Abstract
Background
Loss of subcutaneous (SAT) with sparing of visceral (VAT) adipose tissue (AT) has been documented in HIV + men and women. Intermuscular AT (IMAT) rivals VAT in independent associations with cardiovascular risk.
Objective
To determine whether the size and distribution of IMAT differs in HIV+ vs. HIV- men and/or women.
Design
We used whole-body MRI to measure VAT, IMAT and four SAT compartments and compared them by HIV status using whole-body skeletal muscle (SM) or total AT (TAT) as co-variates in multi-ethnic groups of healthy HIV- (n=86) and stable HIV+ (n=76) men and women.
Results
The sizes of AT depots (adjusting for SM) did not differ by HIV status, except for smaller gluteal SAT (lower trunk, between L4-L5 to greater trochanter) in both sexes (P<0.05). The AT distribution (adjusting for TAT) was significantly different, with larger VAT (P<0.05) and smaller gluteal and limb SAT (P<0.05) in both HIV+ sexes; IMAT increased more with TAT in HIV+ vs. HIV- men (P<0.05 for slope interaction) but there were no significant differences in women. There were significant race by HIV interactions in AT distribution with more pronounced VAT differences in non-Hispanic white men and larger trunk SAT in African Americans HIV+ vs. HIV-.
Conclusion
The AT distribution differed markedly in HIV+ vs. HIV- with limb and lower body SAT representing a smaller proportion of TAT in HIV+ in both sexes and IMAT representing a larger proportion of TAT in HIV+ vs. HIV- men.
Keywords: fat distribution, muscle adipose tissue infiltration, HIV, magnetic resonance imaging
Introduction
Since the advent of highly active anti-retroviral treatment (HAART), the percentage of individuals living with HIV above the age of 50 increased from 17% to 23% between 2001 and 2004 and is predicted to approach 50% by 2015 [1-3]. Individuals with HIV experiencing longer survival will also be affected by metabolic complications known to occur in these circumstances [4]. Such adverse effects include type 2 diabetes mellitus (DM), cardiovascular disease (CVD), osteoporosis, as well as deleterious adipose tissue (AT) distribution [5-6].
Prior cross-sectional reports have characterized changes in fat distribution in HIV+ as a loss of AT from the limbs and face (peripheral) with an increase in central adiposity (the HIV lipodystrophy syndrome) [7-14]. The deleterious effect of increased visceral AT (VAT) and the protective effect of gluteal-femoral AT with regard to metabolic abnormalities and CVD have been established in HIV- populations [15-16]. However the distribution of AT in the lower body is complex with lipid being stored in various compartments: superficial subcutaneous AT, over the muscle fascias (SAT), inter-muscular AT visible by CT or MR imaging between the muscle fibers and underneath the muscle fascia (IMAT) [17] and lipid stored inside the muscle, invisible in a regular MR image but detectable by NMR spectroscopy (extramyocellular lipid or EMCL and intramyocellular lipid or IMCL) [18]. Increased IMCL was correlated strongly with insulin resistance in HIV+ men and with decreased lower body fat by DXA in HIV+ women [18-20]. However much less is known about the distribution of the other AT depots in the lower body, SAT vs. IMAT, in HIV+ men and women. Recently, a larger IMAT was shown to rival VAT in independent associations with cardiovascular risk in HIV- [21-23]. IMAT also represented a larger proportion of whole-body AT (TAT) with increasing age and was proposed as a risk factor for physical limitation and decreased strength in older HIV- adults [24-25]. While we have reported that a relatively larger IMAT and smaller SAT in the lower body correlated with insulin resistance in obese women, independent of HIV status [26], whether IMAT represents a higher proportion of TAT in HIV+ vs. HIV- women or men is not known.
Therefore, the aim of the present study was to determine whether differences in IMAT measured by whole-body MRI exist in a larger cohort of multi-ethnic HIV+ vs. HIV- men and women. We hypothesized that SAT will represent a smaller and IMAT will represent a larger proportion of TAT in HIV+ vs. HIV- individuals. Since HIV/AIDS is diagnosed disproportionately in males versus females in any given year (74% vs. 26% respectively) and since AT distribution is significantly different in men vs. women independent of ethnicity [17, 24] we also examined interactions between HIV status and sex. When examining differences in the regional AT depots we controlled for TAT but also, separately, for the whole-body skeletal muscle (SM), as these two variables may be differentially affected by HIV status and HAART in the current treatment era [14].
Methods
The study involved a retrospective analysis of archived data from HIV+ and HIV- men and women who had been recruited for multiple different studies of body composition that occurred from 1999-2004. All individuals had undergone analysis of body fat depots via whole body MRI while at weight stability, for cross-sectional analyses or as baseline studies prior to interventions. The 162 participants included 35 HIV+ and 26 HIV- men as well as 41 HIV+ and 60 HIV- women.
The HIV+ subjects were stable with or without HAART which was typical of previously used antiretroviral regimens at the time when the studies were conducted (1999-2004). Overall 25% of subjects were HAART-naïve, 75% were taking nucleoside reverse transcriptase inhibitors (NRTI), 43% were taking protease inhibitors (PI) and 37% were taking non-nucleoside reverse transcriptase inhibitors (NNRTI). Twenty-three percent (23%) of men were HAART-naive, 3% were on NRTI-s alone, 37% were on NRT-s plus PI-s, 31% were on NRTI-s plus NNRTI-s and 3% were on a three drug combination. The respective frequencies in women were 15%, 5%, 32%, 24% and 15% and did not differ significantly from the frequencies in men. The ethnicity breakdown in the HIV+ group was 60% African Americans, 13% Non-Hispanic whites, 22 % Hispanics and 5% others.
The HIV- subjects were healthy adults and none were on investigational agents. The ethnicity breakdown was 43% African Americans, 46% Non-Hispanic whites, 8% Hispanics and 3% others.
All studies as well as the current analysis were approved by the Institutional Review Board of St. Luke's Roosevelt Hospital Center.
Magnetic resonance imaging
AT and SM volumes were measured by whole-body MRI as previously reported [26]. Briefly, subjects were placed in a 1.5 T scanner (× 6 Horizon; General Electric, Milwaukee, WI, USA) with their arms extended above their heads. The entire body was visualized on a scout coronal image (6× Horizon) and the axial level of L4-L5 was identified. The scans used to calculate the various depot compartments were acquired by contiguous axial slices of 10-mm thickness at 40-mm intervals below L4-L5 to the toes and above this level to the fingertips. The images were then subsequently analyzed on SLICEOMATIC image analysis software (version 4.2; Tomovision, Montreal, QC, Canada). [25] AT volumes were calculated by measuring the relevant tissue area in each slice with the use of threshold methods and manual delineation to draw boundaries among different tissues. The volume between slices was extrapolated from area measurements. The following fat depots were calculated: total visceral (VAT), IMAT, and 4 SAT compartments – Leg (below greater trochanter), Lower Trunk (greater trochanter to L4-L5), Upper Trunk (above L4-L5 to the shoulders) and Arms (above the shoulders) SAT. IMAT was defined as the AT visible between the muscle groups and beneath the muscle fascia. [26] The gray level intensity (threshold value) of the AT in the SAT region was first determined and used as reference and this value was reduced by 20% to identify the IMAT threshold.
The MRI scans were read at the New York Obesity Research Center Image Reading Center at St. Luke's Roosevelt Hospital Center. The CV on repeated readings of the same 2 scans by observers analyzing the images was 3.8 % for TAT, 3.4% for SAT, 9.7% for VAT, 2.2 % for SM, and 7.3 % for IMAT.
Statistical analysis
Group data are presented as means ± standard error. Differences in age, weight, height, BMI, SM, and TAT by HIV or by sex were analyzed separately by student's t-test. A general linear model (GLM) was used to compare AT depots by sex and HIV status after adjusting for age, height, and either skeletal muscle (SM) or total adipose tissue (TAT). SM was used to determine differences between groups in absolute AT volume, while TAT was used to evaluate differences in relative AT distribution. When interactions between sex and HIV status were significant results were presented separately in the four groups. When interactions were not significant differences were reported by HIV status in men and women combined. Because we have previously shown that AT distribution is influenced by race in HIV- cohorts [17] we conducted additional analyses to assess whether there were any interactions between HIV sex and race in a subset of subjects with races (African American, non-Hispanic white or Hispanic) represented in all the four groups (HIV+ and HIV-, men and women). A P-value <0.05 was considered statistically significant. All analyses were performed with Statistica version 6 for Windows.
Results
Subject characteristics and unadjusted values for the whole-body SM and AT and regional AT volumes are shown in Table 1 while comparisons of absolute AT volumes after adjusting for age, height and SM are shown in Table 2 and after adjusting for age, height and TAT are shown in Table 3.
Table 1.
Subject characteristics and magnetic resonance imaging (MRI) measurements in HIV-positive and HIV-negative men and women.
| Subject characteristic | Men | Women | P-Value* by Sex | P-Value* by HIV status | ||||
|---|---|---|---|---|---|---|---|---|
| HIV+ n=35 | HIV- n=26 | HIV+ n=41 | HIV- n=60 | HIV+ | HIV- | Men | Women | |
| Age (years) | 44.9 ± 1.3 | 45.8 ± 1.5 | 41.5 ± 1.2 | 40.7 ± 1.0 | 0.08 | 0.005 | 0.68 | 0.59 |
| Race (%) ** | 17/48/26/9 | 69/23/8/0 | 10/71/8/0 | 37/52/8/3 | 0.37 | 0.01 | 0.001 | 0.003 |
| HAART (%) # | 23/3/37/31/3 | -------- | 15/5/32/24/15 | --------- | NS | -------- | ------- | ------- |
| Weight (kg) | 74.5 ± 2.5 | 85.4 ± 2.9 | 81.8 ± 2.3 | 86.6 ± 1.9 | 0.02 | 0.76 | 0.0005 | 0.16 |
| BMI (kg/m2) | 24.2 ± 0.8 | 26.7 ± 0.9 | 31.4 ± 0.7 | 32.5 ± 0.6 | <0.00001 | <0.00001 | 0.0001 | 0.32 |
| SM (l) | 27.8±0.7 | 32.3±0.8 | 21.5±0.6 | 22.7±0.5 | <0.00001 | <0.00001 | 0.00001 | 0.15 |
| TAT (l) | 18.4±2.0 | 24.3±2.3 | 38.5±1.8 | 41.9±1.5 | <0.00001 | <0.00001 | 0.006 | 0.21 |
| VAT (l) | 3.3±0.3 | 2.9±0.3 | 2.8±0.3 | 2.5±0.2 | 0.27 | 0.18 | 0.50 | 0.24 |
| IMAT (l) | 1.2±0.1 | 0.9±0.1 | 1.7±0.1 | 1.7±0.1 | 0.002 | <0.00001 | 0.10 | 0.99 |
| Total Sat (l) | 14±1.8 | 20.5±2 | 34±1.6 | 37.7±1.3 | <0.00001 | <0.00001 | 0.0001 | 0.14 |
| Arm Sat (l)1 | 2.3±0.2 | 3.1±0.2 | 3.8±0.2 | 4.3±0.2 | <0.00001 | 0.0002 | 0.0007 | 0.09 |
| Upper Trunk Sat (l)2 | 4.3±0.6 | 5.5±0.7 | 10±0.6 | 10.8±0.5 | <0.00001 | <0.00001 | 0.06 | 0.38 |
| Lower Trunk Sat (l)3 | 1.8±0.4 | 3.5±0.5 | 5.9±0.4 | 7.3±0.3 | <0.00001 | <0.00001 | 0.00008 | 0.02 |
| Leg Sat (l)4 | 5.6±0.8 | 8.5±0.9 | 14.3±0.7 | 15.4±0.6 | <0.00001 | <0.00001 | 0.00003 | 0.30 |
All values are mean ± standard error. BMI, body mass index, SM, skeletal muscle, TAT, total adipose tissue, VAT, visceral adipose tissue; IMAT, inter-muscular adipose tissue; SAT, subcutaneous adipose tissue; HAART: highly-active anti-retroviral therapy;
includes all SAT of the arms above the shoulders;
includes all SAT above L4-L5 up to the shoulders;
includes all SAT between greater trochanter and L4-L5;
includes all SAT below the greater trochanter;
P-values derived by t-test analysis, significant if <0.05
Number of Non-Hispanic whites/African Americans/Hispanics/others as % of total sample in each group, P value shown for the difference between % non-Hispanic whites;
Number of HIV men or women who were treatment-naïve, on NRTI alone, on NRTI-s plus PI, on NRTI-s plus NNRTI, or on 3 drug combination, as % of total sample in each group.
Table 2.
Comparisons of regional AT volumes by whole-body MRI, adjusting for age, height and SM.
| Regional AT volume | Men | Women | P-Value* By HIV | ||
|---|---|---|---|---|---|
| HIV+ | HIV- | HIV+ | HIV- | ||
| VAT (l) | 3.2 ± 0.3 | 2.6 ± 0.4 | 2.9 ± 0.3 | 2.6 ± 0.2 | 0.097 |
| IMAT (l) | 1.2 ± 0.1 | 0.9 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | ---------- |
| Total SAT (l) | 10.8 ± 1.8 | 12.2 ± 2.5 | 38.0 ± 1.7 | 40.5 ± 1.4 | 0.23 |
| Arm SAT (l)1 | 2.0 ± 0.2 | 2.3 ± 0.3 | 4.1 ± 0.2 | 4.5 ± 0.2 | 0.084 |
| Upper Trunk SAT (l)2 | 3.3 ± 0.6 | 2.8 ± 0.8 | 11.3 ± 0.6 | 11.6 ± 0.5 | 0.85 |
| Lower Trunk SAT (l)3 | 1.1 ± 0.4 | 1.7 ± 0.6 | 6.8 ± 0.4 | 7.9 ± 0.3 | 0.025 |
| Leg SAT (l)4 | 4.3 ± 0.8 | 5.5 ± 1.0 | 15.8 ± 0.7 | 16.4 ± 0.6 | 0.21 |
All values are mean ± standard error. SM, whole-body skeletal muscle; VAT, visceral adipose tissue; IMAT, inter-muscular adipose tissue; SAT, subcutaneous adipose tissue;
Arm SAT: includes all SAT of the arms above the shoulders;
Upper Trunk SAT: SAT above L4-L5 up to the shoulders;
Lower Trunk SAT: SAT between greater trochanter and L4-L5;
Leg SAT: SAT below the greater trochanter.
P-value calculated by GLM, significant if <0.05 (adjusted for age, height and SM);
-- HIV by Sex interactions for IMAT (M<F for both HIV+ and HIV- groups, P<0.0006, HIV differences in men P=0.093, in women P=0.475.)
Table 3.
Comparison of regional AT distribution by whole-body MRI, adjusting for age, height and TAT.
| Regional AT volume | Men | Women | P-Value* By HIV | ||
|---|---|---|---|---|---|
| HIV+ | HIV- | HIV+ | HIV- | ||
| VAT (l) | 4.6 ± 0.3 | 3.9 ± 0.3 | 2.2 ± 0.2 | 1.7 ± 0.2 | 0.007 |
| IMAT (l)) | -------- | ------- | 1.8 ± 0.1 | 1.7 ± 0.1 | ------- |
| Total SAT (l) | 27.0 ± 0.3 | 28.2 ± 0.4 | 29.4 ± 0.3 | 30.0 ± 0.2 | 0.00001 |
| Arm SAT (l)1 | 3.6 ± 0.2 | 3.9 ± 0.2 | 3.3 ± 0.1 | 3.5 ± 0.1 | 0.024 |
| Upper Trunk SAT (l)2 | 8.5 ± 0.3 | 8.1 ± 0.4 | 8.5 ± 0.3 | 8.3 ± 0.2 | 0.235 |
| Lower Trunk SAT (l)3 | 4.5 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 | 5.7 ± 0.2 | 0.00001 |
| Leg SAT (l)4 | 10.4 ± 0.5 | 11.3 ± 0.5 | 12.6 ± 0.4 | 12.6 ± 0.3 | 0.25 |
All values are mean ± standard error. TAT, whole-body, total adipose tissue; VAT, visceral adipose tissue; IMAT, inter-muscular adipose tissue; SAT, subcutaneous adipose tissue;
Arm SAT: includes all SAT of the arms above the shoulders;
Upper Trunk SAT: SAT above L4-L5 up to the shoulders;
Lower Trunk SAT: SAT between greater trochanter and L4-L5;
Leg SAT: SAT below the greater trochanter.
P-Value calculated by GLM, significant if <0.05 (adjusted for age, height, and TAT);
---HIV by Sex interactions for IMAT (HIV+>HIV- in men P<0.00001, in women P=0.284.
In our cohort men were older and had lower BMI than women (Table 1). Expected differences in body composition and body fat distribution between men and women were found in both HIV+ and HIV-groups (Table 1-3) with higher SM, lower TAT and relatively more VAT and less Leg SAT in men than in women. The rise in IMAT with SM was steeper in women compared to men (P<0.05), for both the HIV+ and the HIV- groups.
Age did not differ by HIV status (Table 1). HIV+ and HIV- women had similar BMI, SM, TAT and all AT compartments except for Lower Trunk SAT (includes gluteal regional), which was smaller in HIV+ women (P=0.02). HIV+ men had lower BMI, weight and SM than HIV- men (Table 1); SM was not different in HIV+ vs. HIV- men after adjusting for weight and height (P=NS). With regard to the AT, HIV+ men had lower TAT and peripheral SAT (Arms, Lower Trunk and Legs) than HIV- men, but similar VAT and IMAT (Table 1). After adjusting for age, height and SM, the only significant difference by HIV status was observed for Lower Trunk SAT (includes gluteal region) which was smaller in HIV+ vs. HIV-men and women (Table 2). In terms of the relative AT distribution, after adjusting for age, height and TAT, HIV+ men and women had more VAT and less SAT, specifically Arm and Lower Trunk SAT than HIV-counterparts (Table 3). For IMAT, there was a significant sex by HIV interaction (P=0.028). For men, the relationship between IMAT and SM and the mean values for IMAT were similar between HIV+ vs. HIV – (P=0.093) but there was a greater rise in IMAT with TAT for HIV+ than for HIV- (Figure 1, P<0.05). The slope of rise with either SM or TAT and the mean values for IMAT did not differ significantly in HIV+ vs. HIV- women (Tables 2 and 3, Figure 1).
Figure 1.
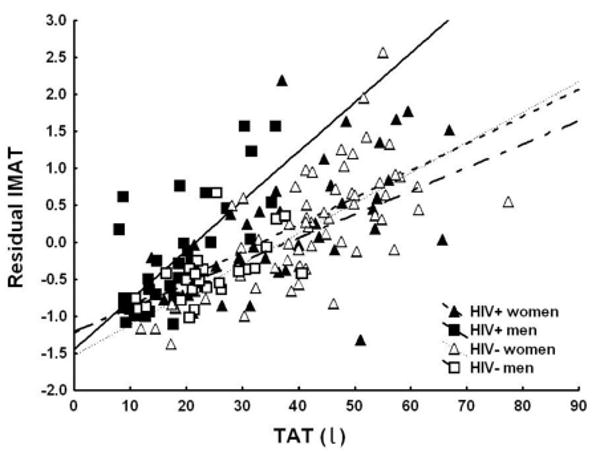
Relationship between residual IMAT and TAT. Filled squares: HIV+ men. Open squares: HIV- men. Filled triangles: HIV+ women. Open triangles: HIV- women. Residual IMAT (intermuscular adipose tissue) is the difference between the observed and the expected values of IMAT, calculated through multiple regressions as a function of age and height. IMAT and TAT (whole-body adipose tissue) were measured by whole-body MRI. For the difference in slopes, P<0.05 men and P=NS for women.
We performed additional analyses to determine whether HAART or race influenced our results. Excluding the HIV+ HAART naïve from the database or using HAART category type as factor (naïve, NRTI, PI or NNRTI) instead of the HIV+ status in similar analyses as presented above did not modify the results. Addition of race to the above presented analyses in a subset of subjects (African Americans, non-Hispanic whites and Hispanics) showed no significant 3-way interactions (race by HIV by sex), but resulted in some changes. In Table 2: a) There were significant race by HIV (P=0.009) and sex by race (P=0.006) interactions for VAT; in post-hoc analyses VAT was significantly higher only in HIV+ vs. HIV- non-Hispanic white men (P<0.00001); b) The sex by HIV interactions for IMAT were not significant; the P value for the main HIV effect on IMAT was 0.05; c) The P value for differences in Lower Trunk SAT changed from 0.025 to 0.082 (NS). In Table 3: a) There was a significant race by HIV (P=0.007) interaction for VAT; in post-hoc analyses VAT was significantly higher only in HIV+ vs. HIV- non-Hispanic whites (P<0.0001); b) The sex by HIV interaction for IMAT was not significant; the P value for the main HIV effect on IMAT was 0.014; c) The P values for differences in Arm SAT changed from 0.024 to 0.052 and for differences in Leg SAT changed from 0.25 to 0.014; d) There was a significant race by HIV (P=0.038) interaction for Upper Trunk SAT; in post-hoc analyses Upper Trunk SAT was significantly higher only in HIV+ vs. HIV- African Americans (P<0.0001).
Discussion
This study investigated differences in AT sizes and distribution between HIV+ and HIV- men and women; in addition to previous reports [11-14, 26-28] it emphasized the newly described depot IMAT. This study is unique in that by using the whole body MRI technique we separated IMAT, the visible AT between the muscle fibers and underneath the muscle fascia, from SAT, the AT between the muscle fascia and the skin. We found no significant differences in IMAT or VAT by HIV status when adjusting for SM alone; in contrast, VAT represented a higher proportion of TAT in HIV+ than in HIV- and the rise in IMAT with TAT was steeper in HIV+ men than in HIV- men or both HIV+ and HIV- women. We also found, as previously described, smaller limb and lower trunk SAT in HIV+ vs. HIV-, by comparing subdivisions of SAT without interference from IMAT or organ and muscle fat, such as when these regional measurements were determined by dual-energy X-ray absorptiometry (DXA) [13-14, 17, 24].
To our knowledge, this is the first report of a difference in IMAT by HIV in men with a wide range of body weight. Whole-body MRI has been used before by us and others [11, 13-14, 26-27, 28] to compare AT depots in HIV+ vs. HIV-; however, IMAT was not separated from SAT in the majority of these previous reports. Our findings suggest that if the loss of fat in HIV+ individuals occurs in the protective AT depots of the lower body, then IMAT, just as VAT, is relatively spared. This relative sparing of IMAT may remain significant in terms of metabolic consequences. Not all AT depots carry equivalent metabolic risk [27]. Enlarged VAT and IMAT have both been associated with an unfavorable metabolic profile and enlarged IMAT was associated with chronic disease incidence and functional decline in old age, independent of the total amount of body fat [23-26]. In addition, short-term inactivity substantially increased IMAT (15-20%), without changing SAT, in healthy young men and women [24-25, 29-31]. Close to 50% of the HIV population is expected to be over the age of 50 by 2015 [2-3]. At present, there is limited knowledge about longitudinal body composition changes with aging in the HIV+ population. Decreased muscle strength, independent of muscle wasting, has been linked to increased cytokines, specifically TNF-α, [32], which in turn correlates with the immune function in HIV+ individuals. [33] Given our findings, studies considering the relationship between cytokines, IMAT accumulation and muscle strength in HIV individuals would be relevant.
We confirmed previous findings that, in the era of antiretroviral therapy, the HIV infection is associated with lipoatrophy i.e. smaller SAT in limbs and lower trunk without loss of VAT and, as we show here first, without loss of IMAT [11, 13-14, 26-28]. Without adjusting for race, whether correcting for SM or TAT, we found that gluteal SAT was the SAT sub compartment most affected by HIV, in both men and women while the leg SAT (below the greater trochanter) was not smaller in HIV+ vs. HIV- women, not unlike some [26] but not all of our previous reports [28]. However when race was added to the analyses adjusted leg SAT means were significantly smaller in both HIV+ vs. HIV- men (10.3±0.5 vs 11.8±0.7 l) and, albeit less so, in HIV+ vs. HIV- women (12.1±0.5 vs. 12.9±0.5 l). Other significant race by HIV interactions were found for VAT and upper trunk SAT, with differences being most pronounced in non-Hispanic white men and African Americans, respectively. Although consistent with known differences in HIV- populations, because of the small number of subjects in our individual data cells when broken down by race, these findings must be confirmed in larger cohorts.
The strength of our study includes the use of whole body MRI with the novel addition of measuring IMAT and SAT sub-compartments in a cohort of HIV+ and HIV- men and women with a wide weight range. The limitations include the use of a convenience sample of HIV+ and HIV- men and women, lack of information with regard to viral load and CD4 counts, lifestyle activity patterns and a heterogeneous HAART regimen which did not allow us to report in detail on the role of the different anti-retrovirals on AT size and distribution. We have previously reported that a regimen of diet and exercise in obese HIV+ women promoted weight loss but that the VAT and IMAT decreases were less than expected from a similar intervention in HIV- obese women [34]. Therefore we hypothesize that differences in physical activity patterns in HIV+ vs. HIV- would have been unlikely to explain the differences in IMAT we have observed. Also, in this study we were unable to attribute the AT findings to a specific HAART regimen. Previous reports have established that AT changes in HIV are mainly associated with the separate or combined use of two major classes of drugs, NRT-s and PI-s [35]. The inclusion or exclusion of HIV+ who were HAART naïve in our study did not modify the results however since they represented a small percentage of the subjects a definitive conclusion on whether the HIV itself was associated with AT depots changes cannot be made. Further studies examining these aspects could provide additional knowledge into the effect of the HIV/HAART on AT distribution.
In conclusion, IMAT and SAT distributions differ in HIV+ vs. HIV-. Our findings strongly suggest that IMAT and SAT should be separated when studying differences in AT distribution in HIV+ vs. HIV-. In-vitro studies investigating the possibility that these AT depots may be differentially affected by HIV/HAART should be conducted.
Acknowledgments
We would like to thank the study participants and the members of the New York Obesity Research Center Body Composition Laboratory and Image Reading Center for the MRI analyses. This project was supported by National Institutes of Health Grants PO1-DK-42612, PO1 DK-42618, RO1-DK-40414, MO1-RR-00645, P30-DK-26687, RO1-HL-65938 and in part by grants from Merck & Co, Agouron Pharmaceuticals and Bristol Myers Squibb.
References
- 1.Patella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) HIV/AIDS surveillance report. Vol. 16. Atlanta (GA): US Department of Health and Human Services, CDC; 2004. 2005 [online] [Google Scholar]
- 3.Smith G. Washington, DC: Senate Special Committee on Aging; 2005. Statement of Senator Gordon H. Smith. Aging hearing: HIV over fifty, exploring the new threat. [online]. Available at: www.aging.senate.gov/public/_files/hr141gs.pdf. [Google Scholar]
- 4.Mack KA, Ory MG. AIDS and older Americans at the end of the twentieth century. J Acquir Immune Defic Syndr. 2003;33 2:S68–75. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- 5.Graham NM. Metabolic disorders among HIV-infected patients treated with protease inhibitors: a review. J Acquir Immune Defic Syndr. 2000;25:S4–11. doi: 10.1097/00042560-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 7.John M, Nolan D, Mallal S. Antiretroviral therapy and the lipodystrophy syndrome. Antivir Ther. 2001;6:9–20. doi: 10.1177/135965350100600102. [DOI] [PubMed] [Google Scholar]
- 8.Nolan D, John M, Mallal S. Antiretroviral therapy and the lipodystrophy syndrome, part 2: concepts in aetiopathogenesis. Antivir Ther. 2001;6:145–60. [PubMed] [Google Scholar]
- 9.Wanke CA, Falutz JM, Shevitz A, Phair JP, Kotler DP. Clinical evaluation and management of metabolic and morphologic abnormalities associated with human immunodeficiency virus. Clin Infect Dis. 2002;34:248–59. doi: 10.1086/324744. [DOI] [PubMed] [Google Scholar]
- 10.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Engelson ES, Kotler DP, Tan YX, et al. Fat distribution in HIV infected patients reporting truncal enlargement quantified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:1–8. doi: 10.1093/ajcn/69.6.1162. [DOI] [PubMed] [Google Scholar]
- 12.Koutkia P, Grinspoon S. HIV-associated lipodystrophy: pathogenesis, prognosis, treatment, and controversies. Ann Rev Med. 2004;55:303–17. doi: 10.1146/annurev.med.55.091902.104412. [DOI] [PubMed] [Google Scholar]
- 13.From the Study of Fat Restribution and Metabolic Change in HIV Infection (FRAM). Fat Distribution in Men with HIV Infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.From the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat Distribution in Women with HIV Infection. J Acquir Immune Defic Synd. 2006;42:562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Despres JP. Is Visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 16.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endo Metab. 2005;90:4573–8. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher D, Kuznia P, Heshka, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torriani M, Thomas BJ, Barlow RB, Librizzi J, Dolan S, Grinspoon S. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J Appl Physiol. 2006;100:609–14. doi: 10.1152/japplphysiol.00797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan SK, Samaras K, Thompson CH, et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 20.Luzi L, Perseghin G, Tambussi G, et al. Intramyocellular lipid accumulation and reduced whole body lipid oxidation in HIV lipodystrophy. Am J Physiol Endocrinol Metab. 2003;284:274–80. doi: 10.1152/ajpendo.00391.2001. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 22.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with increased intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim JE, Heshka S, Albu JB, et al. Intermuscular adipose tissue rival visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes. 2007;31:1400–5. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 26.Albu JB, Kenya S, He Q, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86:100–6. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotler DP, Lopes JL, Engelson ES, Wang J, Agin D, Heymsfield SB. Interactions among sex, HIV infection, and fat redistribution. AIDS Reader. 2000;10:589–94. [PubMed] [Google Scholar]
- 28.Johnson JA, Albu JB, Engleson ES, et al. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2004;286:261–71. doi: 10.1152/ajpendo.00056.2003. [DOI] [PubMed] [Google Scholar]
- 29.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–84. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 30.Visser M. Changes in skeletal muscle and body fat in old age-results from longitudinal studies. Int J Body Comp Res. 2008;6:A60. [Google Scholar]
- 31.Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987;45:225–31. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 32.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–84. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 33.Aukrust P, Muller F, Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 34.Engelson ES, Agin D, Kenja S, Werber-Zion G, Luty B, Albu JB, Kotler DP. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55:1327–36. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Kotler DP, Ionescu G, Johnson JA, Inada Y, Qing H, Engelson ES, Albu JB. Studies of adipose tissue metabolism in human immunodeficiency virus-associated lipodystrophy. Clin Infectious Dis. 2003;37(Suppl. 2):S47–51. doi: 10.1086/375891. [DOI] [PubMed] [Google Scholar]


