Abstract
Neurocognitive decline is a frequent adverse effect of glioblastoma. Antitumor therapies that are efficacious, as measured by traditional endpoints such as objective response (OR) and progression-free survival (PFS), and have beneficial effects on neurocognitive function (NCF) are of clinical benefit to these patients. We evaluated neurocognitive changes across time in 167 patients with recurrent glioblastoma treated with bevacizumab-based therapy in BRAIN, a phase II, randomized, multicenter trial. All patients underwent MRI and neurocognitive testing at baseline and every 6 weeks thereafter. Memory, visuomotor scanning speed, and executive function were evaluated using the Hopkins Verbal Learning Test–Revised, the Trail Making Test, and the Controlled Oral Word Association test, respectively. NCF relative to baseline for patients with an OR, PFS >6 months, or disease progression was evaluated at time of OR, 24 weeks, and time of progression, respectively. For patients with an OR or PFS >6 months, median standardized test scores were examined from baseline to week 24. Most patients with an OR or PFS >6 months had poorer NCF performance compared to the general population at baseline and had improved or stable NCF at the time of response or at the 24-week assessment, respectively; most patients with progressive disease had neurocognitive decline at the time of progression. For patients with an OR or PFS >6 months, median standardized test scores were largely stable across the first 24 weeks on study. Neurocognitive testing was an objective, valid, and feasible method of monitoring NCF in patients with recurrent glioblastoma.
Keywords: bevacizumab, cognitive function, glioblastoma, quality of life
Glioblastoma is a highly malignant and rapidly progressing disease. At the time of diagnosis, patients with glioblastoma frequently suffer from neurocognitive deficits.
Neurocognitive function (NCF) has been shown to be a predictor of survival in patients with recurrent malignant glioma1 and has a direct bearing on health-related quality of life (QoL). Diminished NCF in patients with brain tumors has been associated with reduced independence, difficulty maintaining roles in the home and work environments, decreased ability to participate in daily living activities,2–4 and increased caregiver burden and distress.4,5 Neurocognitive decline often precedes reductions in daily functioning and QoL in these patients1,6–8 and has been shown to occur in advance of radiographic evidence of tumor progression.9,10
Outcome assessment in neuro-oncology is oriented predominantly toward radiographic changes and survival time.11 Radiographic response, in particular, is not always the best indicator of patient status. For instance, with anti-angiogenic therapy, apparent radiographic response may reflect a normalization of tumor vasculature rather than a true antitumor effect. Patient-centered outcomes assessed with neurocognitive testing can provide supportive information about the clinical benefit achieved with increased response rates and survival and may help patients and physicians to make decisions about the potential benefits of treatment. Furthermore, antitumor therapies that have beneficial effects on NCF, in addition to being efficacious in terms of radiological response and survival, would be of value to patients with high-grade gliomas in terms of improved QoL and functional independence.1 To date, there has been no evaluation of NCF in multisite clinical trials of patients with recurrent glioblastoma.
In the phase II BRAIN trial of patients with recurrent glioblastoma,12 the vascular endothelial growth factor (VEGF) inhibitor bevacizumab (BEV; Genentech), either alone or in combination with irinotecan (CPT-11), demonstrated improved objective response (OR) rates and 6-month progression-free survival (PFS) compared with historical controls. As an exploratory outcome in BRAIN, NCF was assessed with a battery of neurocognitive tests widely used in neuropsychological clinical practice and research studies in patients with brain tumors.1,9 We took advantage of the available data to describe NCF in a population of patients with recurrent glioblastoma, evaluate how NCF changed with treatment, and assess the overall feasibility of using neurocognitive testing to monitor NCF in this rapidly progressing disease.
Patients and Methods
Patients
One hundred sixty-seven patients with glioblastoma at first or second relapse were randomized to receive BEV (n= 85) or BEV + CPT-11 (n= 82) in the BRAIN study. BRAIN methodology, including study design, eligibility, treatment, assessments, and analyses, has been published in detail.12 BRAIN is registered at www.clinicaltrials.gov (NCT00345163). The protocol was approved by the institutional review board at each study site, and all patients provided informed consent prior to participation in the study. All patients underwent clinical, laboratory, MRI, and neurocognitive testing at baseline and prior to the beginning of each treatment cycle (i.e., every 6 weeks) up to 52 weeks or until disease progression or discontinuation.
Neuroimaging
Co-primary endpoints of BRAIN, OR rate and 6-month PFS, were assessed by a blinded, independent radiology facility (IRF; RadPharm, Inc., Princeton, NJ) according to World Health Organization Response Evaluation Criteria,13 taking corticosteroid dose into account.14 Non-contrast-enhancing lesions were considered nontarget lesions in tumor assessment. Progression was determined by contrast-enhancing and non-contrast-enhancing lesions. As mandated by the protocol, in the absence of radiographic documentation, clinical progression, assessed by the investigator according to his/her judgment of neurological progression, was used to determine disease progression. (Notably, each determination of disease progression in BRAIN was based on radiographic documentation.) All patients were followed until discontinuation from the study, loss to follow-up, study termination, or death.
Neurocognitive Testing
Memory, visuomotor scanning speed, and executive function were evaluated using 3 objective, standard, valid tests (Table 1). The maximum time to complete each test ranged from 3 to 5 min, for a total evaluation time of ∼25 min.
Table 1.
Overview of neurocognitive tests
| Test | Description | Possible range of score | Reliable change index threshold (from baseline) |
|---|---|---|---|
| Hopkins Verbal Learning est-Revised (HVLT-R) | The Hopkins Verbal Learning Test-Revised (HVLT-R)15 is a learning and memory test, in which the patient is asked to learn and recall a list of 12 words over three trials. | ||
| Total Recall (HVLT-R TR) | 0–36 | ±5 words15 | |
| Delayed Recall (HVLT-R DR) | Spontaneous recall is assessed before and after a delay. Recognition discriminability is also assessed after a delay. Four alternate versions of the test were used to minimize practice effects over time. | 0–12 | ±3 words |
| Delayed Recognition (HVLT-R RECOG) | −12–+12 | ±2 words | |
| Trail Making Test (TMT) | The Trail Making Test (TMT)28 Part A (TMTA) assesses visual scanning and motor tracking requiring focused attention. Patients are required to sequentially connect numbered dots in ascending order that are randomly scattered across the test page. Part B (TMTB) includes a divided attention component requiring mental flexibility (i.e., executive function). On this subtest, dots with numbers and letters are randomly scattered on the test page. Patients are required to alternate between connecting numbers and letters in an ascending sequential order. Both tests require the patient to complete the sequence as fast as possible. TMTA was discontinued after 3 min and TMTB was discontinued after 5 min for patients that had difficulty in order to reduce patient burden. | ||
| Part A (TMTA) | 1–2750 | ±12 s29 | |
| Part B (TMTB) | 1–3750 | ±26 s | |
| Controlled Oral Word Association (COWA) | The Controlled Oral Word Association [COWA]30 test assesses lexical fluency. Given a specific letter of the alphabet, patients are required to produce as many words as possible that begin with that letter. There are two alternate forms of the COWA, each with three unique letter exemplars. | 0 – unlimited | ±12 words17 |
Statistical Analysis
For each NCF test, raw scores and standardized scores (mean = 0, SD = 1) using published normative data from a healthy population15–17 were calculated for analyses. For Trail Making Test Parts A and B, raw scores were prorated (percent prorated: Part A = 4.73%, Part B = 19.38%) if the test was discontinued or the maximal time was reached secondary to a neurological difficulty, according to the method described by Heaton et al.16 If a test was not administered, or the patient was unable to attempt a test, it was excluded from the analysis. The percentage of patients with analyzable data (completed and/or prorated tests) for each NCF test at each assessment was calculated.
At each assessment, change in raw test score relative to baseline was calculated, and neurocognitive status was categorized as improved, stable, or declined, using the Reliable Change Index (RCI).18 The RCI is derived from the standard error of measurement of each test and represents the 90% confidence interval for the difference in raw score from baseline to the next assessment that would be expected if no real change occurred:
 |
where SEdiff is the standard error of difference, SEM is the standard error of measurement, SD is the standard deviation, and rxy is the test-retest reliability statistic. All RCI thresholds were rounded to the nearest whole number. Changes that did not meet the RCI threshold for improvement or decline were categorized as stable performance. Changes (i.e., improvement, decline) from baseline neurocognitive status were confirmed at the next neurocognitive assessment, when available.
To assess the relationship among 3 clinical and radiographic tumor response outcomes and change in NCF, neurocognitive status relative to baseline was evaluated for 3 subgroups of patients at specific timepoints: patients with an IRF-determined OR at the time of first response, patients with IRF-determined PFS >6 months at the Week 24 assessment, and patients with investigator-determined progressive disease at the time of progression. Changes in standardized scores over time were also plotted for the first 2 subgroups of patients. Finally, concomitant medications that could affect NCF were summarized for patients who had an IRF-determined OR.
Results
Patient Demographics and Baseline Characteristics
Between June 2006 and February 2007, 167 patients with glioblastoma in first or second relapse were randomized to receive BEV (n = 85) or BEV + CPT-11 (n= 82) in the BRAIN study (Table 2). Negative mean standardized test scores in all tests indicated that patients were performing below the mean of the general population.
Table 2.
Patient demographics and baseline characteristics
| BEV (n = 85) | BEV + CPT-11 (n = 82) | |
|---|---|---|
| Age in years | ||
| Mean (SD) | 53.8 (11.0) | 55.0 (12.4) |
| Median | 54 | 57 |
| Range | 23–78 | 23–79 |
| % Male | 68.2 | 69.5 |
| % White | 90.6 | 89.0 |
| Education,a years | ||
| Mean (SD) | 15.1 (2.6) | 15.2 (3.0) |
| Median | 16 | 16 |
| Range | 9–22 | 6–22 |
| Relapse, % | ||
| First | 81.2 | 80.5 |
| Second | 18.8 | 19.5 |
| KPS, % | ||
| 90–100 | 44.7 | 37.8 |
| 70–80 | 55.3 | 62.2 |
| Initial surgery, % | ||
| Complete resection | 42.4 | 37.8 |
| Partial resection | 49.4 | 53.7 |
| Biopsy only | 8.2 | 8.5 |
| Medication use at baseline, % | ||
| Anticonvulsants | 21.2 | 36.6 |
| Corticosteroids | 50.6 | 52.4 |
| Opioids | 15.3 | 11.0 |
| Psychostimulants | 12.9 | 4.9 |
| Median months from radiotherapy to study treatment | 6.2 | 6.6 |
| Median months from surgery for recurrent disease to study treatment | 7.4 | 7.9 |
| Standardized neurocognitive test scores at baseline, mean (SD), median | ||
| HVLT-R TR | n = 83, −2.47 (1.85), −2.22 | n = 75, −2.30 (1.89), −2.21 |
| HVLT-R DR | n = 81, −2.72 (2.16), −2.67 | n = 74, −2.43 (2.19), −2.11 |
| HVLT-R RECOG | n = 79, −1.92 (2.61), −1.29 | n = 75, −1.61 (2.44), −0.57 |
| TMTA | n = 82, −4.81 (5.65), −2.65 | n = 73, −12.34 (59.67), −2.54 |
| TMTB | n = 73, −7.52 (11.96), −2.91 | n = 70, −7.70 (12.81), −3.65 |
| COWA | n = 81, −1.67 (1.07), −1.89 | n = 74, −1.55 (1.38), −1.58 |
aBEV n = 64; BEV + CPT-11 n = 62. HVLT-R, Hopkins Verbal Learning Test-Revised; TR, Total Recall; DR, Delayed Recall; RECOG, Delayed Recognition; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; COWA, Controlled Oral Word Association; BEV, bevacizumab; CPT-11, irinotecan.
Neurocognitive Outcomes
Eighty-five to 98% of all patients completed the neurocognitive tests at baseline; and the majority of patients who remained on study completed tests at each assessment. Table 3 shows the percentage of patients who completed individual tests at baseline, Week 6, and Week 24).
Table 3.
Frequency of analyzable neurocognitive dataa
| BEV |
BEV + CPT-11 |
|||||
|---|---|---|---|---|---|---|
| Test, % Completed | Baseline (n = 85) | Week 6 (n = 76) | Week 24 (n = 29) | Baseline (n = 82) | Week 6 (n = 75) | Week 24 (n = 34) |
| HVLT-R TR | 98 | 96 | 93 | 92 | 89 | 91 |
| HVLT-R DR | 95 | 92 | 90 | 90 | 89 | 88 |
| HVLT-R RECOG | 93 | 92 | 90 | 92 | 87 | 88 |
| TMTA | 97 | 95 | 90 | 89 | 87 | 94 |
| TMTB | 86 | 91 | 79 | 85 | 79 | 88 |
| COWA | 95 | 93 | 93 | 90 | 87 | 94 |
aIncludes patients for whom test scores were prorated. n = number of patients eligible (i.e., progression free and still on study) for assessment at time indicated. BEV, bevacizumab; CPT-11, irinotecan; HVLT-R, Hopkins Verbal Learning Test-Revised; TR, Total Recall; DR, Delayed Recall; RECOG, Delayed Recognition; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; COWA, Controlled Oral Word Association.
The majority of patients who had an IRF-determined OR or IRF-determined PFS >6 months had stable or improved performance on all tests relative to baseline at the time of OR (BEV = 75.0%; BEV + CPT-11 = 60.7%) or at the Week 24 assessment (BEV = 70.4%; BEV + CPT-11 = 69.0%), respectively (Table 4). In contrast, the majority of the patients who had investigator-determined disease progression had declined on at least one test at the time of progression (BEV = 69.4%; BEV + CPT-11 = 56.0%; Table 4), and >40% declined on multiple tests.
Table 4.
Neurocognitive status relative to baseline for key patient subgroups
| Patient subgroup | RCI-determined neurocognitive status |
|||
|---|---|---|---|---|
| BEV |
BEV + CPT-11 |
|||
| Stable or improved on all tests | Declined on at least one test | Stable or improved on all tests | Declined on at least one test | |
| Patients with an IRF-determined OR (at time of response)a | 18/24 (75.0%) | 6/24 (25.0%) | 17/28 (60.7%) | 11/28 (39.3%) |
| Patients with IRF-determined PFS >6 months (at Week 24 assessment)b | 19/27 (70.4%) | 8/27 (29.6%) | 20/30 (69.0%) | 9/30 (30.0%) |
| Patients with investigator-determined disease progression (at time of progression)c | 15/49 (30.6%) | 34/49 (69.4%) | 11/25 (44.0%) | 14/25 (56.0%) |
a3 BEV + CPT-11,
b2 BEV and 3 BEV + CPT-11 and
c8 BEV and 21 BEV + CPT-11 patients with missing data were excluded. BEV, bevacizumab; CPT-11, irinotecan; IRF, independent review facility; RCI, Reliable Change Index; OR, objective response; PFS, progression-free survival.
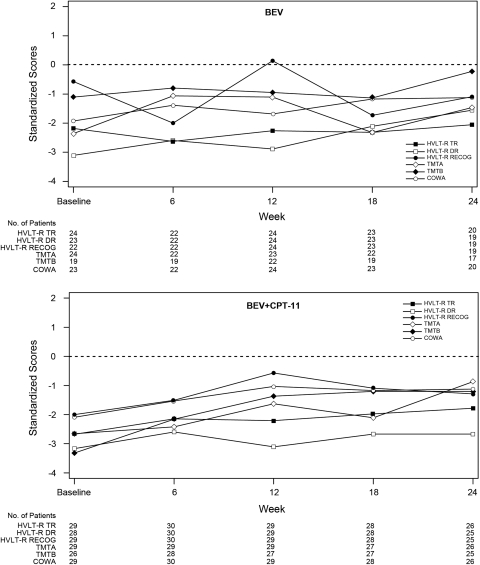
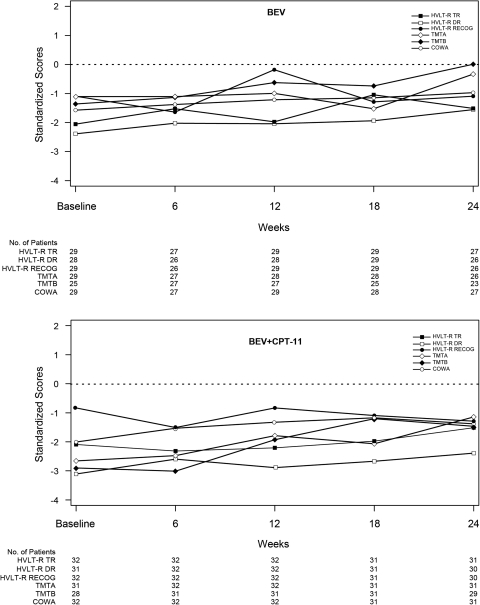
Compared with the general population, patients with an IRF-determined OR or PFS >6 months had poorer performance on all neurocognitive tests at baseline (Figs 1 and 2); and the median standardized scores of these patients remained stable from baseline to Week 24, with trends suggesting improvement in some patients.
Fig. 1.
Neurocognitive test scores across time for patients with an objective response. Values represent median test scores of patients in the BEV (upper panel) and BEV + CPT-11 (lower panel) study groups who had an IRF-determined objective response and completed neurocognitive tests at the indicated time of assessment, standardized using normative data from the general population (i.e., mean = 0, SD = 1). Values under the x-axis represent the number of patients assessed at each corresponding timepoint. BEV, bevacizumab; CPT-11, irinotecan; HVLT-R, Hopkins Verbal Learning Test-Revised; TR, Total Recall; DR, Delayed Recall; RECOG, Delayed Recognition; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; COWA, Controlled Oral Word Association; IRF, independent review facility.
Fig. 2.
Neurocognitive test scores across time for patients with progression-free survival >6 months. Values represent median test scores of patients in the BEV (upper panel) and BEV + CPT-11 (lower panel) study arms with progression-free survival >6 months, according to IRF review, and completed tests at the indicated time of neurocognitive assessment, standardized using normative data from the general population (i.e., mean = 0, SD = 1). Values under the x-axis represent the number of patients assessed at each corresponding timepoint. BEV, bevacizumab; CPT-11, irinotecan; HVLT-R, Hopkins Verbal Learning Test–Revised; TR, Total Recall; DR, Delayed Recall; RECOG, Delayed Recognition; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B; COWA, Controlled Oral Word Association; IRF, independent review facility.
Concomitant Medication
Given the low rate of use, it is unlikely that psychostimulants or opioids affected neurocognitive status at the time of IRF-determined OR. The prevalent use of anticonvulsants and corticosteroids makes it difficult to determine their effects, if any, on NCF in this patient population (Table 5).
Table 5.
Frequency of concomitant medication use at the time of independent review facility-determined objective response
| Concomitant medication | % Patients at time of IRF-determined objective response |
|
|---|---|---|
| BEV (n = 24) | BEV + CPT-11 (n = 31) | |
| Anticonvulsants | 71 | 65 |
| Corticosteroids | 54 | 45 |
| Opioids | 17 | 7 |
| Psychostimulants | 8 | 10 |
IRF, independent review facility; BEV, bevacizumab; CPT-11, irinotecan.
Discussion
The BRAIN study evaluated NCF in the largest sample of patients with progressive glioblastoma to date. Test completion rates were high and consistent with other large multicenter trials,19 indicating that the approximately 25-min assessment was feasible and not overly burdensome for patients with glioblastoma in first or second relapse or study sites.
Neurocognitive tests were sensitive to changes in NCF over time and demonstrated that, relative to baseline, the majority of patients who had an IRF-determined OR or PFS >6 months had improved or stable NCF at the time of response or at the 24-week assessment, respectively; while those who had investigator-determined progressive disease demonstrated evidence of neurocognitive decline at the time of progression.
Median test scores during the first 24 weeks on study for patients with an IRF-determined OR or PFS >6 months were generally stable, with trends suggesting improvement in some patients. While this is consistent with many anecdotal reports of significant improvement in patient NCF while on therapy, the absence of a control arm in the BRAIN study does not permit us to rule out the possibility that the trend suggesting improvement may also reflect practice effects, despite efforts to diminish this possibility by using alternate forms of the tests across time.20
In some patients with recurrent glioblastoma, the degree of neurocognitive impairment can be so great at baseline that it is unlikely that subsequent test scores will exceed the RCI threshold necessary to determine a decline in NCF (i.e., floor effect). Sensitivity analyses in the subset of BEV-group patients who had an OR showed that only one of the neurocognitive tests, the HVLT-R-DR (Hopkins Verbal Learning Test-Revised-Delayed Recognition), showed a potential for floor effects. Even when taking this into account, the majority of responders did not experience a decline in NCF at the time of OR relative to baseline. Thus, although patients were quite cognitively debilitated at baseline relative to a healthy population, change from baseline NCF did not appear to have been influenced by potential floor effects.
Antiangiogenic therapies have complicated the interpretation of classic radiographic outcomes (e.g. van den Bent 2009; Wen et al. 2010),21,11 primarily due to the effects of tumor vasculature normalization confounding true antitumor effects. In the current trial there was evidence of a trend suggesting some degree of consistency between radiographically determined outcomes and NCF outcomes at key radiographic time points. However, in a substantial minority of patients there was discordance between radiographic outcomes and NCF outcomes. Further examination of these discrepancies in the future may enhance our understanding of the clinical impact of different radiographic features/patterns.
In addition to alternative imaging modalities for tumor assessment and determination of response and progression,11 data increasingly demonstrate that patient-centered endpoints, such as NCF, may be used to measure clinical benefit;22 NCF is an attractive endpoint, as it provides a direct, objective, valid, and standardized measure of a cardinal, early, and frequent symptom of brain tumor.22,23 In high-grade glioma, pretreatment NCF24 and NCF 16 months after treatment25 were predictive of survival, even after controlling for age, KPS, histology, and time since diagnosis.1 Changes in NCF can occur in a predictable relationship with evidence of changing lesion burden, as seen in the current analysis; and they have also been demonstrated to occur in advance of radiographic evidence of tumor progression.7,9,10 Additionally, NCF is a direct measure of patient well-being that is associated with functional independence, and subjective QoL2,5,6 is more sensitive to disease progression than self-reported QoL,2,5,6 activities of daily living,1,5,6 and the results of mental status screening tests, such as the Mini-Mental State Examination.26,27 Furthermore, decline in NCF is associated with caregiver distress and burden.4,5 As demonstrated in this analysis of BRAIN participants, integrating NCF testing as an outcome in brain tumor trials is feasible and yields critical information about clinical benefit that may not be captured by radiographic imaging alone. Of note, potential confounding variables, such as treatment toxicities or unrecognized comorbidities (e.g., subclinical seizures) were not taken into account when assessing NCF.
To summarize, NCF testing with objective and valid tests to measure patient functioning was feasible in patients with recurrent glioblastoma. The majority of patients who had an IRF-determined OR or PFS >6 months had improved or stable NCF at the time of response or at the 24-week assessment, respectively; and most patients who had investigator-determined progressive disease demonstrated evidence of neurocognitive decline at the time of progression. Inclusion of a control or comparison arm in future studies will facilitate more detailed interpretations of these data.
Funding
This work was supported by Genentech, Inc.
Acknowledgments
We thank Meghna Samant and Dafeng Chen, Genentech, Inc. for biostatistics support. Genentech, Inc. provided support for manuscript preparation.
Conflict of interest statement. Jeffrey S. Wefel has served as a consultant for Genentech, Roche, and Exelixis and as an advisor for Genentech. Timothy Cloughesy has served as a consultant for Roche, Genentech, Exelixis, Merck, and Astra Zeneca. James L. Zazzali is an employee of Genentech and owns stock in Roche. Maoxia Zheng is an employee of Genentech and owns stock in Roche. Michael Prados has provided uncompensated expert FDA testimony on behalf of Genentech. Patrick Y. Wen has received research support from and has served on an advisory board for Genentech. Tom Mikkelsen has received honoraria and speaking fees from Genentech. David Schiff has served on Genentech advisory boards each of the last 3 years. Lauren E. Abrey has received honoraria and consulting fees from Genentech and Roche. W.K. Alfred Yung has had consulting relationships with Novartis and Merck/Schering Plough and advisory affiliations with Novartis, Eden, and Merck. Nina Paleologos has served as a speaker for Merck and Genentech and a consultant for Merck, Genentech, and Eisai. Martin K. Nicholas has served on an advisory board for Genentech. James Vredenburgh has received consulting fees, honoraria, and speaker fees from Genentech and Roche. Asha Das is an employee of Genentech and owns stock in Roche. Henry S. Friedman has served as a paid consultant and speaker for Genentech.
References
- 1.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 2.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76:562–568. doi: 10.1136/jnnp.2004.036186. doi:10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farace E, Shaffrey ME. Relationship of neurocognitive impairment to QOL in malignant brain tumor patients. Society for Neuro-Oncology Fifth Annual Meeting: November 9–12, 2000; Chicago, IL: 2000. Abstract 61. [Google Scholar]
- 4.Meyers CA, Boake C. Neurobehavioral disorders experienced by brain tumor patients: Rehabilitation strategies. Cancer Bull. 1993;45:362–364. [Google Scholar]
- 5.Tomaszewski FS, Cahn-Weiner DA, Harvey DJ, et al. Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. The Clinical Neuropsychologist. 2008;23:446–461. doi: 10.1080/13854040802360558. doi:10.1080/13854040802360558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Bentzen SM, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. doi:10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 7.Brown PD, Jensen AW, Felten SJ. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24:5427–5433. doi: 10.1200/JCO.2006.08.5605. doi:10.1200/JCO.2006.08.5605. [DOI] [PubMed] [Google Scholar]
- 8.Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9:53–62. doi: 10.1215/15228517-2006-012. doi:10.1215/15228517-2006-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5:89–95. doi: 10.1215/S1522-8517-02-00026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong CL, Goldstein B, Shera D, Ledakis GE, Tallent EM. The predictive value of longitudinal neuropsychologic assessment in the early detection of brain tumor recurrence. Cancer. 2003;97:649–656. doi: 10.1002/cncr.11099. doi:10.1002/cncr.11099. [DOI] [PubMed] [Google Scholar]
- 11.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. doi:10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 12.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, Jr, Calmcross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 15.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 16.Heaton RK, Miller SW, Taylor MJ, et al. Revised comprehensive norms for an expanded Halstead–Reitan battery. Odessa, FL: PAR: 2004. [Google Scholar]
- 17.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 18.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. doi:10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. doi:10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 20.Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20:339–352. doi: 10.1076/jcen.20.3.339.822. doi:10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- 21.van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. doi:10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. doi:10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 23.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–333. doi: 10.1097/00006123-200008000-00011. discussion 333–334 doi:10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61:1796–1798. doi: 10.1212/01.wnl.0000098892.33018.4c. [DOI] [PubMed] [Google Scholar]
- 25.Levin VA, Yung WK, Bruner J, et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53:58–66. doi: 10.1016/s0360-3016(01)02819-x. doi:10.1016/S0360-3016(01)02819-X. [DOI] [PubMed] [Google Scholar]
- 26.Meyers CA, Kudelka AP, Conrad CA, et al. Neurotoxicity of CI-980, a novel mitotic inhibitor. Clin Cancer Res. 1997;3:419–422. [PubMed] [Google Scholar]
- 27.Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21:3557–3558. doi: 10.1200/JCO.2003.07.080. doi:10.1200/JCO.2003.07.080. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Trail Making Test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1958. 1992. [Google Scholar]
- 29.Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18:373–384. doi: 10.1080/1385404049052420. doi:10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 30.Benton AL, Hamsher KDS. Multilingual aphasia examination. Iowa City: AJA Associates; 1989. [Google Scholar]