Abstract
MicroRNAs (miRNAs) are small, noncoding RNAs that play a critical role in developmental and physiological processes and are implicated in the pathogenesis of several human diseases, including cancer. They function by regulating target gene expression post-transcriptionally. In this study, we examined the role of oncogenic mir-21 in the pathogenesis of glioblastoma, the most aggressive form of primary brain tumor. We have previously reported that mir-21 is expressed at higher levels in primary glioblastoma-tissue and glioblastoma-derived cell lines than in normal brain tissue. We demonstrate that downregulation of mir-21 in glioblastoma-derived cell lines results in increased expression of its target, programmed cell death 4 (Pdcd4), a known tumor-suppressor gene. In addition, our data indicate that either downregulation of mir-21 or overexpression of its target, Pdcd4, in glioblastoma-derived cell lines leads to decreased proliferation, increased apoptosis, and decreased colony formation in soft agar. Using a glioblastoma xenograft model in immune-deficient nude mice, we observe that glioblastomaderived cell lines in which mir-21 levels are downregulated or Pdcd4 is over-expressed exhibit decreased tumor formation and growth. Significantly, tumors grow when the glioblastoma-derived cell lines are transfected with anti-mir-21 and siRNA to Pdcd4, confirming that the tumor growth is specifically regulated by Pdcd4. These critical in vivo findings demonstrate an important functional linkage between mir-21 and Pdcd4 and further elucidate the molecular mechanisms by which the known high level of mir-21 expression in glioblastoma can attribute to tumorigenesis—namely, inhibition of Pdcd4 and its tumor-suppressive functions.
Keywords: glioblastoma, mir-21, Pdcd4, tumor inhibition
MiRNAs regulate gene expression after recognition of sequence-specific binding sites, typically in the 3′-untranslated region of target mRNAs, causing mRNA deadenylation and degradation or translational repression.1–3 Alterations in the pattern of gene expression resulting from aberrant expression of miRNAs are associated with numerous diseases, including cancer.4–7 We previously assessed the expression patterns of 241 mature human miRNAs in 59 of the human tumor-derived cell lines that comprise the NCI60 panel and in a set of corresponding normal tissues.8 These tumor-derived cell lines had been established from melanoma, various leukemias, and from cancers of the gastrointestinal tract, kidney, ovary, breast, prostate, lung, and central nervous system (CNS).8 We found that miRNA expression patterns may mark specific biological characteristics of tumors and/or mediate biological activities important for their pathobiology. We also identified miRNAs for which the expression level in specific tumor cell lines was either significantly increased or decreased compared with levels observed in corresponding normal tissue, suggesting that their function was either enhanced or diminished in association with tumorigenesis.
Evaluating cell lines derived from glial tumors of the CNS, we found that 5 miRNAs were expressed at higher levels and 59 miRNAs were expressed at lower levels than those detected in normal brain tissue.8 We designated these miRNAs “candidate oncogenes” or “tumor suppressors,” respectively. CNS tumors of glial origin constitute the majority of primary brain tumors in adults and are called gliomas, the most malignant of which are known as glioblastoma. In this study, we evaluated the role of one candidate oncogenic miRNA, miR-21, in mediating the pathology associated with glioblastoma. MiR-21 has been identified as one of the most commonly overexpressed miRNAs in solid tumors.9,10 In glioblastoma, mir-21 is overexpressed, and its expression correlates with glioma grade.8,10–15 Low levels of mir-21 are expressed in grade II and grade III gliomas, whereas higher levels are observed in glioblastoma.13,15
Pdcd4, a known tumor suppressor gene, has been identified as a functional target of mir-21.16,17 Recently, mir-21 was shown to regulate Pdcd4 in glioblastoma.18 However, the effect of mir-21 regulation of Pdcd4 on specific biological activities of pathologic potential (eg, apoptosis, proliferation, anchorage-independent growth, or more significantly, in vivo growth of glioblastoma xenografts) has, to our knowledge, not been studied. The work presented in this article elucidates the in vitro—and, more importantly, in vivo—tumor suppressive activities of mir-21 that are modulated by its inhibition of Pdcd4.
Materials and Methods
Tissue Samples
Glioblastoma specimens and normal brain tissue samples were from the Neurosurgery Tissue Bank at the University of California–San Francisco. All samples were obtained with informed consent after approval of the human research committees at the University of California and studied after approval of the Committee for the Protection of Human Subjects at Dartmouth-Hitchcock Medical Center.
Cell Lines and Culture Conditions
Glioblastoma-derived cell lines SNB19, U251, U87, and SF767 were cultured in Dulbecco's Modified Eagle's Medium/10% fetal bovine serum (FBS)/1% penicillin (10,000 U/mL)/streptomycin (10, 000 µg/mL). All cells were grown in a humidified incubator in 5% CO2 at 37°C.
Derivation of Stable, Polyclonal Cultures and Monoclonal Cell Lines Expressing Pdcd4
To derive stable Pdcd4-expressing polyclonal cultures, U251 and U87 cell lines were transfected with pcDNA-Pdcd4 (kindly provided by Dr Nancy Colburn, National Cancer Institute, Bethesda, MD) and cells were selected for 3 weeks with Geneticin (Invitrogen), 500 µg/mL. After this cultures were expanded and maintained in Geneticin, 200 µg/mL. To derive stable GFP- and Pdcd4-expressing polyclonal cultures, U87 and U251 cell lines were transfected first with pEGFP (Clontech), and cells were selected for 3 weeks with hygromycin, 100 µg/mL, after which cells were transfected with pcDNA-Pdcd4 and selected as mentioned above. Monoclonal U25Pdcd4 or U87Pdcd4 as well as U251GFP+Pdcd4 or U87GFP+Pdcd4 cultures were derived from single cells seeded in 96-well plates.
Transient Expression of Anti-mirs and siRNAs
Anti-miR-21 miRNA Inhibitor, anti-miR miRNA Inhibitors-Negative/toxicity Control, and FAM dye-labeled Anti-miR were purchased from Applied Biosystems/Ambion. Predesigned siRNA constructs complementary to Pdcd4 were obtained from Ambion (Silencer Select, siRNA ID s26048). Transient transfections were performed using siPORT NeoFX Transfection Agent (Applied Biosystems/Ambion) in accordance with the manufacturer's instructions. During the transfection, cells were cultured in reduced serum OptiMEM medium (Invitrogen).
Real-time Quantification of microRNAs Using Stem-loop Real-time Polymerase Chain Reaction
The expression profiles of 241 microRNAs were measured as described previously.8 This method uses stem-loop primers for reverse transcription followed by real-time PCR (TaqMan MicroRNA Assays; Applied Biosystems). Expression of mature miRNAs was determined by the TaqMan miRNAassay (Applied Biosystems). The Taqman primer-probe for quantification of miR-21 (for the target sequence UAGCU UAUCAGACUGAUGUUGA) was from Applied Biosystems. RNA input was normalized using 4 endogenous controls: 18S rRNA, β2M, glyceraldehyde-3-phosphate dehydrogenase, and β-actin.
Western Blot Analysis to Detect Pdcd4 Protein
To obtain whole-cell lysates, cells were sonicated and then lysed on ice for 30 min in lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.5% NP40, 1 mmol/L phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail mix [Roche]). Protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce). For Western blot analysis, 40 µg of protein was separated on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Pdcd4 was detected using a affinity purified rabbit anti-Pdcd4 antibody (Rockland) at a 1:5000 dilution as the primary antibody, followed by a alkaline phosphatase-linked goat anti-rabbit secondary antibody (Abcam) used at 1:50,000 dilution. To detect β-actin as a loading control, a mouse monoclonal antibody to β-actin (Abcam) was used at 1:5000 as the primary antibody, followed by an alkaline phosphatase–linked rabbit anti-mouse (Abcam) secondary antibody used at 1:5000. After incubation of the membranes with the specific antibodies, proteins were visualized by chemiluminescence (ECL; Amersham). To detect Pdcd4 in tumors, tumor tissues were first homogenized using a sonicator, and proteins were extracted and processed as mentioned above.
Northern Analysis to Detect miRNAs
Total RNA was extracted from glioblastoma specimens, normal brain tissue or glioblastoma-derived cell lines with Trizol Reagent in accordance with the vendor's recommendations. Twenty micrograms of total RNA was separated on a 10% urea-polyacrylamide gel and transferred to a GeneScreen Plus (PerkinElmer). Radioactive-labeled StarFire (Integrated DNA Technologies) oligonucleotide probes were used for miRNA detection. Membranes were stripped by boiling in 0.1% SDS and re-hybridized to U6 probe for to determine loading controls.
Proliferation Assays
Fifty thousand U251 or U87 cells that were untreated or transfected with a nonspecific negative/toxicity anti-mir (NS) control (30 μM) or anti-mir-21 (3, 10, and 30 μM) were seeded onto a 10-cm tissue culture dish at day 0. Growth curves were determined by counting cells every 24 h for 5 days with a hemacytometer.
Anchorage-Independent Growth Assay in Soft Agar
Anchorage-independent growth assays were performed by seeding 1 × 105 cells in 0.4% Noble agar on an 0.8% agar base layer, both of which contained Dulbecco's modified Eagle's medium/10% fetal bovine serum (FBS)/1% penicillin (10,000 U/mL)/streptomycin (10,000 µg/mL). Colonies were counted (>0.1-mm) 2 weeks after seeding, and the data from triplicate determinations were expressed as mean ± standard error of the mean (SEM).
Apoptosis Assays
Cells were cultured on 4-well Lab-TeK II Chamber Slides (Nunc/Thermo Fisher Scientific) and anti-mir and negative/toxicity (NS) control transfections were performed in the chambers. Forty-eight hours after transfection, cells were fixed in 4% paraformaldehyde and the chambers were removed. For labeling nuclei of apoptotic cells, terminal deoxynucleotidyl transferase–mediated nick-end labeling (TUNEL) was done using the DeadEnd fluorometric TUNEL system (Promega) in accordance with the manufacturer's protocol. Cell nuclei were also stained with Hoechst dye. The number of TUNEL-positive cells was divided by the number of Hoechst-stained cells to yield the percent apoptotic nuclei. Four 40× objective fields containing 200 cells each were counted per chamber, with 3 chambers analyzed per condition.
Xenograft Growth in Athymic Nude Mice
Female, 4–6-week-old Nude-Foxn1nu mice (Harlan) were injected with phosphate buffered saline–washed cells (5 × 106 cells) subcutaneously in the flank. Tumor size was measured in 3 dimensions with calipers, and volume was calculated assuming the shape as ellipsoid. All animal studies were conducted using procedures outlined by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Institutional Animal Care and Use Committee (IACUC). The IVIS 200 Xenogen Imaging System (Caliper Life Sciences) was used to detect tumors in vivo.
Statistical Analysis
Data are represented as the mean ± standard deviation (SD) or ± SEM, as indicated. Differences were analyzed by using an unpaired 2-tailed Student t test, and P < .05 was considered statistically significant. All of the experiments were performed at least 3 times.
Results
Down Regulation of mir-21 Expression in Glioblastoma-Derived Cell Lines Results in Enhanced Pdcd4 Expression
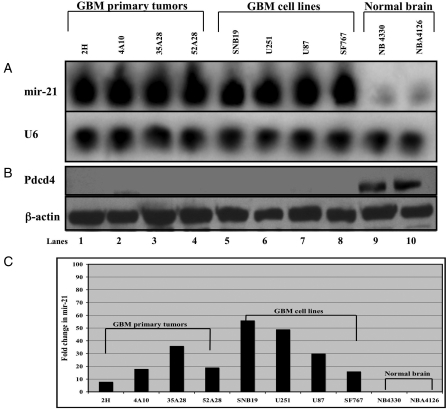
Having identified mir-21 as an oncogenic miRNA that is highly expressed in human glioblastoma-derived cell lines,8 we sought to confirm the observations of others that mir-21 was highly expressed in tissue derived from primary glioblastoma (Fig. 1A, lanes 1–4) as well as in glioblastoma-derived cell lines (Fig. 1A, lanes 5–8), compared with normal brain tissue (Fig. 1A, lanes 9–10).12,18 We obtained comparable results with a highly sensitive assay utilizing stem-loop primers for reverse-transcription followed by PCR (Fig. 1C).19 Elevated levels of mir-21 were observed in all 7 glioblastoma-derived cell lines and in all 13 glioblastoma tumors we examined (Fig. 1) (data not shown).
Fig. 1.
Mir-21 is highly expressed in glioblastoma (GBM) primary tumors and glioblastoma-derived cell lines compared to normal brain tissue. (A) Northern blot analysis of mir-21 in primary tissues (2H, 4A10, 35A28, and 52A28) and GBM-derived cell lines (SNB19, U251, U87, and SF767), compared with normal human brain samples (NB4330 and NB4126). Twenty micrograms of total RNA was loaded in each well. U6 loading controls from the same blot are shown in the lower panel. (B) Western blot analysis of mir-21 in primary tissues (2H, 4A10, 35A28, and 52A28) and GBM-derived cell lines (SNB19, U251, U87, and SF767), compared with normal human brain samples (NB4330 and NB4126). Forty micrograms of protein from whole-cell lysates were loaded in each well for quantifying Pdcd4 protein levels by Western blot analysis using an anti-Pdcd4 antibody. β-Actin loading controls from the same blots are shown in the lower panels. (C) Real-time polymerase chain reaction (PCR) quantification of mir-21 in primary GBM tissues, GBM-derived cell lines, and normal human brain samples. Stem-loop primers were used for reverse transcription followed by real-time PCR. mir-21 levels are shown as fold change versus the level detected in normal brain tissue. RNA input was normalized using 4 endogenous controls: 18S rRNA, β2M, GAPDH, and β-actin.
Although glioblastomas harbor diverse oncogenes and mutated tumor-suppressor genes whose pattern of alteration and expression varies considerably from tumor to tumor,20 most glioblastomas examined to date have high levels of mir-21 expression.11–14,18 Because Pdcd4, a well-characterized tumor-suppressor gene,16,21–25 is a known target of mir-21, we sought to characterize its expression in glioblastoma. We hypothesized that as a target transcript encoding a protein involved in tumor suppression, the expression of Pdcd4 should be decreased in glioblastoma-derived cell lines. By use of Western blot analysis, we observed that Pdcd4 protein levels are undetectable in primary glioblastoma tissues (Fig. 1B, lanes 1–4) and glioblastoma-derived cell lines (Fig. 1B, lanes 5–8), compared with normal human brain samples (Fig. 1B, lanes 9–10).
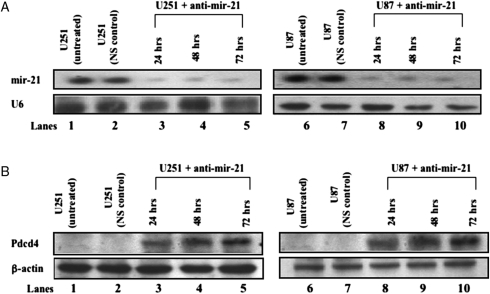
Having observed an inverse correlation between mir-21 and Pdcd4 protein levels (Fig. 1A and B), we then sought additional evidence to corroborate the association of endogenous Pdcd4 protein with endogenous miR-21 in vitro. We assessed Pdcd4 expression in glioblastoma-derived cell lines in which mir-21 expression had been experimentally decreased using an anti-mir-21 (Ambion Anti-miR miRNA inhibitor) that decreases the cellular levels of mir-21 specifically for up to 120 h after transfection (data not shown).We transiently transfected U251 and U87 cells with anti-mir-21 and harvested cells at 24, 48, or 72 h, a time window that is optimized for detection of up-regulation of known mir-21 target proteins.13–16 To ensure that the down-regulation of mir-21 by anti-mir-21 was specific and not due to nonspecific toxicity, we included a nonspecific anti-mir (NS) as a negative/toxicity control for each transfection. In addition, a FAM dye-labeled, nonspecific anti-mir was also used to determine transfection efficiency for each experiment. We routinely achieved 80%–90% transfection efficiency. At each time point examined after transfection, anti-mir-21–treated and control cells were collected and divided into 2 identical aliquots from which we isolated total RNA and nuclear proteins, respectively. Northern blot analyses demonstrated decreased levels of mir-21 in U251 and U87 cells 24, 48, and 72 h after transfection with anti-mir-21 (Fig. 2A, lanes 3–5 and 8–10). Western blot analyses of nuclear proteins isolated from these same U251 and U87 cultures exhibited increased Pdcd4 protein levels 24, 48, and 72 h after anti-mir-21 treatment (Fig. 2B, lanes 3–5 and 8–10, respectively). In both Fig. 2A and B, lanes 1 and 6 evaluate untreated U251 and U87, respectively, and lanes 2 and 7 are isolates of U251 and U87 cells treated with a nonspecific negative toxicity control. As depicted in Fig. 1A, mir-21 is highly expressed (Fig. 2A, lanes 1 and 6) in untreated U251 and U87, whereas Pdcd4 protein levels were undetectable (Fig. 2B, lanes 1 and 6). These data are consistent with the regulation of Pdcd-4 by mir-21. In addition, we compared the miRNA expression levels from the NCI60 glioma derived cell lines to RNA, DNA, and protein expression data for the same cell lines (primary data at http://dtp.nci.nih.gov/webdata.html). In that analysis we found a significant inverse correlation (P = .004) between miR-21 and Pdcd4 protein expression levels (data not shown) similar to the data described above in Fig. 2A and B that demonstrated undetectable levels of Pdcd4 protein in glioblastoma-derived cell lines that expressed high levels of mir-21 and vice-versa.
Fig. 2.
Inhibition of mir-21 expression in glioblastoma-derived cell lines enhances Pdcd4 expression. (A) Northern blot analysis of mir-21 expression levels in U251 and U87 cells following anti-mir-21 (30 μM) transfection. Total RNA was collected 24, 48, and 72 h after transfection, and 20 μg were loaded in each well for quantifying mir-21 levels by Northern blot analysis. U6 loading controls from the same blot are shown in the lower panel. (B) Western blot analysis of Pdcd4 in U251 and U87 cells after anti-mir-21 (30 μM) transfection. Whole-cell lysates were prepared 24, 48, and 72 h after transfection with anti-mir-21, and 40 μg of protein were loaded in each well for quantifying Pdcd4 protein levels by Western blot analysis using an anti-Pdcd4 antibody. β-Actin loading controls from the same blots are shown in the lower panels. In all blots, lane 1 is an untreated controls and lane 2 contains lysates from cells treated with a nonspecific negative/toxicity anti-mir (NS) control.
Inhibiting mir-21 expression in glioblastoma-derived cell lines results in decreased proliferation, increased apoptosis, and diminished anchorage-independent growth
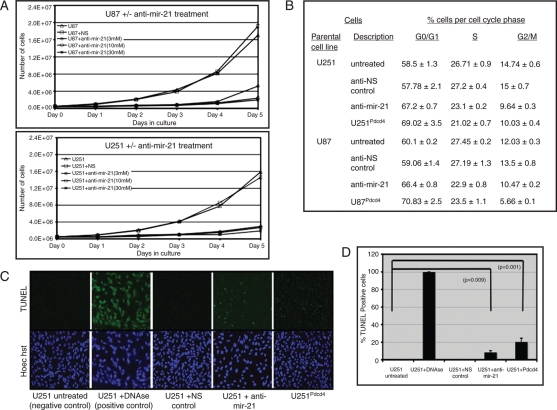
Mir-21 has been shown to act as an anti-apoptotic factor in glioblastoma-derived cell lines,11,12 and conversely, Pdcd4 has been shown to be pro-apoptotic.24 Having observed an inverse correlation between expression of mir-21 and Pdcd4 in glioblastoma, we sought to determine the biological effect of mir-21 expression in glioblastoma-derived cell lines. U251 and U87 cells were transfected with varying concentrations of anti-mir-21 (3, 10, and 30 μM), and the cell count was assessed every 24 h after transfection for 5 days (Fig. 3A). Downregulation of mir-21 in the anti-mir-21–treated cells was confirmed by northern blot analyses on days 1 and 5 (data not shown). We observed decreased cell numbers in cultures treated with anti-mir-21, compared with cultures that were incubated in regular media or transfected with a nonspecific negative toxicity control (Fig. 3A). Also, TUNEL staining revealed enhanced apoptosis in U251 (Fig. 3B) and U87 cells (data not shown) treated with anti-mir-21, compared with cells cultured in either medium alone or with NS control. Although the enhanced apoptosis was easily recognizable in anti-mir-21–treated glioblastoma-derived cells lines, we sought evidence of decreased proliferation in these cultures to better understand the remarkable difference in cell counts in the treated cultures (Fig. 3A). Cell-cycle analysis revealed that anti-mir-21 treatment of U251 and U87 cells significantly increased the number of cells in G0/G while decreasing the fraction of cells present in S-phase (Fig. 3C).
Fig. 3.
Anti-mir-21 treatment inhibits proliferation of glioblastoma-derived cell lines in vitro. (A) U251 and U87 cells that were untreated or transfected with a nonspecific negative/toxicity anti-mir (NS) control (30 μM) or anti-mir-21 (3, 10, and 30 μM) were examined. Fifty thousand cells were plated onto a 10-cm tissue culture dish at day 0 for each condition, and cells were counted daily. (B) Cell-cycle analyses of U251 and U87 cells and cells transfected with non-specific negative/toxicity anti-mir (NS) control (30 μM) or anti-mir-21 (30 μM) as well as U251Pdcd4 and U87Pdcd4 cells. Cells were fixed with 70% ethanol, treated with RNAse and stained with propidium iodide, after which cell-cycle progression was analyzed by flow cytometry. The data are shown as the percentage of cells in various phases of the cell cycle and are mean values ± standard errors of the mean of the percentage of G0/G1, G2/M, or S phase in 3 experiments. (C) TUNEL staining of U251 cells and U251 transfected with anti-mir-21 (30 μM, 72 h) or a nonspecific anti-mir (NS) control (30 μM) and U251Pdcd4 cells. Untreated paraformaldehyde-fixed U251 cells treated with DNAse were included as a positive control. Cell nuclei were also stained with Hoechst dye. (D) Quantification of TUNEL-positive cells per 200 randomly selected cells on 4 different evaluations per condition.
Normal brain tissue expresses Pdcd4.19,26,27 Although Pdcd4 is not detectable in glioblastoma tumors or glioblastoma-derived cell lines,26,27 suppression of mir-21 results in increased Pdcd4 expression in these tissues (Fig. 2A and B). Therefore, beyond determining the biological effects of downregulating mir-21 in glioblastoma, we sought evidence that Pdcd4 was a critical target of mir-21 and mediated the specific effects of mir-21 that could contribute to glioblastoma tumorigenesis. We transfected U251 and U87 cell lines with a Pdcd4 cDNA expression construct (pcDNA-Pdcd4) to generate stable glioblastoma-derived cell cultures that overexpressed Pdcd4 (U251Pdcd4 and U87Pdcd4). Clonal isolates and a polyclonal culture of U251 overexpressing Pdcd-4, U251Pdcd4 demonstrated increased spontaneous apoptosis, as determined by TUNEL staining, compared with the parental cells from which they were derived (Fig. 3B and C). Increased apoptosis in U87 cells treated with anti-mir-21 or stably over expressing Pdcd4 (U87Pdcd4) was also observed (data not shown).
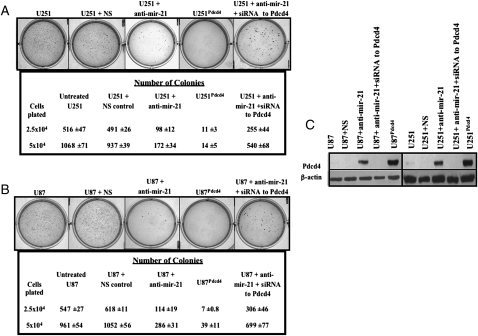
Anchorage-independent growth assayed in soft agar is a characteristic of transformed cells that correlates closely with their tumorigenicity. U251 and U87 can grow in an anchorage-independent manner and form colonies in soft agar. Therefore, to examine the role of mir-21 and its target Pdcd4 in anchorage-independent growth, we examined the effect of inhibiting mir-21 expression on colony formation in soft agar. U251 cells treated with anti-mir-21 for 72 h and plated in soft agar displayed decreased anchorage-independent growth and formed fewer colonies (Fig. 4A and B) when compared to controls. The U251Pdcd4 polyclonal cell culture showed a decrease in colony number compared to cells that did not have elevated Pdcd-4 levels. These data are representative of U251Pdcd4 polyclonal cultures and monoclonal cell lines (data not shown). We also observed decreased numbers of colonies formed by U87 cells that were treated with anti-mir-21 or stably over-expressed Pdcd4 (U87Pdcd4) (Fig. 4B). In 3 independent experiments, the number of colonies formed following exposure to anti-mir-21 decreased by ∼65% (10 μM anti-mir-21) and ∼75% (30 μM anti-mir-21).
Fig. 4.
Suppression of mir-21 inhibits anchorage-independent growth of glioblastoma-derived cell lines. (A) Quantification of colonies formed by (A) U251 and (B) U87 cells treated with anti-mir-21 (30 μM) for 72 h and plated out to grow on soft agar in 6-well plates at either 2.5 × 104 cells/well or 5 × 104 cells/well. Also depicted are U251 and U87 cells that were transfected with anti-mir-21 (30 μM) followed by transfection with siRNA to Pdcd4 (10 μM) for 72 h Untreated U251 or U87 cells, nonspecific negative/toxicity anti-mir (NS) control (30 μM) treated cells, and cells over-expressing Pdcd4 (U251Pdcd4 and U87Pdcd4) were also evaluated. Colony numbers are the averages of 6 determinations per condition ± SEM. (C) Western blot analyses to determine Pdcd4 expression levels in U251 and U87 transfected with anti-mir-21 or NS control (30 μM) only or with anti-mir-21 (30 μM) followed by siRNA to Pdcd4 (10 μM) treatment as well as U251Pdcd4 and U87Pdcd4 cell lines. β-Actin loading controls are shown in the lower panels.
Pdcd4 has a binding site for mir-2111,12 and the inverse correlation between mir-21/Pdcd4 levels (Fig. 2A and B) strongly suggests that mir-21 regulates Pdcd4. However, because mir-21 can potentially target several genes,11 we wanted to establish that the biologic effects we measured were a consequence of Pdcd4 being targeted by mir-21. We evaluated colony formation by U251 or U87 cells that were treated for 72 h with either anti-mir-21 or with anti-mir-21 and an siRNA that inhibits expression of Pdcd4. U251 or U87 cells treated for 72 h with anti-mir-21 formed 70% and 60% fewer colonies, respectively, than did control cells. Compared with cells that were treated with anti-mir-21 only, U251 or U87 that were treated with anti-mir-21 and siRNA to Pdcd4 produced higher number of colonies, similar to those formed by untreated U251 or U87 (Fig. 4A and B). In addition, U251Pdcd4 and U87Pdcd4 cell lines were also examined and demonstrated a decrease in number of colonies formed in soft agar, compared with cells that did not have elevated Pdcd-4 levels (Fig. 4A and 4B). Western blot analyses was used to monitor Pdcd4 levels in U251 or U87 cells that were untreated, treated with the NS anti-mir control, anti-mir-21, or anti-mir-21 and siRNA to Pdcd4 (Fig. 4C).
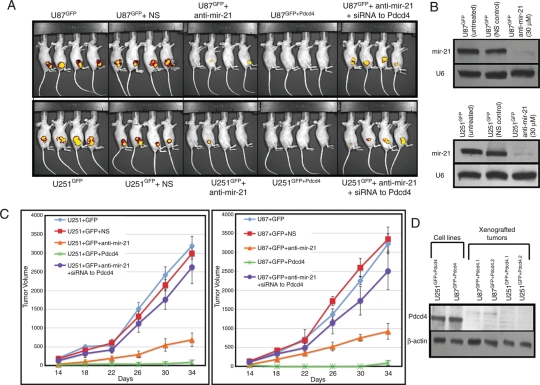
Downregulation of mir-21 or Overexpression of Pdcd4 Results in Reduction of Glioblastoma Xenograft Growth that is Reversed by siRNA to Pdcd4
In addition to being a pro-apoptotic gene, Pdcd4 is a suppressor of malignant transformation and tumor progression and has been shown to inhibit invasion and intravasation by tumor cells.16,22–25 Having established that glioblastoma-derived cell lines displayed decreased proliferation and colony formation in vitro when mir-21 expression was decreased or Pdcd4 was increased (Figs 3 and 4), we sought to identify a role for mir-21 and its target Pdcd4 in glioblastoma tumorigenesis in vivo by evaluating glioblastoma xenografts in immune-deficient mice (Fig. 5A). We prepared polyclonal cultures of U251GFP and U87GFP cells stably expressing green fluorescent protein (GFP). We also prepared U87 and U251 cell lines overexpressing GFP as well as Pdcd4 (U251GFP+Pdcd4 and U87GFP+Pdcd4). Subcutaneous injection of 5 × 106 cells from U251GFP or U87GFP in the flank of immunosuppressed mice gave rise to tumors that were first palpable by day 7 after injection and that developed to ∼1.5 cm3 in size by day 30. We used the Xenogen Imaging System to detect these tumors in vivo (Fig. 5A). We examined animals that received U251GFP or U87GFP lines after transfection with anti-mir-21 as well U251GFP or U87GFP lines after transfection with anti-mir-21 and siRNA to PDCD4 (Fig. 5A). U251GFP or U87GFP lines transfected with anti-mir-21 demonstrated decreased tumor growth in vivo (Fig. 5A). Suppression of mir-21 in the cell lines was confirmed by Northern blot analysis (Fig. 5B). Mice that received cell lines overexpressing Pdcd4, U251GFP+Pdcd4, or U87GFP+Pdcd4 also had tumors that were reduced in size, compared with tumors arising in animals injected with the same cells bearing only GFP and not overexpressing Pdcd4 (Fig. 5A). We monitored tumor growth for up to 6 weeks after the inoculation of these animals with tumor cells. More importantly, animals that received U251GFP or U87GFP lines after transfection with anti-mir-21 and siRNA to PDCD4 gave rise to tumors that developed to ∼1.5 cm3 in size by day 30, comparable to the tumors that developed from the control, untreated glioblastoma-derived cell lines (Fig. 5A). Tumor growth was measured every 4 days starting at day 10 after injection (Fig. 5C). In all of these experiments, tumor growth was reduced by ∼30% at day 30 (P = .01) in mice that were injected with anti-mir-21–transfected U251GFP or U87GFP cells. Similarly, we saw an ∼90% (P = .001) decrease in tumor size or complete lack of tumor growth in mice that were injected with Pdcd4 over-expressing U251GFP+Pdcd4 or U87GFP+Pdcd4 cells. In the 5 independent xenograft experiments we performed examining U251GFP+Pdcd4 or U87GFP+Pdcd4, only 5 of 24 and 6 of 24 mice, respectively, developed tumors detectable by day 30. These tumors were, on average, 90% smaller than the tumors that arose after injection with untreated or nonspecific negative/toxicity control treated U251GFP or U87GFP cells (data not shown). However, the fact that some tumors, albeit much smaller than those formed in the control groups, did develop in U251GFP+Pdcd4- or U87GFP+Pdcd4-derived xenografts provided an opportunity to examine Pdcd4 levels in such tumors (Fig. 5D). Although the xenografts were not of sufficient size to analyze DNA, RNA, and protein, we were able to measure Pdcd4 protein levels and found that Pdcd4 levels were undetectable by Western blot analysis of 4 individual tumors that arose from animals injected with U251GFP+Pdcd4 or U87GFP+Pdcd4 lines (Fig. 5D).
Fig. 5.
Downregulation of mir-21 or overexpression of Pdcd4 results in reduction of glioblastoma xenograft growth that is reversed by siRNA to Pdcd4. (A) U251GFP or U87GFP (5 × 106) cells that were either untreated, transfected with NS control (30 μM) or anti-mir-21 (30 μM), or transfected with anti-mir-21 (30 μM) followed by transfection with siRNA to Pdcd4 (10 μM) for 72 h, as well as cells expressing elevated levels of Pdcd4 (U251GFP+Pdcd4 and U87GFP+Pdcd4), were injected subcutaneously in the flanks of nude mice. The Xenogen Imaging System was used to image tumor growth in vivo. Data are representative of 5 independent xenograft experiments (total n = 24 animals per condition). (B) Northern blot analysis of mir-21 in U251GFP and U87GFP cells prior to their use as xenografts. U6 loading controls are shown in the lower panel. (C) Tumor volume of xenografts. Tumors were measured every 4 days from day 10 to day 34 after injection. Data are averages of 5 independent xenograft experiments (total n = 24 animals per condition) ± SEM. (D) Western blot analysis of Pdcd4 protein in tumors isolated from mice that were injected with U251Pdcd4 or U87Pdcd4 cell lines.
Furthermore, hematoxylin and eoisin staining of tumors (Supplemental Fig. S1) that arose from untreated U251 or U87 or U251 or U87 cell lines transfected with NS control or anti-mir-21 or with anti-mir-21 and siRNA to PDCD4 show similar morphological features of glioblastoma and are indistinguishable from one another. High cellularity and microvascular proliferation (Supplemental Fig. S1) and the presence of necrosis (Supplemental Fig. S2), all of which are defining features of glioblastoma, are evident in these tumors.
Discussion
Glioblastomas are the most malignant brain tumors of glial origin.21,28 They are also among the most resistant of all human tumors to available modalities of treatment, perhaps because of their genetic heterogeneity and invasiveness.28 Molecular biomarkers that are associated with biological mechanisms contributing to malignancy may be important for understanding pathologic attributes of glioblastoma and for designing effective strategies for glioblastoma therapy. miRNAs have recently been described as potential molecular biomarkers. Previous work in our laboratory8 and by others9–14 has demonstrated that miR-21 is a member of a class of miRNAs that are frequently overexpressed in solid tumors and may contribute to oncogenesis. Elevated expression of miR-21 has been documented in several different types of cancer including those arising in breast,9,16 brain,11–15 prostate,29 ovaries,30,31 colon,32 and pancreas,33 as well as cervical carcinoma,34 hepatocellular carcinoma,35 chronic lymphocytic leukemia,36 and acute myeloid leukemia.37 Pdcd4, a described target of mir-21,16–18 has been characterized as both a tumor suppressor16,21–25 and a pro-apoptotic gene that may function by binding eukaryotic initiation factor 4A (eIF4A) and inhibiting translation initiation.38–40 Such an activity could facilitate the regulation of multiple proteins involved in tumor progression at the translational level. Also, mir-21 may play an oncogenic role in glioblastoma acting as an anti-apoptotic factor.11–15 Negative regulation of Pdcd4 by mir-21 has recently been demonstrated in glioblastoma and in several other tumors.41–44 Although mir-21 has been shown to regulate Pdcd4 in glioblastoma,18 the effect of mir-21/Pdcd4 regulation on specific biological activities, such as apoptosis, proliferation, or anchorage- independent growth, has not been studied. More significantly, the effect on in vivo growth of glioblastoma cells of mir-21/Pdcd4 has not been explored. Our work sought to elucidate both in vitro and critical in vivo effects of mir-21 over-expression and the resulting inhibition of Pdcd4 expression.
The high level of mir-21 expression in human glioblastoma-derived cell lines and glioblastoma primary tumors, compared with that observed in normal brain tissue, suggested its potential in contributing to the malignant behavior of glioblastoma (Fig. 1A and C). Furthermore, by assessing endogenous Pdcd4 protein levels in glioblastoma-derived cell lines (Fig. 1B) and in lines in which endogenous mir-21 expression had been inhibited (Fig. 2A and B), we determined that inhibition of mir-21 in glioblastoma-derived cell lines resulted in enhanced Pdcd4 expression. We observed that cells treated with anti-mir-21 had decreased proliferation (Fig. 3A) and also exhibited enhanced apoptosis, compared with untreated cells (Fig. 3B). In addition, glioblastoma-derived cell lines in which mir-21 levels were inhibited displayed decreased anchorage-independent growth (Fig. 4A and B), whereas glioblastoma-derived cell lines expressing Pdcd4 showed increased apoptosis and diminished anchorage-independent growth (Fig. 4A and B). Compared with cells treated with anti-mir-21 only, cells that were treated with anti-mir-21 and siRNA to Pdcd4 produced an increased number of colonies similar to those formed by untreated controls (Fig. 4A and B). The anti-apoptotic activity for mir-21 in glioblastoma lines that we observed is consistent with previously published data.11,12 This is similar to the anti-apoptotic activity of mir-21 in hepatocellular carcinoma where mir-21 has been shown to downregulate PTEN, initiate caspase activity, and lead to decreased apoptosis.35,45 However, our data suggest that, along with suppression of Pdcd4's pro-apoptotic function, downregulation of its tumor-suppressive activity also contributes to the oncogenic role of mir-21.
We also sought to determine the biological significance of mir-21/Pdcd4 regulation in vivo. More importantly, because mir-21 can target several genes,10,11 we sought to determine whether the biologic effects we observed following mir-21 inhibition were a consequence of Pdcd4 inhibition. We observed that glioblastoma-derived cell lines in which mir-21 expression had been inhibited formed fewer tumors and smaller tumors (Fig. 5A) than those that arose after injection of untreated control cell lines. More importantly, we verified that the decreased tumor growth was predominantly a result of Pdcd4 being downregulated by mir-21. Mice that received U251GFP or U87GFP lines after transfection with anti-mir-21 and siRNA to PDCD4 significantly gave rise to tumors that were comparable to the untreated controls (Fig. 5A and 5C), confirming that the tumor growth is specifically regulated by PDCD4 and represents a crucial functional linkage between mir-21 and PDCD4 in vivo. In these in vivo experiments mice that received cell lines overexpressing Pdcd4 showed a substantial decrease in tumor size and/or complete ablation of tumor growth (Fig. 5A). Importantly, Pdcd4 was undetectable in the few, small tumors that did develop from the Pdcd4 overexpressing lines. The in vivo experiments in which these tumors arose were performed using polyclonal cultures of U251Pdcd4 and U87Pdcd4. The finding that Pdcd4 protein levels were not detectable (Fig. 5D) is strong evidence that these tumors were formed by cells not expressing Pdcd4. It is certainly possible that, in rare cells, the dominant selectable marker used to create these lines was intact, whereas the cDNA encoding Pdcd4 was altered, during the plasmid integration event. Alternatively, the transgene encoding Pdcd4 may have been epigenetically inactivated. Such cells would escape Pdcd4 inhibition and give rise to small tumors in mice injected with U251Pdcd4 or U87Pdcd4 (Fig. 5A). Similarly, the small tumors formed by anti-mir-21–treated cells were probably formed by a fraction of glioblastoma-derived cells that had not been transfected in vitro by synthetic anti-mir-21 prior to their injection into mice.
Although an additional major pathway of Pdcd4 inactivation is the increased proteasomal degradation of Pdcd4 due to its phosphorylation by Akt and p70(S6K),46 our findings provide strong evidence that, by inhibition of Pdcd4 by mir-21, over-expression contributes to glioblastoma growth. Interestingly, cells treated with anti-mir-21 and siRNA to Pdcd4 produced ∼85% of the colonies compared to cells treated with anti-mir-21 only. A possible explanation for this finding is that mir-21 also inhibits other genes contributing to the malignant characteristics of glioblastoma-derived cells47,48. For example, miR-21 has been shown to inhibit apoptosis by regulating Bcl-2 in murine breast cancer model49,50 and to inhibit gemcitabine-induced apoptosis by modulating PTEN and the PI3K pathway.51 Consistent with this idea is the observation that mir-21 regulation of 2 important matrix metalloproteinase inhibitors, RECK and TIMP3, may be of importance in vivo.13
Targeting the anti-apoptotic role of mir-21 has been proposed as a therapeutic strategy for the treatment of glioblastoma therapy. The combined treatment of glioblastoma with an miR-21 antagonist and pathotropic neural precursor cell–mediated delivery of tumor necrosis factor–related apoptosis–inducing ligand (TRAIL) to glioblastoma in vivo resulted in a significant reduction in tumor xenograft growth in mice.14 Our finding that high levels of Pdcd4 expression inhibited tumor growth suggests that inhibiting Pdcd4 degradation or enhancing its expression may be a therapeutic strategy of importance for glioblastoma.
Supplementary Material
Funding
This work was supported by a grant from the Theodora Betz foundation to M.A.I. and by federal funds from the National Cancer Institute, National Institutes of Health (S.L.H. and N.H.C.).
Supplementary Material
Acknowledgments
We thank members of the Israel laboratory for their helpful discussions. We are also grateful for the help received from Sarah Purdy in imaging the tumor xenografts. These data were presented at the 13th Annual Scientific Meeting of the Society for Neuro-Oncology. The content of this publication does not necessarily reflect the views or the policies of the US Department of Health and Human Services nor does the mention of trade names, commercial products or organizations imply endorsement by the US government.
Conflict of interest statement. None declared.
References
- 1.Standart N, Jackson RJ. MicroRNAs repress translation of m7Gppp-capped target mRNAs in vitro by inhibiting initiation and promoting deadenylation. Genes Dev. 2007;21:1975–1982. doi: 10.1101/gad.1591507. doi:10.1101/gad.1591507. [DOI] [PubMed] [Google Scholar]
- 2.de Moor CH, Meijer H, Lissenden S. Mechanisms of translational control by the 3′ UTR in development and differentiation. Semin Cell Dev Biol. 2005;16:49–58. doi: 10.1016/j.semcdb.2004.11.007. doi:10.1016/j.semcdb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–289. doi: 10.1002/jcp.20869. doi:10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- 4.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. doi:10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Sevignani C, Calin GA, Siracusa LD, Croce CM. Mammalian microRNAs: a small world for fine-tuning gene expression. Mamm Genome. 2006;17:189–202. doi: 10.1007/s00335-005-0066-3. doi:10.1007/s00335-005-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. doi:10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. doi:10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Gaur A, Jewell DA, Liang Y, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. doi:10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 9.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. doi:10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–925. doi: 10.1042/BST0370918. doi:10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 11.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. doi:10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. doi:10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 13.Gabriely G, Wurdinger T, Kesari S, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. doi:10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. doi:10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 15.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. doi:10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. doi:10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. doi:10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Liu W, Chao T, et al. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008;272:197–205. doi: 10.1016/j.canlet.2008.06.034. doi:10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. doi:10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. doi:10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 21.Talotta F, Cimmino A, Matarazzo MR, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. doi:10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 22.Young MR, Yang HS, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-kappa B and Pdcd4. Trends Mol Med. 2003;9:36–41. doi: 10.1016/s1471-4914(02)00009-6. doi:10.1016/S1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 23.Allgayer H. Pdcd4, a colon cancer prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol. 2009;73:185–191. doi: 10.1016/j.critrevonc.2009.09.001. doi:10.1016/j.critrevonc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell. 2009;101:309–317. doi: 10.1042/BC20080191. doi:10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 25.Göke R, Gregel C, Göke A, Arnold R, Schmidt H, Lankat-Buttgereit B. Programmed cell death protein 4 (PDCD4) acts as a tumor suppressor in neuroendocrine tumor cells. Ann N Y Acad Sci. 2004;1014:220–221. doi: 10.1196/annals.1294.024. doi:10.1196/annals.1294.024. [DOI] [PubMed] [Google Scholar]
- 26.Gao F, Wang X, Zhu F, et al. PDCD4 gene silencing in gliomas is associated with 5′CpG island methylation and unfavorable prognosis. J Cell Mol Med. 2008;13:4257–4267. doi: 10.1111/j.1582-4934.2008.00497.x. doi:10.1111/j.1582-4934.2008.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao F, Zhang P, Zhou C, et al. Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep. 2007;17:123–128. [PubMed] [Google Scholar]
- 28.Thaker NG, Pollack IF. Molecularly targeted therapies for malignant glioma: rationale for combinatorial strategies. Expert Rev Neurother. 2009;9:1815–1836. doi: 10.1586/ern.09.116. doi:10.1586/ern.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383:280–285. doi: 10.1016/j.bbrc.2009.03.077. doi:10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 30.Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. doi:10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 31.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. doi:10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 32.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. doi:10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 33.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is over expressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. doi:10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel smallRNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. doi:10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 35.Kutay H, Bai S, Datta J, et al. Downregulation of miR-122 in rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–678. doi: 10.1002/jcb.20982. doi:10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Fulci V, Chiaretti S, Goldoni M, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4951. doi: 10.1182/blood-2006-12-062398. doi:10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 37.Jongen-Lavrencic M, Sun SM, Dijkstra MK, Valk PJ, Lowenberg B. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111:5078–5085. doi: 10.1182/blood-2008-01-133355. doi:10.1182/blood-2008-01-133355. [DOI] [PubMed] [Google Scholar]
- 38.Yang HS, Jansen AP, Komar AA, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. doi:10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaRonde-LeBlanc N, Santhanam AN, Baker AR, Wlodawer A, Colburn NH. Structural basis for inhibition of translation by the tumor suppressor Pdcd4. Mol Cell Biol. 2007;27:147–156. doi: 10.1128/MCB.00867-06. doi:10.1128/MCB.00867-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang JH, Cho YH, Sohn SY, et al. Crystal structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci USA. 2009;106:3148–3153. doi: 10.1073/pnas.0808275106. doi:10.1073/pnas.0808275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. doi:10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 42.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C (epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. doi:10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selaru FM, Olaru AV, Kan T, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49:1595–1601. doi: 10.1002/hep.22838. doi:10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. doi:10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 45.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. doi:10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–1260. doi: 10.1158/0008-5472.CAN-07-1719. doi:10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- 47.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor genetropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. doi:10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 48.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. doi:10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 49.Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. doi:10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. doi:10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Ren Y, Moore L, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. doi:10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.