators and nonaspirators’ handgrip strength (p > .05), although handgrip strength was correlated with posterior tongue strength (r = .34, p = .005).
Conclusions.
Lower anterior and posterior isometric and swallowing tongue strength were dependent on aspiration status. Lower lingual strength in healthy adults may predispose them to aspiration. The correlation between tongue and handgrip strength is consistent with the hypothesis that impaired oropharyngeal strength reflects global age-related declines in muscle strength.
Keywords: Swallowing, Tongue, Handgrip, Aspiration, Healthy
WE recently observed an approximate 30% incidence of aspiration in a cohort of healthy older adults (ie, >65 years of age) compared with 0% incidence in young adults during a flexible endoscopic evaluation of swallowing (FEES). Furthermore, the majority of the aspiration episodes were silent (ie, no cough reflex) (1–3). The physiologic reason for aspiration in healthy older adults is unknown.
We also found that pharyngeal peak pressure (ie, strength) was significantly lower in healthy adult aspirators versus nonaspirators (4). Low pharyngeal strength is only one of the components comprising the pathophysiological changes found in those who aspirate. Given that lingual bolus propulsion is one of the key components of the safe swallow (5–10), tongue strength may be a likely indicator of pharyngeal pathophysiology in healthy older adults as well. It has been previously demonstrated that older adults generate less isometric (11–16) but not swallowing (11,13–15) tongue pressures than young adults. We hypothesized healthy older adults who aspirate would have less isometric tongue strength than healthy older adults who do not aspirate. Furthermore, as progressive skeletal muscle weakness is one of the global manifestations of aging, we hypothesized that tongue strength would be correlated with handgrip strength. The purpose of this study was to independently assess tongue (ie, anterior and posterior) and handgrip strength as a function of aspiration status (ie, nonaspirator vs aspirator) and to determine the correlation between tongue and handgrip strength.
METHODS
Participants
Seventy-eight community-dwelling adults were enrolled into the study via advertisement (see Table 1). However, two endoscopic swallowing study digitized files were corrupted and not obtainable, and tongue strength was not acquired on three participants due to equipment malfunction. Handgrip strength was not acquired on six participants due to arthritis, hand pain, or hand surgery in the 3 months prior to testing. Thus, there were 73 complete data sets for the tongue strength and aspiration analyses and 70 for the handgrip and tongue strength analyses. Participants reported a negative history of swallowing, speech, and voice problems and known otolaryngologic, end-stage pulmonary, or neurological disease. All participants were ambulatory and in good health. Participants were recruited by bulletins approved by the Wake Forest University Health Sciences Institutional Review Board. Informed consent was obtained.
Table 1.
Demographics Characteristics of Study Participants as a Function of Aspiration Status
| Aspirator n (%) |
Nonaspirator n (%) |
|||||
| All | Female | Male | All | Female | Male | |
| Total | 28 (38.4) | 13 (46.4) | 15 (53.6) | 45(62.0) | 22 (48.9) | 23 (51.1) |
| Age (y) | ||||||
| 61–70 | 5 (17.9) | 2 (15.4) | 3 (20.0) | 13 (26.9) | 7 (31.8) | 6 (26.1) |
| 71–80 | 9 (32.1) | 3 (23.1) | 6 (40.0) | 16 (32.7) | 7 (31.8) | 9 (39.1) |
| 81–90 | 14 (50.0) | 8 (61.5) | 6 (40.0) | 16 (40.4) | 8 (36.4) | 8 (34.8) |
| Race | ||||||
| Caucasian | 26 (93.0) | 11 (84.6) | 15 (100) | 36 (80.0) | 17 (72.3) | 19 (82.6) |
| African American | 2 (7.1) | 2 (15.4) | 0 (0) | 8 (17.8) | 4 (18.2) | 4 (17.4) |
| Other (Middle East) | 0 (0) | 0 (0) | 0 (0) | 1 (2.2) | 1 (4.5) | 0 (0) |
| Weight (kg) (M/SD) | 78.3 (23.3) | 66.9 (9.8) | 88.3 (27.2) | 80.4 (18.9) | 69.7 (15.4) | 92.7 (15.5) |
| BMI (M/SD) | 26.8 (3.3) | 27.3 (4.3) | 26.4 (2.3) | 28.3 (5.2) | 27.2 (5.6) | 29.6 (4.7) |
| Total MMSE Score (M/SD) | 27.0 (2.5) | 26.5 (2.8) | 27.3 (2.4) | 27.1 (2.9) | 27.5 (2.2) | 26.8 (3.5) |
| Smoking | ||||||
| Never | 15 (53.6) | 10 (76.9) | 5 (33.3) | 17 (37.8) | 10 (45.5) | 7 (30.4) |
| Current | 3 (10.7) | 0 (0) | 3 (20.0) | 1 (2.2) | 0 (0) | 1 (4.3) |
| Former | 10 (35.7) | 3 (23.1) | 7 (47.70) | 27 (60.0) | 12 (54.5) | 15 (65.2) |
| Intubation in last 3 mo | ||||||
| Yes | 1 (3.6) | 1 (7.7) | 0 (0) | 1 (2.2) | 1 (4.5) | 0 (0) |
| No | 27 (96.4) | 12 (92.3) | 15 (100) | 44 (97.8) | 21 (95.5) | 23 (100) |
| Lung disease (eg, COPD, emphysema) | ||||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No | 28 (100) | 13 (100) | 15 (100) | 45 (100) | 22 (100) | 23 (100) |
| Treated for cancer in last 3 mo | ||||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No | 28(100) | 7 (100) | 14 (100) | 45 (100) | 28 (100) | 24 (100) |
| Medication number* | ||||||
| None | 4 (14.8) | 3 (27.3) | 1 (6.0) | 4 (9.3) | 1 (4.3) | 3 (15.0) |
| 1–3 | 10 (37.0) | 3 (27.3) | 7 (25.0) | 19 (44.2) | 13 (56.5) | 6 (30.0) |
| 4–6 | 10 (37.0) | 4 (36.4) | 6 (43.8) | 11 (25.6) | 5 (21.7) | 6 (30.0) |
| >6 | 3 (11.1) | 1 (9.1) | 2 (12.5) | 9 (20.9) | 4 (17.4) | 5 (25.0) |
Notes: BMI = body mass index; COPD = Chronic Obstructive Pulmonary Disease; MMSE = Mini-Mental State Examination.
Medication data were missing for three participants.
Determining Aspiration Status
A KayPENTAX Swallowing Workstation (KayPENTAX, Inc., Lincoln Park, NJ) was utilized for the endoscopic swallowing examinations, as described previously (1–3). Participants underwent FEES while sitting in the upright position. A 3.1-mm digital flexible endoscope was lubricated with Surgilube (Altana Inc., Melville, NY) and passed transnasally, typically on the floor of the nose, by the first author to obtain a superior view of the hypopharynx. The endoscope was moved throughout the study between swallowing and post-swallow positions to collect the data.
Swallowing position required that the distal end of the endoscope was just above the top of the epiglottis so that the entire base of tongue, the tip of the epiglottis, posterior pharyngeal wall, lateral pharyngeal walls (eg, lateral channels), and laryngeal vestibule were visualized prior to bolus administration. The endoscope was maintained in swallowing position throughout bolus administration and was moved only for the few seconds during which the scope was advanced to post-swallow position following a bolus presentation. To obtain post-swallow position, the distal end of the scope was advanced lower into the pharynx, past the tip of the epiglottis, and into the upper portion of the laryngeal vestibule where the glottis and trachea below could be well visualized. Post-swallow position was only held long enough to determine the Penetration Aspiration Scale (PAS) score and then the scope was pulled back into swallowing position.
Nine conditions (5, 10, 15, 20 mL of water, skim milk, 2% milk and whole milk via cup and 5, 10, 15, 20 mL of water, skim milk, 2% milk, whole milk, and soy milk via straw) were studied to determine aspiration status. Thus, each participant contributed 36 swallows for determination of aspiration status. At the beginning of the study, participants were instructed to swallow the water or milk when they were ready. All boluses were dyed with green food coloring to offer better endoscopic visualization.
Swallows were reviewed in real time, slow motion, and frame-by-frame to assign the corresponding PAS score in accordance with previously published methods (1–3,17,18). Higher PAS scores reflect more abnormal swallows. A PAS score of 1 is a normal swallow with no material in the airway, scores between 2 and 5 indicate that material entered the laryngeal vestibule (ie, penetration), and scores of 6–8 indicate that material passed below the vocal cords into the trachea (ie, aspiration). Aspirated swallows were also analyzed as to whether the aspiration occurred before, during, or after the swallow. Aspiration before the swallow was operationally defined as visualization of the bolus entering into the trachea (below the level of the true vocal folds) prior to the whiteout/obscured image associated with the swallow. Aspiration during the swallow was defined as visualization of the bolus in the trachea immediate to the completion of whiteout/obscured image that was not present prior to the whiteout. Aspiration after the swallow was defined as observed entry of penetrated material or residue into the trachea after the swallow. Other signs of aspiration (eg, wet voice) were not evaluated.
Tongue Strength
The same KayPENTAX Swallowing Workstation (KayPENTAX, Inc.) was utilized for the lingual pressure acquisition. Lingual pressure was measured from three small air-filled bulbs (KayPENTAX Inc.) similar to that previously described (19). The examiner positioned the bulb strip anteriorly to posteriorly along the midline of the hard palate of each participant. The examiner assessed correct strip placement between each trial.
Isometric tongue strength.—
Participants were instructed to “press your entire tongue against the roof of your mouth as hard as you possibly can when I say go.” Once, the examiner instructed the participant to “go,” the examiner immediately coached the participant, “press, press, press, okay and rest.” A 30-second rest period was provided, and the sequence was repeated two additional times for a total of three trials.
Swallowing tongue strength.—
Participants were instructed to swallow with the bulb array on their tongue when they were ready. Three trials were obtained with a 1-minute time lapse between the previous swallow and the cue to swallow for the next trial.
Peak pressures (millimeters of mercury) as a function of time for the anterior and posterior bulbs only were measured off-line. Data from the middle bulb were not analyzed given that data acquired from the middle bulb are highly variable across participants (20), given varying palatal arch height between participants.
Reproducibility of tongue strength measures.—
Four separate one-factor repeated measures analysis of variance were undertaken to examine the effect of trial (n = 3) on anterior and posterior isometric and swallowing tongue strength measures. There was no effect of trial on both anterior and posterior swallowing or anterior isometric tongue strength measures (p > .05). To examine the source of the significant effect of trial on posterior tongue strength (p = .004), two orthogonal single-df contrasts were undertaken. Trials one and three were not statistically different than each other; they were, however, significantly higher than the second trial (p < .0001). Considering the effect of trial was negligible on all swallowing strength measures, the three trials across measures were averaged for all further analyses.
Handgrip Strength
An adjustable handheld dynamometer (Jamar Hydraulic Hand Dynamometer, Model No. BK-7498; Fred Sammons, Inc., Burr Ridge, IL) was used to obtain handgrip strength measurements. An examiner, blinded to aspiration status, measured grip strength in both hands. Two trials with brief pauses were conducted for each hand. If a person reported current flare-up of pain in the wrist or hand or had undergone surgery of the upper extremity in the past 3 months, the affected hand was not tested and results of the other hand was used, if applicable. Handgrip strength was not acquired on three participants (ie, nonaspirators) due to arthritis, hand pain, or hand surgery in the 3 months prior to testing. The maximum pressure generated between the left and right hands were used in analyses. Test–retest reliability for grip strength has been shown to be high [eg, intraclass correlation coefficients of .96 and .91 for the left and right hands, respectively (21)].
Statistical Analysis
Means and SEs were calculated for tongue strength by aspiration status and bulb locations and handgrip strength by aspiration status. Repeated measures analysis of covariance (ANCOVA) was performed to investigate isometric tongue strength as a function of aspiration status, bulb location, adjusting for age and gender. Least squared means of tongue strength by aspiration status and bulb location were estimated from the same repeated measures ANCOVA model. ANCOVA was used to compare handgrip strength by aspiration status, adjusting for age and gender. Finally, Pearson partial correlation coefficients were calculated among handgrip strength and tongue strength, adjusting for age and gender. Significance level was set at 0.05 for all analyses.
RESULTS
Aspiration Status
We evaluated 2,628 swallows across the 73 participants. Participants’ demographic characteristics are presented in Table 1. Aspiration (ie, a PAS score ≥ 6) was observed during 91 swallows (3.5%) across 28 aspirators (38% of participants). PAS scores of 6, 7, and 8 were observed on 3%, 38%, and 58%, respectively, of the aspirated swallows. Twelve participants aspirated more than once. With respect to the timing of the aspiration events, 7 occurred before, 82 during, and 1 after the swallow. There were no instances of aspiration occurring in more than one category (eg, aspiration before and during the swallow). There were significantly more episodes of aspiration with milk versus water boluses (3).
Reproducibility of aspiration status was evaluated approximately 1 year following the original endoscopic swallowing evaluation. Nine previously identified aspirators and nonaspirators, respectively, participated in an endoscopic swallowing examination that consisted of 20 mL liquid boluses comprised of 10 water and 10 milk swallows divided equally by cup and straw. All 18 (100%) participants maintained their previously identified aspiration/nonaspiration status, indicating excellent reproducibility of aspiration status.
Isometric Tongue Strength
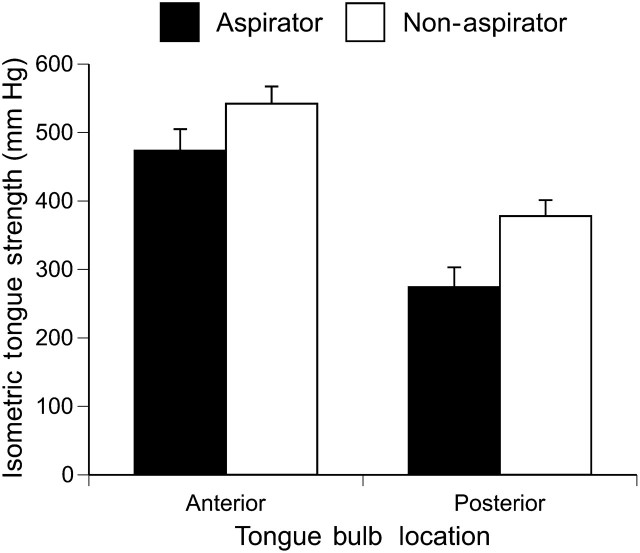
The unadjusted mean anterior and posterior tongue strengths by aspiration status, age, and gender are presented in Table 2. From the ANCOVA model, significant main effects of isometric tongue strength location and aspiration status were found such that anterior yielded greater pressure than posterior tongue (506.07 vs 328.34 mmHg, p < .0001). Aspirators had significantly less tongue strength than the nonaspirators at both the anterior (463.72 vs 548.42 mmHg) and posterior (285.99 vs 370.69 mmHg) bulb locations (p = .01; see Figure 1), after adjusting for other covariates in the model.
Table 2.
Unadjusted Mean Grip, Anterior and Posterior Isometric Tongue Strength as a Function of Age, Gender, and Aspiration Status (M [SE])
| Grip Strength (kg) | Anterior Tongue Strength (mmHg) | Posterior Tongue Strength (mmHg) | |
| Age | |||
| 61–70 y | 35.00 (2.68) | 549.57 (30.62) | 361.31 (44.28) |
| 71–80 y | 33.96 (2.67) | 462.44 (38.03) | 344.40 (33.84) |
| 81–90 y | 27.09 (1.92) | 540.14 (30.28) | 318.99 (25.93) |
| Gender | |||
| Female | 22.09 (1.09) | 535.11 (29.55) | 330.58 (25.21) |
| Male | 40.28 (1.43) | 498.12 (26.64) | 345.08 (28.40) |
| Aspiration status | |||
| Aspirator | 30.46 (2.32) | 473.56 (31.40) | 274.19 (28.86) |
| Nonaspirator | 31.83 (1.84) | 542.18 (24.98) | 377.91 (23.34) |
Figure 1.
Mean isometric tongue strength (millimeters of mercury) as a function of aspiration status and tongue bulb location. Error bars represent ±1 SEM.
Swallowing Tongue Strength
The unadjusted mean anterior and posterior tongue strengths by aspiration status, age, and gender are presented in Table 3. From the ANCOVA model, a significant main effect of swallowing tongue strength location was found such that anterior yielded greater pressure than posterior tongue strength (293.9 vs 243.7 mmHg, p = .0008) after adjusting for other covariates in the model. Aspirators had significantly less swallowing tongue strength than the nonaspirators at both the anterior (270.6 vs 317.2 mmHg) and posterior (220.4 vs 267.0 mmHg) bulb locations (p = .04), after adjusting for other covariates in the model. Females had higher swallowing tongue strength than male (291.5 vs 246.1 mmHg, p = .04). There was no interaction between bulb location and aspiration status.
Handgrip Strength
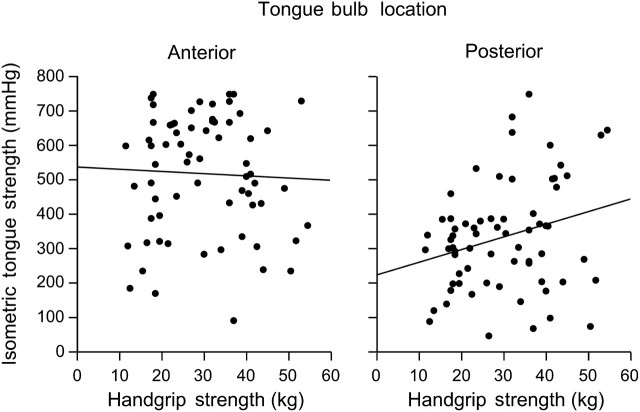
The unadjusted mean handgrip strength by aspiration status, age, and gender are presented in Table 2. In the ANCOVA model, as expected, handgrip strength was lower with advanced age (β = −.52, p < .001) and in women (21.9 kg) compared with men (40.2 kg) (p < .001). After adjusting for age and gender, handgrip strength did not differ between aspirators (30.7 kg) and nonaspirators (31.4 kg, p = .71). The relationship between isometric tongue strength and handgrip strength was examined with partial correlation analysis. Though handgrip strength was not dependent on aspiration status, it was moderately and significantly correlated with posterior (r = .34, p = .005) but not anterior (r = .02, p = .86) isometric tongue strength after adjusting for age and gender as depicted in Figure 2.
Figure 2.
Bivariate scatterplots and regression lines for isometric tongue strength (millimeters of mercury) and maximum handgrip strength (kilopascal) as a function of tongue position.
DISCUSSION
Aspiration was identified in 38% of healthy community-dwelling older adults. Aspirators generated significantly less anterior and posterior isometric and swallowing tongue strength compared with healthy nonaspirators. Although handgrip strength did not differ as a function of aspiration status, there was a significant positive association between posterior tongue strength and handgrip strength.
A decline in isometric tongue strength with aging has been previously reported (11–16). Young and older adults, however, generate similar lingual pressures during swallowing (11,14,15). Because young and older adults swallow with similar tongue pressures, it is reasonable to hypothesize lower isometric tongue strength may not be a risk factor for dysphagia, but rather simply reflects normal effects of aging. However, it has been suggested that lower isometric tongue strength might represent diminished functional reserve, which may increase risk for dysphagia once an insult known to cause dysphagia (eg, cerebrovascular accident) occurs (13). There is some evidence that patients with dysphagia (and not necessarily with aspiration) have lower isometric tongue strength than controls (16,22,23). We used an objective measure of swallowing function (ie, FEES) in this study and found corroborating evidence that lower isometric tongue strength is dependent on aspiration status in healthy older adults.
Additionally, swallowing tongue strength of the aspirators was significantly lower than the nonaspirators at both the anterior and posterior tongue locations. Diminished tongue strength could have contributed to the aspiration observed. In this cohort of healthy older adults, most aspiration events occurred during the swallow. Aspiration during the swallow is easily identified endoscopically by exclusion. If the bolus was not visualized in the trachea prior to “whiteout” and is present in the trachea at the completion of whiteout, it is reasonable to deduce that aspiration occurred during the swallow. Aspiration during the swallow may indicate delayed or decreased laryngeal closure, decreased lingual bolus containment, and/or decreased lingual bolus propulsion as one of the physiologic components of breakdown. Some of these physiologic mechanisms are difficult to assess endoscopically. However, given the sensitivity of FEES in detecting aspiration, a combined approach of utilizing FEES with an instrumental assessment of tongue strength may be warranted. Assessing tongue strength, both anteriorly and posteriorly may provide insight into the role of the tongue in the physiologic breakdown of the swallow as well as provide a measurable goal for swallowing rehabilitative efforts. Toward this line of reasoning, an investigation of tongue strengthening as a potential intervention to manage aspiration is warranted. The tongue is amenable to therapy, and employing a lingual strength-training program can increase tongue strength (24–28).
As depicted in Figure 2, we found posterior isometric tongue and handgrip strength were associated. This observation is intriguing because it suggests that declines in oropharyngeal musculature strength may reflect more global phenomena of age-related declines in muscle strength and progressive disability. If so, the prevalence of frailty-related signs and symptoms might be higher among silent aspirators, a hypothesis that we are now investigating. Similarly, the high prevalence of silent aspiration may help to explain in part the age-related increase in the occurrence of community-acquired pneumonia, by providing a mechanism to introduce a higher microbial load into the airways. A complete understanding of the clinical importance of silent aspiration will require additional study.
Although both anterior and posterior isometric and swallowing tongue strength were significantly lower in aspirators versus nonaspirators, only posterior tongue strength was significantly associated with handgrip strength. This finding calls into question the varying internal architecture relative to muscle, connective tissue, and adipose of the anterior and posterior portions of the tongue and possible similarities between the posterior tongue and the hand. Reports of differences in distribution of tongue adipose tissue as a function of anterior and posterior tongue locations have been equivocal. Miller and colleagues (29) found greater muscle tissue and corresponding less adipose tissue in the posterior versus anterior portions of the tongue, whereas Nashi and colleagues (30), with markedly more participants, found greater adipose tissue in the posterior versus anterior portion of the tongue. Baseline tongue composition may not be important but how tongue composition changes relative to effects of aging may be. If the posterior tongue, for example, has a greater density of adipose tissue at baseline, it may be more susceptible to sarcopenia, predisposing some older adults to decreased tongue strength and aspiration. This may also explain in part why we found no significant correlation between anterior tongue and handgrip strength.
Although reduced isometric and swallowing tongue strength evidenced in aspirators implicate changes in the periphery (eg, tongue adiposity), compared those who do not aspirator, additional central nervous system changes may also be involved. For example, Levine and colleagues (31) reported a significantly higher number of magnetic resonance imaging, cortical unidentified bright objects of older adults who demonstrated slower swallowing durations as measured fluoroscopically. Similarly, Humbert and colleagues (32), using functional magnetic resonance imaging, found greater cortical activation in healthy older adults with fluoroscopically delayed pharyngeal response and residue compared with young adults. In a study specifically related to tongue strength, Robbins and colleagues (13) found greater cerebral atrophy and higher magnetic resonance imaging scores of periventricular white matter lesions in reportedly healthy older adults who generated less isometric tongue strength than the younger adults. Thus, there is mounting evidence of the possible association between central nervous system changes and decreased oropharyngeal strength and functioning in older adults. This, however, needs continued systematic investigation before cause–effect relationships can be determined relative to decreased tongue strength found in healthy older adults and in healthy older adult aspirators versus nonaspirators.
Some previous investigators have used isolated tasks of obtaining peak pressure measurements at the anterior, middle, and posterior regions of the tongue separately (11–13,15,16) to assess tongue strength. This practice was necessitated by the use of a one-bulb measurement device. Testing in this way isolates each region of the tongue; however, swallowing requires a synergistic recruitment of all aspects of the tongue, not just one region. Thus, having the participant do a tongue press with the entire tongue reflects the maximum regional recruitment available when the entire tongue is being contracted/recruited for a swallow. This will more likely reflect the functional reserves available at both anterior and posterior tongue locations during swallowing. Furthermore, the one-bulb device allows the examiner to place the bulb at the region of the tongue being tested. Seemingly, ideal for some measurements, it may introduce potential error in placement. That is, the examiner subjectively determines where the anterior, middle, and posterior regions of the tongue begin and end. Placement error may also be a problem with the contemporary three-bulb array. The fixed bulb locations are not consistent on tongues of different lengths. The anterior bulb should be relatively immune from this issue as the anterior bulb is placed just posterior to the central incisors at the alveolar ridge thus targeting the anterior portion of the tongue in all participants. On the other hand, the acquisition of posterior tongue strength data may be vulnerable to variations in tongue length as the posterior bulb will approximate a more anterior location in a person with a long lingual length versus shorter lingual length and vice versa. However, even with that possibility, it appears the potential variability was negligible in our cohort (see standard deviations between anterior and posterior bulb locations in Table 3 and Table 4). A limitation of this study is that participants’ medical history was self-reported. Although all participants identified as aspirators reported good general health and a negative history of neurological disease, it is possible that an undiagnosed condition could have existed. No formal neurological testing was conducted.
Table 3.
Unadjusted Anterior and Posterior Swallowing Tongue Strength as a Function of Age, Gender, and Aspiration Status (M [SE])
| Anterior Tongue Strength (mmHg) | Posterior Tongue Strength (mmHg) | |
| Age | ||
| 61–70 y | 311.07 (21.07) | 263.41 (22.42) |
| 71–80 y | 319.61 (29.59) | 261.05 (23.31) |
| 81–90 y | 269.42 (22.98) | 220.99 (15.35) |
| Gender | ||
| Female | 324.75 (22.46) | 265.20 (17.02) |
| Male | 274.12 (19.23) | 227.89 (15.79) |
| Aspiration Status | ||
| Aspirator | 275.96 (19.50) | 208.54 (17.70) |
| Nonaspirator | 311.78 (20.89) | 269.03 (14.55) |
In conclusion, subclinical aspiration in healthy older adults, while previously unrecognized, has now been documented in this and three previous independent samples (1–3). The phenomenon may be the result of a generalized weakening of the oropharyngeal musculature. The link between tongue and grip strength may mean lower tongue strength is another manifestation of age-related loss of muscle function; thus, aspiration may be a previously unrecognized consequence of progressive frailty in older adults. If so, one might expect aspiration to be related to other aspects of progressive frailty, a hypothesis to be tested at a later time. Finally, in some cases, it seems plausible that aspiration may be reversible with appropriate tongue strengthening therapy. Studies exploring this hypothesis are also needed.
FUNDING
This work was supported by NIDCD R03 DC009875, by the Wake Forest School of Medicine Claude D. Pepper Older Americans Independence Center (P30 AG21332), and by the GCRC grant of Wake Forest Baptist Medical Center (M01-RR07122).
Acknowledgments
We thank Karen Potvin Klein, MA, ELS (Research Support Core, Wake Forest University Health Sciences), for her editorial contributions to this manuscript. This paper was presented at the Eighteenth Annual Meeting of the Dysphagia Research Society, March 5–7, 2010, San Diego, CA.
References
- 1.Butler SG, Stuart A, Markley L, Rees C. Penetration and aspiration in healthy older adults as assessed during endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2009;118(3):190–198. doi: 10.1177/000348940911800306. [DOI] [PubMed] [Google Scholar]
- 2.Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009;118(2):99–106. doi: 10.1177/000348940911800204. [DOI] [PubMed] [Google Scholar]
- 3.Butler SG, Stuart A, Leng X, et al. Factors influencing aspiration during swallowing in healthy older adults. Laryngoscope. 2010;120(11):2147–2152. doi: 10.1002/lary.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler SG, Stuart A, Wilhelm E. The effects of aspiration status, liquid type, and bolus volume on pharyngeal peak pressure in healthy older adults. Dysphagia. doi: 10.1007/s00455-010-9290-4. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi-Fishman G, Stone M, McCall GN. Lingual action in normal sequential swallowing. J Speech Lang Hear Res. 1998;41(4):771–785. doi: 10.1044/jslhr.4104.771. [DOI] [PubMed] [Google Scholar]
- 6.Steele CM, Van Lieshout P. Tongue movements during water swallowing in healthy young and older adults. J Speech Lang Hear Res. 2009;52(5):1255–1267. doi: 10.1044/1092-4388(2009/08-0131). [DOI] [PubMed] [Google Scholar]
- 7.Steele CM, Van Lieshout PH. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia. 2004;19(3):192–206. doi: 10.1007/s00455-004-0006-5. [DOI] [PubMed] [Google Scholar]
- 8.Steele CM, Van Lieshout PH. The dynamics of lingual-mandibular coordination during liquid swallowing. Dysphagia. 2008;23(1):33–46. doi: 10.1007/s00455-007-9093-4. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JW, van Lieshout PH, Steele CM. Tongue control for speech and swallowing in healthy younger and older subjects. Int J Orofacial Myology. 2007;33:5–18. [PubMed] [Google Scholar]
- 10.Ono T, Hori K, Nokubi T. Pattern of tongue pressure on hard palate during swallowing. Dysphagia. 2004;19(4):259–264. doi: 10.1007/s00455-004-0010-9. [DOI] [PubMed] [Google Scholar]
- 11.Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009;24(1):57–65. doi: 10.1007/s00455-008-9171-2. [DOI] [PubMed] [Google Scholar]
- 12.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci. 1996;51(5):M247–M250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- 13.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 14.Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 15.Youmans SR, Stierwalt JA. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21(2):102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- 16.Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- 17.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 18.Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17(4):308–315. doi: 10.1007/s00455-002-0073-4. [DOI] [PubMed] [Google Scholar]
- 19.Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. J Speech Lang Hear Res. 2004;47(3):572–583. doi: 10.1044/1092-4388(2004/044). [DOI] [PubMed] [Google Scholar]
- 20.Steele CM, Bailey GL, Molfenter SM. Tongue pressure modulation during swallowing: water vs. nectar-thick liquids. J Speech Lang Hear Res. 2009 doi: 10.1044/1092-4388(2009/09-0076). [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18(4):426–427. doi: 10.1197/j.jht.2005.07.003. quiz 428. [DOI] [PubMed] [Google Scholar]
- 22.Robinovitch SN, Hershler C, Romilly DP. A tongue force measurement system for the assessment of oral-phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42. [PubMed] [Google Scholar]
- 23.Yoshida M, Kikutani T, Tsuga K, Utanohara Y, Hayashi R, Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21(1):61–65. doi: 10.1007/s00455-005-9011-6. [DOI] [PubMed] [Google Scholar]
- 24.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 25.Clark HM, O’Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047. doi: 10.1044/1092-4388(2009/08-0062). [DOI] [PubMed] [Google Scholar]
- 26.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 27.Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3(4):735–747. doi: 10.2147/cia.s3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res. 2002;45(1):51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- 30.Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117(8):1467–1473. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- 31.Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia. 1992;7(3):142–147. doi: 10.1007/BF02493446. [DOI] [PubMed] [Google Scholar]
- 32.Humbert IA, Fitzgerald ME, McLaren DG, et al. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44(3):982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]