Abstract
Recent data suggest that the β-catenin/Tcf-4 signaling pathway plays an important role in human cancer tumorigenesis. However, the mechanism of β-catenin/Tcf-4 signaling in tumorigenesis is poorly understood. In this study, we show that Tcf-4 protein levels were significantly elevated in high-grade gliomas in comparison with low-grade gliomas and that Tcf-4 levels correlated with levels of AKT2. Reduction of β-catenin/Tcf-4 activity inhibited glioma cell proliferation and invasion in vitro and tumor growth in vivo. This effect of β-catenin/Tcf-4 activity was mediated by AKT2, and in vivo binding of β-catenin/Tcf-4 to the AKT2 promoter was validated using the chromatin immunoprecipitation assay and luciferase reporter assays. Taken together, we have demonstrated that Tcf-4 is associated with glioma progression and that AKT2 is a new member of the genes that are regulated by β-catenin/Tcf-4.
Keywords: glioma, β-catenin, Tcf-4, AKT2
Gliomas are the most frequent primary tumors that arise in the brain. The most malignant form of glioma, glioblastoma multiforme (GBM, grade IV), is one of the most aggressive human cancers and is a challenging disease to treat, with a median survival of 1 year.1 Despite recent advances in cancer treatment, this statistic has not changed significantly over many years.2,3 Therefore, it is essential to investigate the mechanisms underlying the development and progression of gliomas.
The Wnt/β-catenin/Tcf signaling pathway is a crucial factor in the development of many cancers.4–6 β-catenin and Tcf-4 are the core components of the canonical Wnt/β-catenin/Tcf pathway.7 β-catenin accumulates in the nucleus, where it interacts with coregulators of transcription, including Tcf-4 and Lef-1, to form a β-catenin/Lef/Tcf complex. This complex regulates the transcription of multiple genes involved in cellular proliferation, differentiation, survival, and apoptosis, such as Fra-1, c-myc, and Cyclin D.8–10 Recently, several reports have shown that aberrant activation of Wnt/β-catenin/Tcf signaling is an important contributing factor in glioma development.11–13 β-catenin and Tcf-4 were upregulated in glioma tissues in comparison with normal brain tissues, while knockdown of β-catenin by small interfering RNA (siRNA) in human glioma cells inhibited cell proliferation and invasive ability, induced apoptotic cell death, and delayed tumor growth. However, the mechanism of β-catenin and Tcf-4 involvement in gliomagenesis is poorly understood.
Previous studies in our laboratory have demonstrated that downregulation of the Wnt/β-catenin signaling pathway decreased PI3K expression and Akt phosphorylation, indicating an interplay between Wnt/β-catenin and Akt signaling cascades.12 In this study, we examined the mechanism by which the Wnt/β-catenin/Tcf pathway regulates Akt signaling. Tcf-4 levels increased significantly with increased pathological grade of gliomas and were positively correlated with AKT2 protein levels. Moreover, reduction of β-catenin/Tcf-4 activity inhibited glioma growth in vitro and in vivo and repressed AKT2 expression by directly binding to the AKT2 promoter.
Materials and Methods
Patients and Samples
A glioma tissue microarray was obtained from Shanxi Chaoying Biotechnology. Pathological grade tumors were defined according to the 2000 WHO criteria as follows: 15 cases with grade I, 15 cases with grade II, 15 cases with grade III, and 15 cases with grade IV. The array dot diameter was 1.5 mm and each dot represented a tissue spot from 1 individual specimen that was selected and pathologically confirmed. All microarrays were stored in the dark at 4°C.
Immunohistochemistry
Immunostaining was performed on 6-μm paraffin sections of tumor specimens by the avidin-biotin-complex (ABC) method, as previously described.14 Briefly, sections were incubated with primary antibody (1:100 dilution) overnight at 4°C, then incubated with a biotinylated secondary antibody (1:200 dilution) at room temperature for 1 h, followed by incubation with ABC-peroxidase reagent for an additional 1 h. After washing with Tris-buffer, the sections were stained with DAB (30 mg 3,3 diaminobenzidine dissolved in 100 mL Tris-buffer containing 0.03% H2O2) for 5 min, rinsed in water and counterstained with hematoxylin. The antibodies used in this study were Tcf-4 and AKT2 (Santa Cruz Biotechnology).
Negative controls were obtained by substituting primary antibodies with non-immune serum. Sections with no labeling or with fewer than 5% labeled cells were scored as 0. Sections with 5%–30% of cells labeled were scored as 1, with 31%–70% of cells labeled as 2, and with labeling of ≥71% as 3. The staining intensity was scored similarly, with 0 used for negative staining, 1 for weakly positive, 2 for moderately positive, and 3 for strongly positive. The scores for the percentage of positive tumor cells and for the staining intensity were added to generate an immunoreactive score for each specimen. The product of the quantity and intensity scores were calculated such that a final score of 0–1 indicated negative expression (−), 2–3 indicated weak expression (+), 4–5 indicated moderate expression (++), and 6 indicated strong expression (+++). Each sample was examined separately and scored by 2 pathologists. Cases with discrepancies in the scores were discussed to reach a consensus.
Cell Culture, Transfection, and Aspirin Treatment
Human glioblastoma cell lines (LN229, U251, SNB19, and A172) were obtained from China Academia Sinica cell repository, Shanghai, China. Human glioblastoma cell lines (TJ899 and TJ905) were established in our laboratory.15 Cells were maintained in Dulbecco's modified Eagle's medium ( Gibco) supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C with 5% CO2. Tcf-4 siRNA and nonsense siRNA were both synthesized by Shanghai Genepharma; Tcf-4 siRNA directed sequence: 5′-TTCTCCGAACGTGTCACGT-3′, nonsense siRNA directed sequence: 5′-AGACGAGGGCGAACAGGAG-3′. The vectors expressing human β-catenin and Tcf-4 were both kind gifts from Jinquan Cheng (H. Lee Moffitt Cancer Center and Research Institute), and the recombinant adenoviruses Ad.CMV-β-catenin and Ad.CMV-Tcf-4 were generated using the Adxsi system (Sinogenomax). Antisense AKT2 plasmid, pLXSN-As-AKT2, was also kindly provided by Jinquan Cheng. Aspirin (acetylsalicylic acid) was purchased from Sigma. A 1-M stock solution was prepared in acetone. Cells were cultured in the presence or the absence of increasing concentrations (0.5, 1, 5, 10, or 20 mM) of aspirin for 1, 2, 6, 12, or 24 h.
Western Blot Analysis
Parental and transfected cells were washed with prechilled phosphate buffered saline (PBS) 3 times. The cells were then solubilized in 1% Nonidet P-40 lysis buffer (20-mM Tris, pH 8.0, 137-mM NaCl, 1% Nonidet P-40, 10% glycerol, 1-mM CaCl2, 1-mM MgCl2, 1-mM phenylmethylsulfonyl fluoride, 1-mM sodium fluoride, 1-mM sodium orthovanadate, and a protease inhibitor mixture). Total protein lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes. Blots were incubated with Tcf-4 (Santa Cruz Biotechnology), β-catenin (Cell Signaling), p-β-catenin (Santa Cruz Biotechnology), AKT2 (Cell Signaling), p-AKT (Santa Cruz Biotechnology) or Fra-1 (Santa Cruz Biotechnology) primary antibodies, followed by incubation with a horseradish peroxidase–conjugated secondary antibody. The specific protein was detected using a super signal protein detection kit (Pierce). After washing with stripping buffer, PVDF membranes were reprobed with antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; (Santa Cruz Biotechnology).
Luciferase Reporter Assay
To evaluate β-catenin/Tcf-4 transcriptional activity, we used a pair of luciferase reporter constructs, TOP-FLASH and FOP-FLASH (Upstate Biotechnology). Plasmids of TOP-FLASH (with 3 repeats of the Tcf-binding site) or FOP-FLASH (with 3 repeats of a mutated Tcf-binding site) were transfected into cells treated with aspirin, as instructed by the suppliers. Luciferase activity was measured with the dual-luciferase reporter assay system, with Renilla luciferase activity as an internal control, 48 h after transfection. In addition, pGL3-WT1-AKT2-promoter and pGL3-WT2-AKT2-promoter reporters were created by ligation of β-catenin/
Tcf-4 – t binding sites in the AKT2 promoter into the BglII site of the pGL3 control vector (Promega). pGL3-MUT1-AKT2-promoter and pGL3-MUT2-AKT2-promoter reporters were generated from the wild-type AKT2-promoter reporters by deleting the binding sites.
Cell Cycle Analysis
Cells were harvested, washed with PBS, fixed with 75% ethanol overnight at 4°C, and then incubated with RNase at 37°C for 30 min. Cell nuclei were stained with propidium iodide for 30 min. A total of 104 nuclei were examined in a FACSCalibur flow cytometer (Becton-Dickinson), and DNA histograms were analyzed by Modifit software. Results are presented as the percentage of cells in each phase.
Transwell Invasion Assay
Transwell membranes coated with Matrigel (Becton-Dickinson) were used to assay invasion of glioma cells in vitro. Cells were plated at 5 × 104 per well in the upper chamber in serum-free medium; 20% FBS was added to the medium in the lower chamber. After incubating for 24 h, non-invading cells were removed from the top well with a cotton swab, while the bottom cells were fixed in 95% ethanol, stained with hematoxylin, and photographed in 3 independent fields for each well. Three independent experiments were done and used to calculate fold migration relative to blank control.
Chromatin Immunoprecipitation
The assays for chromatin immunoprecipitation (ChIP) were performed using reagents commercially obtained from Upstate Biotechnology and conducted essentially according to the manufacturer's instructions. Briefly, cells were maintained in 100-mm cell culture plates and were then fixed with formaldehyde for 10 min. Cells were lysed in SDS lysis buffer, and the chromatin DNA was extracted and sonicated into 200–1000 bp fragments. Immunoprecipitation was performed with anti-Tcf-4 (Upstate Biotechnology), anti-RNA polymerase II (positive control), and immunoglobulin G (IgG; negative control). Purified DNA was used for PCR amplification, and primer sets were designed flanking the putative β-catenin/Tcf-4–binding sites. Binding site 1: forward, 5′-TAACTCCCGCTCCGGGGTCC-3′, and reverse, 5′-GGCTACGCAGGCGCACTAGG-3′. Binding site 2: forward, 5′- TCTCGCTGTGTTGCCCTGCC-3′, and reverse, 5′- TGGCCCTACTTTTGTGCCCTATGT-3′.
Subcutaneous Tumor Assay
BALB/c-A nude mice at 6 weeks of age were purchased from the animal center of the Cancer Institute of the Chinese Academy of Medical Science. All experimental procedures were carried out according to the regulations and internal biosafety and bioethics guidelines of Tianjin Medical University and the Tianjin Municipal Science and Technology Commission. An LN229 glioma subcutaneous model was established, as previously described,9 and the mice were randomly divided into 3 groups (6 subcutaneous tumors per group). Tcf-4 siRNA group: A mixture of 20-µL Lipofectamine and Tcf-4 siRNA (50 nmol/L × 10 µL) mixture was injected into the xenograft tumor model in a multisite injection manner. Nonsense group: A mixture of 20-µL Lipofectamine and nonsense siRNA (50 nmol/L × 10 µL) mixture was injected into the xenograft tumor model in a multisite injection manner. Control group: 20 μL PBS was injected into the xenograft tumor model in a multisite injection manner. Treatment was conducted every 2 days, and the tumor volume was measured with a caliper every 2 days, using the formula volume = length × width2/2.16 After being observed for 20 days, the mice bearing xenograft tumors were sacrificed and the tumor tissues were removed for formalin fixation and the preparation of paraffin embedded sections for immunohistochemical analysis.
Statistical Analysis
Data were analyzed with SPSS 10.0. Analysis of variance, t-test, chi square test, and Pearson correlation were used to analyze the significance between groups. Statistical significance was assigned to P values <0.05.
Results
Tcf-4 Expression and its Association with the Pathological Grade of Gliomas
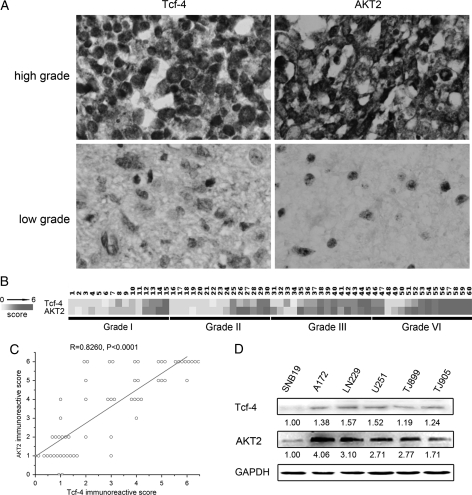
Immunostaining analysis revealed the presence of Tcf-4 protein in gliomas, and the overall positive rate was 60% (36/60). The levels of Tcf-4 protein were markedly higher in high-grade gliomas (WHO grades III and IV) in comparison with low-grade gliomas (WHO grades I and II). Indeed, 23/30 high-grade gliomas exhibited detectable levels of Tcf-4, while 17/30 low-grade gliomas exhibited undetectable levels of the protein (P< .05) (Fig. 1A and B). There were no significant differences between Tcf-4 protein levels in grade III and grade IV gliomas.
Fig. 1.
Expression of Tcf-4 and AKT2 in glioma. (A) Levels of Tcf-4 and AKT2 were detected in glioma tissues by immunostaining. The levels of Tcf-4 and AKT2 protein were markedly higher in high-grade gliomas (WHO grade III and IV) in comparison with low-grade gliomas (WHO grade I and II). (B) Heat map representing protein levels is depicted on a linear scale with a maximum value of 6 to highlight differences within this range. (C) Positive correlation of Tcf-4 expression with AKT2 expression by Pearson correlation analysis. A trend line is provided in each plot, which represents a “best fit,” as determined by simple linear regression. (D) The levels of Tcf-4 and AKT2 were measured in glioma cell lines by Western blot analysis. The quantitative data under the bands show the relative protein levels (normalized to the level of GAPDH).
Positive Correlation Between Levels of Tcf-4 and AKT2 in Gliomas
To explore the relationship between levels of Tcf-4 and AKT2 in gliomas, AKT2 was evaluated by immunostaining (Fig. 1A). Levels of AKT2 were significantly elevated in high-grade gliomas compared with low-grade gliomas. A heat map representing protein levels is shown in Fig. 1B. Pearson correlation showed that a significant positive correlation existed between levels of Tcf-4 and AKT2 (R = 0.8260, P< .0001) (Fig. 1C). We also detected Tcf-4 and AKT2 in 6 glioma cell lines by Western blot analysis. A similar trend between levels of Tcf-4 and AKT2 was observed (Fig. 1D).
Effect of β-Catenin/Tcf-4 Activity on Glioma Cell Proliferation and Invasion
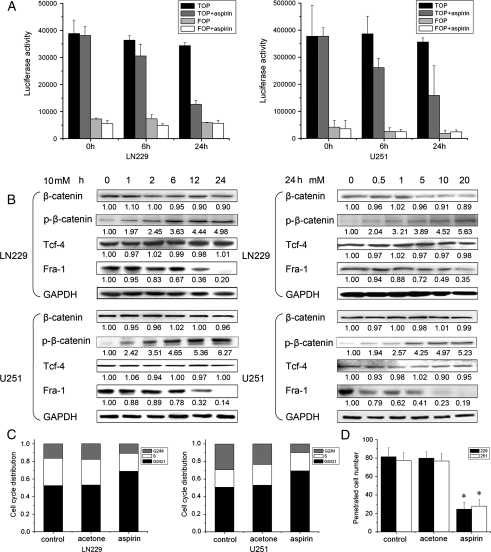
Previous studies in colorectal cancer cells have demonstrated that β-catenin/Tcf-4 transcription is downregulated by aspirin through an increased stabilization of β-catenin phosphorylation.17 Thus, we assessed whether β-catenin/Tcf-4 transcription is also repressed by aspirin in glioma cells using a β-catenin/Tcf-4–responsive luciferase reporter (TOP-FLASH/FOP-FLASH reporter assays). In LN229 cells, activation of the reporter gene was reduced by about 20% within 6 h of exposure to aspirin compared with untreated cells harvested at the same time points (Fig. 2A). After 24 h, aspirin treatment resulted in a 60% inhibition of luciferase activation. To confirm these data, we determined luciferase activation after aspirin treatment in U251 cells, and similar results were obtained. Western blot analysis showed that aspirin did not have an effect on levels of β-catenin and Tcf-4 at any time and dose points in U251 cells; however, a small reduction of β-catenin levels in LN229 cells was observed. Notably, aspirin led to an increase of phosphorylated β-catenin in glioma cells. In addition, a reduction in the level of the β-catenin/Tcf-4 transcription target gene, Fra-1, was found (Fig. 2B). Together, these data indicate that aspirin can inhibit the transcription status of β-catenin/Tcf-4 in glioma cells, which is consistent with previous data.17
Fig. 2.
Effect of β-catenin/Tcf-4 activity on glioma cell proliferation and invasion. (A) 24 h after transfection with TOP or FOP luciferase reporter constructs, the cells were treated with 10-mM aspirin for the indicated times. β-catenin/Tcf-4–dependent transcription was analyzed by measuring luciferase activity. Inhibition of β-catenin/Tcf-4–dependent transcription in response to aspirin treatment was the strongest at the 24 h time point in both LN229 and U251 cells. (B) Cells were treated with increasing concentrations of aspirin for different time periods and levels of β-catenin, p-β-catenin, Tcf-4, and Fra-1 were observed by Western blot analysis. The quantitative data under the bands show protein levels (normalized to levels of GAPDH) relative to the control group. (C) Aspirin induced cell cycle G0/G1 arrest. Cells were treated with 10-mM aspirin for 72 h and subjected to cell cycle analyses by fluorescence-activated cell sorting. (D) In vitro transwell invasion assay, the proportion of penetrating cells was significantly decreased in the aspirin group. Data are presented as the means of triplicate experiments. *P< .05 compared with the control group.
To investigate the effects of low β-catenin/Tcf-4 transcriptional activity on glioma cell proliferation and invasion, cell cycle and in vitro Matrigel invasion assays were performed after aspirin treatment. As shown in Fig. 2C, the percentages of LN229 cells in G0/G1 phase for control, acetone, and aspirin groups were 52.6%, 53.3%, and 68.9%, respectively. The S phase fractions in control, acetone, and aspirin groups were 30.9%, 29.0%, and 20.3%, respectively. In addition, the percentages of cells in G2/M phase in control, acetone, and aspirin groups were 16.5%, 17.7%, and 10.8%, respectively. A similar cell cycle distribution trend was detected in U251 cells. As observed in the Matrigel invasion assay, the number of LN229 cells that had penetrated the Matrigel were 81.4 ± 9.6, 79.9 ± 6.8, and 24.6 ± 7.5, respectively, for control, acetone, and aspirin groups (P < .05). Significant numbers of U251 cells that penetrated the Matrigel were also observed (Fig. 2D). These results suggest that β-catenin/Tcf-4 activity is critical for glioma cell proliferation and invasion.
AKT2 Levels are Regulated by β-Catenin/Tcf-4 Activity
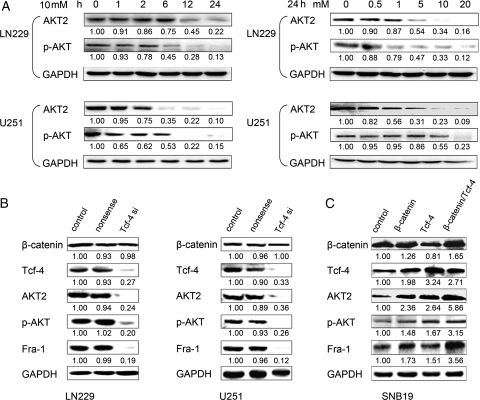
We have previously shown interplay between Wnt/β-catenin and PI3K/Akt signaling cascades in gliomas. To investigate in detail the PI3K/Akt pathway affected by β-catenin/Tcf-4 activity, we employed Western blot analysis. Figure 3A depicts the time- and dose-dependent effects of aspirin on AKT2 levels and on Akt phosphorylation in LN229 and U251 cells. Interestingly, the time and dose dependency of AKT2 downregulation in glioma cells was the same as for the β-catenin/Tcf-4 target gene, Fra-1. We also downregulated β-catenin/Tcf-4 activity using Tcf-4 siRNA. Indeed, a significant reduction of AKT2 levels and phosphorylation of Akt was detected after knockdown of Tcf-4, while Fra-1 expression was also repressed (Fig. 3B). In addition, SNB19 cells, which have low levels of β-catenin and Tcf-4, were transfected with Ad.β-catenin and/or Ad.Tcf-4 recombinant adenovirus. Overexpression of β-catenin and Tcf-4 in SNB19 cells induced an increase in the levels of AKT2 and Fra-1 and an increase in phosphorylation of Akt (Fig. 3C). Therefore, we hypothesized that AKT2 transcription might be a direct target of the β-catenin/Tcf-4 complex in glioma cells.
Fig. 3.
AKT2 expression is regulated by β-catenin/Tcf-4 activity. (A) Cells were treated with increasing concentrations of aspirin for different time periods. Western blot analysis showed a clear reduction of AKT2 levels and of Akt phosphorylation in a time- and dose-dependent manner. (B) Tcf-4 downregulation by siRNA triggered a significant repression of AKT2 levels in LN229 and U251 cells. (C) Overexpression of β-catenin and/or Tcf-4 induced an increase of AKT2 levels. The quantitative data under the bands show protein levels (normalized to levels of GAPDH) relative to the control group. Data are presented as the means of triplicate experiments.
The AKT2 Promoter is Directly Regulated by β-Catenin/Tcf-4
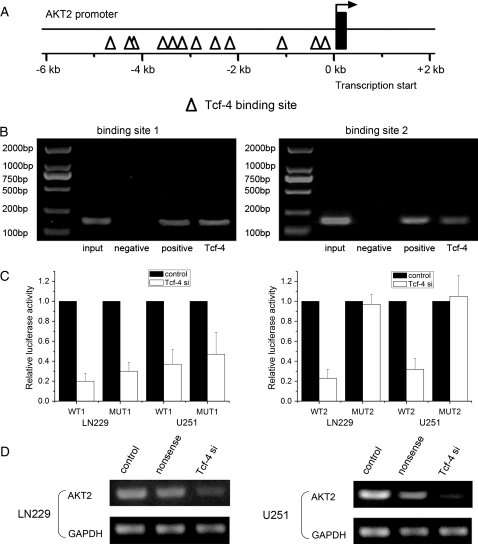
To investigate the functional interaction between β-catenin/Tcf-4 signaling and AKT2 gene expression, we scanned the AKT2 gene promoter region for regulatory DNA-binding elements. Searching the GenBank nucleotide database revealed the presence of 12 putative β-catenin/Tcf-4–binding sites in the AKT2 gene (Fig. 4A) that showed a high degree of homology to the core consensus sequence (CTTTG or CAAAG). To determine in vivo binding of β-catenin/Tcf-4 to the AKT2 promoter we performed ChIP assays in LN229 cells. In vivo cross-linked DNA-protein complexes were immunoprecipitated with appropriate antibodies. Cross-linking was then reversed and PCR amplification was performed using primers flanking the consensus binding sites at −349/−343 bp and −3491/−3497 bp in the AKT2 promoter. Agarose gel electrophoresis of PCR products showed that AKT2 promoter DNA was immunoprecipitated by an anti-Tcf-4 antibody (Fig. 4B). Moreover, we created pGL3-WT1-AKT2-promoter, pGL3-MUT1-AKT2-promoter, pGL3-WT2-AKT2-promoter, and pGL3-MUT2-AKT2-promoter plasmids for the AKT2 promoter consensus binding sites located at −349/−343 bp and −3491/−3497 bp, respectively. Both of the wild-type AKT2 promoters demonstrated low activity after reduction of Tcf-4. However, only mutations on the binding site located at −3491/−3497 bp could abolish the inhibitory effect of Tcf-4 siRNA on luciferase activity (Fig. 4C). In addition, reduction of Tcf-4 inhibited AKT2 mRNA expression (Fig. 4D). These data indicate that β-catenin/Tcf-4 regulates AKT2 through binding AKT2 promoter at −3491/−3497 bp at the transcriptional level.
Fig. 4.
AKT2 is a direct target of β-catenin/Tcf-4. (A) The promoter region of the AKT2 gene was analyzed for consensus β-catenin/Tcf-4–binding sites. Diagram of the AKT2 promoter showing the location of β-catenin/Tcf-4–binding sites. (B) Untreated LN229 cells were grown to confluency and lysed. ChIP was performed on cell lysates using equal portions of anti-Tcf-4, anti-RNA polymerase II (positive control), and IgG (negative control) as described in Materials and Methods. Input samples are DNAs amplified from lysates before immunoprecipitation. Results for binding site 1 and 2 (located at −349/−343 bp and −3491/−3497 bp in the AKT2 promoter, respectively) are shown. (C) pGL3-WT1-AKT2-promoter, pGL3-MUT1-AKT2-promoter, pGL3-WT2-AKT2-promoter, and pGL3-MUT2-AKT2-promoter reporters were transfected into LN229 cells, which were then treated with Tcf-4 siRNA. Luciferase activity was determined 48 h after transfection. Luciferase activity of control group was normalized as 1 in WT group and MUT group, respectively. (D) LN229 and U251 cells were transfected with Tcf-4 siRNA, and AKT2 mRNA levels were detected by RT-PCR assay. GAPDH was regarded as an endogenous normalizer. Error bars represent standard deviation and were obtained from 3 independent experiments. *P< .05 compared with the control group.
Reduction of AKT2 Inhibits Glioma Cell Proliferation and Invasion
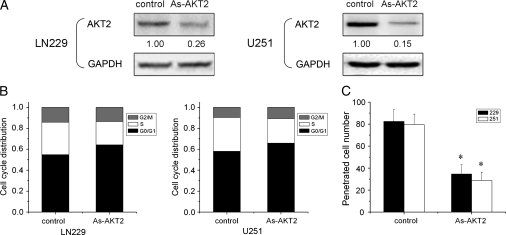
To verify an AKT2-mediated effect of β-catenin/Tcf-4 activity on glioma cell proliferation and invasion, the biological function of AKT2 was evaluated. Reduction of AKT2 by antisense AKT2 caused a cell cycle defect in LN229 and U251 cells with a significant increase in the percentage of cells in the G0/G1 phase (Fig. 5A and B). Furthermore, an in vitro transwell invasion assay showed that an average of 60% of cells in the antisense AKT2 group were defective in migrating through Matrigel compared with cells in control groups (Fig. 5C). The results suggest that the β-catenin/Tcf-4 activity that regulates glioma cell proliferation and invasion is, in part, mediated by AKT2.
Fig. 5.
Reduction of AKT2 decreases glioma cell proliferation and invasion. (A) LN229 and U251 cells were transfected with antisense AKT2, and AKT2 expression was detected by Western blot analysis. GAPDH was regarded as an endogenous normalizer. (B) Cells were treated with antisense AKT2 for 72 h, and subjected to cell cycle analyses by fluorescence-activated cell sorting. Reduction of AKT2 induced cell cycle arrest in G0/G1. (C) Downregulation of AKT2 expression caused a substantial reduction in the proportion of penetrating cells in the in vitro transwell invasion assay. Data are presented as the means of triplicate experiments. *P< .05 compared with the control group.
Reduction of Tcf-4 inhibits glioma growth in vivo
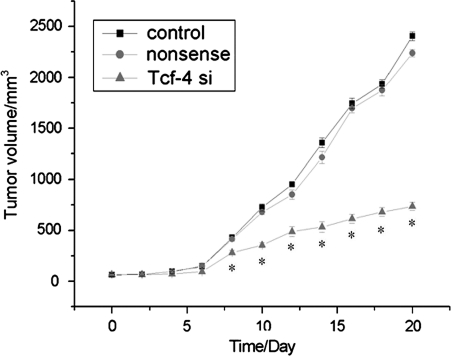
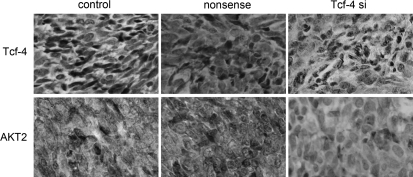
To explore the effect of β-catenin/Tcf-4 activity on tumor growth in vivo, we employed a xenograft mouse model using LN229 glioma cells treated with Tcf-4 siRNA. On day 8, the tumor size of the Tcf-4 siRNA group started to reach statistical significance compared with control groups (P< .05). At the termination of the study, there was a marked reduction in tumor mass between the Tcf-4 siRNA group and the control groups (Fig. 6). After observation for 20 days, tumor samples were dissected from mice, and paraffin-embedded sections were prepared for immunohistopathological examination. Similar to the results obtained from the in vitro study, the expression of Tcf-4 and AKT2 in tumor specimens of the Tcf-4 siRNA group was highly downregulated (Fig. 7).
Fig. 6.
Reduction of Tcf-4 inhibits glioma growth in vivo. After establishment of subcutaneous tumors, Tcf-4 siRNA was injected in a multisite injection manner. Tumor volumes were measured every 2 days during treatment. Downregulation of Tcf-4 expression efficiently decreased tumor growth in vivo. *P< .05 compared with the control group.
Fig. 7.
Reduction of Tcf-4 represses levels of AKT2 in vivo. Immunohistochemistry was performed on xenograft tumors after Tcf-4 siRNA treatment. The levels of Tcf-4 and AKT2 were suppressed in LN229 xenograft tumors of the Tcf-4 siRNA group.
Discussion
We have shown that the level of Tcf-4 significantly increases with increased pathological grade of gliomas and that it correlates positively with AKT2 expression. Reduction of β-catenin/Tcf-4 activity inhibited glioma growth in vitro and in vivo. Moreover, levels of AKT2 were regulated by β-catenin/Tcf-4 activity and the β-catenin/Tcf-4 complex bound directly to the AKT2 promoter.
High-grade gliomas are the most common human brain tumors and are essentially incurable. Thus, advances on all scientific and clinical fronts are needed. In an attempt to better understand gliomas, many groups have turned to high-dimensional profiling studies. The Cancer Genome Atlas Network catalogs recurrent genomic abnormalities in GBM and describes a robust gene expression-based molecular classification of GBM into proneural, neural, classical, and mesenchymal subtypes and integrates multidimensional genomic data to establish patterns of somatic mutations and DNA copy number.18,19 Their analysis illustrates that a survival advantage in patients treated heavily with temozolomide and radiation varies by subtype, with classical or mesenchymal subtypes having significantly delayed mortality that was not observed in the proneural subtype. The proneural signature contains several proneural developmental genes, such as SOX genes, as well as Tcf-4. It has been reported that Tcf-4, the oligodendrocyte-related transcription factor, is required for maturation of oligodendrocyte progenitors.20 In addition, Tcf-4 interacts with β-catenin in the nucleus and forms a β-catenin/Tcf-4 complex, thereby regulating transcription of multiple genes involved in cell proliferation, metastatic potential, and tumorigenesis. These data suggest that β-catenin and Tcf-4 play an important role in glioma progression and prognosis or prediction of response to therapy. In our study, reduction of β-catenin/Tcf-4 activity inhibited glioma cell proliferation and invasion in vitro and glioma growth in vivo.
Akt, a serine/threonine kinase, also known as protein kinase B, functions as a key mediator of the PI3K/Akt pathway. Activated Akt is phosphorylated at Thr308 and Ser473 and subsequently regulates β-catenin by inducing phosphorylation of GSK3β at Ser9.21,22 In addition, Akt can regulate β-catenin directly by inducing phosphorylation at Ser552, resulting in β-catenin translocation from the cytosol into the nucleus, increasing Tcf-4 transcriptional activity.23 In turn, AKT1, an isoform of Akt, is regulated by the β-catenin/Tcf-4 complex at the transcriptional level. Aspirin induces a decrease of AKT1 expression, which is regulated by the β-catenin/Tcf-4 complex as revealed by a reporter assay.24 AKT2 and AKT3 are closely related and highly conserved homologs of AKT1; therefore, whether AKT2 and AKT3 are targets of Wnt/β-catenin signaling awaits further investigation. In our study, aspirin inhibited AKT2 expression by the modulation of β-catenin/Tcf-4 activity in a time- and concentration- dependent manner. Downregulation of Tcf-4 expression decreased levels of AKT2, whereas upregulation of β-catenin and/or Tcf-4 expression increased AKT2 levels. Furthermore, 12 putative β-catenin/Tcf binding sites were identified in the promoter region of the AKT2 gene, and in vivo binding of β-catenin/Tcf-4 to the AKT2 promoter was validated by ChIP and luciferase reporter assays. These data indicate that Akt activation increases the nuclear accumulation of β-catenin, while Akt is regulated by β-catenin/Tcf-4 at the transcriptional level, leading to a positive feedback loop between β-catenin/Tcf-4 signaling and Akt signaling.
AKT2 expression is associated with more advanced and highly aggressive gliomas.25–27 Our previous data have revealed that levels of AKT2 are greatly increased with ascending tumor grade and correlated positively with the proliferation activity of gliomas, in contrast to normal brain tissues.14,28 Reduction of AKT2 by antisense AKT2 inhibits C6 glioma cell growth in vitro and in vivo.29 In addition, AKT2 contributes to glioma cell migration and invasion by regulating the formation of cytoskeleton, influencing adhesion, and increasing expression of MMP9.30 In this study, downregulation of AKT2 in LN229 and U251 glioma cells induced cell cycle G0/G1 phase arrest and inhibited cell invasion. These results suggest that β-catenin/Tcf-4 activity can regulate glioma cell proliferation and invasion, in part, mediated by AKT2.
In conclusion, the results of the present study indicate that Tcf-4 is associated with the progression of glioma malignancy. Levels of Tcf-4 increase significantly with the ascending pathological grade of gliomas and correlate positively with levels of AKT2. Moreover, β-catenin/Tcf-4 activity regulates expression of AKT2 by binding to the AKT2 promoter, thereby modulating glioma cell growth. To our knowledge, this is the first demonstration that AKT2 is regulated by β-catenin/Tcf-4 at the transcriptional level. In summary, our data add AKT2 to the list of β-catenin/Tcf-4 target genes and propose a model by which aberrant Wnt/β-catenin/Tcf-4 signaling is involved in glioma progression.
Funding
This work was supported by the China National Natural Scientific Fund (30971136, 30872657), the Tianjin Science and Technology Committee (09JCZDJC17600), the Program for New Century Excellent Talents in University (NCET-07-0615), Jiangsu Province's Medical Major Talent program (RC2007061), and Jiangsu Province's Natural Science Foundation (BK2008475). Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Acknowledgments
We thank Prof. Jinquan Cheng for experimental support, discussions, and suggestions.
Conflict of interest statement. None declared.
References
- 1.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. doi:10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 2.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279–283. doi: 10.1001/archneurol.2010.5. doi:10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Page M, Solheim K, et al. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11:330–339. doi: 10.1215/15228517-2008-093. doi:10.1215/15228517-2008-093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ying Y, Tao Q. Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics. 2009;4:307–312. doi: 10.4161/epi.4.5.9371. doi:10.4161/epi.4.5.9371. [DOI] [PubMed] [Google Scholar]
- 5.Huang K, Zhang JX, Han L, et al. MicroRNA roles in beta-catenin pathway. Mol Cancer. 2010;9:252. doi: 10.1186/1476-4598-9-252. doi:10.1186/1476-4598-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei W, Chua MS, Grepper S, et al. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76. doi: 10.1186/1476-4598-8-76. doi:10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. doi:10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HL, Wang J, Xiao SY, et al. Elevated protein expression of cyclin D1 and Fra-1 but decreased expression of c-Myc in human colorectal adenocarcinomas overexpressing beta-catenin. Int J Cancer. 2002;101:301–310. doi: 10.1002/ijc.10630. doi:10.1002/ijc.10630. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Ren Y, Moore L, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. doi:10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 10.Yochum GS, Cleland R, Goodman RH. A genome-wide screen for beta-catenin binding sites identifies a downstream enhancer element that controls c-Myc gene expression. Mol Cell Biol. 2008;28:7368–7379. doi: 10.1128/MCB.00744-08. doi:10.1128/MCB.00744-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue X, Lan F, Yang W, et al. Interruption of β-catenin suppresses the EGFR pathway by blocking multiple oncogenic targets in human glioma cells. Brain Res. 2010;1366:27–37. doi: 10.1016/j.brainres.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Pu P, Zhang Z, Kang C, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–361. doi: 10.1038/cgt.2008.78. doi:10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 13.Sareddy GR, Panigrahi M, Challa S, et al. Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int. 2009;55:307–317. doi: 10.1016/j.neuint.2009.03.016. doi:10.1016/j.neuint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Han L, Zhang A, et al. AKT2 expression is associated with glioma malignant progression and required for cell survival and invasion. Oncol Rep. 2010;24:65–72. doi: 10.3892/or_00000829. [DOI] [PubMed] [Google Scholar]
- 15.Wang SL, Li F, Pu PY, et al. The establishment and characteristics of human glioblastoma cell lines TJ899 and TJ905. J Tianjin Med. 1996;24:416–418. [Google Scholar]
- 16.You Y, Geng X, Zhao P, et al. Evaluation of combination gene therapy with PTEN and antisense hTERT for malignant glioma in vitro and xenografts. Cell Mol Life Sci. 2007;64:621–631. doi: 10.1007/s00018-007-6424-4. doi:10.1007/s00018-007-6424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dihlmann S, Klein S, Doeberitz Mv MK. Reduction of beta-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated beta-catenin. Mol Cancer Ther. 2003;2:509–516. [PubMed] [Google Scholar]
- 18.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. doi:10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG Island Methylator Phenotype that Defines a Distinct Subgroup of Glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu H, Cai J, Clevers H, et al. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–11408. doi: 10.1523/JNEUROSCI.0160-09.2009. doi:10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. doi:10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 22.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. doi:10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. doi:10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dihlmann S, Kloor M, Fallsehr C, et al. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26:1503–1512. doi: 10.1093/carcin/bgi120. doi:10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- 25.Mure H, Matsuzaki K, Kitazato KT, et al. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol. 2010;12:221–232. doi: 10.1093/neuonc/nop026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pu P, Kang C, Li J, et al. Antisense and dominant-negative AKT2 cDNA inhibits glioma cell invasion. Tumour Biol. 2004;25:172–178. doi: 10.1159/000081099. doi:10.1159/000081099. [DOI] [PubMed] [Google Scholar]
- 27.Fortin SP, Ennis MJ, Savitch BA, et al. Tumor necrosis factor-like weak inducer of apoptosis stimulation of glioma cell survival is dependent on Akt2 function. Mol Cancer Res. 2009;7:1871–1881. doi: 10.1158/1541-7786.MCR-09-0194. doi:10.1158/1541-7786.MCR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G, Kang C, Pu P. Increased expression of Akt2 and activity of PI3K and cell proliferation with the ascending of tumor grade of human gliomas. Clin Neurol Neurosurg. 2010;112:324–327. doi: 10.1016/j.clineuro.2010.01.003. doi:10.1016/j.clineuro.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Pu P, Kang C, Li J, et al. The effects of antisense AKT2 RNA on the inhibition of malignant glioma cell growth in vitro and in vivo. J Neurooncol. 2006;76:1–11. doi: 10.1007/s11060-005-3029-3. doi:10.1007/s11060-005-3029-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Gu F, She C, et al. Reduction of Akt2 inhibits migration and invasion of glioma cells. Int J Cancer. 2009;125:585–595. doi: 10.1002/ijc.24314. doi:10.1002/ijc.24314. [DOI] [PubMed] [Google Scholar]