Abstract
The main reason why tumours are not controlled by the immune system is that, unlike pathogens, they do not express potent tumour rejection antigens (TRAs). Tumour vaccination aims at stimulating a systemic immune response targeted to, mostly weak, antigens expressed in the disseminated tumour lesions. Main challenges in developing effective vaccination protocols are the identification of potent and broadly expressed TRAs1–3 and effective adjuvants to stimulate a robust and durable immune response4–6. Here we describe an alternative approach in which the expression of new, and thereby potent, antigens are induced in tumour cells by inhibiting nonsense-mediated messenger RNA decay (NMD)7–10. Small interfering RNA (siRNA)-mediated inhibition of NMD in tumour cells led to the expression of new antigenic determinants and their immune-mediated rejection. In subcutaneous and metastatic tumour models, tumour-targeted delivery of NMD factor-specific siRNAs conjugated to oligonucleotide aptamer ligands led to significant inhibition of tumour growth that was superior to that of vaccination with granulocyte–macrophage colony-stimulating factor (GM-CSF)-expressing irradiated tumour cells11, and could be further enhanced by co-stimulation. Tumour-targeted NMD inhibition forms the basis of a simple, broadly useful, and clinically feasible approach to enhance the antigenicity of disseminated tumours leading to their immune recognition and rejection. The cell-free chemically synthesized oligonucleotide backbone of aptamer–siRNAs reduces the risk of immunogenicity and enhances the feasibility of generating reagents suitable for clinical use.
Disseminated metastatic disease is the primary cause of death among cancer patients. Cancer vaccination stimulates a systemic immune response against judiciously chosen tumour antigens expressed in the tumour cells that seeks out and destroys the disseminated tumour lesions. The development of effective cancer vaccines will require the identification of potent and broadly expressed TRAs1–3 as well as effective adjuvants to stimulate a robust and durable immune response4–6. An alternative approach to vaccination is to express new, and hence potent, antigens in tumour cells in situ. How to express new antigens in the disseminated tumour lesions, but not in normal tissue, have precluded the development of such strategies so far. NMD is an evolutionarily conserved surveillance mechanism in eukaryotic cells that prevents the expression of mRNAs containing a premature termination codon (PTC)8–10. Inhibition of NMD in cultured human cell lines using siRNAs targeted to any of its factors, SMG1, UPF1, UPF2 or UPF3, results in the upregulation of several products encoded by the PTC-containing mRNAs (see, for example, refs 12–15). Many of these products, resulting from aberrant splicing or NMD-dependent autoregulated alternative splicing7,8,16, encode new peptides that have not induced tolerance (see Supplementary Discussion). We proposed that the upregulation of such products when NMD is inhibited in tumour cells will elicit an immune response against (some of) the new products, and that the immune response will inhibit tumour growth. Moreover, there is evidence that frameshift mutations in cancer cells exhibiting DNA mismatch repair generate PTC-containing transcripts that are negatively controlled by NMD17. Inhibiting NMD could, therefore, further augment the production of such tumour-specific antigens (see Supplementary Discussion).
To determine whether NMD inhibition in tumour cells can stimulate protective anti-tumour immunity, we tested whether the stable expression of NMD factor short hairpin RNAs (shRNAs) in tumour cells inhibits their growth potential in mice. CT26 colon carcinoma tumour cells were transduced with a lentiviral vector (PTIG-U6tetOshRNA) encoding Smg1 or Upf2 shRNAs expressed from a tet-regulated U6 promoter18. shRNA expression can be upregulated in vitro by adding doxycycline to the culture medium, and in vivo by providing doxycycline in the drinking water. Doxycycline-induced Smg1 and Upf2 shRNA expression in cultured CT26 cells results in downregulation of the corresponding mRNA (Supplementary Fig. 1a) and inhibition of NMD (Supplementary Fig. 1b). Long-term inhibition of NMD, or other functions controlled by SMG1 or UPF2, had no measurable effects on the viability or proliferative capacity of the CT26 cells in vitro (data not shown).
To determine whether siRNA inhibition of NMD in the tumour-bearing mice can stimulate immune responses against products that are normally under NMD control, we measured the intratumoral accumulation of T cells recognizing a model tumour antigen that is suppressed as a result of NMD. B16/F10 tumour cells containing the doxycycline-inducible Smg1, Upf2 or control shRNA were stably transfected with an NMD reporter plasmid encoding the dominant major histocompatibility complex (MHC) class I epitope of the chicken ovalbumin gene (OVA) upstream of a PTC (diagrams in Fig. 1a and Supplementary Fig. 1a). Tumour-bearing mice were infused with OT-I transgenic CD8+ T cells that recognize the OVA MHC class I-restricted epitope20, or with Pmel-1 transgenic CD8+ T cells that recognize an MHC class I-restricted epitope in the endogenous gp100 tumour antigen expressed in B16 tumour cells19. gp100 expression is not under NMD control. As shown in Fig. 1a, unlike Pmel-1 T cells, the OT-I T cells failed to accumulate to significant levels in the OVA-negative B16/F10 tumours or in tumours transfected with the PTC-containing β-globin-OVA construct encoding but not expressing Smg1 or Upf2 shRNA. However, upregulation of Smg1 or Upf2 shRNA, but not control shRNA (doxycycline in the drinking water) resulted in a significant accumulation of OT-I T cells in the tumours. This experiment shows that siRNA inhibition of NMD in tumour cells can induce an immune response in vivo against an antigen that is under NMD control.
Figure 1. Expression of Upf2 or Smg1 shRNA in CT26 tumour cells leads to immune-mediated inhibition of tumour growth.
a, Intratumoral accumulation of OVA-specific OT-I T cells in response to NMD inhibition. B16/F10 tumour cells transduced with shRNA-encoding lentiviral vectors (described in Supplementary Fig. 1a) were stably transfected with an NMD reporter plasmid (described in Supplementary Fig. 1b) containing the class I-restricted epitope of chicken ovalbumin (OVA). Mice were implanted subcutaneously with parental tumour cells (wild-type (WT) B16) or with the lentivirus-transduced tumour cells, and either received or did not receive doxycycline in their drinking water. When tumours became palpable, mice were injected with either OT-I or Pmel-1 transgenic CD8+T cells (three mice per group). Six days later, tumours were excised and analysed for OT-I and Pmel-1 T-cell content by flow cytometry. Ctrl, control. n = 2 b, Balb/c mice were implanted subcutaneously with CT26 tumour cells stably transduced with the shRNA inducible lentiviral vector encoding Smg1, Upf2 and control shRNA (ten mice per group). Each group was divided into two subgroups receiving (filled circles) or not receiving (open circles) doxycycline in the drinking water. n = 2. c, Same as b except that tumour cells were injected into immune-deficient nude mice. n = 1.
To determine whether siRNA-mediated inhibition of NMD affects tumour growth, the lentiviral-transduced CT26 cells expressing a control, Smg1 or Upf2 shRNA were implanted subcutaneously into mice and tumour growth was monitored in the presence or absence of doxycycline administered in the drinking water. Figure 1b shows that tumour cells expressing Smg1 or Upf2 shRNA, but not control shRNA, grew initially but failed to progress. Tumour inhibition was immune-mediated because the tumours grew in nude mice (Fig. 1c), and mice that rejected the tumours shown in Fig. 1b, but not age-matched control mice, resisted a second challenge with parental tumour cells (not shown). Delaying doxycycline treatment of mice expressing Smg1 shRNA diminished the tumour inhibitory effect that was completely lost when drug treatment was delayed for 6 days (Supplementary Fig. 2). Tumour rejection correlated with the induction of T-cell responses against tumour cells expressing Smg1 shRNA. No T-cell responses were detected against tumour cells that did not express Smg1 shRNA or against normal tissues including liver, colon and prostate (Supplementary Fig. 3). This is consistent with the hypothesis that tumour rejection was mediated by the induction of immune responses against NMD-controlled products that were upregulated when NMD was inhibited in the tumour cells.
In the experiment shown in Fig. 1b, tumour growth was completely prevented when NMD was inhibited in all tumour cells from the time of tumour implantation. Simulating a more relevant clinical model, we tested whether inhibition of NMD in pre-existing tumours can induce therapeutically useful tumour immunity. To preclude NMD inhibition in normal cells, the NMD factor siRNAs were targeted to tumour cells using oligonucleotide aptamer ligands21,22. Smg1 and Upf2 siRNA were conjugated to an oligonucleotide aptamer that binds to prostate-specific membrane antigen (PSMA)23 as shown in Supplementary Fig. 4. PSMA-expressing CT26 and B16 tumour cell lines were generated by transduction with a PSMA-encoding expression vector, and PSMA expression was confirmed by flow cytometry (not shown). The PSMA-conjugated siRNAs bound to and were taken up by PSMA-expressing, but not parental, tumour cells (Supplementary Fig. 5), leading to the downregulation of their target RNAs (Supplementary Fig. 6).
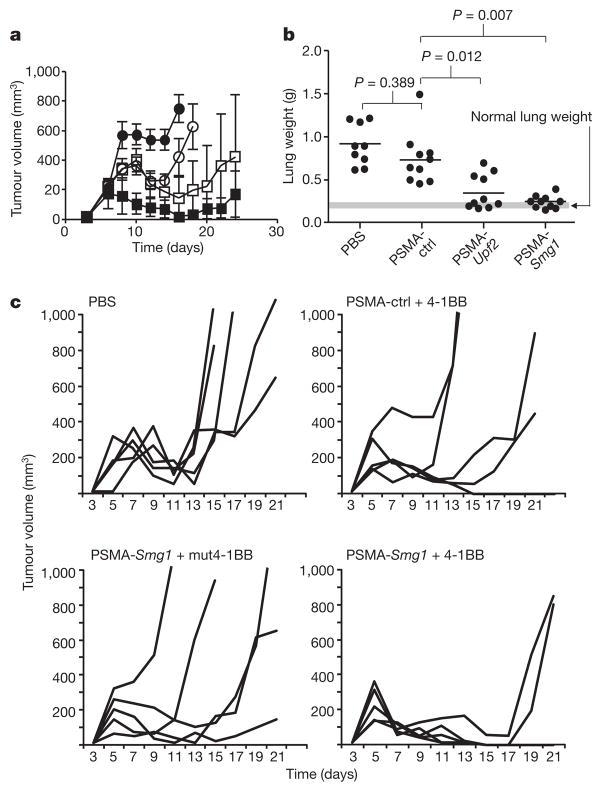
We next tested whether systemic administration of PSMA aptamer–siRNA conjugates by tail vein injection can inhibit tumour growth. As shown in Fig. 2a, treatment of day 3 subcutaneously implanted PSMA-CT26 tumour cells with PSMA-conjugated Smg1 siRNA, and to a lesser extent Upf2 siRNA, significantly inhibited tumour growth. Two out of seven mice treated with the PSMA aptamer–Smg1 siRNA conjugate rejected the implanted tumours and remained tumour-free (Supplementary Fig. 7). When treatment intensity was increased by doubling the dose of the aptamer–siRNA conjugate and extending treatment to seven injections, six out of seven of mice rejected the tumour long term. Treatment with PSMA aptamer conjugated to control siRNA had a small inhibitory effect that could have resulted from the binding of the PSMA aptamer–siRNA to the tumour cells, or be due to non-specific immune stimulatory effects of the oligonucleotide24,25. We found no increase in IFNα levels in the serum of mice treated with PSMA aptamer–control or Smg1 siRNA conjugates (data not shown). As shown in Fig. 2b, the treatment of day 5 PSMA-B16/F10 tumour-implanted mice with PSMA aptamer-conjugated Upf2 or Smg1 siRNA inhibited the development of lung metastasis that was more profound in the SMG1 group. To determine whether the anti-tumour response elicited by NMD inhibition can be further enhanced by co-stimulation, PSMA-CT26 tumour-bearing mice were treated with PSMA aptamer–Smg1 siRNA and an agonistic 4-1BB aptamer dimer26. The stringency of NMD inhibition and 4-1BB co-stimulation was adjusted to elicit a limited anti-tumour effect when applied separately by delaying treatment with PSMA aptamer–siRNA conjugates from days 3 to 5 and administering a single dose of 4-1BB aptamer on day 6. As shown in Fig. 2c, combination therapy with PSMA aptamer–Smg1 siRNA and 4-1BB aptamer was more than additive.
Figure 2. Inhibition of tumour growth in mice treated with PSMA aptamer targeted Upf2 and Smg1 siRNAs.
a, Balb/c mice were implanted subcutaneously with PSMA-CT26 tumour cells and 3 days later injected via the tail vein with PBS (filled circles) or with PSMA aptamer–siRNA conjugates (open circles, control siRNA; open squares, Upf2 siRNA; filled squares, Smg1 siRNA) (5 mice per group). n = 2. b, C57BL/6 mice were implanted with PSMA-B16/F10 tumour cells by tail vein injection, and 5 days later were injected with PSMA aptamer–siRNA conjugates (ten mice per group). Metastatic load was determined by measuring lung weight at the time of euthanization. n = 2. c, Combination immunotherapy using NMD inhibition and 4-1BB co-stimulation. PSMA-CT26 tumour-bearing mice (five mice per group) were treated with various combinations of PSMA aptamer conjugated to Smg1 or control siRNA and an agonistic or co-stimulation-deficient 4-1BB aptamer dimer26 (mut4-1BB) and monitored for tumour growth. n = 1.
To determine whether tumour inhibition shown in Fig. 2 is a result of aptamer targeting of siRNA to PSMA-expressing tumour cells, mice were implanted in opposite flanks with PSMA-expressing and parental CT26 tumour cells and PSMA aptamer conjugated to control or Smg1 siRNA was administered systemically by tail vein injection (Fig. 3a). Figure 3b shows that 32P-labelled PSMA aptamer–Smg1 siRNA conjugate accumulated preferentially in PSMA-expressing tumour cells. Figure 3c shows that systemic administration of PSMA aptamer-conjugated Smg1, but not control, siRNA inhibited the growth of PSMA-expressing CT26 tumour cells but not the contralaterally implanted parental CT26 tumour cells. Supplementary Fig. 8 shows a snapshot of the tumour-bearing mice at the day of euthanization.
Figure 3. PSMA aptamer–Smg1 siRNA rejection of PSMA-expressing, but not parental, CT26 tumour cells.
a, Mice were co-implanted subcutaneously with PSMA-expressing (left flank) and parental (right flank) CT26 tumour cells and injected with PSMA aptamer–Smg1 siRNA via the tail vein. b, Fifteen days after tumour inoculation, 32P-labelled aptamer–siRNA was injected, and 3 or 24 h later tumours were excised and the 32P content determined. n = 3. c, Three days after tumour inoculation, mice were injected with aptamer–siRNA conjugate (eight mice per group) as described in Fig. 2a and tumour growth was monitored. Open circles, parental CT26; filled circles, PSMA-CT26. n = 2.
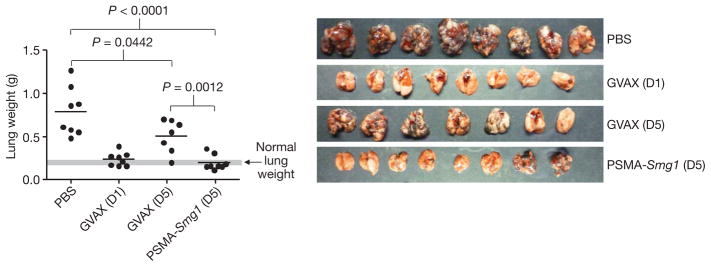
To assess the potency of tumour-targeted NMD inhibition, we compared the anti-tumour effects of treating tumour-bearing mice with PSMA aptamer–Smg1 siRNA conjugate and vaccination with GM-CSF-expressing irradiated syngeneic tumour cells (GVAX)11,27. In therapeutic protocols when vaccination is initiated 2–4 days after tumour inoculation, the anti-tumour impact of GVAX is limited, unless combined with other treatments such as CTLA-4 blockade28 or T-regulatory cell depletion29. As shown in Fig. 4, in the B16 lung metastasis model described in Fig. 2b, GVAX treatment of day 1 tumour bearing mice significantly inhibited metastasis, whereas treatment of day 5 tumour bearing mice had a limited anti-metastatic effect that barely reached statistical significance. By comparison, treatment of day-5 tumour-bearing mice with PSMA aptamer–Smg1 siRNAs inhibited metastasis to an extent comparable to that of administering GVAX at day 1. Given that these are first generation aptamer–siRNA conjugates and the dose and schedule of aptamer–siRNA treatment have not been optimized, these results indicate that tumour-targeted siRNA-mediated NMD inhibition is more effective than a commonly used vaccination protocol.
Figure 4. Comparison of PSMA aptamer–Smg1 siRNA treatment to vaccination with GM-CSF expressing irradiated tumour cells.
C57BL/6 mice were injected intravenously with B16/F10 tumour cells and treated with PSMA aptamer–siRNA conjugates starting at day 5 as described in Fig. 2b, or vaccinated with GM-CSF-expressing irradiated B16/F10 tumour cells (GVAX) starting at days (D) 1 or 5 using the protocol described previously29. n = 1.
Tumour-targeted NMD inhibition is a new approach to stimulate protective anti-tumour immunity. Instead of stimulating or potentiating immune responses against existing, often weak, antigens expressed in the tumour cells—the goal of current tumour vaccination protocols—NMD inhibition generates new antigenic determinants in situ in the disseminated tumour lesions. It should be noted that NMD control of gene expression is ‘leaky’. In addition to the first round of translation, known as pioneer translation, the efficiency of nonsense-mediated degradation varies among individual mRNA targets8–10. Immune recognition is, therefore, a consequence of upregulation of NMD-controlled products above a certain threshold that was set by the natural immune tolerance mechanisms. The NMD inhibition strategy described in this study is simple, consisting of a single reagent that can be synthesized by a cell-free chemical process, it obviates the need to identify TRAs or adjuvants, and is broadly applicable as it targets a common pathway in all tumours. The potency of the NMD inhibition approach was suggested when compared to GVAX vaccination. Arguably, these first generation aptamer–siRNA conjugates and the dose and treatment schedule can be further optimized. It would be of interest to determine in future studies whether the NMD-induced antigens are cross-reactive among different tumours, and if so to identify the dominant antigens induced by NMD inhibition.
METHODS SUMMARY
Tumour immunotherapy studies
Three-hundred-thousand parental or pTIG-U6tetOshRNA transduced CT26 tumour cells were implanted subcutaneously in Balb/c or Nude mice. At the day of tumour implantation, mice started receiving water supplemented with 10% sucrose with or without 2 mg ml−1 doxycycline (Sigma).
To evaluate the anti-tumour effects of PSMA aptamer–siRNAs, mice were implanted with 1 × 106 PSMA-CT26 tumour cells and injected with 400 pmoles of aptamer–siRNA in 100 μl PBS via the tail vein at days 3, 5, 7, 9, 11 and 13. In combination therapy, treatment with PSMA aptamer–siRNA was administered at days 5, 7, 9, 11 and 13, and a single dose of 500 pmoles of 4-1BB aptamer dimer was administered on day 6.
To monitor metastasis, C57BL/6 mice were implanted with 105 B16-PSMA transduced cells by the tail vein and injected with 400 pmoles of aptamer-siRNA conjugates at days 5, 8, 11, 14 and 17. When about half of the mice in the control groups had shown signs of morbidity (approximately days 25–28), the mice were euthanized and their lungs were weighed. GM-CSF-expressing B16/F10 tumour cells, provided by G. Dranoff, were irradiated (50 Gy) and 5 × 105 cells were injected subcutaneously at days 1, 4 and 7, or days 5, 8 and 11, as described previously29.
For statistical analysis, P values were calculated using a Student’s t-test.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Acknowledgments
We thank J. Zhang for assistance in the mouse studies, A.-M. Jegg for technical assistance in characterizing Smg1 siRNAs, J. Rossi for advising in the design of aptamer–siRNA conjugates, and S. Nair and D. Boczkowski for advice in performing T-cell assays. This work was supported by the Dodson foundation and the Sylvester Comprehensive Cancer Center (Medical School, University of Miami).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions F.P. suggested the approach and was responsible for designing the aptamer–siRNA conjugates and interpreting the results, D.K. was responsible for the mouse studies, P.H.G. helped design the aptamer–siRNA conjugates, and E.G. oversaw experimental design, data analysis, and wrote the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 2.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20:276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilboa E. The promise of cancer vaccines. Nature Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 5.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nature Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 7.Frischmeyer PA, Dietz HC. Nonsense-mediated mRNA decay in health and disease. Hum Mol Genet. 1999;8:1893–1900. doi: 10.1093/hmg/8.10.1893. [DOI] [PubMed] [Google Scholar]
- 8.Behm-Ansmant I, et al. mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett. 2007;581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nature Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 10.Mühlemann O, Eberle AB, Stalder L, Zamudio Orozco R. Recognition and elimination of nonsense mRNA. Biochim Biophys Acta. 2008;1779:538–549. doi: 10.1016/j.bbagrm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–298. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Bchiri J, et al. Nonsense-mediated mRNA decay impacts MSI-driven carcinogenesis and anti-tumor immunity in colorectal cancers. PLoS One. 2008;3:e2583. doi: 10.1371/journal.pone.0002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nature Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 14.Usuki F, et al. Specific inhibition of nonsense-mediated mRNA decay components, SMG-1 or Upf1, rescues the phenotype of Ullrich disease fibroblasts. Mol Ther. 2006;14:351–360. doi: 10.1016/j.ymthe.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann J, Hol EM, Jack H. M hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol Cell Biol. 2006;26:1272–1287. doi: 10.1128/MCB.26.4.1272-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isken O, Maquat LE. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nature Rev Genet. 2008;9:699–712. doi: 10.1038/nrg2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62:2447–2454. [PubMed] [Google Scholar]
- 18.Aagaard L, et al. A facile lentiviral vector system for expression of doxycycline-inducible shRNAs: knockdown of the pre-miRNA processing enzyme Drosha. Mol Ther. 2007;15:938–945. doi: 10.1038/sj.mt.6300118. [DOI] [PubMed] [Google Scholar]
- 19.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Gold L. Oligonucleotides as research, diagnostic, and therapeutic agents. J Biol Chem. 1995;270:13581–13584. doi: 10.1074/jbc.270.23.13581. [DOI] [PubMed] [Google Scholar]
- 22.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 23.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 24.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Judge AD, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 26.McNamara JO, et al. Multivalent 4-1BB binding aptamers costimulate CD8 T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.