Abstract
In animal studies, caloric restriction resulting in increased longevity is associated with a reduction in body temperature, which is strain specific and likely under genetic control. Small studies in humans have suggested that temperatures may be lower among elderly populations, usually attributed to loss of thermoregulation. We analyzed cross-sectional data from 18,630 white adults aged 20–98 years (mean 58.3 years) who underwent oral temperature measurement as part of a standardized health appraisal at a large U.S. health maintenance organization. Overall, women had higher mean temperatures (97.5 ± 1.2°F) than men (97.2 ± 1.1°F; p < .0001). Mean temperature decreased with age, with a difference of 0.3°F between oldest and youngest groups after controlling for sex, body mass index, and white blood cell count. The results are consistent with low body temperature as a biomarker for longevity. Prospective studies are needed to confirm whether this represents a survival advantage associated with lifetime low steady state temperature.
Keywords: Lower body temperature, Biomarkers, Longevity
STUDIES of body temperature in large cohorts of human participants are rare. Although body temperature is recognized as a clinically useful physiological parameter in the context of infection or extreme environmental exposures, few epidemiological studies have included body temperature as a routine measurement. One consistent observation that has emerged from studies of human body temperature, however, has been that advanced age is associated with lower body temperature. In the landmark cross-sectional studies of Wunderlich in the 1860s—which included 25,000 participants and established the “normal” body temperature as 98.6°F—lower body temperatures among the elderly participants were observed (1). The largest reported longitudinal study of body temperature in humans, reported a decade ago among men in the Baltimore Longitudinal Study of Aging, found that participants with core body temperatures in the lower 50% of the study population had significantly lower mortality than those with body temperatures in the upper 50% over 25 years of follow-up, suggesting an element of selection (2). Alternatively, lower body temperatures at older ages may reflect a loss of thermoregulation, which has been demonstrated with aging and is associated with shorter life spans in mice (3,4).
Most studies of longevity and body temperature in animals have been performed in the context of caloric restriction. Studies in a variety of mammalian homeothermic species have shown that caloric restriction is associated with both lengthened life span and lower core body temperature (5). The effect of enforced reduced dietary intake on lowering temperature in mice appears to be under genetic control (6,7). Other studies in mice have demonstrated that the association of lower body temperature and increased longevity can occur even in the absence of caloric restriction (8).
We have studied the distribution of body temperature across the age spectrum in more than 18,000 participants who underwent oral body temperature determination by a defined protocol (an accurate measure of core body temperature in humans [9]) as part of a standardized health appraisal visit. An extensive associated database allowed us to explore associations of other physiological parameters with oral temperature.
METHODS
The study was conducted using a cohort recruited from the Kaiser San Diego Health Appraisal Clinic 1998–2001 and consenting to use of their data and blood samples for genetic and other studies as approved by the Institutional Review Boards of both Kaiser Permanente Southern California and The Scripps Research Institute. An annual health appraisal at the clinic is available to all members of Kaiser San Diego. All patients seen at the clinic answered a 400-item questionnaire regarding their medical history and underwent a standard series of screening tests, including measurement of complete blood cell count, thyroid-stimulating hormone, and vital signs, including oral temperature, by standard protocols (Appendix). At the time of the data collection for this study, the clinic was seeing more than 45,000 patients per year. A total of 27,852 patients consented to participate in the study.
Body temperature was measured by medical assistants trained in Health Appraisal Clinic–specific procedures according to a standardized protocol using an oral thermometer with a digital display. Participants were instructed not to eat, drink, or smoke for 15 minutes prior to the temperature measurement. The thermometer's covered probe was positioned in the sublingual pocket until the final display tone was heard. Of the 27,852 participants in the cohort, measurements were not available for 1,114 participants (4.0%). We further limited our analysis to participants with normal thyroid-stimulating hormone (0.3–3.0 ng/mL) and self-designated “white” ethnicity, so that a total of 18,630 participants were included in the analysis.
All statistical analyses were performed using SAS 9.1 (Cary, N. C.), and all analyses were done separately for men and women. Temperature was analyzed as a continuous variable. Age categories were defined by 10-year intervals beginning with 20–29 years and ending with 80 years and older. Body mass index (BMI) measured in kilogram per square meter was categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30) according to definitions recommended by the National Heart Lung and Blood Institute. White blood cell (WBC) count was divided by quartiles separately in males and females.
Mean and standard deviation of temperature were calculated by age group. One-way analysis of variance was used to compare mean body temperatures by age group. Pair-wise comparisons were made with the youngest age group (20–29 years) as the control using Dunnett's correction for multiple comparisons. Univariate associations of BMI and WBC with body temperature were assessed in each age group by one-way analysis of variance and pair-wise comparisons again using Dunnett's correction. Analysis of covariance using SAS Proc GLM was used to assess the relationship of age to body temperature controlling for BMI and WBC count. Associations with body temperature were also examined in multiple linear regression models using age, BMI, and WBC count as continuous variables.
RESULTS
The study cohort consisted of 18,630 white participants aged 20–98 years. Mean age was 58.0 years with approximately equal numbers of men and women (Table 1). Overall mean body temperature for the cohort was 97.3°F. The mean temperature in women (97.5 ± 1.2°F) was higher than in men (97.2 ± 1.1°F; p < .0001).
Table 1.
Characteristics of Cohort
| Women | Men | |
| N | 9,227 | 9,403 |
| Mean age (SD, y) | 57.3 (13.5) | 58.6 (13.3) |
| Range (y) | (20–98) | (20–92) |
| Mean body temperature (SD, °F) | 97.5 (1.2) | 97.2 (1.1) |
Mean body temperature decreased with age in both men and women (Table 2). Among women, temperatures were significantly lower in all age groups more than 40 years old compared with the 20- to 29-year-old age group. In men, differences were significant only when the oldest two groups were compared with the youngest group. Further comparison by age showed that temperatures less than 96°F (5th percentile of the overall group) were significantly more common and temperatures more than 98°F (95th percentile of the overall group) were significantly less so in older (≥60 years) compared with younger participants (59 years and younger; Table 3 and Figure 1).
Table 2.
Mean Body Temperature (°F) by Age and Sex
| Age Group (y) | Women |
Men |
||
| N | M (SD) | N | M (SD) | |
| 20–29 | 210 | 97.70 (1.08) | 162 | 97.28 (0.99) |
| 30–39 | 729 | 97.77 (1.11) | 617 | 97.25 (1.13) |
| 40–49 | 1,724 | 97.73 (1.15) | 1,571 | 97.26 (1.28) |
| 50–59 | 2,316 | 97.45 (1.12)* | 2,482 | 97.20 (1.07) |
| 60–69 | 2,403 | 97.31 (1.17)* | 2,458 | 97.11 (1.08) |
| 70–79 | 1,505 | 97.32 (1.14)* | 1,691 | 97.06 (1.09)* |
| 80+ | 339 | 97.36 (1.30)* | 422 | 97.01 (1.13)* |
Notes: *p < .05 compared with youngest age group (20–29 y) of the same sex corrected with Dunnett's test for multiple comparisons.
Table 3.
Frequency of Temperatures <96°F and >98°F by Age and Sex
| Age Group (y) | Women |
Men |
||||
| N | <96°F, n (%) | % >98°F, n (%) | N | <96°F, n (%) | >98°F, n (%) | |
| 20–59 | 4,979 | 345 (6.9) | 1,929 (38.7) | 4,832 | 569 (11.8) | 1,179 (24.4) |
| 60+ | 4,247 | 456 (10.7)* | 1,167 (27.5)* | 4,571 | 623 (13.6)† | 850 (18.6)* |
Notes: *p < .001 compared with younger age group of the same sex by chi-square.
p = .007 compared with younger age group of the same sex by chi-square.
Figure 1.
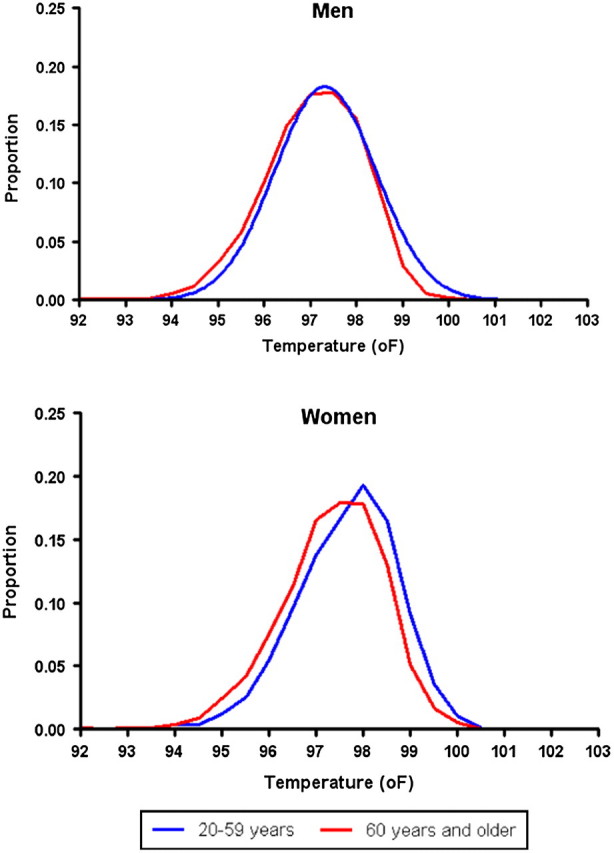
Distribution of body temperatures by age and sex.
Obesity was associated with higher mean body temperatures in both men and women (Figure 2). Obese participants had a mean temperature of approximately 0.3–0.5°F higher than normal weight participants of the same sex and age, a difference which was statistically significant for all groups except men aged 30–39 years and women aged 80 years and older.
Figure 2.
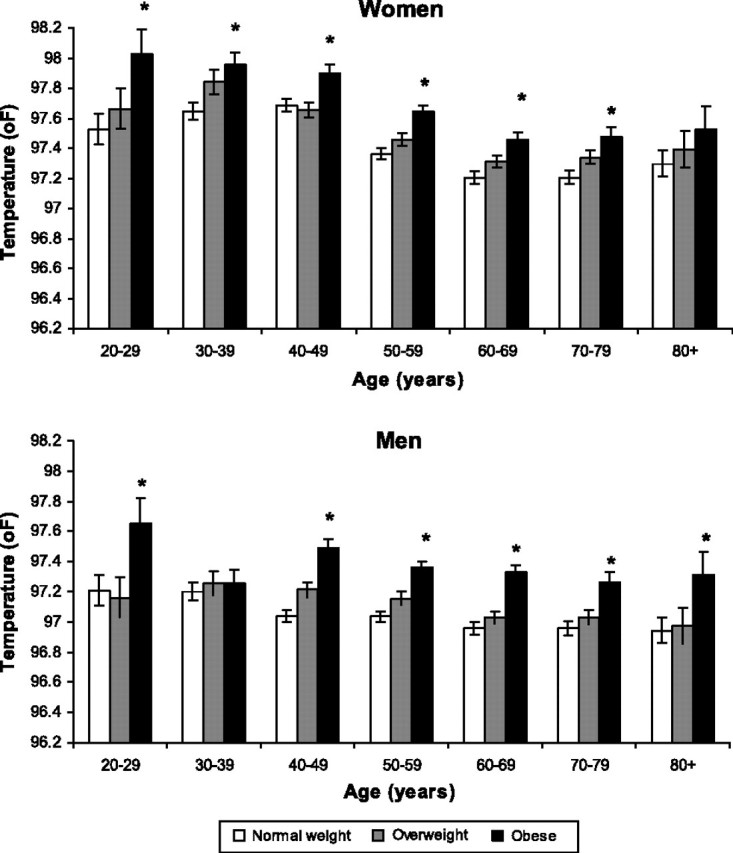
Mean body temperature by body mass index, age, and sex. Error bars = ±standard error of the mean. *p < .05 compared with normal weight group of same age category corrected with Dunnett's test for multiple comparisons.
A higher WBC count was also associated with higher mean body temperature across age groups (Figure 3). The trend was more consistent for men, with participants in the highest WBC quartile having mean body temperatures that were approximately 0.4°F higher than participants with the lowest WBC quartile of the same age group, a difference that was significant in all age groups except the youngest and oldest. For women, the difference in mean temperature between the participants in the highest and lowest WBC quartiles reached statistical significance only in the 20- 29-, 40- 49-, and 50- to 59-year-old groups.
Figure 3.
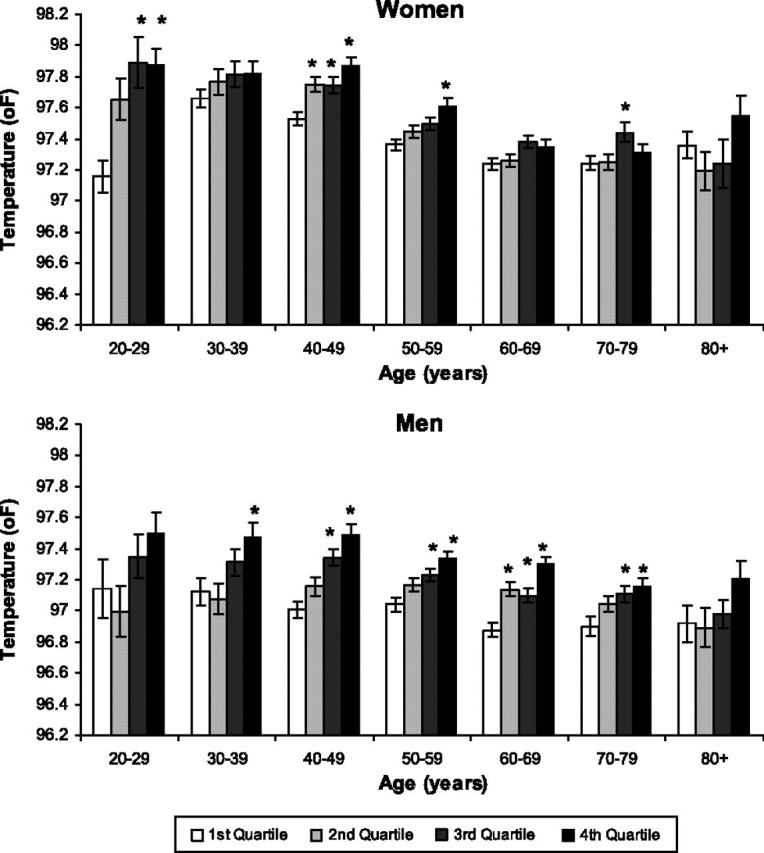
Mean body temperature by white blood cell (WBC) count, age, and sex in white participants with normal thyroid-stimulating hormone. WBC quartiles defined by cutoff values (in 106/mL) of 5.3, 6.4, and 7.5 in men and 5.5, 6.5, and 7.6 in women, respectively. Error bars = ±standard error of the mean. *p < .05 compared with the lowest WBC quartile of the same sex and ethnic group corrected with Dunnett's test for multiple comparisons.
Thyroid-stimulating hormone levels within the normal range were not significantly associated with body temperature (data not shown).
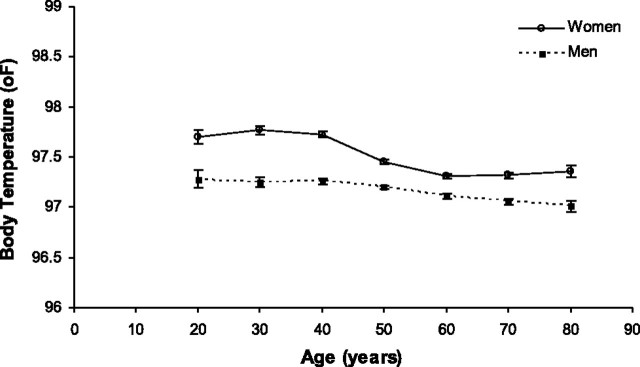
In sex-specific multivariable linear regression models including age, BMI, and WBC, all three variables remained statistically significantly associated with body temperature in both men and women (p < .0001 for each variable). The adjusted mean values controlling for BMI and WBC were within 0.01°F of the unadjusted means for each age group. The decrease in body temperature with age in men was linear across the age range, whereas the decrease among women occurred primarily between ages 40 and 60 years, with no statistically significant change in temperature across the younger and older age ranges (Figure 4). The greatest difference in mean body temperature between men and women was observed in the 20–40 year age range in which women's mean temperature was approximately 0.45°F higher than men’s.
Figure 4.
Mean body temperature by age adjusted for body mass index and white blood cell count in white participants with normal thyroid-stimulating hormone. Error bars = ±standard error of the mean.
DISCUSSION
In this large study of asymptomatic participants attending a health appraisal clinic and undergoing standardized vital sign measurement, mean body temperature of the population overall was 97.3°F. This observation is consistent with other studies in a variety of populations that have found that normal body temperature defined as the population mean is lower than the long-standing accepted norm of 98.6°F (1,10). Our findings of higher mean temperatures in women compared with men with differences in the range of 0.3–0.4°F are also consistent with the findings of others (1,11). Our most significant observation is that mean body temperature varies by age, being significantly lower in the oldest age groups compared with the youngest in both men and women.
Obesity was associated with higher mean body temperatures in every age group for both men and women. Little has been published regarding the relationship of BMI to core body temperature. Some have hypothesized that obesity is associated with lower body temperature, consistent with a lower resting metabolic rate (12). Of the five identified studies examining temperature and body weight, three (encompassing a total of 334 participants) found lower values (11,13,14) while two, with a total of 866 individuals, reported higher body temperatures associated with increased BMI (15,16). Our data gathered in a far larger population than the sum of all the prior analyses clearly indicate that obesity is associated with higher body temperature in an almost quantitative manner. It is possible that the association is based on the function of adipose tissue as an inflammatory organ with adipose mass related to total inflammatory mediator production. However, values for serum cytokine and chemokine concentrations were not available.
In view of the emerging literature on caloric restriction, lower body temperature and longevity in animals, of particular interest were our results regarding the relationship between age and body temperature. The decrease with age observed in our study is consistent with the general trend observed in 27 studies of body temperature (encompassing a total of <4,000 individuals), although many of the included studies were small and had a narrower age range (10). Two general explanations are possible for the inverse relationship between age and body temperature: Body temperature may decrease in individuals with aging due to decompensation of temperature regulatory mechanisms with age. Alternatively, individuals who maintain a lower steady state body temperature may have a survival advantage. The cross-sectional nature of our study does not allow us to distinguish between the two. Of note, the largest decrease in mean body temperature among women was observed in the perimenopausal age range, raising the possibility that lower temperatures in older women may be related to changes in hormone levels. However, it is well known that menopausal symptoms are associated with transient increases in core body temperature in contrast to the overall decrease in core body temperature among women in this age group seen in our study (17). Thus the hormonal influences on thermoregulation with aging in women are likely to be complex, perhaps even independent of the short-term effects, which themselves are not yet well understood.
The support for a relationship between longevity and lower body temperature, independent of caloric restriction, comes from recent studies showing that reducing core body temperature of mice by 0.5–0.6°C through genetic manipulation of the body temperature control centers of the hypothalamus resulted in life span that was 20% longer than in control mice (8). Interestingly, the transgenic mice in this study gained body weight without a significant change in food intake, effectively dissociating the longevity effect of lower body temperature from that of caloric restriction. An accompanying editorial speculated that “if life-span extension could be this simple, one might wonder whether 37°C is indeed the optimal body temperature for humans, and why evolution has not selected for a lower body temperature and longer life span (18).” Our results indicate that this selection may in fact be operative to some degree in humans. The cross-sectional nature of our study does not allow us to directly test whether the results are due to a survival advantage. Support for the notion that lower body temperature leads to longer life spans in humans comes from the Baltimore Longitudinal Study of Aging in which men with body temperatures below the median were shown to have significantly higher survival rates than those with body temperatures above the median temperature over 25 years of follow-up (2).
Our analysis shows that in every age cohort up to age 50, there is a small discrete subset of individuals with body temperatures less than 96°F. The representation of that population increases with increasing age. Conversely, the proportion of individuals with body temperatures more than 98°F is stable until age 50 and drops after age 60.
Our multivariable models showing persistence of the significant association between age and body temperature controlling for BMI are consistent with the idea that the effects are at least partially independent. This is particularly important to establish given the decreased prevalence of obesity in the older age groups, which may have contributed to the lower mean body temperatures independent of a survival advantage of the lower temperature itself.
There is indirect support for the association of lower body temperature and longer life spans from numerous studies of caloric restriction in animals (5,6). In these studies, animals of a wide variety of species, maintained on diets typically containing 30–40% fewer calories than normal, exhibit both lower body temperatures and life spans of up to 50% longer than normal. Though much more limited in number and scale, studies also suggest the same phenomenon occurs in humans. In preliminary results of a randomized clinical trial testing the effects of 25% calorie reduction in 150 human participants, core body temperature was significantly reduced by about 0.4°F after 6 months (19). The association between the lower body temperature and lengthening of life span as a result of caloric restriction has led to the use of lower body temperature as a “biomarker” of extended survival not only in studies of caloric restriction directly but also in studies in the newly emerging field of “calorie restriction mimetics (20).”
A potential limitation of our analysis is the fact that body temperature measurements were made under clinical conditions with the time of the measurement varying among the participants. The diurnal pattern of body temperature within individuals has been well established, with lowest temperatures occurring in the morning and the highest temperatures occurring in late afternoon, although the range of the differences can vary widely between individuals. In this study, the time of temperature measurement was not consistent between patients and was not recorded. However, given the structure of the clinic, most measurements could be expected to have been made between 10 AM and 2 PM, a time frame which avoids the daily extremes of body temperature. In addition, the large sample sizes serve to in effect average out such potential differences.
Further understanding of the relationship between body temperature and longevity in humans will likely benefit from animal studies that are identifying genetic influences on body temperature as well as the effect of body temperature on multiple cellular processes, such as protein structure and enzymatic activity, that may contribute to aging. Interestingly, the murine candidate “longevity genes,” including the Prop-1 and Pit-1 loci, are associated with reduced body temperature (21). Furthermore, the classical chaperone system is known to be induced by “heat shock.” It is possible that a lower body temperature reduces the total load of misfolded proteins that over a lifetime may have a significant mortality effect. Involvement of the insulin and insulin-like growth factor (IGF) signaling pathway may also be critical. It has been found that IGF and insulin have hyperthermic effects in experimental animals and that hemizygous IGFr knockout animals have decreased body temperatures, longer life spans, and an enhanced capacity to deal with the Aβ aggregates associated with Alzheimer’s disease (22–24). From the findings of our study of significant differences in mean body temperature associated with multiple factors in addition to age, including sex, ethnicity, BMI, and WBC, it is clear that it will be important for future studies examining the relationship of age to life span to take these factors into account.
FUNDING
This manuscript is 21055-MEM. Dr. Waalen is supported by a grant from the National Institutes of Health AR054901-01A2.
Appendix
Preventive Medicine Test Guidelines for Participants Attending the Kaiser San Diego Health Appraisal Clinic
| Test | Females |
Males |
||
| <50 years old | >50 years old | <50 years old | >50 years old | |
| Height | X | X | X | X |
| Weight | X | X | X | X |
| Blood pressure | X | X | X | X |
| Oral body temperature | X | X | X | X |
| Visual acuity | X | X | X | X |
| Spirometry | X | X | X | X |
| Fasting glucose | X | X | X | X |
| Complete blood count | X | X | X | X |
| Total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol | X | X | X | X |
| Creatinine | X | X | X | X |
| Syphilis enzyme immunoassay | X | X | X | X |
| Thyroid-stimulating hormone | X | X | X | X |
| Fecal occult blood test | X | X | ||
| Clock Face Test | >65 years | >65 years | ||
| Calcium | X | |||
| Urine CT/GC | X | X | X | X |
| Transferrin saturation, serum ferritin | ||||
| Prostate specific antigen | All members, once in a lifetime | Offered at 50–70 years | ||
| Purified protein derivative skin test for tuberculosis | County Health Department guidelines for San Diego County | |||
| Chest x-ray | All new purified protein derivative skin test for tuberculosis converters | |||
| Potassium | All patients on diuretics, hypertension medications, or potassium supplements | |||
| Audiometry | All members aged 55 years and older and those complaining of hearing loss or requesting an exam | |||
| Mammogram | All women aged 40–74 years every 2 years | |||
| Women aged 40 years and older every year if positive family history for breast cancer in relative older than 50 years | ||||
| Women aged 35 years and older if positive family history for breast cancer in relative younger than 50 years old | ||||
| Electrocardiogram | Baseline at age 50 | |||
| Dual energy x-ray absorptiometry | Per National Osteoporosis Foundation guidelines | |||
| Pap smear | Per American College of Obstetrics and Gynecology guidelines | |||
| Hemoglobin A1C | All diabetics | |||
| Tonometry | All diabetics | |||
| Urinanalysis | All diabetics | |||
| Retinal photos | All diabetics | |||
| Monofilament | All diabetics | |||
References
- 1.Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578–1580. [PubMed] [Google Scholar]
- 2.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales P, Rikke BA. Thermoregulation in mice exhibits genetic variability early in senescence. Age (Dordr) 2010;32:31–37. doi: 10.1007/s11357-009-9109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds MA, Ingram DK, Talan M. Relationship of body temperature stability to mortality in aging mice. Mech Ageing Dev. 1985;30:143–52. doi: 10.1016/0047-6374(85)90003-x. [DOI] [PubMed] [Google Scholar]
- 5.Rikke BA, Johnson TE. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp Gerontol. 2004;39:927–930. doi: 10.1016/j.exger.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Rikke BA, Yerg JE, III, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 7.Rikke BA, Yerg JE, III, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Quantitative trait loci specifying the response of body temperature to dietary restriction. J Gerontol A Biol Sci Med Sci. 2004;59:118–125. doi: 10.1093/gerona/59.2.b118. [DOI] [PubMed] [Google Scholar]
- 8.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 9.Hooper VD, Andrews JO. Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science. Biol Res Nurs. 2006;8:24–34. doi: 10.1177/1099800406289151. [DOI] [PubMed] [Google Scholar]
- 10.Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci. 2002;16:122–128. doi: 10.1046/j.1471-6712.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Richardson C, Roberts J, Gren L, Lyon JL. Cold hands, warm heart. Lancet. 1998;351:1492. doi: 10.1016/S0140-6736(05)78875-9. [DOI] [PubMed] [Google Scholar]
- 12.Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Is obesity associated with lower body temperatures? Core temperature: a forgotten variable in energy balance. Metabolism. 2009;58:871–876. doi: 10.1016/j.metabol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Adam K. Human body temperature is inversely correlated with body mass. Eur J Appl Physiol Occup Physiol. 1989;58:471–475. doi: 10.1007/BF02330699. [DOI] [PubMed] [Google Scholar]
- 14.Rising R, Keys A, Ravussin E, Bogardus C. Conconmitant inter individual variation in body temperature and metabolic rate. Am J Physiol. 1992;263:E730–734. doi: 10.1152/ajpendo.1992.263.4.E730. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson H, Svardsudd K, Larsson B, Welin L, Ohlson LO, Wilhelmsen L. Body temperature in general population samples. The study of men born in 1913 and 1923. Acta Med Scand. 1985;217:347–352. [PubMed] [Google Scholar]
- 16.Rising R, Fontvieille AM, Larson DE, Spraul M, Bogardus C, Ravussin E. Racial differences in body core temperature between Pima Indians and Caucasian men. Int J Obes. 1995;19:1–5. [PubMed] [Google Scholar]
- 17.Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. doi: 10.1016/j.ajog.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Saper CB. Biomedicine. Life, the universe, and body temperature. Science. 2006;314:773–774. doi: 10.1126/science.1135375. [DOI] [PubMed] [Google Scholar]
- 19.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008;94:643–648. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram DK, Zhu M, Mamczarz J, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 21.Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 23.Cohen E, Paulsson JF, Blinder P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Alavez M, Tabarean IV, Osborn O, et al. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]