Abstract
A high-speed counter-current chromatography (HSCCC) technique in a preparative scale has been applied to separate and purify cordycepin from the extract of Cordyceps militaris(L.) Link by a one-step separation. A high efficiency of HSCCC separation was achieved on a two-phase solvent system of n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v) by eluting the lower mobile phase at a flow rate of 2 ml/min under a revolution speed of 850 rpm. HSCCC separation of 216.2 mg crude sample (contained cordycepin at 44.7% purity after 732 cation-exchange resin clean-up) yielded 64.8 mg cordycepin with purity of 98.9% and 91.7% recovery. Identification of the target compound was performed by UV, IR, MS, 1H NMR and 13C NMR.
Keywords: Countercurrent chromatography, Preparative chromatography, Cordycepin, Cordyceps militaris(L.) Link
1. Introduction
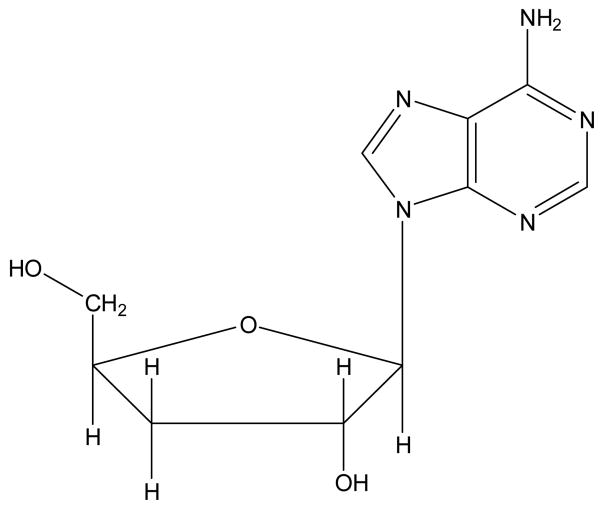
Cordyceps is a well-known traditional Chinese medicinal mushroom. Cordyceps militaris (Clavicipitaceae, Ascomycotina), is also known as the Chinese caterpillar fungus because it is a parasitic organism that grows on a rare caterpillar, belongs to the Dong-Chong-Xia-Cao group in Chinese herbs [1]. From ancient time, this fungus has widely used as a folk tonic food or invigorant by the Chinese as well as its usage as a crude drug preparation for increased longevity, endurance and vitality. Recent studies have demonstrated that the extracts of C. militaris have multiple pharmacological actions such as hemostatic, mycolytic, anti-asthmatic, and hypoglycemic [2,3]. The major active compounds are considered to be cordycepin, ophiocordin and some polysaccharides[4]. Cordycepin is a nucleoside analogue, which has a broad spectrum of biological activity. Now pharmacological tests revealed that it inhibited de novo biosynthesis of purine, regulated the production of interleukins in T lymphocytes, and demonstrated anti-fungal, anti-tumor and anti-viral effect [5-7]. Its structure is shown in Fig.1.
Fig.1.
The chemical structure of cordycepin (C10H13N5O3).
In view of these beneficial effects, a large quantity of pure materials is urgently needed for further pharmacological studies and as “marker compound” for the chemical evaluation or standardization of cordyceps and its medical products. The preparative separation of cordycepin from cordyceps militaris by classical methods are tedious, time consuming, requiring multiple chromatographic steps on silica gel, polyamide column, etc. and have the peril of loss of compounds due to the violent adsorptive effect of the solid matrix[8]. So, an efficient method for the preparative separation and isolation of cordycepin from natural and cultured cordyceps is warranted. High-speed countercurrent chromatography (HSCCC), is a from of liquid-liquid partition chromatographic technique that uses no support matrix and the liquid stationary phase is immobilized by centrifugal force, eliminates irreversible adsorption of sample onto the solid support used in the conventional chromatographic column, has an excellent sample recovery. The method permits directly introduction of crude samples into the column without more preparation, so it has been successfully applied to isolate and purify a number of natural products[9-12], but there have no reports of using HSCCC to isolate and separate cordycepin from cordyceps militaris.
The aim of the present paper, therefore, was first to develop an efficient method to isolate and purify cordycepin from C. militaris by an analytical-scale HSCCC. Then, a preparative-scale HSCCC was used to isolate and separate the target from the crude extract of C. militaris.
2. Experimental
2.1. Instrumentation
The analytical HSCCC instrument employed was a Model GS 20 analytical high-speed counter-current chromatography designed and constructed in Beijing UE Biotechnology, Beijing, China. The apparatus holds a pair of column holders symmetrically on the rotary frame at a distance of 5 cm from the central axis of the centrifuge. The multilayer coil separation column was prepared by winding a 50 m long, 0.85 mm I.D. PTFE (polytetrafluoroethylene) tube directly onto the holder hub forming multiple coiled layers with a total capacity of 40 ml. The β value varied from 0.4 at the internal terminal to 0.7 at the external terminal (β = r/R, where r is the distance from the coil to the holder shaft, and R, the revolution radius or the distance between the holder axis and central axis of the centrifuge). The HSCCC system was equipped with a model S constant-flow Pump, a UV detector operating at 254 nm and a portable recorder. Although the revolution speed of the apparatus could be regulated with a speed controller in a range between 0 and 2000 rpm, the optimum speed of 1600 rpm was used in the present study.
The preparative HSCCC was performed using a Model GS10A multilayer coil planet centrifuge (Beijing UE Biotechnology) equipped with a PTFE multilayer coil of 110 m in length and 1.6 mm in I.D. with a total capacity of 230 ml. The β value of this preparative column ranges from 0.5 to 0.8. The HSCCC system was equipped with a model S constant-flow Pump, a UV detector operating at 254 nm and a portable recorder. Although the revolution speed of the apparatus could be regulated with a speed controller in a range between 0 and 1000 rpm, the optimum speed of 850 rpm was used in the present studies.
The high-performance liquid chromatography (HPLC) equipment used was a Shimadzu LC-10A system including two LC-10ATVP pumps, a SPD-M10AVP photodiode array detector, a SCL-10AVP system controller and a LC Solution workstation (Shimadzu, Kyoto, Japan).
The nuclear magnetic resonance (NMR) spectrometer used here was a Mercury Plus 400 NMR system (Varian Inc., USA). The mass electro-spray spectrometer used here was a LCQ Deca XP Max system (Finnigan, USA). 732 cation-exchange resin was purchased from the Huizhi Resin Plant (Shanghai, China).
2.2. Materials
All organic solvent used for preparation of crude sample and HSCCC separation was of analytical grade (Damao Chemical Reagent Factory, Tianjin, China). Methanol used for HPLC was chromatographic grade (Merck, Germany), and water was distilled water.
The C. militaris was donated by Zhongshan LEAGUE Cordyceps Manufactured Product Co. LTD, Zhongshan, Guangdong Province, China.
2.3. Preparation of the crude sample
The C. militaris was carried out reflux distillation with 75% ethanol three times. United the filter and evaporated to no ethanol under reduced pressure at 60° and the residue was obtained, then the residue was redissolved in water (total volume 800ml) and was added 0.5 mol/L HCl aqueous solution to pH at 3.0, which was applied to a glass column (6 cm × 80 cm, contained 1.0 kg 732 cation-exchange resin). 2000 ml HCl aqueous solution (pH 3.0) was first used to elute the resin in order to remove some un-target chemicals, which have no or little retention on 732 cation-exchange resin. Then 3000 ml 0.15 mol/L ammonia aqueous solution was used to elute the target compound, and the eluant was collected and evaporated to dryness under reduced pressure at 60°, which was used for further HSCCC isolation and separation.
2.4. Preparation of two-phase solvent system and sample solution
In the present study, several kinds of solvent systems including n-hexane–ethyl acetate–methanol–water, ethyl acetate–methanol–water, n-hexane–n-butanol–methanol–water at different volume ratios were mixed, respectively, and thoroughly equilibrated in a separated funnel at room temperature. The two phases were separated shortly before use. The sample solution was prepared by dissolving 216.2 mg of the crude sample in the 10 ml lower phase of solvent system for isolation and purification.
2.5. HSCCC separation procedure
HSCCC was performed as follows: the multilayer coiled column was first entirely filled with the upper phase. The lower phase was then pumped into the head end of the column at a flow rate of 1.0 ml/min (for analytical instrument) or 2.0 ml/min (for preparative instrument), while the apparatus was run at 1600 rpm (for analytical instrument) or 850 rpm (for preparative instrument). After hydrodynamic equilibrium was reached, indicated by a clear mobile phase eluting at the tail outlet, 1 ml (for analytical instrument) or 10 ml (for preparative instrument) of sample solution was injected through the sample port. The effluent from the tail end of the column was continuously monitored with a UV detector at 254 nm. Each peak fraction was manually collected according to the chromatogram and evaporated under reduced pressure. The residuals were dissolved in methanol for subsequent HPLC analysis. The retention of the stationary phase relative to the total column capacity was calculated from the volume of the stationary phase collected from the column.
2.6. HPLC analysis and identification of HSCCC peak fractions
The crude extract, the sample after cleaning-up by cation-exchange resin and the peak fraction obtained by HSCCC were analyzed by HPLC. A satisfactory separation of the target compound was obtained with a reversed-phase Prodigy C18 column (4.6 mm ×250 mm i.d., 5μm, Phenomenex, USA) under the following conditions: the mobile phase composed of methanol/water (15:85, v/v) was eluted isocratically at a flow rate of 1.0 ml/min, column temperature is 30°, and the effluent was monitored at 260 nm by a photodiode array detector. No complex gradient of mobile phase and no buffer were necessary.
Identification of the HSCCC peak fraction was carried out by UV, IR, MS, 1H NMR and 13C NMR.
3. Results and discussion
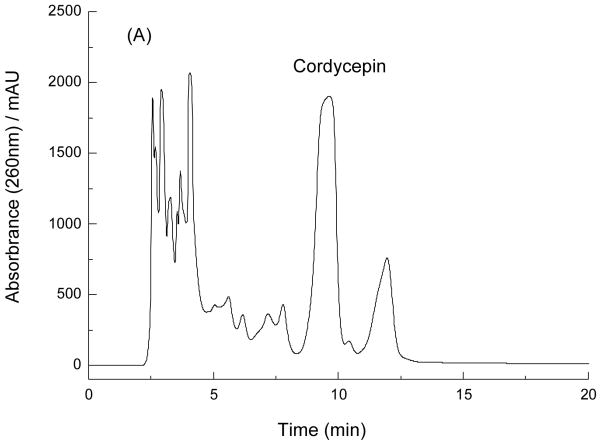
In our present study, 732 cation-exchange resin was used to obtain crude samples in place of organic extraction, which are unfriendly to our environment. The result indicted that target compound mainly existed in 0.15 mol/L ammonia aqueous solution. So, HCl aqueous solution (pH 3.0) was first used to elute the resin in order to remove some un-target chemicals, which have no or little retention on 732 cation-exchange resin. Second, 0.15 mol/L ammonia aqueous solution was used to elute the target compound and the crude sample was obtained for further isolation and purification. The crude extract (Fig. 2A), the sample after cleaning-up by cation-exchange resin (Fig. 2B) were analyzed by HPLC. The purity of cordycepin, which was adsorbed on the 732 cation-exchange resin column and then eluted with 0.15 mol/L ammonia aqueous solution, was increased from 29.4% to 44.7%. The efficiency of separation and purification was very remarkable.
Fig.2.
HPLC chromatograms of the crude extract from C. militaris and the sample after cleaning-up by 732 cation-exchange resin. Column: reversed-phase Prodigy C18 column (4.6 mm ×250 mm i.d., 5 μm); mobile phase: methanol/water (15:85, v/v); flow rate: 1.0 ml/min; detection wavelength: 260 nm; column temperature: 30°; A: crude extract; B: the sample after cleaning-up by 732 cation-exchange resin.
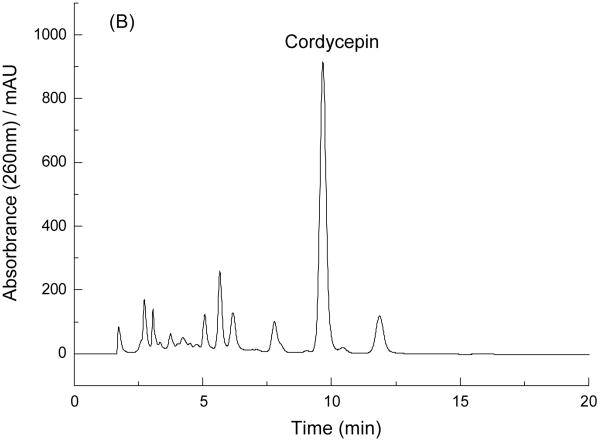
Successful separation by HSCCC largely depends on the selection of a suitable two-phase solvent system. So, some two-phase solvent systems were test by analytical HSCCC, including n-hexane–ethyl acetate–methanol–water, ethyl acetate–methanol–water, and n-hexane–n-butanol–methanol–water at different volume ratios, to determine the optimal two-phase solvent system for the preparative HSCCC separation. The results indicated that n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v) was suitable for the separation of cordycepin from the crude extract (Fig. 3).
Fig.3.
Analytical HSCCC separation of the crude sample obtained from C. militaris after cleaning-up by 732 cation-exchange resin. Solvent system: n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v); stationary phase: upper phase; mobile phase: lower phase; flow rate: 1.0 ml/min; evolution speed: 1600 rpm; sample: 4.2 mg dissolved in 2 ml of the lower phase; retention of the stationary phase: 49%; detection wavelength: 254 nm. Peak c: cordycepin.
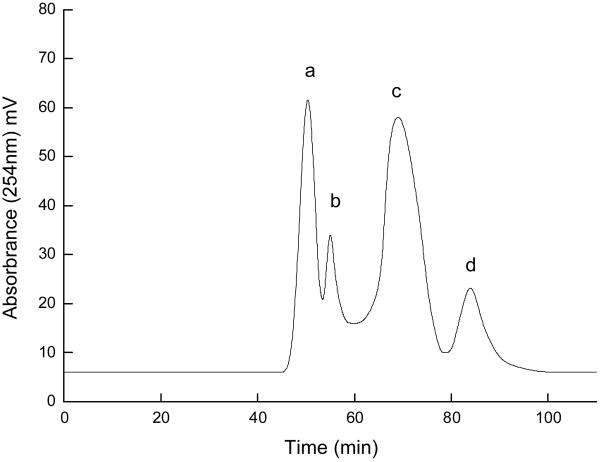
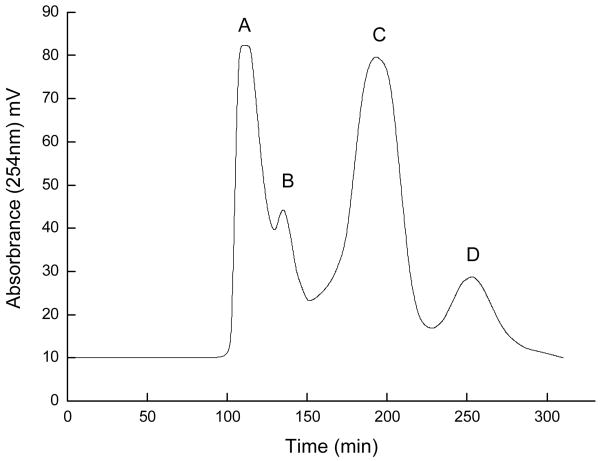
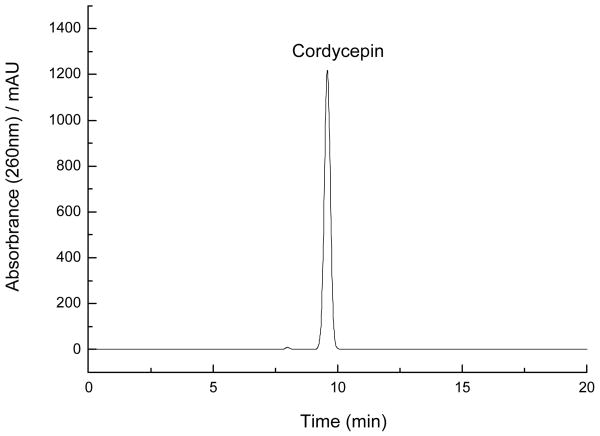
Preparative HSCCC was performed in the optimal two-phase solvent system, n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v), which produced four peak fractions A, B, C and D in Fig. 4, and retention percentage of the stationary phase was 54%. As shown in Fig.5, the HPLC analysis peak C of preparative HSCCC revealed that the pure cordycepin could be obtained from the crude sample. The purity of peak C (cordycepin) was 98.9%, as determined by the peak area percent of HPLC. This HSCCC separation yielded 64.8 mg cordycepin from 216.2 mg of crude sample. The recovery of the separation was as high as 91.7%.
Fig.4.
Preparative HSCCC separation of the crude sample obtained from C. militaris after cleaning-up by 732 cation-exchange resin. Solvent system: n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v); stationary phase: upper phase; mobile phase: lower phase; flow rate: 2.0 ml/min; revolution speed: 850 rpm; separation temperature: 30°; sample: 216.2 mg dissolved in 10 ml of the lower phase; retention percentage of stationary phase: 54%; detection wavelength: 254 nm. Peak C: cordycepin.
Fig.5.
HPLC chromatograms of the cordycepin fraction obtained by preparative HSCCC. Column: reversed-phase Prodigy C18 column (4.6 mm ×250 mm i.d., 5 μm); mobile phase: methanol/water (15:85, v/v); flow rate: 1.0 ml/min; detection wavelength: 260 nm; column temperature: 30°.
The structural identification of the isolated component was performed UV, IR, ESI-MS, 1H NMR and 13C NMR as follows. Peak C (cordycepin), which in accord with the literature [13,14]: UV: λnm (EtOH) 204, 259 nm. IR: νmax (KBr): 3416, 3331, 3142, 2923, 1675, 1609, 1576, 1479, 1420, 1385, 1341, 1300, 1208, 1176, 1115, 1039, 959, 835, 726, 518 cm-1. ESI-MS: m/z 252 [M+H]+; ESI-MS/MS: m/z 136 [M+H-116]+, 252 [M+H]+. 1H NMR (400MHz, CD3OD-d4) δ ppm: 8.31(s, 1H, 3-H), 8.22(s, 1H, 8-H), 6.07(d, 1H, 1′-H), 4.70(m, 1H, 4′-H), 3.75(t, 1H, 5′-H), 3.92(t, 1H, 5′-H), 2.33(m, 1H, 3′-H), 2.22(m, 1H, 3′-H). 13C NMR (400MHz, CD3OD-d4) δ ppm: 157.42(C-1), 153.64(C-3), 149.91(C-5), 120.68(C-6), 141.12(C-8), 93.60(C-1′), 76.59(C-2′), 34.51(C-3′), 82.55(C-4′), 64.20(C-5′).
4. Conclusion
The result of our studies showed that HSCCC is a useful tool for the preparative separation of the target compound yielding high purity and high recovery from the crude sample of natural products. Using HSCCC cordycepin is separated from C. militaris with a two-phase solvent system of n-hexane–n-butanol–methanol–water (23:80:30:155, v/v/v/v), 64.8 mg cordycepin was obtained from 216.2 mg crude extract in less than 320 min. The method is simple, fast, and without complex solvent system or gradient elution.
Acknowledgments
The authors thank Zhongshan LEAGUE Cordyceps Manufactured Product Co. LTD, for donating C. militaris.
References
- 1.Rao YK, Chou CH, Tzeng YM. Anal Chim Acta. 2006;566:253–258. [Google Scholar]
- 2.Pegler DN, Yao YJ, Li Y. Mycologia. 1994;8:3–5. [Google Scholar]
- 3.Mizuno T. Int J Med Mushr. 1999;1:251–261. [Google Scholar]
- 4.Mina M, Eriko U, Akihiko S, Mikio S. Enzyme Microb Tech. 2006;39:641–646. [Google Scholar]
- 5.Sugar AM, McCaffrey RP. Antimicrob Agents Ch. 1998;42:1424–1427. doi: 10.1128/aac.42.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson LS, Ting RC, Gallo RC, Wu AM. Int J Cancer. 1975;15:451–456. doi: 10.1002/ijc.2910150311. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Meyer CU, Schmidtke P, Zepp F. Eur J Pharmacol. 2002;453:309–317. doi: 10.1016/s0014-2999(02)02359-2. [DOI] [PubMed] [Google Scholar]
- 8.Peng JY, Fan GR, Hong ZY, Chai YF, Wu YT. J Chromatogr A. 2005;1074:111–115. doi: 10.1016/j.chroma.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 9.Liu RM, Feng L, Sun AL, Kong LY. J Chromatogr A. 2004;1057:89–94. doi: 10.1016/j.chroma.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Li AF, Sun AL, Liu RM. J Chromatogr A. 2005;1076:193–197. doi: 10.1016/j.chroma.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Peng JY, Fan GR, Chai YF, Wu YT. J Chromatogr A. 2006;1102:44–50. doi: 10.1016/j.chroma.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Ma XF, Zhang TY, Zhang YB, Liu QH, Ito Y. J Chromatogr A. 2007;1151:180–182. doi: 10.1016/j.chroma.2007.02.105. [DOI] [PubMed] [Google Scholar]
- 13.Frederiken S, Matting H, Ktenow H. Biochem Biophys Acta. 1965;95:189. [Google Scholar]
- 14.Liu JM, Zhong YR, Yang Z, Cui SL, Wang FH. China J Chin Mat Med. 1989;14:608–609. [PubMed] [Google Scholar]