Abstract
This study was designed to confirm the previously observed favorable survival rates and prognostic factors in young children with nonmetastatic medulloblastoma (MB) treated with postoperative chemotherapy alone. Patients who received a diagnosis during the period January 2001 through December 2005 and who were aged <4 years received 3 cycles of postoperative systemic multiagent chemotherapy and intraventricular methotrexate. In cases of complete remission, treatment was terminated after 2 additional cycles of chemotherapy. Otherwise, secondary surgery, radiotherapy, and consolidation chemotherapy were recommended. At a median follow-up of 4.5 years, the 5-year event-free survival (EFS) and overall survival (OS) rates (± standard error) for 45 patients (median age, 2.5 years) were 57% ± 8% and 80% ± 6%, respectively. Nineteen patients with desmoplastic/nodular MB variants had better 5-year EFS and OS rates (90% ± 7% and 100% ± 0%, respectively) than did 23 patients with classic MB (30% ± 11% and 68% ± 10%, respectively; P < .001 for EFS; P = .008 for OS). Five-year EFS and OS rates for 3 children with anaplastic MB were 33% ± 27%. Desmoplastic/nodular histology was an independent prognostic factor for EFS. Twenty-nine of 30 patients without postoperative residual tumor remained in continuous complete remission.
Our results confirm that histology of MB variants is a strong prognostic factor in this age group. Sustained tumor control can be achieved by this chemotherapy regimen in young children with desmoplastic/nodular MB variants. For children with non-desmoplastic/nonnodular MB variants, for which predominantly local relapses lead to less favorable survival rates, local radiotherapy has been introduced after chemotherapy since 2006.
Keywords: brain tumor, chemotherapy, children, medulloblastoma, treatment
One-third of cases of medulloblastoma (MB), the most common malignant brain tumor of childhood, occur during the first 3 years of life,1 and ∼50% of MB cases are diagnosed before the age of 5 years.1 Although the prognosis of older children with MB has been improved by combined strategies using surgery, craniospinal radiotherapy (CSI), and chemotherapy, the survival rates for children aged <3 years have been historically poor for decades and did not exceed 25%–45% until the past decade.2,3 The unfavorable prognosis might be partly explained by frequent occurrence of metastases and by the different biology of MB in young children.2,4,5 Moreover, the immature brain is particularly susceptible to radiotherapy-induced neurocognitive deficits,6 resulting in reluctance to use radiotherapy in young children with MB.2,7–9
Treatment strategies for young children with MB have been explored, with the goal of improving survival while decreasing neurologic sequelae.2,7–9 Most of the studies aimed to delay or avoid radiotherapy by different approaches using conventional systemic chemotherapy and radiotherapy7,10–13 or high-dose chemotherapy with autologous stem cell rescue, given either during primary treatment or at relapse.9,14–16
The pilot trial HIT-SKK’87 of the Society of Pediatric Oncology and Hematology in Germany, Austria, and Switzerland (GPOH) for children aged <3 years confirmed that postoperative chemotherapy may successfully delay the start of radiotherapy.17 With the aim of reducing the poor neuropsychologic outcome of children after chemotherapy and CSI, intraventricular methotrexate was introduced as a substitute for radiotherapy in the subsequent HIT-SKK’92 trial.13 Treatment was completed without radiotherapy or additional chemotherapy after 6 months of intensive systemic chemotherapy (with cyclophosphamide, vincristine, methotrexate, carboplatin, and etoposide) and intraventricular methotrexate (36 single doses; 2mg/dose), if complete remission (CR) was achieved. In that study, favorable survival rates have been obtained, especially for young patients with desmoplastic/nodular MB (DMB) (5-year progression-free survival [PFS] rate (± standard error) 85% ± 8%; 5-year overall survival [OS] rate (± standard error) 95% ± 5%),13 and compared with the HIT-SKK’87 trial, neurocognitive deficits were less pronounced. DMB and MB with extensive nodularity (MBEN), closely related to DMB but differing from DMB by expanded lobular architecture and reduced internodular reticulin-rich components, were confirmed to have a first peak incidence during early childhood in a meta-analysis involving 5 study groups,18 accounting for 30%–50% of cases of early childhood MB in the different study groups analyzed. Both DMB and MBEN were defined as separate variants in the latest World Health Organization classification of brain tumors.19
Here, we report the results of the trial HIT-SKK’2000BIS4 for patients who received diagnoses before 2006. The trial was designed to confirm or further improve the favorable survival rates obtained in the previous HIT-SKK’92 trial for children aged <3 years13 but for patients <4 years of age, with use of a chemotherapy regimen that was extended by 2 additional cycles of systemic chemotherapy.
Patients and Methods
Eligibility
Criteria for inclusion were diagnosis of a primary intracranial tumor, centrally reviewed and histologically confirmed diagnosis of MB, no MB classified as secondary malignancy, age <4 years at time of diagnosis, no medical contraindication to protocol therapy, and no history of previous radiotherapy or chemotherapy. Patients were to have no evidence of disseminated disease confirmed by centrally reviewed cerebrospinal fluid (CSF) cytology and craniospinal MRI at diagnosis. To be included in the study, patients must have survived the first day of postoperative chemotherapy. All institutions participating in the study had received approval from their institutional review boards, and informed consent was obtained from legal representatives of all patients.
Patients, Surgery, and Pathological Analysis
Forty-eight children with newly diagnosed nondisseminated MB, who were aged <4 years at the time of initial tumor surgery, and who had received a diagnosis during the period January 2001 through December 2005 at 27 of 88 participating centers in Germany, Austria, and Switzerland were registered in the prospective multicenter study HIT-SKK’2000BIS4 (ClinicalTrials.gov/NCT00303810). The maximum possible safe surgical removal of the primary tumor was recommended. After histological confirmation of diagnosis, an intraventricular access device (Ommaya or Rickham reservoir) was implanted. Central pathologic review was performed for all patients with MB by an experienced neuropathologist (T.P.), and findings were classified according to the World Health Organization classification of brain tumors19 as classic MB (CMB; n = 25), DMB (n = 14), MBEN (n = 6), and anaplastic MB (AMB; n = 3).
Treatment
After surgery, HIT-SKK chemotherapy was initiated within 2–4 weeks. Patients received 3 cycles of chemotherapy consisting of intravenous cyclophosphamide, vincristine, methotrexate (followed by leucovorin rescue after 42 h), carboplatin, and etoposide and concomitant intraventricular methotrexate (36 single doses; 2 mg/dose), for a total duration of 6 months, as described elsewhere.13,17 Doses of cyclophosphamide, vincristine, and carboplatin were calculated on the basis of body surface area and were adjusted for age (for patients aged ≤6 months, 2/3 of the full dose; for those aged 7–12 months, 4/5 of the full dose). Intraventricular administration of methotrexate was not performed for children with suspected infection of the central nervous system, thrombocytopenia (platelet count, <30, 000 platelets/mm3), evidence of disturbed circulation of CSF, presence of a ventriculoperitoneal or ventriculoatrial shunt, trough CSF methotrexate levels ≥5 μmol/L 24 h after intraventricular application of methotrexate, or CSF protein levels ≥80 mg/dL. Treatment was terminated after application of 2 additional cycles of chemotherapy (with cyclophosphamide, vincristine, carboplatin, and etoposide) in ABAB sequence, if a complete remission was achieved after the previous chemotherapy. Cycle A comprised an 1-h infusion of cyclophosphamide (800 mg/m2 per day) on day 1–3 and intravenous vincristine (1.5 mg/m2) on day 1. Cycle B consisted of an 1-h infusion of carboplatin (200 mg/m2 per day) on day 1–3 and a 30-min infusion of etoposide (150 mg/m2 per day) on day 1–3. In case of noncomplete remission, secondary surgery, CSI (24 Gy with a boost up to 54.6 Gy to the posterior fossa, if patients were aged ≥18 months), and 4 cycles of consolidation chemotherapy with cisplatin, CCNU (lomustine), and vincristine were recommended.
Evaluation and Follow-Up
Staging included pre- and postoperative cranial MRI, spinal MRI, and evaluation of CSF to exclude CSF spread. Postoperative imaging was recommended to be performed within 48 (72) h after initial tumor resection.20 Extent of surgical resection was measured on axial plane (≤1.5 cm2 or >1.5 cm2). Lumbar CSF sampling was recommended ≥14 days after surgery (before the start of chemotherapy). Central review of histological data has been performed for all patients with MB. Twenty-four of 45 patients analyzed in this study had complete central review of CSF and imaging. In 21 patients, who also lacked evidence of metastatic disease, the quality of diagnostic evaluations was insufficient or central review was incomplete for the following reasons: ventricular and no lumbar CSF specimens were examined (n = 10), lumbar CSF examination revealed >50% cytolytic cells (n = 1), central review of lumbar CSF specimens was performed 4 days after the start of systemic chemotherapy (n = 1), central review of CSF specimens (n = 6), preoperative and postoperative MRI scans (n = 1), or CSF and MRI scans (n = 3) were of poor quality or incomplete. Characteristics of 24 fully and 21 incompletely assessable patients (Table 1) were similar with regard to histological findings, sex, and age. Postoperative residual tumors were observed more frequently among incompletely assessable children than among fully assessable patients.
Table 1.
Demographic characteristics and disease characteristics of patients
| Characteristic | Complete review (n = 24) | Incomplete review (n = 21) | All patients (n = 45) |
|---|---|---|---|
| Sex | |||
| Male | 19 | 14 | 33 |
| Female | 5 | 7 | 12 |
| Median age at diagnosis, years (range) | 2.6 (0.5–4.0) | 2.2 (0.3–3.8) | 2.5 (0.3–4.0) |
| Histology | |||
| CMB | 13 | 10 | 23 |
| DMB | 7 | 6 | 13 |
| MBEN | 3 | 3 | 6 |
| AMB | 1 | 2 | 3 |
| Postoperative residual tumor | |||
| R– (no residual tumor) | 20 | 10 | 30 |
| R+ (any residual tumor) | 4 | 11 | 15 |
| Residual tumor ≤1.5 cm2 | 22 | 17 | 39 |
| Residual tumor >1.5 cm2 | 2 | 4 | 6 |
| Duration of follow-up for surviving patients, median years (range) | 4.5 (1.3–8.2) | 4.6 (2.8–7.4) | 4.5 (1.3–8.2) |
| Secondary surgery | |||
| Performed | 4 | 5 | 9 |
| Not performed | 21 | 15 | 36 |
AMB, anaplastic medulloblastoma (MB); CMB, classic MB; DMB, desmoplastic MB; MBEN, MB with extensive nodularity; R+, any residual tumor; R–, no residual tumor.
After each cycle of chemotherapy, cranial, and spinal MRI scans were performed. CR was defined as the total disappearance of visible residual tumor.20 A continuous CR (CCR) was defined as an absence of any evaluable disease, after complete tumor resection, during the entire observation time. Partial response (PR) was defined as a ≥50% decrease in the tumor volume, improvement (IMP) was defined as a 25%–50% decrease in tumor volume, stable disease (SD) was defined as a <25% decrease or increase, and progressive disease (PD) was defined as a ≥25% increase, as assessed by MRI.20 Before any treatment element was administered, complete blood count, electrolyte level, creatinine level, and liver function parameters were assessed. Serum levels of methotrexate were determined at regular intervals until levels were <0.25 μmol/L. Imaging was to be repeated every 3–4 months in the first 2 years after treatment, every 6–9 months up to 5 years after treatment, and annually thereafter.
Statistical Analysis
Data were collected at the HIT 2000 study center at the Children's University Hospital of Hamburg-Eppendorf (the HIT 2000 study center was located until 2009 at the Children's University Hospital of Wuerzburg), data from patients from Austria were collected at the Children's University Hospital of Graz. Radiotherapy data were collected at the Department of Radiation Oncology, University of Leipzig. Survival was estimated by the Kaplan-Meier method, and a log-rank test was used for comparisons of survival in different groups.21 OS was defined as date of diagnosis to death of any cause or to the date of last visit; EFS was defined as date of diagnosis to date of first progression, relapse, occurrence of secondary malignancy, death of any cause, or last contact; radiotherapy-free survival and CSI-free survival were defined as date of diagnosis to start of any radiotherapy (radiotherapy-free survival) or to start of CSI (CSI-free survival), respectively, or to date of death of any cause, both of which were considered to be treatment failures. To analyze the impact of response after 3 cycles of chemotherapy, OS was defined as the time from evaluation of response to date of death or to the date of the last visit. For multivariable analysis, Cox regression models with forward- and backward-stepwise selection (the inclusion criterion was a P value of the score-test ≤.05; the exclusion criterion was P value of the likelihood-ratio test ≥.10) were used to analyze the impact of histology (CMB vs DMB or MBEN vs AMB) and residual disease (>1.5 cm2 vs ≤1.5 cm2) on EFS; OS was not assessable because of the low number of deaths reported.22 For the purpose of statistical analysis, DMB and MBEN were considered to be one entity (DMB/MBEN) as appropriate. Because of the expected registration rate per year (<10 patients/year), the study HIT-SKK’2000BIS4 was designed as a prospective multicenter observational study to be compared with the previous study HIT-SKK’92 as historical control. No primary end point was defined. A log-rank test was performed to compare survival rates of eligible (intent-to-treat) nonmetastatic patients treated in the HIT-SKK’92 with children aged <3 years treated in the HIT-SKK’2000BIS4 trial. All P values are considered to be explorative, no significance level was fixed. Analysis was performed with SPSS software, version 17.0 (SPSS).
Results
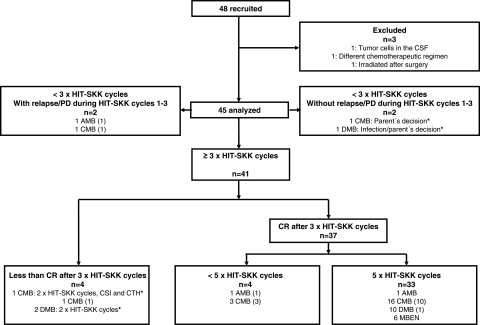
Between January 2001 to December 2005, 48 (36 male and 12 female) children aged <4 years with nondisseminated MB were enrolled. Three patients were excluded, all of whom were male; 2 had CMB, and 1 had DMB. One patient had tumor cells in the CSF (central review), 1 patient received a different chemotherapeutic regimen, and 1 patient underwent irradiation after surgery. Demographic and disease characteristics of the 45 evaluable patients are listed in Table 1.
Tumor Location, Response to Chemotherapy, and Progressive Disease During Therapy
Tumor localization was midline in 38 children (84%) and involved cerebellar hemispheres in 7 children (16%) (Fig. 1). The tumor resection was complete (R0) in 30 patients and incomplete (R+) in 15 patients. Response evaluation after 3 cycles of HIT-SKK chemotherapy demonstrated CCR in 29 (97%) of 30 patients and objective response in 13 (87%) of 15 children, respectively (Fig. 1). One of 30 patients (AMB) had a local relapse (Fig. 1). Two children received <3 cycles of HIT-SKK chemotherapy without relapse during that period (Fig. 2). Forty-one patients received ≥3 cycles of HIT-SKK chemotherapy and had no relapse during this induction treatment. Thirty-seven children with CR after 3 cycles of chemotherapy received the additional chemotherapy cycles as recommended. Protocol noncompliance was observed in 5 patients (Fig. 2). The 5-year OS among 38 patients without measurable disease after 3 cycles of HIT-SKK chemotherapy (CCR and CR) was higher than that of 7 patients (4 of whom had CMB, 2 of whom had DMB, and 1 of whom had AMB) who achieved less than a CR after these 3 cycles (5-year overall survival rate ± standard error, 84% ± 6% versus 57% ± 19%; P = .052).
Fig. 1.
Participant flow. AMB, anaplastic medulloblastoma (MB); CCR, continuous complete remission; CMB, classic MB; CR, complete response; CSI, craniospinal radiotherapy; DMB, desmoplastic MB; IMP, improvement; local RT, local radiotherapy; MBEN, MB with extensive nodularity; PD, progressive disease; PR, partial response; R0, complete resection; R+, incomplete resection; SD, stable disease.
Fig. 2.
CONSORT flow chart showing primary treatment of patients. Numbers in parentheses indicate the number of patients with relapse or progressive disease. AMB, anaplastic medulloblastoma (MB); CMB, classic MB; CR, complete response; CSF, cerebrospinal fluid; CSI, craniospinal radiotherapy; DMB, desmoplastic MB; MBEN, MB with extensive nodularity; PD, progressive disease. *Protocol noncompliance was observed in 5 patients.
Pattern of Relapse
At the time of analysis, data on relapse or tumor progression were reported for 18 patients at a median time of 1.3 years after primary surgery (range, 0.20–4.49 years; only 1 child with CMB experienced relapse [at a distant site] >2 years after primary surgery) (Fig. 1). Time to relapse was similar for patients with postoperative residual disease (7 of 15 patients; median, 1.25 years after primary surgery; range, 0.62–1.63 years), compared with patients who did not have postoperative residual disease (11 of 30 patients; median, 1.35 years after primary surgery; range, 0.20–4.49 years;). Twelve (67%) of the 18 treatment failures (10 in patients with CMB, and 1 each in patients with DMB and AMB) observed were isolated local relapses (Fig. 1). Treatment failures of fully assessable patients with MB (n = 24) were also predominantly local (6 local and 2 distant failures; median time after primary surgery, 1.32 years; range, 0.20–1.72 years).
EFS, OS, and Salvage Therapy
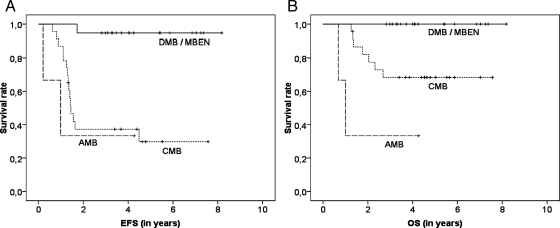
The estimated 5-year EFS and OS (± standard error) for the 45 patients were 57% ± 8% and 80% ± 6%, respectively (Fig. 3A and 3B). There was no difference between patients with complete and incomplete central reference assessments (5-year EFS, 66% ± 10% vs 46% ± 13% [P = .382]; 5-year OS, 83% ± 8% vs 76% ± 9% [P = .576]). Rates of radiotherapy-free and CSI-free survival at 5 years were 59% ± 7% (Fig. 3C). Only 1 patient underwent local irradiation (it was an individual decision for a patient with residual disease) (Fig. 1); the patient had relapse at distant site and died 2 years after diagnosis. Two patients with residual disease after 3 cycles of HIT-SKK chemotherapy and 9 patients who experienced relapse were treated with CSI (Fig. 1). Nineteen patients (13 of whom had DMB and 6 of whom had MBEN) had better EFS and OS rates (5-year rates, 95% ± 5% and 100% ± 0%, respectively) than 23 patients with CMB (5-year rates, 30% ± 11% [P < .001] and 68 ± 10% [P = .008], respectively) (Fig. 4A and 4B). EFS and OS rates for 3 patients with AMB were 33% ± 27% (5-year rates). One of 14 patients with DMB without postoperative residual disease experienced a local relapse 1.7 years after diagnosis; he is presently alive and has been in his second CR for >2 years after salvage treatment (secondary surgery, systemic chemotherapy, and CSI), with a duration of follow-up of 4.1 years (Fig. 2). All 6 patients with MBEN are still event-free. Two children with AMB experienced a relapse and died after salvage therapy (systemic chemotherapy, 1 patient; CSI, 1 patient). After relapse, additional therapy was reported for 14 of 15 patients with CMB who underwent (n = 6) or did not undergo (n = 8) secondary surgery (Fig. 2). Eight children with CMB experienced relapse and are still alive after receiving additional treatment (CSI and systemic chemotherapy, 2 patients; CSI and secondary surgery, 1; CSI, systemic chemotherapy, and secondary surgery, 3; systemic chemotherapy and high-dose chemotherapy, 1; and CSI, systemic chemotherapy, and high-dose chemotherapy, 1). Seven children with CMB experienced a relapse and died. Six received further treatment (systemic chemotherapy, 2 patients; local radiotherapy and systemic chemotherapy, 1; CSI and systemic chemotherapy, 1; secondary surgery and systemic chemotherapy, 1; and secondary surgery, systemic chemotherapy, and high-dose chemotherapy, 1). After relapse, no additional therapy was reported for 1 patient. The EFS and OS rates for patients with (n = 15) and without (n = 30) any postoperative residual disease were not different (5-year EFS rate, 53% ± 13% vs 59% ± 10% [P = .531]; 5-year OS rate, 80% ± 10% vs 80% ± 8% [P = .957]). Patients without or with residual disease ≤1.5 cm2 (n = 39) had better OS rates (5-year OS rate, 84% ± 6% vs 50% ± 20%; P = .044) and a trend toward a better EFS rate (5-year EFS rate, 60% ± 9% vs 33% ± 19%; P = .110) (Fig. 5) than patients with residual disease >1.5 cm2 (n = 6; all patients with CMB).
Fig. 3.
Survival of patients with nonmetastatic medulloblastoma (MB). Kaplan-Meier estimates for event-free survival (EFS; panel A), overall survival (OS; panel B), and radiation (RT)–free survival (panel C).
Fig. 4.
Survival according to histology. Nineteen patients (13 patients with desmoplastic medulloblastoma [DMB)] and 6 patients with medulloblastoma with extensive nodularity [MBEN]) had better event-free survival (EFS; panel A) and overall survival (OS; panel B) rates (5-year rates, 95% ± 5% and 100% ± 0%, respectively) than did 23 patients with classic medulloblastoma (CMB; 30% ± 11% [P < .001] and 68% ± 10% [P = .008], respectively).
Fig. 5.
Survival according to postoperative residual tumor. Thirty-nine patients without residual tumor or with small residual tumor (≤1.5 cm2) had slightly better rates of event-free survival (EFS; 5-year rates, 60% ± 9% vs 33% ± 19%; P = .110) (panel A) and overall survival (OS; 5-year rates, 84% ± 6% vs 50% ± 20%; P = .044) (panel B), compared with 6 patients with residual tumor >1.5 cm2.
When comparing children aged <2.5 years (median age) at diagnosis with those aged 2.5–4 years, we did not notice different OS rates (5-year rate, 83% ± 8% vs 77% ± 9%; P = .609) or EFS rates (5-year rate, 70% ± 10% vs 43% ± 13%; P = .190). Of note, frequency of histological subtypes differed in children aged <2.5 years (CMB, 10 patients; DMB, 9; MBEN, 4; and AMB, 0), compared with children aged ≥2.5 years (CMB, 13 patients; DMB, 4; MBEN, 2; and AMB, 3). When comparing patients aged <3 years (the age limit of the previous HIT-SKK’92 trial) with those aged 3–4 years, we found similar rates for OS (5-year rate, 82% ± 6% vs 71% ± 14%; P = .420) and EFS (5-year rate, 56% ± 9% vs 62% ± 15%; P = .981) and different frequencies of histological subtypes (for patients aged <3 years, CMB, 17; DMB, 10; MBEN, 6; and AMB, 1; for patients aged ≥3 years, CMB, 6; DMB, 3; MBEN, 0; and AMB, 2).
There was no difference between patients aged <3 years treated in the HIT-SKK’2000BIS4 trial (n = 34) and patients without metastases treated in the previous HIT-SKK’92 trial (n = 24; 5-year EFS rate, 56% ± 9% vs 71% ± 9% [P = .323]; 5-year OS rate, 82% ± 7% vs 83% ± 8% [P = .853]). There were no differences between survival rates for the groups of histological subtypes from both trials (Table 2).
Table 2.
Event-free survival (EFS) and overall survival (OS) rates for children aged <3 years at diagnosis, according to histology
| Histology | 5-year EFS rates (%) ± SE (%) (number of patients) |
P value | 5-year OS rates (%) ± SE (%) (number of patients) |
P value | ||
|---|---|---|---|---|---|---|
| HIT-SKK’92 | HIT-SKK’2000BIS4 | HIT-SKK’92 | HIT-SKK’2000BIS4 | |||
| CMB | 55 ± 15 (11) | 27 ± 12 (17) | 0.222 | 73 ± 13 (11) | 71 ± 11 (17) | 0.851 |
| DMB | 67 ± 19 (6) | 90 ± 10 (10) | 0.216 | 83 ± 15 (6) | 100 ± 0 (10) | 0.197 |
| MBEN | 100 ± 0 (7) | 100 ± 0 (6) | NA | 100 ± 0 (7) | 100 ± 0 (6) | NA |
| AMB | (0) | 0 ± 0 (1) | NA | (0) | 0 ± 0 (1) | NA |
Univariable analyses were performed for 58 patients with nonmetastatic medulloblastoma (MB); (HIT-SKK’92, 24 patients; HIT-SKK’2000BIS4, 34 patients). AMB, anaplastic MB; CMB, classic MB; DMB, desmoplastic MB; MBEN, MB with extensive nodularity; NA, not applicable.
In a multivariable Cox regression analysis that tested the variables histology and residual disease (>1.5 cm2 vs ≤1.5 cm2), only the histologic variants had an influence on EFS. Patients with DMB or MBEN had a 95% lower risk of experiencing an event than did patients with CMB (hazard ratio, 0.05; 95% confidence interval, 0.01–0.39; P < .001); patients with AMB and patients with CMB had a similar risk of having an event.
Treatment Toxicity and Feasibility of Intraventricular Methotrexate Administration
Table 3 shows the cumulative rate of toxicities over the entire courses of the HIT-SKK chemotherapy (data were assessable for 43 of 45 patients for HIT-SKK cycles 1–3 and 36 of 40 patients for HIT-SKK cycles 4–5). Four patients received <3 cycles (Fig. 2). Myelotoxicity was frequent; 19 children required red blood cell transfusions during the first 3 cycles, and 17 patients received red blood cell transfusions during the 2 additional cycles. Similarly, 20 patients underwent platelet transfusions during the cycles 1–3, and 17 patients received platelet transfusions during cycles 4–5. No renal or audiological grade 3 or grade 4 toxicities were reported. No treatment-related deaths or lethal infections occurred.
Table 3.
Cumulative number of patients who had grade 3 and 4 toxicities during HIT-SKK chemotherapy
| Cycles 1–3 | Cycles 4–5 | |
|---|---|---|
| Cumulative number of patients | 43 of 45 | 36 of 45 |
| White blood cells | ||
| Grade 3 | 2 | – |
| Grade 4 | 28 | 23 |
| Platelets | ||
| Grade 3 | 3 | 6 |
| Grade 4 | 21 | 13 |
| Nervous system | ||
| Grade 3 | – | 1 |
| Grade 4 | 4 | 1 |
| Infection | ||
| Grade 3 | 9 | 8 |
| Grade 4 | – | – |
| Vomiting | ||
| Grade 3 | 1 | 1 |
| Grade 4 | 1 | – |
| Mucositis/stomatitis | ||
| Grade 3 | 2 | 1 |
| Grade 4 | 3 | – |
| Methotrexate toxicity | ||
| Infection/fever | 4 | – |
| Othera | 24 | 1 |
| Other | ||
| Grade 3 | 5 | – |
| Grade 4 | 4 | 1 |
aOther methotrexate associated toxicities include myelotoxicity, hepatotoxicity, mucositis, and diarrhea.
In the majority of children, the administration of intraventricular methotrexate was feasible, without unexpected potentially methotrexate associated toxicities (Table 4). Reasons for methotrexate dose modifications are listed in Table 5. One child did not receive any intraventricular methotrexate. Revision of reservoir was performed in 8 children (because of CSF infection with Staphylococcus aureus in 1 patient and reservoir malfunction in 7 patients). Elevated methotrexate levels were measured in 7 (1. cycle), in 5 (2. cycle), and in 2 patients (3. cycle), and no temporally related toxicities (eg, seizures) were reported in these asymptomatic cases.
Table 4.
Number of applications of intraventricular methotrexate (MTX) during the first 3 cycles
| No. of patients |
|||
|---|---|---|---|
| Number of MTX applications | Cycle 1 | Cycle 2 | Cycle 3 |
| 0–4 MTX applications | 5 | 3 | 6 |
| 5–8 MTX applications | 10 | 2 | 6 |
| >9 MTX applications | 28 | 37 | 31 |
| Unknown | 2 | 3 | 2 |
Table 5.
Reasons of intraventricular methotrexate (MTX) dose modifications during HIT-SKK chemotherapy cycles 1–3
| No. of patients |
|||
|---|---|---|---|
| Reason for MTX dose modification | Cycle 1 | Cycle 2 | Cycle 3 |
| Elevated MTX level | 7 (without symptoms) | 5 (without symptoms) | 2 (without symptoms) |
| Reservoir malfunction | 5 | 2 | 2 |
| Cerebrospinal fluid infection | 1 (Staphylococcus aureus) | – | – |
| Fever | 3 | – | – |
| Disturbed cerebrospinal fluid circulation | 2 | – | – |
| Delayed implantation of reservoir | 4 | – | – |
| Leukoencephalopathy | 1 | 1 | 1 |
| Ventriculoperitoneal shunt | 1 | 1 | 1 |
| Seizure | 2 | – | 1 |
Discussion
This multi-institutional prospective study was designed to confirm or further improve the favorable survival rates previously obtained13 by the same 6-month intravenous, multiple-agent chemotherapy and intraventricular methotrexate regimen, prolonged by further systemic chemotherapy for children in CR.
The estimated survival rates among the 45 patients with nondisseminated MB aged <4 years (5-year EFS rate, 57% ± 8%; 5-year OS rate, 80% ± 6%) and the estimated survival rates of the 34 patients with nondisseminated MB aged <3 years (5-year EFS rate, 56% ± 9%; 5-year OS rate, 82% ± 7%) compare favorably with results of other studies and are in the range for the 24 patients with nondisseminated MB from the previous HIT-SKK’92 trial of children aged <3 years.13 The frequencies of the histological subtypes (CMB and DMB) among children aged 3–4 years and their survival rates are not different from those observed in children aged <3 years. All children with MBEN were aged <3 years at diagnosis. It is not possible to prove an additional benefit of the newly introduced prolonged chemotherapy for children who were in CR after 3 cycles of chemotherapy. The 5-year CSI-free survival rate was 59% ± 7% and was comparable with the 5-year CSI-free survival rate for 64 young patients with nonmetastatic MB treated with postoperative chemotherapy alone (57% ± 12%) as reported in the previous SFOP study, which included high-dose chemotherapy as part of salvage treatment.16
It has been shown that DMB/MBEN account for >40% of cases of early childhood MB.13,17,18,23,24 MBEN is associated with a favorable outcome.18,23,24 Moreover, the previous HIT-SKK’92 trial demonstrated that 20 patients with DMB had a better outcome than 23 patients with CMB,13 independent of presence or absence of metastases or postoperative residual tumor. The results of the current trial confirm the high relative frequency of DMB/MBEN and the prognostic impact of histology in this age group. Nineteen patients with DMB/MBEN had better EFS and OS rates (5-year rates, 95% ± 5% and 100% ± 0%, respectively) than did 23 patients with CMB (5-year rates, 30% ± 11% [P < .001] and 68 ± 10% [P = .008], respectively). In addition, desmoplastic/nodular histology was an independent prognostic factor for EFS in our series. Previous studies provided evidence that the ability to achieve a gross-total resection of the tumor may represent a positive prognostic indicator for survival.2,9,10,13,16 In contrast, other large studies found that incomplete resection did not significantly affect survival.17,25–27 In our study, patients without a postoperative residual tumor >1.5 cm2 (n = 39) appeared to have better EFS and OS rates (5-year rates, 60% ± 9% and 84% ± 6%, respectively) than did patients with a postoperative residual tumor >1.5 cm2 (n = 6; 33% ± 19% and 50% ± 20%, respectively). In addition, 10 of 23 patients with CMB and 5 of 19 patients with DMB/MBEN had evidence of postoperative residual disease, suggesting that gross total resection appears to be more common and feasible for patients with DMB or MBEN, as was reported recently.18 Biology may provide explanations for the better resectability and outcome of DMB and MBEN. At present, MB can be separated at least into 4 molecular subtypes, with differences in signaling pathway activation, age, histology, and clinical outcome.28–32 DMB is more common among young children and adults and is characterized by pathological activation of the sonic-hedgehog pathway.28,32 In the multivariable Cox regression analysis in our study, histology but not extent of resection was identified as an independent prognostic factor (P < .001).
The high frequency of CCR and objective response after 3 cycles of chemotherapy (97% in patients without and 87% in patients with postoperative residual tumor) compares favorably with response rates reported elsewhere.10,13,16,33,34 After 3 cycles of chemotherapy, we observed 7 patients (4 of 23 with CMB, 2 of 13 with DMB, 0 of 6 with MBEN, and 1 of 3 with AMB) with less than CR, suggesting that non-DMB/non-MBEN respond less favorably to chemotherapy. Disease recurrence and progressive disease in young patients with MB have been reported to occur early (within 1–2 years of therapy). Of note, compared with the current analysis, relapses did occur in a similar time frame (median, 1.3 years) as in the previous HIT-SKK’92 trial.13 Taken together, most of the relapses occur during the first 2 years; late relapses (>2 years after initial diagnosis) are rare events (1 patient with CMB in the current analysis and 2 in the previous HIT-SKK’92 trial).13
In summary, our results confirm that this postoperative chemotherapy alone appears to be a promising treatment for young patients with DMB or MBEN. In addition, DMB/MBEN histology has been shown to be a strong independent favorable prognostic factor. However, it seems unlikely that the 2 additional cycles of modified HIT-SKK chemotherapy can further improve outcome. Local radiotherapy was introduced since January 2006—after confirmation of poor survival rates and frequent local relapses of patients with non-DMB/non-MBEN—for all children aged >18 months with CMB, AMB, or large-cell MB.
Funding
This work was supported by the German Children's Cancer Foundation (Deutsche Kinderkrebsstiftung).
Acknowledgments
We thank the participating centers for their valuable cooperation. Special thanks to Wiebke Treulieb and Christine Lindow (HIT data center) for their excellent data management.
Conflict of interest statement. None declared.
References
- 1.Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92:3155–3164. doi: 10.1002/1097-0142(20011215)92:12<3155::aid-cncr10158>3.0.co;2-c. doi:10.1002/1097-0142(20011215)92:12<3155::AID-CNCR10158>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski S. Current treatment approaches to early childhood medulloblastoma. Expert Rev Neurother. 2006;6:1211–1221. doi: 10.1586/14737175.6.8.1211. doi:10.1586/14737175.6.8.1211. [DOI] [PubMed] [Google Scholar]
- 3.Kellie SJ. Chemotherapy of central nervous system tumours in infants. Childs Nerv Syst. 1999;15:592–612. doi: 10.1007/s003810050548. doi:10.1007/s003810050548. [DOI] [PubMed] [Google Scholar]
- 4.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–67. doi: 10.1159/000071316. doi:10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 6.Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28:348–354. doi: 10.1002/(sici)1096-911x(199705)28:5<348::aid-mpo4>3.0.co;2-h. doi:10.1002/(SICI)1096-911X(199705)28:5<348::AID-MPO4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Rutkowski S, Cohen B, Finlay J, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54:635–637. doi: 10.1002/pbc.22372. doi:10.1002/pbc.22372. [DOI] [PubMed] [Google Scholar]
- 8.Warren KE, Packer RJ. Current approaches to CNS tumors in infants and very young children. Expert Rev Neurother. 2004;4:681–690. doi: 10.1586/14737175.4.4.681. doi:10.1586/14737175.4.4.681. [DOI] [PubMed] [Google Scholar]
- 9.Kalifa C, Grill J. The therapy of infantile malignant brain tumors: current status? J Neurooncol. 2005;75:279–285. doi: 10.1007/s11060-005-6752-x. [DOI] [PubMed] [Google Scholar]
- 10.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. doi:10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 11.Gajjar A, Mulhern RK, Heideman RL, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol. 1994;12:1212–1216. doi: 10.1200/JCO.1994.12.6.1212. [DOI] [PubMed] [Google Scholar]
- 12.Geyer JR, Zeltzer PM, Boyett JM, et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12:1607–1615. doi: 10.1200/JCO.1994.12.8.1607. [DOI] [PubMed] [Google Scholar]
- 13.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. doi:10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 14.Chi SN, Gardner SL, Levy AS, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–4887. doi: 10.1200/JCO.2004.12.126. doi:10.1200/JCO.2004.12.126. [DOI] [PubMed] [Google Scholar]
- 15.Mason WP, Grovas A, Halpern S, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 16.Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–580. doi: 10.1016/S1470-2045(05)70252-7. doi:10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski S, Gerber NU, von Hoff K, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11:201–210. doi: 10.1215/15228517-2008-084. doi:10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28:4961–4968. doi: 10.1200/JCO.2010.30.2299. doi:10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of tumours of the central nervous system. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warmuth-Metz M, Bison B, Leykamm S. Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol. 2009;19:263–273. doi: 10.1007/s00062-009-9029-5. doi:10.1007/s00062-009-9029-5. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi:10.2307/2281868. [Google Scholar]
- 22.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. doi:10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 23.Garre ML, Cama A, Bagnasco F, et al. Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome—a new clinical perspective. Clin Cancer Res. 2009;15:2463–2471. doi: 10.1158/1078-0432.CCR-08-2023. doi:10.1158/1078-0432.CCR-08-2023. [DOI] [PubMed] [Google Scholar]
- 24.Giangaspero F, Perilongo G, Fondelli MP, et al. Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg. 1999;91:971–977. doi: 10.3171/jns.1999.91.6.0971. doi:10.3171/jns.1999.91.6.0971. [DOI] [PubMed] [Google Scholar]
- 25.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 26.Johnston DL, Keene D, Bartels U, et al. Medulloblastoma in children under the age of three years: a retrospective Canadian review. J Neurooncol. 2009;94:51–56. doi: 10.1007/s11060-009-9799-2. doi:10.1007/s11060-009-9799-2. [DOI] [PubMed] [Google Scholar]
- 27.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. doi:10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 28.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. doi:10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. doi:10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 30.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. doi:10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 31.Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–472. doi: 10.1038/ng.336. doi:10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants [published online ahead of print September 7, 2010] J Clin Oncol. 2010 doi: 10.1200/JCO.2009.27.4324. doi:10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ater JL, van Eys J, Woo SY, Moore B, 3rd, Copeland DR, Bruner J. MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol. 1997;32:243–252. doi: 10.1023/a:1005744527443. doi:10.1023/A:1005744527443. [DOI] [PubMed] [Google Scholar]
- 34.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro-oncol. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]