Abstract
There is a paucity of knowledge from population data about sex differences and their age variation in physiological determinants of longevity. This study fills this gap using nationally representative samples of 38,000 individuals aged 17+ from the National Health and Nutrition Examination Survey (1988–2006). It examines sex differences in the age trajectories of 14 markers of physiological functions across multiple systems and three summary indices including inflammation burden, metabolic syndrome, and allostatic load. Statistical analyses show substantial sex differences, age variations, and sex by age interaction effects for all variables examined. These patterns remain robust after adjustment of risk factors and shed light on the biological base of the reduction of sex difference in mortality in the post-reproductive life span.
Keywords: Physiological dysregulation, Sex difference, Age variation, Inflammation, Allostatic load
THE male survival disadvantages at all ages have been observed across human populations and even species. However, a full understanding of how the sex gap evolves with individual aging is still lacking (1). Both historical and contemporary human mortality data suggest that the sex gap in mortality is more pronounced in young adulthood (2) and decreases in postmenopausal ages due to a faster mortality rate acceleration for women after middle age that coincides with female fecundity decline (3). Studies of cause-specific mortality have further documented that cardiovascular diseases (CVD) account for the majority of the sex gap in adult mortality and the decline of this gap at old ages (3–5). The sex differences in age- and cause-specific mortality suggest the hypothesis that the reduction in male survival disadvantage in the old ages has a biological base, which cannot be directly tested using mortality data alone. To further understand age variations in sex differentials in mortality, it is essential to compare age trajectories of physiological functions between males and females that may be linked to sex-specific mortality patterns (3,6).
Systemic inflammation and the metabolic disorders are important pathogenic mechanisms in a host of age-related conditions, such as arterial disease, diabetes, malignancies, and Alzheimer’s disease (7–10) and strongly predict mortality (11–13). In addition, the aging and frailty process is characterized by a progressive dysregulation of the homeostatic network and accelerated decline in function across multiple regulatory systems. The allostatic load (AL), a count of high-risk biological parameters across systems, has been increasingly utilized as a generalized indicator of cumulative burden of physiological dysregulation (14,15) and a powerful summary index of population frailty predicting major health outcomes and mortality in the oldest ages (16,17). Sex and age differences in the lifetime burden of physiological dysregulation, therefore, should be examined as the major physiological mechanisms underlying sex differences in mortality and their age variations.
Although there are compelling theoretical explanations for sexual dimorphism in immune, metabolic, and multiple systems, extant empirical evidence mostly is based on small animal or clinical samples of single sex and/or limited age ranges (1,2,18–24). Recent population-based studies also suggest substantial sex differentials in several biomarkers (13,25–32), but they are largely focused on individual rather than overall burden of physiological disorders and are often restricted to small samples that are homogeneous in health status, demographic, or geographic characteristics. Age variations in sex differences in biological functions contribute to the complexity of explanations but have not been systematically examined or rigorously modeled in population-based samples. There is little knowledge from large nationally representative community samples of the distributions and patterns of variations in major biological measures of inflammation, metabolic disorders, and cumulative physiological dysregulation among subgroups by sex and age within the same study population.
This study fills these gaps through a precise characterization of the sex differences in inflammation, metabolic syndrome (MetS), and AL across the adult life course in population data. We model both sex and age differences in these physiological variables to better understand the narrowing sex gap in mortality in post-reproductive life span. We test the hypothesis that women enjoy immunological and metabolic advantages until menopause, but these advantages decrease in older ages. We focus on the sex and age patterns in biological parameters while adjusting for major social, behavioral, and morbidity factors frequently examined in previous research (8,15,29,31). The pathways by which these factors operate over the life course may be complicated and are topics of future examinations of the interconnections between social and biological processes producing sex phenotypic differences throughout the life span.
METHODS
Study Population
The data come from the National Health and Nutrition Examination Survey (NHANES) conducted by the National Center for Health Statistics between 1988 and 1994 (III) and 1999 and 2006 (IV) that used a multistage stratified sampling design and include a representative cross-sectional sample of the noninstitutionalized U.S. population, with an oversample of older persons and minorities (33). This study includes about 38,000 individuals aged 17 years and older (age ranges 20+ for NHANES III and 17+ for NHANES IV) for whom interview, clinical examination, and laboratory tests are available. We examined 14 markers of physiological functions listed in Table 1 including 3 markers of inflammation, 8 markers of metabolic functions, and 3 additional markers: serum homocysteine—an amino acid shown to be related to health and frailty (16); lung function—peak flow; and urinary function—creatinine clearance. The laboratory measurements and assay procedures for all these markers have been described elsewhere (35,36).
Table 1.
Sample Size, High-Risk Cutoff Point, and Weighted Descriptive Statistics of Biological Variables in NHANES (1988—2006)
| Variable | High-Risk Cutoff Point | Men |
Women |
Difference, p Value* | ||||||
| N | M | SD | % High Risk | N | M | SD | % High Risk | |||
| Inflammation | ||||||||||
| C-reactive protein | >3.0 mg/dL | 18,052 | 0.3 | 0.8 | 19.8 | 19,909 | 0.5 | 0.8 | 34.4 | <.001 |
| Plasma fibrinogen | ≥341/411 mg/dL† | 7,289 | 340.4 | 85.0 | 21.5 | 7,751 | 355.5 | 85.9 | 29.1 | <.001 |
| Urinary albumin | ≤3.5 μg/mL | 18,241 | 19.7 | 54.2 | 22.0 | 20,251 | 17.7 | 47.7 | 27.0 | <.001 |
| Inflammation index (age 40+) | 6,952 | 0.7 | 0.8 | 7,239 | 1.0 | 0.9 | <.001 | |||
| Metabolic factors | ||||||||||
| Waist circumference | >102/88 cm‡ | 18,139 | 98.2 | 15.0 | 36.6 | 19,942 | 92.2 | 16.0 | 55.3 | <.001 |
| Systolic blood pressure | ≥130 mmHg | 17,264 | 124.3 | 16.5 | 15.5 | 18,950 | 121.4 | 21.6 | 17.8 | <.001 |
| Diastolic blood pressure | ≥85 mmHg | 17,263 | 73.1 | 13.2 | 9.0 | 18,949 | 69.5 | 12.9 | 4.9 | <.001 |
| Serum triglycerides | ≥150 mg/dL | 14,607 | 154.6 | 160.8 | 34.8 | 16,155 | 126.6 | 102.9 | 25.6 | <.001 |
| HDL cholesterol | <40 mg/dL | 18,026 | 46.9 | 13.3 | 30.2 | 19,845 | 57.3 | 16.1 | 10.7 | <.001 |
| Fasting glucose | ≥110 mg/dL | 12,438 | 102.6 | 29.8 | 15.7 | 13,727 | 98.6 | 30.7 | 11.8 | <.001 |
| Body mass index | ≥30 kg/m2 | 18,883 | 27.6 | 5.6 | 26.9 | 20,935 | 27.8 | 7.0 | 31.3 | <.001 |
| Glycated hemoglobin (HbA1c) | ≥6.4% | 18,258 | 5.5 | 0.9 | 6.6 | 20,204 | 5.4 | 0.9 | 5.8 | .031 |
| Metabolic syndrome | 9,317 | 0.2 | 0.4 | 10,163 | 0.1 | 0.4 | <.001 | |||
| Other physiological functions | <.001 | |||||||||
| Serum homocysteine | ≥15 μmol/L | 13,099 | 9.4 | 4.2 | 5.0 | 14,882 | 8.0 | 4.1 | 3.8 | <.001 |
| Peak flow (largest value) | <2113 mL | 7,724 | 3,447.7 | 1,484.1 | 19.4 | 8,735 | 2,691.8 | 1,179.5 | 32.5 | <.001 |
| Creatinine clearance | <66.7 mg/dL | 18,484 | 152.6 | 85.6 | 16.0 | 20,401 | 111.2 | 75.8 | 33.8 | <.001 |
| Allostatic load | 6,775 | 2.3 | 1.9 | 7,710 | 2.6 | 1.9 | <.001 | |||
Notes: Fibrinogen was only measured for respondents aged 40 years and older. The glucose measures require fasting and have smaller samples. Homocysteine was assayed only in the second half of NHANES III (1991–1994). And peak flow was not available for NHANES IV. NHANES = National Health and Nutrition Examination Survey. HDL = High Density Lipoprotein.
Top quartile high-risk cutoff points for NHANES III and IV, respectively.
Clinical high-risk cutoff points for men and women, respectively—the clinical criteria for high risk are only sex specific (34).
Test for sex difference in the means (two sided). An α level of .0027 is used to adjust for multiple comparisons using the Bonferroni method. All sex differences are significant with the exception of mean HbA1c. Results for sex difference in the % high risk are similar.
The cutoff points for high-risk levels are based on clinical practice for 11 markers and empirically defined for 3 (fibrinogen, peak flow, and creatinine clearance) as the top quartile at risk based on previous studies (16,26). The assays used to measure C-reactive protein (CRP) and fibrinogen differed at the two study periods (NHANES IV values are higher). The laboratory doing the assays for CRP performed an adjustment of NHANES IV values that produced highly comparable CRP values (33). We are not aware of a similar adjustment for fibrinogen, so we adopted wave-specific top quartile cutoff points. We found no difference in results using different cutoff points for CRP (3.0 and 4.0 mg/L) and the same cutoff point for fibrinogen (top quartile for NHANES III), which suggests low sensitivity of findings to the choice of cutoff points. We also conducted robustness analyses by allowing the high-risk cutoff points vary with age in order to take into account of the possibility that the common at-risk levels may not apply to (ie, be too high for) the oldest ages. First, we compared the current cutoff points with those published in previous studies of older adults aged 40+ (26) and 65+ years (37) from the same NHANES data and older adults aged 70–79 years from a different data set (17). The cutoff points are the same for most biomarkers common to all studies, with the exception of blood pressure, triglycerides, and HbA1C whose cutoff points are actually slightly lower in our study. We changed these cutoff points to those used in previous studies (diastolic blood pressure ≥90, systolic blood pressure ≥ 140, triglycerides ≥200, and HbA1C ≥7.1). Second, we empirically defined the cutoff points as the top quartile at risk for all markers in age-specific groups (<60 and ≥60). Third, because the potential change in risk level mostly pertains to the oldest ages due to increasing debility and impending mortality, we eliminated respondents aged 85+ years from the samples. None of the above analysis showed any substantive change in the results. Based on common practice and for comparability, we used the cutoff points shown in Table 1 for all ages in the final analyses reported here.
We constructed summary indices based on the aforementioned high-risk cutoff points. The burden of inflammation index is the sum of the positive indicators and ranges from 0 to 3. The MetS is defined based on the National Cholesterol Education Program/Adult Treatment Panel III criteria (38) as positive for those having three or more of five metabolic disorders: abdominal obesity, high blood pressure, hypertriglyceridemia, low High Density Lipoprotein cholesterol, and high fasting glucose. Following previous studies, we operationalized the AL as a count of high-risk biological parameters across multiple systems, including markers of inflammation, cardiovascular and metabolic functions, lung function, and renal function, that is, a sum of positive indicators of all 14 markers above. We present results using the 13-count AL (without fibrinogen) to include all ages (additional analyses show no difference in results from those using the 14-count AL).
In addition to sex and age variables, covariates in the final analysis are shown in Table 2 and include social demographic characteristics, such as race/ethnicity and marital status; socioeconomic status indicated by education and family income in 1991 dollars; health behaviors indicated by cigarette smoking and alcohol use; morbidity indicated by the number of chronic conditions including 14 self-reported chronic illnesses: angina, arthritis, asthma, bronchitis, diabetes, emphysema, heart attack, heart failure, cancer, stroke, hip fracture, osteoporosis, spine fracture, and wrist fracture; and medications including female hormone therapy, hypertension, and cholesterol medication. Sample characteristics (weighted) based on these covariates for all and by age and sex are shown in Table 2. Respondents who lacked measures of the biomarkers for summary indices and covariates are excluded from the analytic samples. Compared to those with complete data on biomarkers, those with missing data were older, more likely to be widowed, and had more chronic illnesses. These individuals either had difficulties completing parts of the examination due to incapacity or simply refused to provide samples (33). In all, the combined NHANES samples provide sufficient numbers of observations for multivariate analyses. And the exclusion of older and frailer persons produces more conservative estimates.
Table 2.
Characteristics (weighted) of the Allostatic Load Sample* in National Health and Nutrition Examination Survey (1988–2006)
| Variable | All (N = 11,600) | Men |
Women |
||||
| All Ages (N = 5,347) | 17–59 y (N = 3,531) | 60+ y (N = 1,816) | All Ages (N = 6,253) | 17–59 y (N = 4,375) | 60+ y (N = 1,878) | ||
| Race % | |||||||
| Non-Hispanic White | 73.9 (43.9) | 74.4 (43.6) | 72.0 (44.9) | 84.1 (36.6) | 73.4 (44.2) | 70.3 (45.7) | 83.3 (37.3) |
| Non-Hispanic Black | 10.2 (30.2) | 9.4 (29.3) | 10.3 (30.4) | 6.3 (24.4) | 10.8 (31.1) | 11.9 (32.4) | 7.7 (26.6) |
| Mexican American | 6.7 (25.1) | 7.5 (26.3) | 8.6 (28.0) | 3.2 (17.6) | 6.1 (23.8) | 7.0 (25.6) | 2.9 (16.8) |
| Other | 9.1 (28.8) | 8.6 (28.1) | 9.2 (28.9) | 6.4 (24.5) | 9.6 (29.5) | 10.8 (3.1) | 6.1 (23.9) |
| Education % | |||||||
| 0–8 y | 3.3 (18.0) | 3.6 (18.5) | 2.8 (16.4) | 6.6 (24.8) | 3.2 (17.6) | 2.1 (14.3) | 6.7 (25.0) |
| 9–12 y | 43.7 (49.6) | 43.7 (49.6) | 43.2 (49.5) | 46.0 (49.9) | 43.7 (49.6) | 40.1 (49.0) | 55.3 (49.7) |
| 13+ y | 53.0 (49.9) | 52.3 (49.9) | 54.1 (49.8) | 47.4 (49.9) | 53.1 (49.9) | 57.8 (49.4) | 38.0 (48.6) |
| Family income (median in 1991 $) | 30,283 | 35,961 | 37,500 | 30,283 | 30,283 | 32,500 | 22,712 |
| Marital status % | |||||||
| Married | 66.5 (47.2) | 70.1 (45.8) | 67.4 (46.9) | 81.0 (39.3) | 62.9 (48.3) | 66.3 (47.3) | 52.1 (50.0) |
| Widowed | 5.8 (23.5) | 2.2 (14.7) | 0.6 (7.5) | 8.7 (28.2) | 9.4 (29.2) | 1.8 (13.1) | 33.9 (47.4) |
| Divorced/separated | 11.6 (32.0) | 9.3 (29.0) | 9.6 (29.4) | 8.1 (27.2) | 13.9 (34.6) | 15.0 (35.7) | 10.3 (30.5) |
| Never married | 16.1 (36.7) | 18.4 (38.7) | 22.5 (41.8) | 2.2 (14.8) | 13.8 (34.5) | 17.0 (37.6) | 3.6 (18.6) |
| Cigarette smoking % | |||||||
| Never | 48.3 (50.0) | 40.2 (49.0) | 43.4 (49.6) | 27.7 (44.8) | 56.2 (49.6) | 56.4 (49.6) | 55.3 (49.7) |
| Former | 25.7 (43.7) | 30.2 (45.9) | 23.3 (42.3) | 57.4 (49.4) | 21.4 (41.0) | 18.1 (38.5) | 32.1 (46.7) |
| Current | 25.9 (43.8) | 29.6 (45.6) | 33.3 (47.2) | 14.8 (35.5) | 22.4 (41.7) | 25.5 (43.6) | 12.7 (33.3) |
| Alcohol use (mean days per month) | 5.1 (8.2) | 6.8 (9.2) | 6.6 (8.7) | 7.6 (10.9) | 3.3 (6.6) | 3.2 (6.1) | 3.6 (7.9) |
| Number of chronic conditions | 0.9 (1.2) | 0.8 (1.2) | 0.7 (1.0) | 1.6 (1.5) | 1.0 (1.2) | 0.7 (1.0) | 1.8 (1.5) |
| Female hormone therapy % | 25.4 (43.5) | 25.3 (43.5) | 18.2 (38.6) | 48.3 (50.0) | |||
| Hypertension medication % | 17.5 (38.0) | 18.9 (39.1) | 9.9 (29.8) | 40.6 (49.1) | 16.2 (36.8) | 10.4 (30.6) | 46.0 (50.0) |
| Cholesterol medication % | 10.0 (30.1) | 10.8 (31.0) | 7.1 (25.6) | 25.3 (43.5) | 9.3 (29.0) | 4.9 (21.7) | 23.3 (42.2) |
Characteristics for the inflammation index and metabolic syndrome samples are similar and not presented; only variables that are common across two waves are included.
Statistical Analysis
We first conducted descriptive analyses to examine distributions of measured biological functions by sex using the t test and by sex and age using analysis of variance and the χ2 test. We then estimated multivariate regression models to assess the parametric relationships of sex and age with biological variables. We used log transformations of continuous outcomes to account for skewed sampling distributions. Results show improved model fit to data on all markers using the log transformation. We estimated ordinal logit and Poisson regressions for the inflammation index, logistic regression models for MetS, and Poisson and negative binomial models for the AL. We used various codings of the age variable (continuous and categorical) and tested for its polynomial functional forms. We conducted stepwise regressions that brought in measures one at a time. None of these analyses show substantively different results with regard to the directions and magnitudes of regression coefficients and significance tests on either coefficients or the models as a whole. We chose the best model specifications based on tests of statistical significance of coefficient estimates and model fit statistics using Bayes Information Criterion, a generalized test of model fit adjusting the impact of model dimensions on model deviance (smaller is better). We further examined social, behavioral, and morbidity factors in relation to summary biological indices to understand how they may account for sex and age differences observed. All statistical analyses were performed using Stata 10.0. We used the Bonferroni method for adjustment of p values due to the multiple tests that were conducted (39). And we adjusted for the complex survey designs using sampling weights for descriptive analysis and the “svy” procedures for the regression analysis.
RESULTS
Both descriptive and multivariate regression analyses show substantial and highly significant sex differences, nonlinear age variations, and sex differences in age trajectories for all individual markers and summary indices of physiological dysregulation. The results on individual markers of inflammation, metabolic, and other functions are largely consistent with those on the corresponding indices. We focus on the modeling results for the summary indices and refer interested readers to the online supplement for detailed results for all 14 individual markers. A brief description of the Supplementary Figures is in order here. Supplementary Figures A–C present the observed data on biomarkers of inflammation, metabolic functions, and other functions together with smoothed age curves from the best-fitting models using polynomials of age (eg, age, age2, age3, etc.), sex, and their interactions. The regression coefficients for these effects (not shown) are all highly significant and do not differ significantly by wave. Results hold after adjusting for other covariates.
Table 3 presents the regression coefficient estimates from the best-fitting models of summary indices, including ordinal logit regressions of the inflammation index, the logistic regressions of the MetS, and the negative binomial regressions of the AL. Models 1a–3a present the crude associations of age, sex, and age by sex interaction with outcome variables. Models 1b–3b present the adjusted associations controlling for social, behavioral, and morbidity risk factors. There are significant quadratic age effects for all three indices, suggesting increases in physiological disorders with age that decelerate at older ages. The age patterns of sex differentials in physiological dysregulation generally show gradual decreases of the sex gaps starting in the early 60’s and support the hypothesis of a postmenopausal reduction of female physiological advantages. The patterns of sex difference and age variation vary by biological functions and other risk factors (detailed results of all the control variables are available upon request).
Table 3.
Model Estimates of Age Patterns, Sex Differences, and Age Changes in Sex Differences in Summary Indices of Physiological Dysregulation in NHANES (1988–2006)
| Variables | Inflammation Index (N = 6,864) |
MetS (N = 15143) |
AL (N = 11,600) |
|||||||||
| Model 1a |
Model 1b |
Model 2a |
Model 2b |
Model 3a |
Model 3b | |||||||
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Incidence Rate Ratio (95% CI) | p Value | Incidence Rate Ratio (95% CI) | p Value | |
| Age | 2.56 (1.78–3.68) | <.001 | 2.34 (1.32–4.16) | .004 | 3.90 (3.15–4.84) | <.001 | 4.40 (3.33–5.80) | <.001 | 1.65 (1.56–1.74) | <.001 | 1.61 (1.50–1.71) | <.001 |
| Age2 | 0.93 (0.90–0.96) | <.001 | 0.94 (0.90–0.98) | .005 | 0.92 (0.90–0.94) | <.001 | 0.90 (0.88–0.92) | <.001 | 0.97 (0.96–0.97) | <.001 | 0.96 (0.96–0.97) | <.001 |
| Sex (male = 1) | 0.24 (0.15–0.38) | <.001 | 0.26 (0.12–0.58) | .001 | 4.38 (2.91–6.59) | <.001 | 9.37 (5.56–15.80) | <.001 | 0.92 (0.89–0.95) | <.001 | 0.96 (0.92–0.99) | .023 |
| Sex × Age | 1.13 (1.05–1.22) | .001 | 1.13 (1.01–1.28) | .041 | 0.82 (0.76–0.88) | <.001 | 0.77 (0.70–0.84) | <.001 | 0.95 (0.92–0.99) | .225 | 0.96 (0.92–1.00) | .156 |
| NHANES (IV = 1) | 0.86 (0.79–0.94) | .001 | 0.71 (0.61–0.84) | <.001 | 1.02 (0.92–1.13) | .722 | 0.99 (0.85–1.15) | .891 | 0.87 (0.84–0.90) | <.001 | 0.88 (0.85–0.92) | <.001 |
| Model fit: BIC | 33,080.98 | 16,069.39 | 16,948.88 | 13,150.63 | 56,057.36 | 45,065.12 | ||||||
Notes: AL = allostatic load; BIC = Bayes Information Criterion; CI = confidence interval; MetS = metabolic syndrome; NHANES = National Health and Nutrition Examination Survey.
Note 1: All statistical tests are two sided. An α level of .01 is used for each model to adjust for multiple comparisons using the Bonferroni method.
Note 2: Models 1b, 2b, and 3b all control for race, education, family income, marital status, cigarette smoking, alcohol use, and chronic conditions. Model 1b also controls for obesity, MetS, Sex × Smoking, Age × Smoking, Sex × Obesity, Age × Obesity. Model 2b also controls for obesity, C-reactive protein, albumin, hypertension medication, cholesterol medication, Sex × Smoking, Age × Smoking. Model 3b also controls for hypertension medication and cholesterol medication. Female hormone use is included in sex-stratified analysis for women only but is not significantly associated with any outcome when other factors are controlled. Interactions of covariates with sex and age were tested but included only when statistically significant.
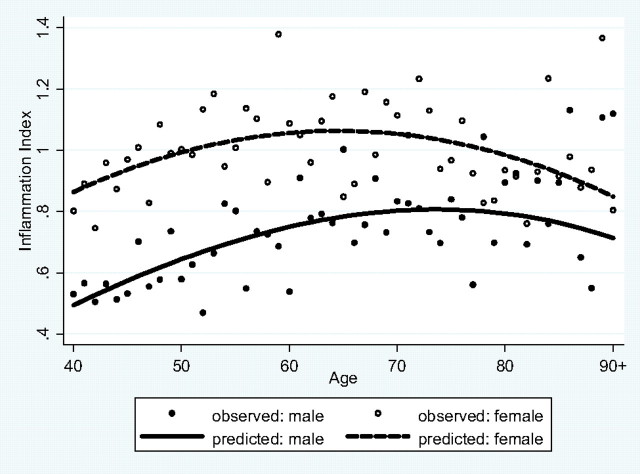
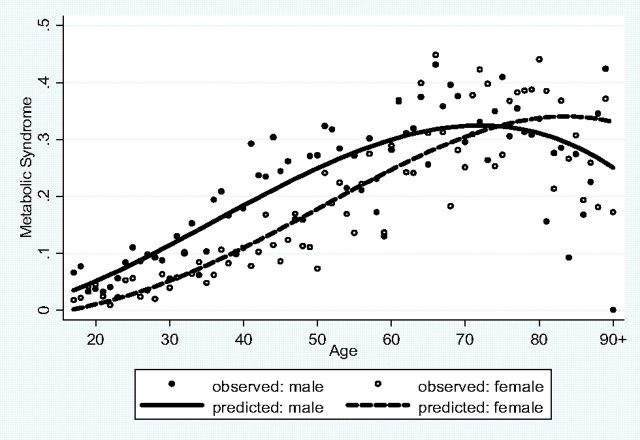
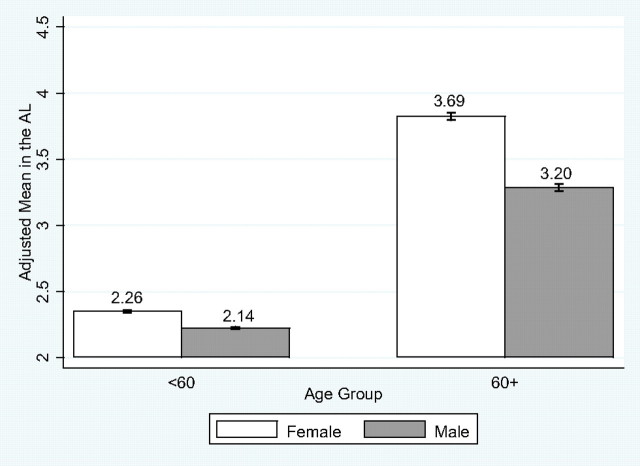
Model 1a shows that men have a lower inflammation burden than do women (odds ratio = 0.24, 95% confidence interval : 0.15–0.38, p < .001), but the sex gap decreases with age, as indicated by the sex by age interaction (p = .001) and the converging sex gaps in the age curves in Figure 1. Model 1b shows that adjusting for other covariates reduces the age, sex, and age by sex interaction effects in magnitude and/or significance level. Model 2a shows that the odds of experiencing the MetS are more than four times higher for men than for women (odds ratio = 4.38, 95% confidence interval = 2.91–6.59, p < .001), but this difference decreases with age (p < .001 for the sex by age interaction), as illustrated by Figure 2 in which the sex gap in the probability of MetS converges and reverses later in life. The results hold and become stronger in Model 2b with adjustment of covariates. Model 3a shows that men are expected to have lower AL than women, holding age constant (incidence rate ratio = 0.92, 95% confidence interval = 0.89–0.95, p < .001). The sex difference decreases somewhat after adjusting for other covariates (Model 3b). Although the sex by age interaction term is not statistically significant in this model of continuous age effects, it is highly significant in the model using a dichotomous age variable (age < 60 vs 60+, results not shown), suggesting a widening sex gap in persons aged 60 years and older (p = .008 for the sex by age interaction). Figure 3 further compares the adjusted means by sex and age groups. It shows that the AL increases with age more for women than for men, leading to a larger female excess in postmenopausal ages that persists after adjustment of other covariates (p = .025 for the sex by age interaction).
Figure 1.
Sex difference and age variation in inflammation index. Note: Instead of the individual observations that would preoccupy the graph due to their large number, the observed data represent the mean for each age. The same applies to Figures 2 and Supplementary Figures A–C.
Figure 2.
Sex difference and age variation in probability of metabolic syndrome.
Figure 3.
Sex difference and age variation in allostatic load (AL).
Models 1b–3b also adjusted for interactions of risk factors with sex and age that appear significant (such as obesity and smoking) and show improved model fit over Models 1a–3a, respectively, but the residual sex differences and age variations remain statistically significant. We examined additional covariates, including health insurance, diet and nutrition, physical activity, and social integration (religious attendance, ties with friends and family relatives, and membership in social organizations) in these models. Because variables of social integration are not available in the NHANES IV, models including these variables can only be estimated using the NHANES III data. The age and sex effects remain robust in models of inflammation, MetS, and AL even after adjusting for additional covariates. Excluding persons with CRP levels less than 10 mg/L (indicating acute infections) and controlling for the effect of estrogen medications in women does not change the results.
DISCUSSION
It is unclear from previous studies whether genetic and hormonal influences contribute to greater male or female preponderance in major physiological determinants of human longevity and how they vary with age. In addition, few studies have attempted at simultaneous assessments of sex and age differences in multiple domains of biological functions with statistical adjustments for other risk factors. This study addresses these issues and provides population-based evidence for important sex differences in the age trajectories of major markers of immune and metabolic systems and the AL.
Women show higher mean levels of inflammatory markers and overall burden but slower rates of increase in inflammation with age. This suggests the complicated nature of the interaction of sex-specific reproductive anatomy and functions with vascular inflammatory processes. The female sex hormone estrogen has been hypothesized to have a protective anti-inflammatory effect that may improve host resistance to degenerative diseases (24). It has also been proposed that endogenous estrogens may reduce the risk of CVD in females by modulation of the fibrinolytic factors much more than by affecting the levels of inflammatory markers or coagulation factors (24). The female reproductive senescence due to the exhaustion of ovarian oocytes and ovarian steroid loss may interact with these processes and contribute to the age changes in inflammation. The higher inflammation but lower risk of CVD in women than in men also seems to suggest the use of sex-specific high-risk cutoff points for inflammatory markers in future research (25,27).
There are large male excesses in a host of metabolic disorders and the overall MetS that disappear in late life. Recent research points to the importance of long-lasting effects of female sex hormone changes. Fluctuations in estrogens especially in 17β-estradiol (E2) during the menstrual cycle and pregnancy induce endocrine and vascular challenges in women that decrease vascular resistance and arterial blood pressure, increase cardiac output by as much as 40%, and create optimal cardiovascular compliance comparable with the effects of exercise and even the circulatory efforts of athletes (24). The “jogging female heart” may thus protect women against CVD risks during reproductive years. Postmenopausal increase in these risks in women then follow their physiological changes with age as a result of reductions of estrogen and increased fat storage and deposition of fat in abdominal areas. There is a lag of 5–10 years between the average age of menopause and noticeable accelerations of female physiological dysregulation in our data. Although the wide use of estrogen replacement therapies among U.S. women could have contributed to the delayed deterioration (1,3), the fact that the timing of change is consistent with those in mortality shown from historical data on other industrialized countries (3) suggests that this may reflect the latency of physiological disorders in the absence of estrogen and related modulating factors.
Women exhibit a higher cumulative burden of physiological dysregulation across multiple systems indicated by the AL than men. Although such difference is small before menopause, it grows larger afterward. The relationship between the AL and age can be used to characterize the rate of biological aging (17). A faster rate of increase in the AL with age in women compared with men indicates the lack of a female biological aging superiority hypothesized in a previous study of Japanese clinical sample (32). In fact, this finding, together with that of the MetS, indicates the loss of female advantages in various biological functions at older ages that are highly consistent with the reduction of sex difference in all-cause and CVD mortality with age. Reduction of the female excess in inflammation with age, on the other hand, may be one key factor that contributes to a persistent female advantage in survival into the old age. We would like to caution that the AL should not be interpreted as merely a sum or linear combination of individual systems it encompasses, but rather as an indicator of biological complexity of the comorbidity process and general vulnerability to stress, as suggested by recent literature on the measurement of frailty (40,41). The summary indices of inflammation, MetS, and AL, therefore, should be considered as distinct aspects of physiological dysregulation and results on their sex and age variations should be interpreted accordingly.
These findings on sex differences in age-specific changes in physiological dysregulation also have implications for profound sex differences in prevalence of functional disability in old ages (42). Chronic inflammation, metabolic disorders, and a high AL impose burdens on physical functions, the accumulation of which initiate the progressive disablement process which then leads to physical disability as the end product (43). And higher levels and faster rates of deterioration of all three indices in older ages correspond well to the higher prevalence of disability and steeper declines in physical functions for women than for men documented in previous research. Given the longer female life expectancy, interventions aimed at promoting healthy aging and quality of life for women should thus be targeted at eliminating these specific predisease pathways to disability.
We find that differential exposures and vulnerabilities to social status and behaviors partially account for the sex differences in age patterns of various biological functions. In addition, the fact that the associations of behavioral factors such as smoking and obesity with certain indicators of physiological dysregulation such as inflammation and MetS vary by sex and/or age groups should be taken into account in future investigations. A considerable amount of sex and age variation in most physiological parameters is unexplained by the inclusion of the above and other factors, however. This provides a most compelling reason for more in-depth examination of the biological base for sex differences and their age changes. For example, recent biological aging research builds on the free radical theory of aging to elucidate the mechanism by which estrogens may affect longevity. There is evidence of more measured oxidative stress damage to DNA in males than in females (24) and that 17β-estradiol upregulates antioxidant gene expression and thus protects females against aging-related diseases (44,45). These are promising directions for future studies of longevity. It is challenging, however, to test the mechanisms found at the cellular level with data measured at the population level as it involves studies across levels of organization (1). Because these hypotheses have been generated from studies of animals, human cells, and organs in experimental settings that are largely detached from social life experiences, the question of how sex differences arise from inevitable interactions between the physical and social worlds in human populations remains to be addressed.
This study is based on cross-sectional data as prospective data containing multiple biomeasures are rare. The age trajectories revealed here then represent the distributions by age of the surviving population from cohorts born earlier. There are two caveats in the interpretation of results. First, the mortality risk and hence force of selection with respect to physiological status is greater with the increase of age. Therefore, the acceleration of physiological dysregulation with age is reduced by selective survival. Selective survival is not the only explanation, however, because the age patterns vary by physiological measures and adjustment of other risk factors. If selection slowed down the age increase in frailty for all groups, one should have observed similar downward age patterns in both sexes. But this is not observed in findings of MetS or AL. A related issue is that selection may have decreased population heterogeneity later in life (41,46). If this is the case, one should have observed smaller gaps in men and women in old age. But instead the gap increases for the AL after the age of 60. Second, the age variations may be confounded with cohort differences. There is evidence that recent cohorts (especially those born after 1955) show increases in obesity rates (47), which likely inflates the age-specific estimates of metabolic disorders for the younger age groups in the current study samples. Modeling the age, period, and cohort effects is a difficult methodological issue and requires additional time periods of data (48). In sum, collection and analysis of longitudinal data on biomarkers should be a priority for future research because they would facilitate the test of selection effect and produce estimates of within-cohort age trajectories that represent true developmental changes with age and hence help to distinguish aging and cohort effects.
Limitations in the measurement of biological variables invite future investigations using a broader spectrum of markers. Although the NHANES is among the few national surveys that offer a wide range of indicators of biological functions, many other biomarkers are not currently included such as other proinflammatory cytokines (eg, interleukin-6) and physiological stress responses in terms of stress hormones regulated by the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system. There may be sex differences in biobehavioral response to stress (“fight-or-flight” for males vs “tend-and-befriend” for females) that have a neuroendocrine basis which may modulate risks for stress-related disorders and survival (49). Including measurement of these responses is essential to a more comprehensive characterization of sex difference in physiology in population.
This study presents some initial evidence of the potential physiological pathways through which age changes in sex mortality gaps occur. The full establishment of the links between physiological processes and sex differences in age-specific mortality at the individual level requires additional analysis of mortality follow-up data, which we now are conducting.
FUNDING
This work was supported by National Institute of Aging Grant No. K01AG036745 awarded to Y.Y and University Cancer Research Funds at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgments
We are grateful to those who provided helpful comments for this paper when it was presented at the 2010 Annual Meetings of the Population Association of America, biodemography of aging session.
References
- 1.Institute of Medicine. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Weden MM, Brown RA. Historical and life course timing of the male mortality disadvantage in Europe. Biodemography Soc Biol. 2008;53:61–79. doi: 10.1080/19485565.2006.9989117. [DOI] [PubMed] [Google Scholar]
- 3.Horiuchi S. Postmenopausal acceleration of age-related mortality increase. J Gerontol A Biol Sci. 1997;52A:B78–B92. doi: 10.1093/gerona/52a.1.b78. [DOI] [PubMed] [Google Scholar]
- 4.Travato F, Lalu NM. Contribution of cause-specific mortality to changing sex differences in life expectancy: seven nations case study. Soc Biol. 1998;45:1–20. doi: 10.1080/19485565.1998.9988961. [DOI] [PubMed] [Google Scholar]
- 5.Waldron I. What do we know about causes of sex differences in mortality? A review of the literature. Popul Bull. 1985;18:59–76. [PubMed] [Google Scholar]
- 6.Manton KG, Woodbury MA, Stallard E. Sex differences in human mortality and aging at late ages: the effect of mortality selection and state dynamics. Gerontologist. 1995;35:597–608. doi: 10.1093/geront/35.5.597. [DOI] [PubMed] [Google Scholar]
- 7.Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Life Spans. Amsterdam, The Netherlands: Elsevier; 2007. [Google Scholar]
- 8.Hermes GL, Rosenthal L, Montag A, et al. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290:R273–R282. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Qiao Q, Laatikainen T, Zethelius B, et al. Comparison of definitions of metabolic syndrome in relation to the risk of developing stroke and coronary heart disease in Finnish and Swedish cohorts. Stroke. 2009;40:337–343. doi: 10.1161/STROKEAHA.108.518878. [DOI] [PubMed] [Google Scholar]
- 11.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 15.Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. Population and Development Review. 2004;30(suppl):89–107. [Google Scholar]
- 16.Crimmins EM, Johnston M, Hayward M, et al. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol. 2003;38:731–723. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 17.Seeman TE, McEwen BS, Rowe JW, et al. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;90(8):4770–4774. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuurs AHWM, Verheul HAM. Effects of gender and sex steroids on the immune response. J Steroid Biochem Mol Biol. 1990;35(2):157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 19.Washburn TC, Medearis DN, Jr, Childs B. Sex differences in susceptibility to infections. Pediatrics. 1965;35:57–64. [PubMed] [Google Scholar]
- 20.Da Silva JA. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–117. doi: 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 21.Goran MI. Energy metabolism and obesity. Med Clin North Am. 2000;84:347–362. doi: 10.1016/s0025-7125(05)70225-x. [DOI] [PubMed] [Google Scholar]
- 22.Björntorp PA. The regulation of adipose tissue distribution in humans. Int J Obes (Lond) 1996;20:291–302. [PubMed] [Google Scholar]
- 23.Laws A, Hoen HM, Selby JV, et al. Differences in insulin suppression of free fatty acid levels by gender and glucose tolerance status. Arterioscler Thromb Vasc Biol. 1997;17:64–71. doi: 10.1161/01.atv.17.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Eskes T, Haanen C. Why do women live longer than men? Eu J Obstetr Rep Biol. 2007;133:126–133. doi: 10.1016/j.ejogrb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Kim JK, Alley D, Seeman T, et al. Recent changes in cardiovascular risk factors among women and men. J Womens Health (Larchmt) 2006;15:734–746. doi: 10.1089/jwh.2006.15.734. [DOI] [PubMed] [Google Scholar]
- 27.Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) Cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Loucks EB, Berkman LF, Gruenewald TL, et al. Relation of social integration to inflammatory marker concentrations in men and women 70–79 years. Am J Cardiol. 2006;97:1010–1016. doi: 10.1016/j.amjcard.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Halvorson DS, Johnsen SH, Mathiesen EB, et al. The association between inflammatory markers and cartoid atherosclerosis is sex dependent: the Tromso Study. Cerebrovasc Dis. 2009;27:392–397. doi: 10.1159/000207443. [DOI] [PubMed] [Google Scholar]
- 30.Goldman NM, Weinstein M, Cornman J, et al. Sex differentials in biological risk factors for chronic disease: estimates from population-based surveys. J Womens Health (Larchmt) 2004;13(4):393–403. doi: 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- 31.Seeman TE, Crimmins E, Huang M, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura E, Miyao K. Sex differences in human biological aging. J Gerontol A Biol Sci Med Sci. 2008;63A:936–944. doi: 10.1093/gerona/63.9.936. [DOI] [PubMed] [Google Scholar]
- 33.CDC. NHANES III and IV Documentation. http://www.cdc.gov/nchs/nhanes.htm. Accessed July 1, 2009. [Google Scholar]
- 34.Boronat M, Saavedra P, Varillas VF, et al. Differences in traditional and emerging cardiovascular risk factors of subjects discordantly classified by metabolic syndrome definitions of the international diabetes Federation and the National Cholesterol Education Program. Nutr Metab Cardiovasc Dis. 2009;19(6):409–416. doi: 10.1016/j.numecd.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Laboratory Procedures Used for NHANES III, 1988–1994. 1996. http://www/cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. Accessed July 1, 2009. [Google Scholar]
- 36.CDC. NHANES IV Laboratory Methods, 1999–2000. http://www/cdc.gov/nchs/methods99_00.htm. Accessed July 1, 2009. [Google Scholar]
- 37.Crimmins EM, Alley D, Reynolds SL, et al. Changes in biological markers of health: older Americans in the 1990s. J Gerontol A Biol Sci Med Sci. 2005;60A:1409–1413. doi: 10.1093/gerona/60.11.1409. [DOI] [PubMed] [Google Scholar]
- 38.National Institute of Health. Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, MD: National Institute of Health; 2001. (Executive Summary) (NIH Publication No. 01-3670) [PubMed] [Google Scholar]
- 39.Wasserman L. All of Statistics: A Concise Course in Statistical Inference. New York: Springer; 2004. [Google Scholar]
- 40.Fried LP, Xue Q, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64A:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leveille SG, Penninx BWJH, Melzer M, et al. Sex differences in the prevalence of disability in old age: the dynamics of incidence, recovery, and mortality. J Gerontol Soc Sci. 2000;55B:S41–S50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, Guralnik JM, Simonsick E, et al. Progressive versus catastrophic disability: a longitudinal view of the disablement process. J Gerontol Med Sci. 1996;51A:M123–M130. doi: 10.1093/gerona/51a.3.m123. [DOI] [PubMed] [Google Scholar]
- 44.Borras C, Gambini J, Gomez-Cabrera MC, et al. 17ß-oestradiol upregulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 45.Viña J, Borras C, Gambini J, et al. Why females live longer than males: control of longevity by sex hormones. Sci Aging Knowledge Environ. 2005;23:pe17. doi: 10.1126/sageke.2005.23.pe17. [DOI] [PubMed] [Google Scholar]
- 46.Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- 47.Reither E, Hauser R, Yang Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the U.S. Soc Sci Med. 2009;69:1439–1448. doi: 10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Land KC. Age-period-cohort analysis of repeated cross-section surveys: fixed or random effects? Soc Methods Res. 2008;36:297–232. [Google Scholar]
- 49.Taylor SE, Klein LC, Lewis BP, et al. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.