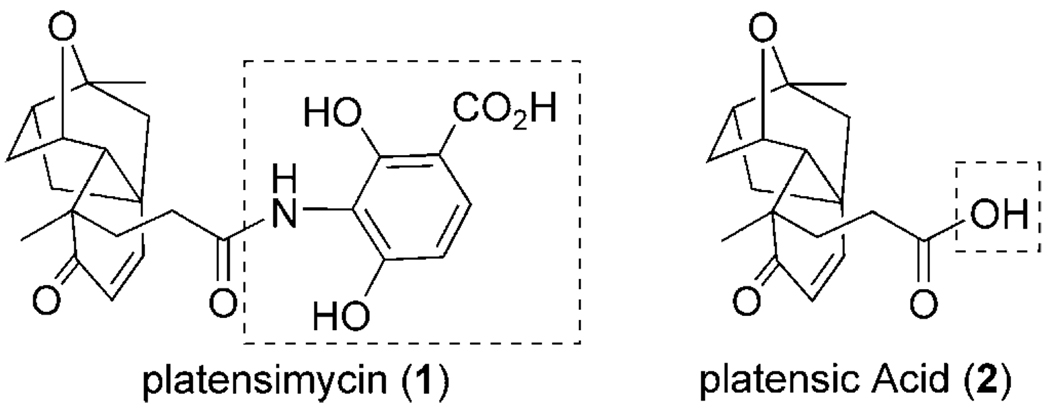
Recently, researchers at Merck disclosed a new natural product, platensimycin (1; Figure 1),[1] which was obtained by screening a large collection of South African soil samples using a novel antibiotic assay approach. Characterization revealed a unique compact core connected to a highly oxygenated and unusual aromatic ring through a propionate tether. Platensimycin has a novel mechanism of action, inhibiting the β-ketoacyl-(acyl carrier protein) synthase (FabF) in the bacterial fatty acid synthetic pathway.[2] Several new members of this class have since been reported.[3] These differ only in functionalization of the carboxylate terminus. This attractive natural product target has also encouraged researchers to engineer strains to improve its production.[4]
Figure 1.
The structures of platensimycin and platensic acid.
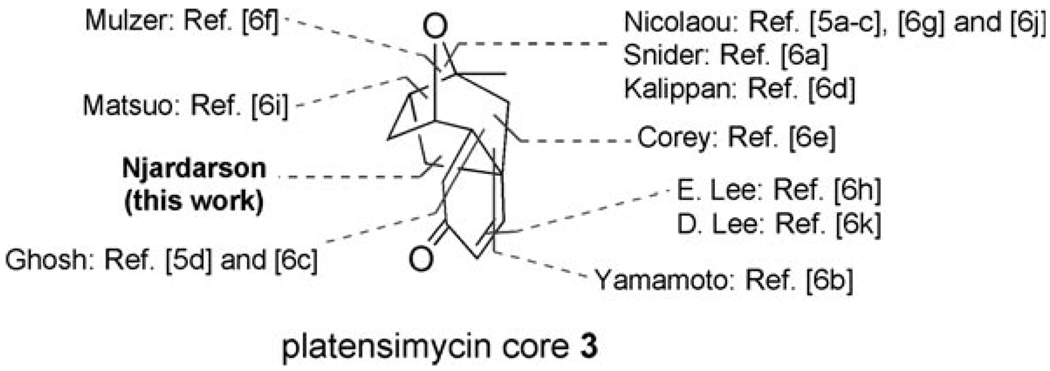
Despite a flurry of synthetic activity, only two groups have completed the total syntheses of platensimycin (1).[5] All other reported efforts have focused on constructing the platensimycin core 3.[6] To highlight the diversity of these synthetic approaches we have chosen to emphasize the last bond formed to complete the platensimycin core as reported by each research group (Figure 2). Recently, a series of derivatives obtained by modifying platensimycin have been reported.[7] Alternatively, several research groups have developed analogues of platensimycin,[8] some of which were equipotent with the natural product.[8a–c] These results bode well for analogue approaches utilizing diverted total synthetic strategies.[9]
Figure 2.
Synthetic approaches to complete the platensimycin core.
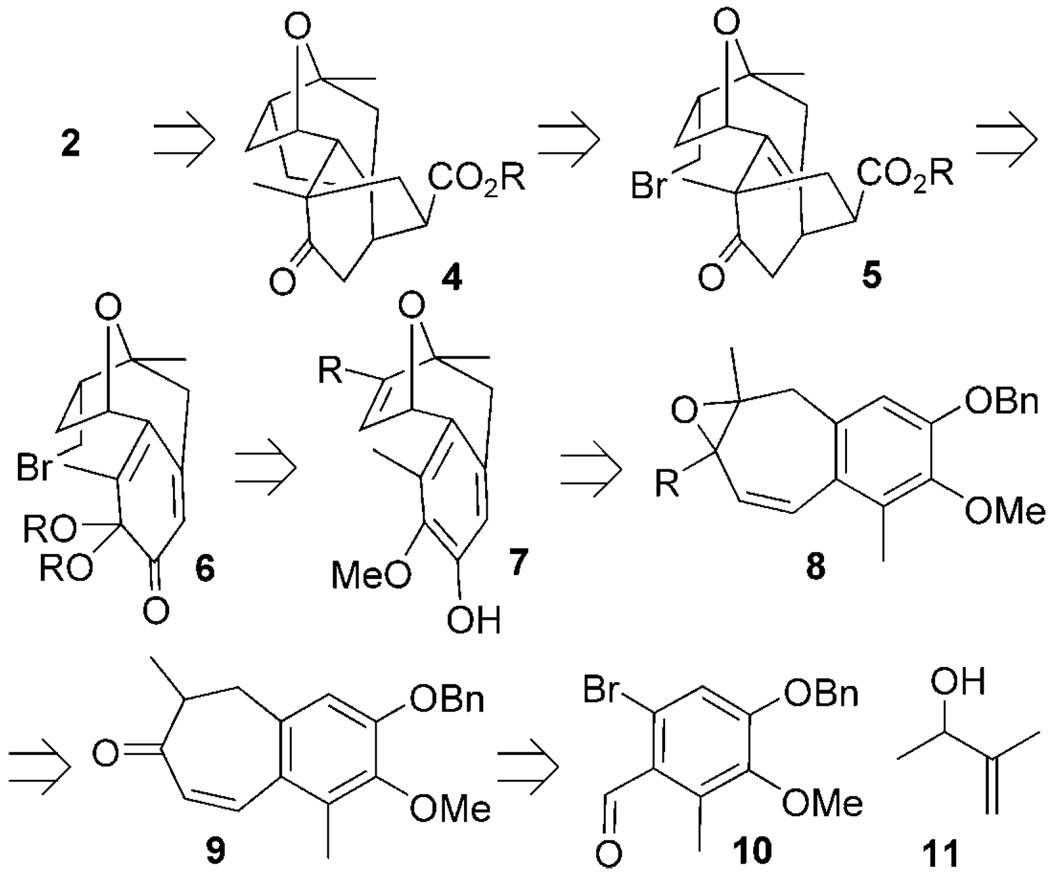
We envisioned a concise retrosynthetic plan for the total synthesis of platensimycin (Scheme 1). Platensic acid (2) was our immediate target as it serves later as the branch point for accessing all the other members of this natural product family. We proposed that 2 could be accessed from 4 by a retro-Michael ring-opening reaction and subsequent hydrolysis of the resulting ester. Radical cyclization of bromide 5 would then be expected to afford the platensimycin carbocyclic core 4. Oxidative dearomatization of 7 and in situ trapping of the ketal 6 with methyl acrylate was expected to provide 5 as the only cycloadduct. In this one remarkable transformation, the aromatic core would be unraveled and primed for the subsequent cyclization step. At the same time, the quaternary center bearing the side chain with the desired oxidation state would be installed by a substrate and regiocontrolled Diels–Alder cycloaddition. Oxatropane 7 would originate from vinyl oxirane 8 using our newly described copper-catalyzed ring-expansion protocol.[10] Epoxidation of the diene obtained from enone 9 could also serve as the asymmetric entry point for this synthesis, which in turn would be assembled in two steps from 10[11] and 11 using a Heck coupling and then an intramolecular condensation.
Scheme 1.
Platensimycin retrosynthetic analysis. Bn = benzyl.
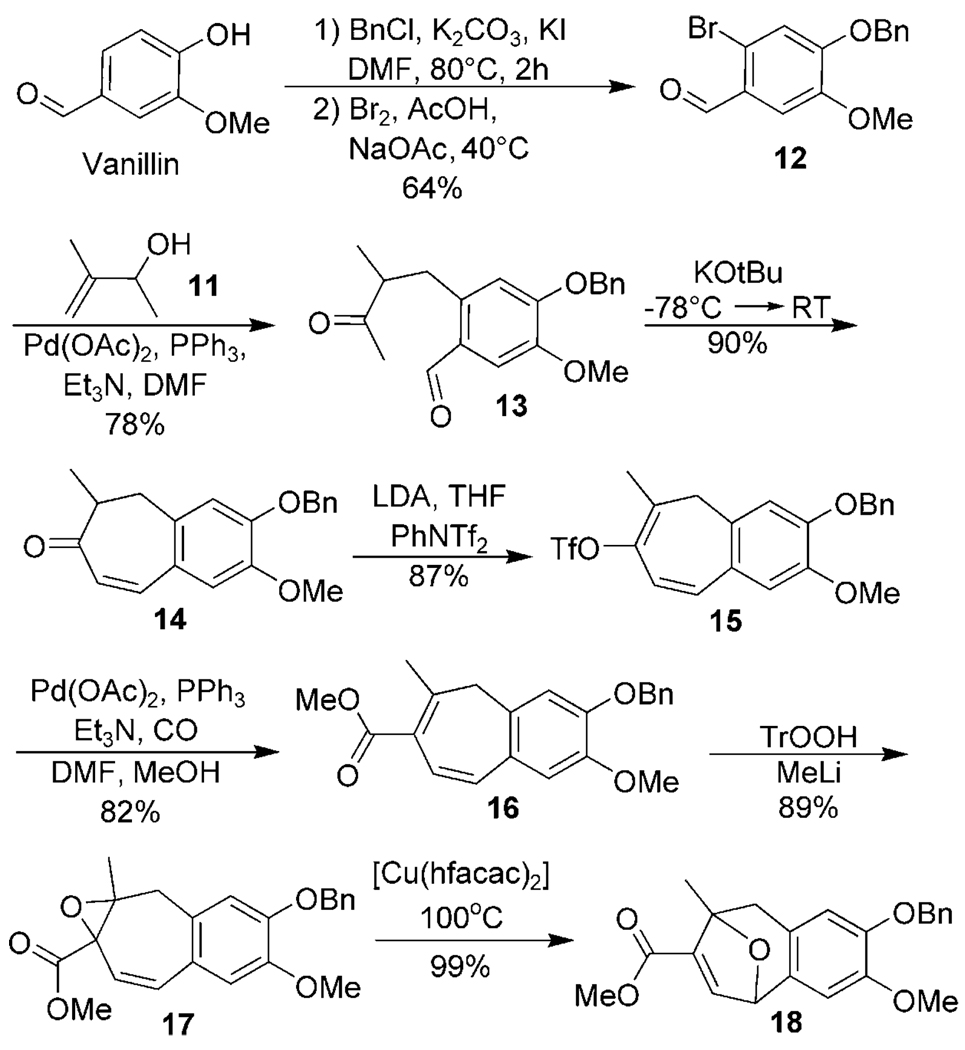
Our synthetic efforts commenced with vanillin[12] which was regioselectively brominated and protected by using known procedures (12, Scheme 2).[13] This substrate was subjected to Heck coupling conditions in the presence of allylic alcohol 11, which furnished keto aldehyde 13.[14] The methyl branching is key to the rapid assembly of the fused ring system 14, ensuring that under the thermodynamic conditions employed, the initial five-membered ring aldol product cannot dehydrate and instead undergoes a retro aldol to give exclusively the seven-membered ring enone. Triflate formation to give 15 was accomplished by deprotonation with LDA[15] and trapping of the resulting enolate with N-phenyl-triflamide. Palladium-mediated carbonylation afforded dienoate 16 in excellent yield.[16] Regioselective epoxidation was accomplished using the highly reactive trityl hydroperoxide,[17] and our new copper-catalyzed ring-expansion protocol formed oxatropane 18.
Scheme 2.
Synthesis of the functionalized aryl-fused oxatropane. DMF = N,N-dimethylformamide, LDA = lithium diisopropylamide, Tf = trifluorosulfonyl, Tr = triphenylmethyl, hfacac = hexafluoroacetyl acetonate.
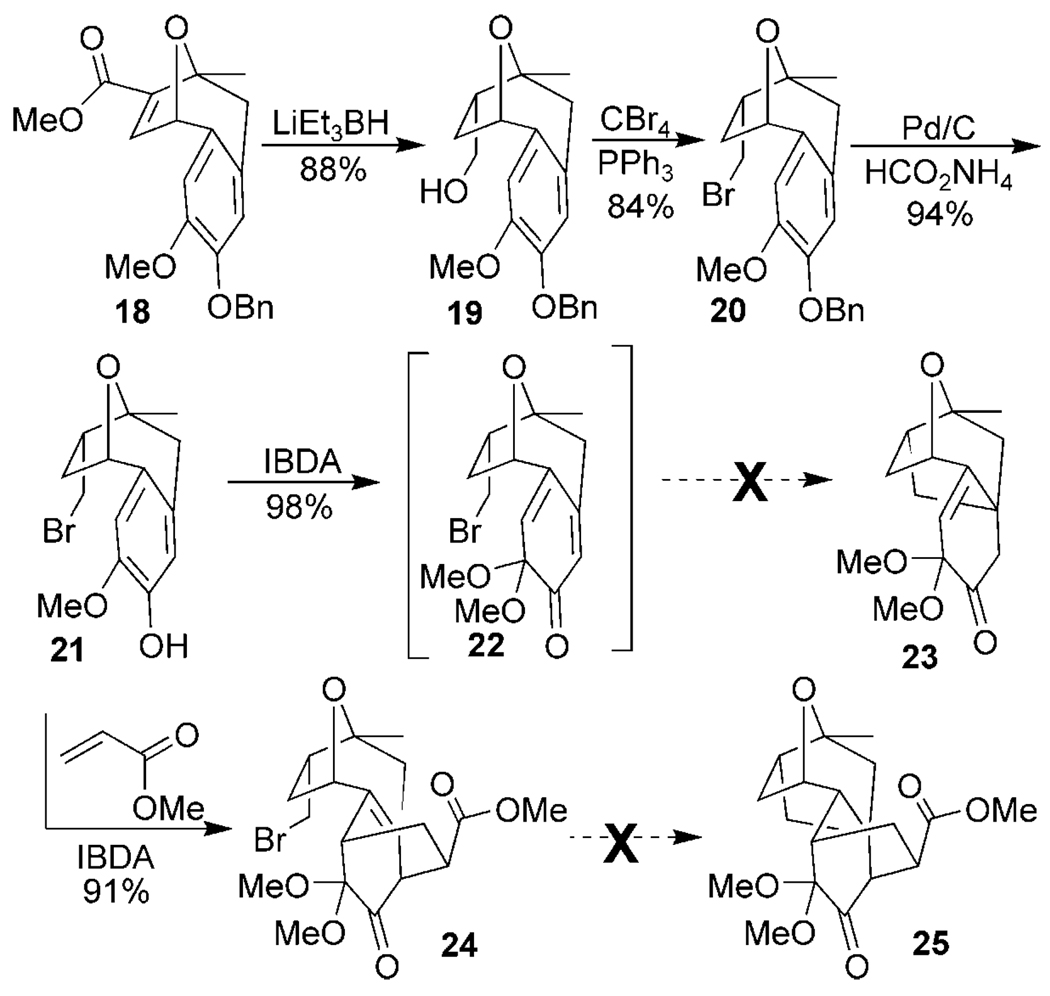
Methyl enolate 18 (Scheme 3) was fully reduced stereo-selectively to form 19 using lithium triethyl borohydride. Primary bromide 20 was accessed using carbon tetrabromide and triphenylphosphine, and the arylbenzyl ether was removed by employing a transfer-hydrogenolysis protocol. Oxidation of 21 using IBDA afforded dimethyl ketal 22 which underwent a facile Diels–Alder dimerization. This process was slow enough, however, to test the proposed cyclization. Unfortunately, all efforts to access 23 using either radical or anionic conditions did not form the desired core, giving instead only the product of bromide reduction and no C–C bond formation.[18] Regardless, diene 22 could be trapped in situ after oxidative dearomatization with methyl acrylate to give the desired cycloadduct 24. Our calculations had indicated that the structure of the new six-membered ring would bring the radical-accepting olefin into closer proximity with the primary radical, and therefore make the cyclization more likely. Unfortunately all attempts to form 25 were not successful, again giving only the product of bromide reduction.
Scheme 3.
Oxidative dearomatization/cyclization attempts. IBDA = iodobenzene diacetate.
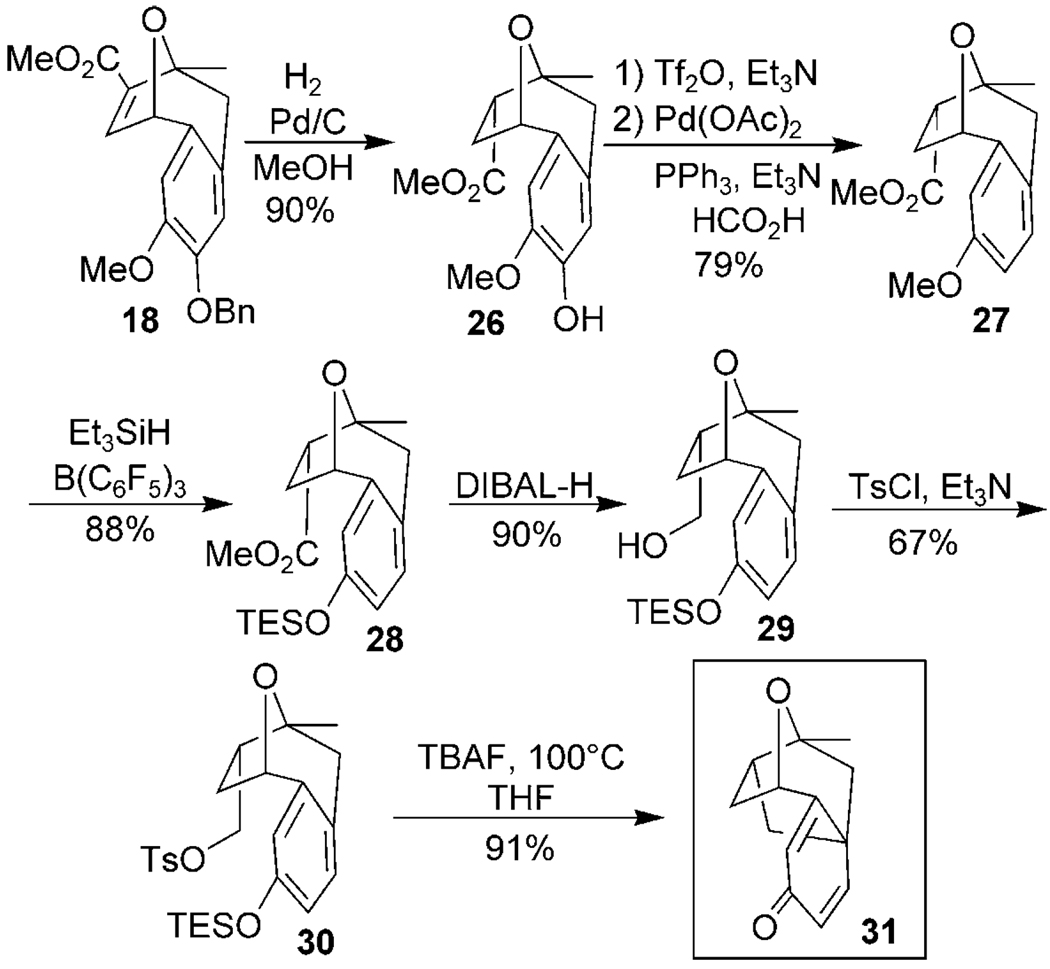
We decided to evaluate a slightly different substrate to determine whether our proposed C–C cyclization strategy to form the platensimycin core was feasible (Scheme 4). To this end oxatropane 18 was converted into 26 by alkene reduction and hydrogenolysis of the benzyl protecting group. Deoxygenation was then accomplished by converting 26 into an aryl triflate and then effecting reductive cleavage using palladium and formic acid to give 27. A remarkably mild phenolic silylation of the remaining arylmethyl ether with triethylsilane and tris(pentafluorophenyl)borane furnished TES-protected phenol 28.[19] The ester was reduced with DIBAL-H to give primary alcohol 29, which was directly activated for displacement by conversion into the tosylate 30. When this cyclization precursor was heated with TBAF it rapidly underwent the alkylative cyclization in excellent yield to form the platensimycin core 31. The 1H and 13C NMR spectra of 31 are identical to those previously reported,[6e,f] thus completing our formal synthesis of platensimycin.
Scheme 4.
Intramolecular alkylative dearomatization. DIBAL-H = diisobutylaluminum hydride, TES = triethylsilyl, Ts = 4-toluenesulfonyl, TBAF = tetra-n-butylammonium fluoride.
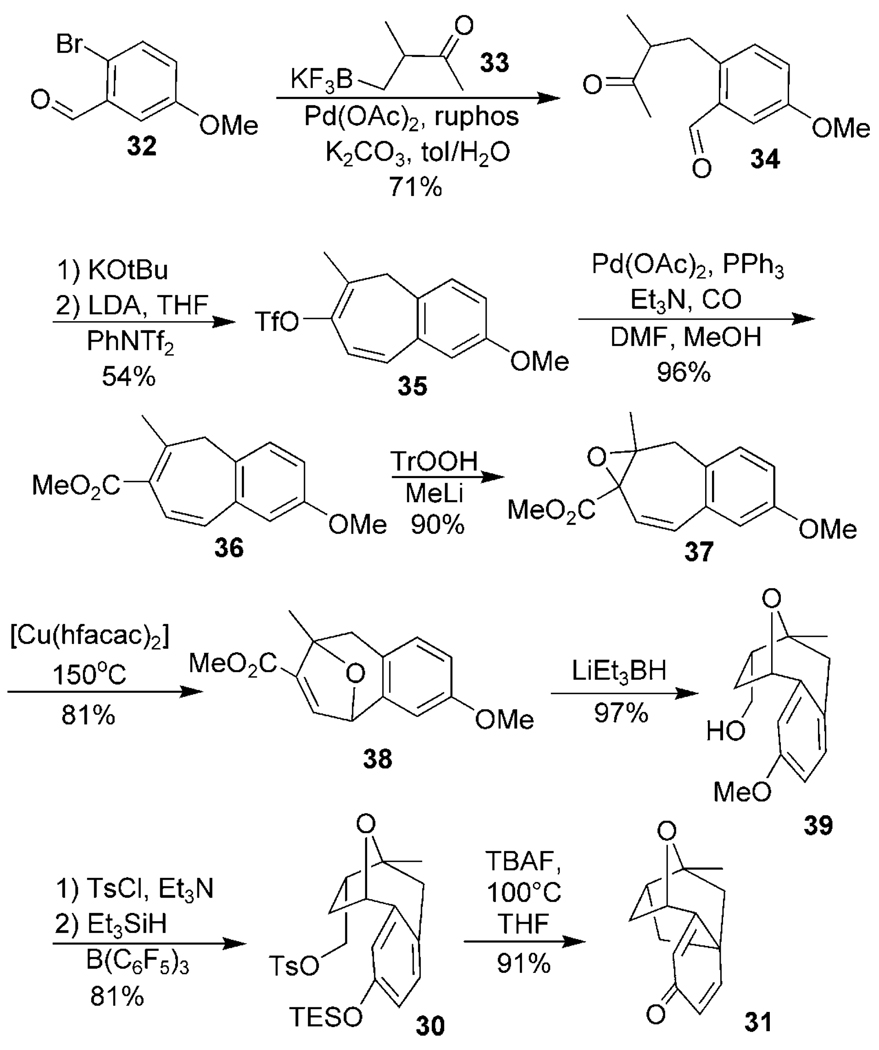
This success inspired us to improve the synthetic approach by starting with brominated anisaldehyde 32 rather than the vanillin analogue 12 (Scheme 5). This approach would allow us to alleviate the steps associated with the late stage deoxygenation necessary in Scheme 4. To that end, commercially available bromide 32 was converted into 34, using the cross-coupling strategy reported by Molander and Petrillo, to directly install the requisite ketone chain.[20] This keto aldehyde cleanly underwent the analogous condensation, triflate formation, and carbonylation using the previously optimized conditions to give 36. Nucleophilic epoxidation with trityl hydroperoxide allowed access to vinyl oxirane 37, which subsequently ring-expanded to oxatropane 38 when subjected to our [Cu(hfacac)2] conditions. Substrate-controlled reduction afforded primary alcohol 39 which was converted into the tosylate and again hydrosilated to give TES-protected phenol 30. The platensimycin core 31 was again accessed by alkylative dearomatization, this time completing the formal synthesis in only ten steps from commercially available precursor 32.
Scheme 5.
Efficient synthesis of the platensimycin core. ruphos = 2-dicyclohexylphosphino-2′,6′-diisopropoxylbiphenyl.
In summary, we have developed a very efficient route to the compact platensimycin core. Our architectural assembly relied on the use of a new copper-catalyzed oxirane ring-expansion in combination with an alkylative dearomatization to complete the core. Other notable features of this synthetic approach include an underutilized phenol ether deprotection, nucleophilic enoate epoxidation, and a mild introduction of a substituted alkyl ketone using a trifluoroborate cross-coupling.
Supplementary Material
Footnotes
We would like to thank NIH-NIGMS (RO1 GM086584) and Cornell University for support of this synthetic program. Fellowships from an NIH Chemical Biology Interface Grant (NAM) and the Hunter Rawlings Presidential Scholar Program (EAB) are gratefully acknowledged.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200903347.
References
- 1.a) Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]; b) Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. J. Am. Chem. Soc. 2006;128:11916–11920. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]; c) Häbich D, von Nussbaum F. ChemMedChem. 2006;1:951–954. doi: 10.1002/cmdc.200600145. [DOI] [PubMed] [Google Scholar]; d) Herath KB, Attygalle AB, Singh SB. J. Am. Chem. Soc. 2007;129:15422–15423. doi: 10.1021/ja0758943. [DOI] [PubMed] [Google Scholar]
- 2. Zhang YM, White SW, Rock CO. J. Biol. Chem. 2006;281:17541–17544. doi: 10.1074/jbc.R600004200. Young K, Jayasuriya H, Ondeyka JG, Herath K, Zhang C, Kodali S, Galgoci A, Painter R, Brown-Driver B, Yamamoto R, Silver LL, Zheng Y, Ventura JI, Sigmund J, Ha S, Basilo A, Vicente F, Tormo JR, Pelaez F, Youngman P, Cully D, Barret JF, Schmatz D, Singh SB, Wang J. Antimicrob. Agents Chemother. 2006;50:519–526. doi: 10.1128/AAC.50.2.519-526.2006. For a review on antibiotics including platensimycin, see: Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Angew. Chem. 2009;121:670–732. doi: 10.1002/anie.200801695. Angew. Chem. Int. Ed.2009, 48, 660 – 719.
- 3.a) Herath KB, Zhang C, Jayasuriya H, Ondeyka JG, Zink DL, Burgess B, Wang J, Singh SB. Org. Lett. 2008;10:1699–1702. doi: 10.1021/ol800251v. [DOI] [PubMed] [Google Scholar]; b) Jayasuriya H, Herath KB, Ondeyka JG, Zink DL, Burgess B, Wang J, Singh SB. Tetrahedron Lett. 2008;49:3648–3651. [Google Scholar]; c) Zhang C, Ondeyka J, Zink DL, Burgess B, Wang J, Singh SB. Chem. Commun. 2008:5034–5036. doi: 10.1039/b810113b. [DOI] [PubMed] [Google Scholar]
- 4.Smanski MJ, Peterson RM, Rajski SR, Shen B. Antimicrob. Agents Chemother. 2009;53:1299–1304. doi: 10.1128/AAC.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Nicolaou KC, Li A, Edmonds DJ. Angew. Chem. 2006;118:7244–7248. Angew. Chem. Int. Ed.2006, 45, 7086 – 7090. [Google Scholar]; b) Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew. Chem. 2007;119:4016–4019. doi: 10.1002/anie.200700586. Angew. Chem. Int. Ed.2007, 46, 3942 – 3945. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Pappo D, Tsang KY, Gibe R, Chen DY-K. Angew. Chem. 2008;120:958–960. doi: 10.1002/anie.200705080. Angew. Chem. Int. Ed.2008, 47, 944 – 946. [DOI] [PubMed] [Google Scholar]; d) Ghosh AK, Xi K. J. Org. Chem. 2009;74:1163–1170. doi: 10.1021/jo802261f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou Y, Chen C-H, Taylor CD, Foxman BM, Snider BB. Org. Lett. 2007;9:1825–1828. doi: 10.1021/ol070563g. Li P, Payette JN, Yamamoto H. J. Am. Chem. Soc. 2007;129:953–954. doi: 10.1021/ja073547n. Ghosh AK, Xi K. Org. Lett. 2007;9:4013–4016. doi: 10.1021/ol701783z. Kaliappan KP, Ravikumar V. Org. Lett. 2007;9:2417–2419. doi: 10.1021/ol070848t. Lalic G, Corey EJ. Org. Lett. 2007;9:4921–4923. doi: 10.1021/ol702323s. Tiefenbacher K, Mulzer J. Angew. Chem. 2007;119:8220–8221. Angew. Chem. Int. Ed.2007, 46, 8074 – 8075. Nicolaou KC, Tang Y, Wang J. Chem. Commun. 2007:1922–1923. doi: 10.1039/b704589a. Kim CH, Jang KP, Choi SY, Chung YK, Lee E. Angew. Chem. 2008;120:4073–4075. doi: 10.1002/anie.200800568. Angew. Chem. Int. Ed.2008, 47, 4009 – 4011. Matsuo J-I, Takeuchi K, Ishhibashi H. Org. Lett. 2008;10:4049–4052. doi: 10.1021/ol801584r. Nicolaou KC, Lee A, Ellery SP, Edmonds DJ. Angew. Chem. 2009;121:6411–6413. Angew. Chem. Int. Ed.2009, 48, 6293 – 6295. Yun SY, Zheng J-C, Lee D. J. Am. Chem. Soc. 2009;131:8413–8415. doi: 10.1021/ja903526g. Review of synthetic approaches: Tiefenbacher K, Mulzer J. Angew. Chem. 2008;120:2582–2590. Angew. Chem. Int. Ed.2008, 47, 2548 – 2555.
- 7.a) Singh SB, Herath KB, Wang J, Tsou N, Ball RG. Tetrahedron Lett. 2007;48:5429–5433. [Google Scholar]; b) Krauss J, Knorr V, Manhardt V, Scheffles S, Bracher F. Arch. Pharm. Chem. Life. Sci. 2008;341:386–392. doi: 10.1002/ardp.200700177. [DOI] [PubMed] [Google Scholar]; c) Shen HC, Ding F-X, Singh SB, Parthasarathy G, Soisson SM, Ha SN, Chen X, Kodali S, Wang J, Dorso K, Tata JR, Hammond ML, MacCoss M, Colletti SL. Bioorg. Med. Chem. Lett. 2009;19:1623–1627. doi: 10.1016/j.bmcl.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8. Nicolaou KC, Lister T, Denton RM, Montero A, Edmonds DJ. Angew. Chem. 2007;119:4796–4798. doi: 10.1002/anie.200701548. Angew. Chem. Int. Ed.2007, 46, 4712 – 4714. Nicolaou KC, Tang Y, Wang J, Stepan AF, Li A, Montero A. J. Am. Chem. Soc. 2007;129:14850–14851. doi: 10.1021/ja076126e. Nicolaou KC, Stepan AF, Lister T, Li A, Montero A, Tria GS, Turner CI, Tang Y, Wang J, Denton RM, Edmonds DJ. J. Am. Chem. Soc. 2008;130:13110–13119. doi: 10.1021/ja8044376. For other core mimics, see: Yeung Y-Y, Corey EJ. Org. Lett. 2008;10:3877–3878. doi: 10.1021/ol801400a. Wang J, Lee V, Sintim HO. Chem. Eur. J. 2009;15:2747–2750. doi: 10.1002/chem.200802568.
- 9.Njardarson JT, Gaul G, Shan D, Huang X-Y, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]
- 10.Batory LA, McInnis CE, Njardarson JT. J. Am. Chem. Soc. 2006;128:16054–16055. doi: 10.1021/ja067073o. [DOI] [PubMed] [Google Scholar]
- 11.Cook SP, Danishefsky SJ. Org. Lett. 2006;8:5693–5695. doi: 10.1021/ol062067i. [DOI] [PubMed] [Google Scholar]
- 12.Although vanillin lacks the requisite methyl group from our retrosynthetic analysis, it’s availability made it a nice model system.
- 13.a) Kametani T, Terui T, Ogino T, Fukumoto K. J. Chem. Soc. C. 1969:874–878. [Google Scholar]; b) Martin vP. Helv. Chim. Acta. 1989;72:1554–1582. [Google Scholar]
- 14.Sundar N, Bhat SV. Synth. Commun. 1998;28:2311–2316. [Google Scholar]
- 15.Aujard I, Rome D, Arzel E, Johansson M, de Vos D, Sterner O. Bioorg. Med. Chem. Lett. 2005;13:6145–6150. doi: 10.1016/j.bmc.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Nagamitsu T, Sunazuka T, Obata R, Tomoda H, Tanaka H, Harigaya Y, Omura S, Smith AB., III J. Org. Chem. 1995;60:8126–8127. [Google Scholar]
- 17.Li C, Pace EA, Liang M-C, Lobkovsky E, Gilmore T, Porco JA., Jr J. Am. Chem. Soc. 2001;123:11308–11309. doi: 10.1021/ja0169769. [DOI] [PubMed] [Google Scholar]
- 18.The reactivity of this type of diene towards dimerization could be suppressed by using lead(IV) acetate instead of IBDA. The resulting mixed ketal did not dimerize and therefore provided us with more opportunities to explore the 5-exo cyclization route to 23. Our studies with this mixed ketal substrate were unfortunately in agreement with the results obtained for 22.
- 19.Gevorgyan V, Liu J-X, Rubin M, Benson S, Yamamoto Y. Tetrahedron Lett. 1999;40:8919–8922. [Google Scholar]
- 20.Molander GA, Petrillo DE. Org. Lett. 2008;10:1795–1798. doi: 10.1021/ol800357c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.