Abstract
Insights into the molecular pathogenesis of glioblastoma have not yet resulted in relevant clinical improvement. With standard therapy, which consists of surgical resection with concomitant temozolomide in addition to radiotherapy followed by adjuvant temozolomide, the median duration of survival is 12–14 months. Therefore, the identification of novel molecular targets and inhibitory agents has become a focus of research for glioblastoma treatment. Recent results of bevacizumab may represent a proof of principle that treatment with targeted agents can result in clinical benefits for patients with glioblastoma. This review discusses limitations in the existing therapy for glioblastoma and provides an overview of current efforts to identify molecular targets using large-scale screening of glioblastoma cell lines and tumor samples. We discuss preclinical and clinical data for several novel molecular targets, including growth factor receptors, phosphatidylinositol-3 kinase, SRC-family kinases, integrins, and CD95 ligand and agents that inhibit these targets, including erlotinib, enzastaurin, dasatinib, sorafenib, cilengitide, AMG102, and APG101. By combining advances in tumor screening with novel targeted therapies, it is hoped that new treatment options will emerge for this challenging tumor type.
Keywords: integrins, PI3 kinase, receptor tyrosine kinase, SRC-family kinases, VEGF signaling
Glioblastoma is the most common primary central nervous system tumor, accounting for ∼60% of the 17, 000 primary brain tumors diagnosed annually in the United States.1 Patients who receive a diagnosis of glioblastoma have a dismal prognosis, typically dying within 3 months if untreated. Standard treatment increases median survival to 12 months, although disease tends to progress within 6–9 months and the 2-year survival rate is <25%.2 In this review, we discuss the limitations of existing therapies for glioblastoma before summarizing ongoing efforts to identify novel molecular targets and develop novel targeted agents for this disease.
Search Strategy and Selection Criteria
Relevant publications in the PubMed database published during the period from January 1995 through February 2010 were identified using the search terms “glioblastoma,” “glioma,” “VEGF,” “EGFR,” “PI3K,” “SRC,” “PDGFR,” “integrin,” “CD95,” “TRAIL,” and “c-MET.” Only articles published in English were reviewed. Relevant clinical trials were identified by searching http://www.clinicaltrials.gov/ using the search terms “glioblastoma” and “glioma.”
Current Glioblastoma Therapy
The current standard of care for newly diagnosed glioblastoma is surgical resection with concomitant daily temozolomide (TMZ; 75 mg/m2) and radiotherapy, followed by 6 cycles of adjuvant TMZ (150–200 mg/m2) for 5 days during each 28-day cycle.3 However, almost all patients with glioblastoma experience disease recurrence. Because no standard treatment option exists after recurrence, rechallenging with TMZ or switching to an alternative TMZ dosing regimen has become common practice. In a retrospective analysis (n = 80), the 6-month progression-free survival (PFS) rate was similar for patients with recurrent glioblastoma or anaplastic astrocytoma after TMZ regimen change (26%) or rechallenge (29%).4 The Canadian RESCUE study showed similar results using a low-dose metronomic TMZ schedule, reporting a smaller benefit from rechallenge if prior TMZ exposure exceeded 6 months.5 Ongoing studies are investigating alternative TMZ regimens in first-line and second-line settings, including the Neurooncology Working Group (NOA)-08, Radiation Therapy Oncology Group (RTOG) 0525, and DIRECTOR trials.
Targeted Therapy for Glioblastoma
The introduction of molecularly targeted agents is one of the most significant advances in cancer therapy in recent years. Targeted therapies block activation of oncogenic pathways, either at the ligand–receptor interaction level or by inhibiting downstream signal transduction pathways, thereby inhibiting growth and progression of cancer. Because of their specificity, targeted therapies should theoretically have better efficacy and safety profiles than systemic cytotoxic chemotherapy or radiotherapy.
Because of the substantial neovascularization seen in glioblastoma, targeted antiangiogenic therapies have received considerable attention.6 The main rationale for using antiangiogenic therapies in glioblastoma is to normalize the vasculature, restoring the selective permeability of the blood-brain barrier (BBB), rather than starving tumors of oxygen and growth factors as originally proposed.7 However, animal models of glioblastoma have shown that antiangiogenic therapies may reduce the effectiveness of TMZ.8 The sequence of combination regimens and effects of specific antiangiogenic therapies on the BBB should be fully characterized to optimize therapy.
Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), has shown unusually high response rates in recurrent grade 3 and 4 gliomas (6-month PFS rate, 46%; 6-month overall survival (OS rate), 77%),9 which led to US approval for glioblastoma. Whether these response rates are a valid surrogate for PFS and OS remains a matter of debate.10 Recently, the worry of more distant recurrences with bevacizumab treatment was not substantiated in a matched-pair analysis.11
Identifying Novel Therapeutic Targets in Glioblastoma
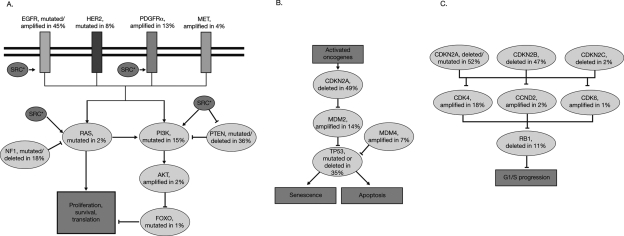
Identifying biological mechanisms contributing to glioblastoma oncogenesis will help researchers and physicians to develop and select appropriate targeted therapies to improve patient outcomes. In a large-scale multidimensional analysis performed by the Cancer Genome Atlas involving 206 glioblastoma samples, 91 of which were also analyzed to identify nucleotide sequence aberrations, the most frequent gene amplifications were: epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR)α, 2 transmembrane receptors with tyrosine kinase activity; cyclin-dependent kinase 4 (CDK4), a promoter of cell-cycle progression; and murine double minute (MDM)2 and MDM4, suppressors of P53 activity.12 The most frequent homozygous gene deletions were CDKN2A, CDKN2B, and CDKN2C, which encode tumor suppressor proteins that suppress activation of CDK4 and CDK6; phosphatase and tensin homolog (PTEN), a tumor suppressor that inhibits phosphatidylinositol-3 kinase (PI3K) signaling; retinoblastoma (RB1), a cell-cycle inhibitor; PARK2, a regulator of dopaminergic cell death; and neurofibromin (NF)1, a negative regulator of the RAS signal transduction pathway. The most frequently mutated genes were P53, PTEN, NF1, EGFR, human epidermal growth factor receptor 2 (HER2), RB1, and PIK3R1 and PIK3CA—2 components/regulators of the PI3K signaling pathway. This study shows that signaling pathways involving receptor tyrosine kinases/PI3K, regulators of the cell cycle, such as P53 and the cyclin/RB1 pathway, are considerably altered in glioblastoma (Fig. 1).
Fig. 1.
Genetic alterations in glioblastoma signal transduction pathways (adapted from Parsons et al13). (A) Proliferation and survival signaling is altered in 88% of glioblastomas. (B) p53 signaling is altered in 87% of glioblastomas. (C) RB signaling is altered in 78% of glioblastomas. CCND2 indicates cyclin-D2; CDK indicates cyclin-dependent kinase; CDKN indicates cyclin-dependent kinase inhibitor; EGFR indicates epidermal growth factor receptor; FOXO indicates forkhead box-O; HER2 indicates human epidermal growth factor receptor-2; NF1 indicates neurofibromin; PDGFR indicates platelet-derived growth factor receptor; PI3K indicates phosphatidylinositol 3-kinase; PTEN indicates phosphatase and tensin homolog; RB1 indicates retinoblastoma protein-1; and SRC* indicates activated (phosphorylated) SRC. Figure 1 is adapted from The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455(7216):1061–1068.
A similar study has identified characteristic mutations in the active site of isocitrate dehydrogenase 1 (IDH1) in 12% of patients with glioblastoma. IDH1 mutations occurred in a high proportion of young patients and in the majority of secondary glioblastoma cases and were associated with increased OS (3.8 years), compared with wild-type IDH1 (1.1 years).13 This may be due to increased tumor sensitivity to chemotherapy,14 although a large controlled series in the German Glioma Network did not find any association between prolonged survival of patients with tumors with IDH1 mutations and administration of a specific therapy.15 Mutation of the IDH1 active site prevents conversion of isocitrate to α-ketoglutarate but allows the mutated enzyme to catalyze the nicotinamide dinucleotide phosphate-dependent reduction of α-ketoglutarate to R(-)-2-hydroxyglutarate (2HG). Accumulated 2HG appears to act as an oncometabolite that contributes to glioma formation and malignant progression. This observation is supported by data from patients with inherited 2-hydroxyglutaric aciduria, in whom deficient 2HG dehydrogenase causes an accumulation of brain 2HG. These patients have an increased risk of developing brain tumors, possibly because of increased production of reactive oxygen species.16 Although of particular interest, neither compounds nor trials are available that target IDH1 or NF1 thus far.
Increased tyrosine kinase activity has also been associated with glioblastoma oncogenesis. In a tyrosine kinase activation catalog covering 130 human cancer cell lines, the most frequently activated tyrosine kinases were EGFR, fibroblast growth factor receptor 3 (FGFR3), protein tyrosine kinase 2 (PTK2, also known as focal adhesion kinase, or FAK), and SRC, LCK, and LYN, 3 members of the SRC-family kinases (SFK).17 SRC and SFKs mediate downstream signaling from several growth factor receptors, and SRC is a key binding partner of FAK.18 Screening of 31 primary glioblastoma samples showed similar patterns of tyrosine kinase activation, including SRC activation in 61% of the samples.17 Overexpression of SFKs has been reported in previous studies,19 although the Cancer Genome Atlas study did not identify any focal amplification or somatic missense mutations of SRC or SFKs.12
Studies have already been performed using novel agents that inhibit targets identified by screening methods discussed above or that are based on preclinical studies and experience in other tumors (Table 1). However, further analyses of clinical and molecular data derived from these trials (Table 2)20–44 are necessary to verify the relevance of these targets to glioblastoma.
Table 1.
Targeted therapies in clinical trials for glioblastoma
| Agent | Target molecules | Approved cancer indications | Combination treatments under investigation |
|---|---|---|---|
| APG101 | CD95 | None | RT |
| AMG102 | c-MET | None | Monotherapy |
| Cetuximab | EGFR | CRC, HNSCC | TMZ + RT |
| Erlotinib | EGFR | NSCLC, pancreatic | TMZ + RT |
| CCNU, | |||
| carboplatin, | |||
| sorafenib, | |||
| sirolimus, | |||
| temsirolimus, | |||
| bevacizumab | |||
| Gefitinib | EGFR | NSCLC | Monotherapy |
| BIBW2992 | EGFR, HER2 | None | Monotherapy, |
| TMZ | |||
| TMZ + RT | |||
| Lapatinib | EGFR, HER2 | MBC | Monotherapy, |
| pazopanib | |||
| Cilengitide | αV integrins | None | TMZ + RT |
| Imatinib | PDGFR | CML, Ph+ ALL, KIT + GIST | Vatalanib + hydroxyurea, |
| TMZ, | |||
| hydroxyurea | |||
| Tandutinib | PDGFR | None | Monotherapy, |
| bevacizumab | |||
| NVP-BKM120 | PI3K | None | Monotherapy |
| Enzastaurin | PKC/PI3K/AKT | None | Monotherapy, |
| RT | |||
| TMZ + RT | |||
| Dasatinib | SRC | CML, Ph+ ALL | Monotherapy, |
| TMZ | |||
| TMZ + RT, | |||
| CCNU, | |||
| erlotinib | |||
| Bevacizumab | VEGF | CRC, MBC, GBM, RCC, NSCLC | Cetuximab + irinotecan |
| Cediranib | VEGFR | None | Monotherapy, |
| TMZ, | |||
| CCNU, | |||
| bevacizumab, | |||
| cilengitide, | |||
| Vandetanib | VEGFR, EGFR | None | Monotherapy, |
| TMZ, | |||
| carboplatin, | |||
| imatinib, | |||
| sirolimus, | |||
| etoposide | |||
| Vorisnostat | HDAC I/II | T cell lymphoma | Monotherapy |
| TMZ | |||
| bevacizumab, | |||
| irinotecan, | |||
| bortezomib | |||
| Sorafenib | VEGFR, PDGFR, MAPK | RCC, HCC | Monotherapy, |
| TMZ + RT, | |||
| TMZ, | |||
| temsirolimus, | |||
| erlotinib, | |||
| bevacizumab |
CCNU indicates lomustine; CML indicates chronic myeloid leukemia; CRC indicates colorectal carcinoma; EGFR indicates epidermal growth factor receptor; GBM indicates glioblastoma multiforme; HCC indicates hepatocellular carcinoma; HDAC indicates histone deacetylase; HER2 indicates human epidermal growth factor receptor 2; HNSCC indicates head and neck squamous cell carcinoma; MAPK indicates mitogen-activated protein kinase signaling; MBC indicates metastatic breast cancer; NSCLC indicates nonsmall cell lung carcinoma; PDGFR indicates platelet-derived growth factor receptor; Ph+ ALL indicates Philadelphia chromosome-positive acute lymphoblastic leukemia; PI3K indicates phosphatidylinositol 3-kinase; PKC indicates protein kinase C; RCC indicates renal cell carcinoma; RT indicates radiotherapy; TMZ indicates temozolomide; and VEGFR indicates vascular endothelial growth factor receptor.
Table 2.
Published clinical data for targeted therapies in glioblastoma
| Agents | Phase | Primary/recurrent disease | No. of patients | Primary outcomes | Positive prognostic indicator(s) | Reference |
|---|---|---|---|---|---|---|
| Cetuximab + TMZ + RT | I/II | Primary | 39 | 12-month OS , 89%; 24-month OS , 42%; 6-month PFS , 76%; 12-month PFS , 45% | EGFR and PTEN coexpression significantly correlated with PFS (P = .005) | 20 |
| Cilengitide + TMZ + RT | I/IIa | Primary | 52 | 6-month PFS , 69% | MGMT promoter methylation | 21 |
| Erlotinib + TMZ + RT | I/II | Primary | 97 | OS , 15.3 months; | None | 22 |
| median PFS , 7.2 months | ||||||
| Erlotinib + TMZ + RT | II | Primary | 65 | Median OS , 19.3 months; | MGMT promoter methylation and PTEN+ (P = .04) | 23 |
| median PFS , 8.2 months | ||||||
| RT + TMZ → TMZ + sorafenib | II | Primary | 47 | Median PFS , 6 months (95% CI 3.7 to 7.0 months); | NA | 24 |
| 12-month PFS , 16% | ||||||
| Talampanel | II | Primary | 72 | OS , 18.3 months (median) | NR | 25 |
| AMG102 | II | Recurrent | 20 | Response rate by Macdonald criteria: | NA | 26 |
| 1 cPR, 2 SD, 14 PD, | ||||||
| 1 minor response | ||||||
| Cediranib ± CCNU | III | Recurrent | 300 | PFS Cediranib 30 mg , 3 months; | NA | 47 |
| Cediranib 20 mg + CCNU , 4 months; | ||||||
| CCNU , 2.7 months | ||||||
| Cilengitide | IIa | Recurrent | 81 | 6-month PFS , 16% | NA | 27 |
| Enzastaurin | II | Recurrent | 85 | Objective radiographic responses in 14 patients (10 GBM), including 1 CR; | NA | 28 |
| 6-month PFS , 7% | ||||||
| Enzastaurin | III | Recurrent | 266 | No significant effect on PFS (1.5 vs 1.6 months), OS (6.6 vs 7.1 months), 6-month PFS (P = .13), SD (38.5 vs 35.9%), or OR (2.9 vs 4.3%) respectively for enzastaurin vs lomustine | NA | 29 |
| Erlotinib+ temsirolimus | I/II | Recurrent | 22 (phI) | 6-month PFS , 12.5% | NR | 30 |
| 56 (phII) | ||||||
| Erlotinib + TMZ/CCNU | II | Recurrent | 110 | 6-month PFS , 11.4% | Low phospho-AKT | 31 |
| (P = .068) | ||||||
| Erlotinib + bevacizumab | II | Recurrent | 24 (MG) | 6-month PFS , 25% | NR | 32 |
| 32 (AG) | ||||||
| Erlotinib + carboplatin | II | Recurrent | 43 | Median PFS , 9 weeks;, 6-month PFS , 14% | None | 33 |
| Erlotinib + sirolimus | II | Recurrent | 32 | 6-month PFS , 3.1% | Increased phospho-AKT (P = .045) | 34 |
| Gefitinib | II | Recurrent | 28 | 6-month PFS , 14.3% | None | 35 |
| Imatinib + vatalanib + hydroxyurea | I | Recurrent | 37 | Vatalanib MTD , 1000 mg BID; | NA | 36 |
| DLTs , hematologic, GI, renal, and hepatic; | ||||||
| 6-month PFS , 25% | ||||||
| Imatinib | II | Recurrent | 39 | 6-month PFS , 24% | NA | 37 |
| Imatinib | II | Recurrent | 50/55 | MTD , 800 mg/day | NA | 38 |
| 2 PRs, 6 SDs (GBM) | ||||||
| 0 PRs, 5 SDs (AG); | ||||||
| 6-month PFS , 3% (GBM), 10% (AG) | ||||||
| Imatinib | II | Recurrent | 112 | PR , 5 (3 GBM); | NA | 39 |
| 6-month PFS rate , 16% (95% CI, 8.0% to 34.0%) in GBM | ||||||
| Imatinib | II | Recurrent | 231 | Radiographic response rate , 3.4%; | NA | 40 |
| 6-month PFS , 10.6% | ||||||
| Hydroxyurea ± Imatinib | III | Recurrent | 240 | Median PFS , 6 weeks (both arms); | NA | 41 |
| 6-month PFS , 7% (combination) | ||||||
| Lapatinib | I/II | Recurrent | 7 (phI) | DLT , none; efficacy (SD , 4; PD , 13) | None | 42 |
| 17 (phII) | ||||||
| Sorafenib + erlotinib | I/II | Recurrent | 17 (phI) | 6-month PFS , 16% | NR | 43 |
| 19 (phII) | ||||||
| Talampanel | II | Recurrent | 30 (22 GBM) | 6-month PFS , 4.6% | NA | 44 |
| Vorinostat | II | Recurrent | 66 | 6-month PFS , 15.2%; | NA | 45 |
| OS, 5.7 months | ||||||
| Temsirolimus | II | Recurrent | 65 | 6-month PFS , 7.8%; | p70S6 kinase | 46 |
| OS, 5.7 months |
AG indicates anaplastic glioma; BID indicates twice daily; CCNU indicates lomustine; CI indicates confidence interval; cPR indicates confirmed partial response; CR indicates complete response; GI indicates gastrointestinal; DLT indicates dose-limiting toxicities; EGFR indicates epidermal growth factor receptor; GBM indicates glioblastoma multiforme; MG indicates malignant glioma; MGMT indicates methyl guanine methyltransferase; MTD indicates maximum tolerated dose; NA indicates not available; NR indicates not recorded; OR indicates objective response; OS indicates overall survival; ph indicates phase; PD indicates progressive disease; PFS indicates progression-free survival; PR indicates partial response; PTEN indicates phosphatase and tensin homolog; RT indicates radiotherapy; SD indicates stable disease; and TMZ indicates temozolomide.
Therapeutic Inhibition of Novel Molecular Targets in Glioblastoma
VEGF Signaling
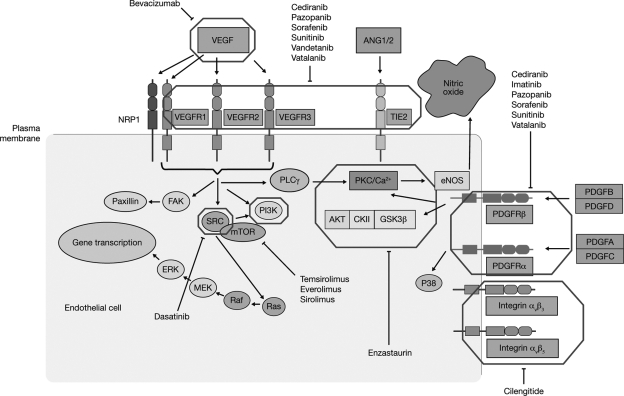
Approval of the anti-VEGF antibody bevacizumab for glioblastoma has highlighted the potential for other antiangiogenic agents in glioblastoma therapy (Fig. 2). Cediranib is a potent, orally available, small-molecule inhibitor of VEGF-receptor (VEGFR) tyrosine kinase activity that rapidly normalizes tumor blood vessels in patients with glioblastoma, leading to a clinical improvement in cerebral edema.47 In mouse models, improvement in edema was associated with increased survival, despite continued tumor growth.48 The first clinical data of the REGAL trial of cediranib plus lomustine (CCNU) to investigate whether preclinical findings will translate into improvements for patients with recurrent glioma have been negative.49 Six other clinical trials are underway to assess cediranib as either a monotherapy or in combination with other agents (Table 3).
Fig. 2.
Molecular targets of antiangiogenic therapies investigated in glioblastoma. ANG indicates angiopoietin; CKII indicates casein kinase II; eNOS indicates endothelial nitric oxide synthase; ERK indicates extracellular signal-regulated kinases; FAK indicates focal adhesion kinase; GSK3β indicates glycogen synthase kinase 3β; MEK indicates mitogen-activated protein kinase kinase; mTOR indicates mammalian target of rapamycin; PDGF(R) indicates platelet-derived growth factor (receptor); PI3K indicates phosphatidylinositol 3-kinase; PKC indicates protein kinase C; PLCγ indicates phopholipase Cγ; and VEGF(R) indicates vascular endothelial growth factor (receptor).
Table 3.
Ongoing clinical trials of targeted therapies
| Treatment | Trial phase | Primary/recurrent disease | No. of patients | Primary outcome(s) | Trial identifier |
|---|---|---|---|---|---|
| ABT-888 + TMZ + RT | I | Pimary | Safety, OS | NCT00770471 | |
| APG101 + RT | II | Recurrent | 83 | 6-month PFS | NCT01071837 |
| BIBW2992 + RT + TMZ | I | Primary | 38 | NA | NCT00977431 |
| BIBW2992 + TMZ | I/II | Recurrent | 140 | NA | NCT00727506 |
| BIBW2992 | II | Recurrent | 60 | NA | NCT00875433 |
| BSI-201 (phase I: after TMZ + RT together with TMZ; phase II: together with TMZ + RT) | I/II | Primary | 100 | Safety, OS | NCT00687765 |
| Bevacizumab + Dasatinib | I/II | Recurrent | 183 | Safety, PFS, OS | NCT00892177 |
| Bevacizumab + TMZ + RT vs TMZ + RT | III | Primary | 942 | PFS, OS | NCT00884741 |
| Bevacizumab + TMZ + RT vs TMZ + RT | III | Primary | 920 | PFS, OS | NCT00943826 |
| Cediranib + TMZ | I/II | Primary | 80 | Safety, PFS | NCT00662506 |
| Cediranib | I | Any | 55 | MTD, DLT | NCT00326664 |
| Cediranib + bevacizumab | I | Recurrent | 51 | MTD, PK, toxicity | NCT00458731 |
| Cediranib + cilengitide | I | Recurrent | 52 | Safety | NCT00979862 |
| Cetuximab + bevacizumab + irinotecan | II | Recurrent | 32 | NA | NCT00463073 |
| Cilengitide + TMZ + RT vs TMZ + RT | II | Primary | 177 | PFS | NCT01062425 |
| Cilengitide + TMZ + RT vs TMZ + RT | III | Primary | 504 | OS | NCT00689221 |
| Dasatinib + TMZ + RT vs TMZ + RT | I/II | Primary | 217 | Safety/OS | NCT00869401 |
| Dasatinib + TMZ + RT | I/II | Primary | 72 | MTD/OS | NCT00895960 |
| Dasatinib + erlotinib | I | Recurrent | 48 | MTD, DLT | NCT00609999 |
| Dasatinib + CCNU | I/II | Recurrent | 108 | Safety/PFS | NCT00948389 |
| Dasatinib | II | Recurrent | 113 | 6-month PFS | NCT00423735 |
| Erlotinib + TMZ + RT | II | Primary | 30 | 6-month PFS | NCT00274833 |
| Erlotinib + sirolimus | I/II | Recurrent | 99 | NA | NCT00509431 |
| Erlotinib + sirolimus | II | Recurrent | 20 | 6-month PFS | NCT00672243 |
| Erlotinib + sorafenib | II | Recurrent | 56 | OS | NCT00445588 |
| Everolimus + TMZ + RT | I/II | Primary | 108 | MTD/OS | NCT00553150 |
| Imatinib + TMZ | I | Any | 40 | Safety, PK, antitumor activity | NCT00354068 |
| Imatinib + TMZ | I | Recurrent | 40 | Safety, PK, antitumor activity | NCT00354068 |
| Imatinib + hydroxyurea | I | Recurrent | 48 | MTD, DLT | NCT00613054 |
| Imatinib + hydroxyurea | I | Recurrent | 72 | MTD, DLT | NCT00613132 |
| Imatinib | II | Recurrent | 77 | 6-month PFS | NCT00039364 |
| Imatinib + hydroxyurea | II | Recurrent | 21 | 6-month PFS | NCT00611234 |
| Imatinib + hydroxyurea | II | Recurrent | 64 | 12-month PFS | NCT00615927 |
| Lapatinib + pazopanib | I/II | Recurrent | 105 | NA | NCT00350727 |
| Lapatinib | II | Recurrent | 44 | NA | NCT00103129 |
| Sorafenib | I | Primary | 18 | NA | NCT00884416 |
| Sorafenib + RT + TMZ | I/II | Primary | 51 | NA | NCT00734526 |
| TMZ→TMZ + RT + sorafenib | II | Primary | 46 | NA | NCT00544817 |
| Sorafenib | I | Recurrent | 36 | NA | NCT00093613 |
| Sorafenib + temsirolimus | I/II | Recurrent | 141 | NA | NCT00329719 |
| Sorafenib | II | Recurrent | 32 | NA | NCT00597493 |
| Sorafenib + bevacizumab | II | Recurrent | 53 | NA | NCT00621686 |
| Tandutinib | I/II | Any | 85 | MTD, safety, response | NCT00379080 |
| Tandutinib + bevacizumab | II | Any | 80 | 6-month PFS | NCT00667394 |
| Vandetanib + TMZ | I/II | Primary | 114 | NA | NCT00441142 |
| Vandetanib + etoposide | I | Recurrent | 48 | NA | NCT00613223 |
| Vandetanib + imatinib + hydroxyurea | I | Recurrent | 48 | NA | NCT00613054 |
| Vandetanib + sirolimus | I | Recurrent | 33 | NA | NCT00821080 |
| Vandetanib | I/II | Recurrent | 94 | NA | NCT00293566 |
| Vandetanib + carboplatin vs vandetanib → carboplatin | II | Recurrent | 128 | NA | NCT00995007 |
| Vorinostat | II | Recurrent | 94 | PFS | NCT00238303 |
| Vorinostat + RT + TMZ | I/II | Primary | 132 | Safety/OS | NCT00731731 |
| Vorinostat + bevacizumab + irinotecan | I | Recurrent | 21 | NA | NCT00762255 |
| Vandetanib + bevacizumab vs bevacizumab | I/II | Recurrent | 108 | Safety/PFS | NCT01266031 |
| Vorinostat + TMZ | I/II | Recurrent | 52 | Safety/PFS | NCT00939991 |
| Vorinostat + TMZ | I | Recurrent | 77 | NA | NCT00268385 |
| Vorinostat + Bortezomib | II | Recurrent | 68 | PFS | NCT00641706 |
| XL184 + RT + TMZ | I | Primary | 85 | Safety | NCT00960492 |
| XL765 + TMZ | I | Maintainance | 80 | Safety | NCT00704080 |
CCNU indicates lomustine; DLT indicates dose-limiting toxicities; MTD indicates maximum tolerated dose; NA indicates not available; OS indicates overall survival; PFS indicates progression-free survival; PK indicates pharmacokinetics; RT indicates radiotherapy; and TMZ indicates temozolomide.
EGFR Family
Approximately 50% of glioblastomas overexpress EGFR and 25% express a constitutively active mutated form of EGFR.50 EGFR overexpression and immunoreactivity are more common in primary tumors than in secondary glioblastomas.51 These observations—in addition to the large body of preclinical data in glioblastoma52 and successful targeting of EGFR in other tumors—make EGFR an attractive target for glioblastoma therapy. However, caution is needed with EGFR inhibitors, because hypoxia and low glucose levels might convert the cytotoxic effects of EGFR inhibition into a cytoprotective effect.53
One agent that has been the subject of many clinical trials is erlotinib, an orally active inhibitor of the EGFR tyrosine kinase approved for treating some forms of non–small cell lung cancer and pancreatic cancer. In a phase I study, patients with gliomas expressing high levels of EGFR and low levels of activated AKT had better responses to erlotinib (ie, a 50% decrease in tumor cross-sectional area) than did those with low EGFR expression and high levels of activated AKT.54 However, phase II trials have thus far shown limited clinical benefit of erlotinib in patients with either recurrent or newly diagnosed glioblastoma (Table 2), either in combination regimens22,23,33,34 or as monotherapy.31 Studies to identify markers predicting response to EGFR inhibitors in patients with recurrent glioblastoma have shown significant correlation of response to therapy with coexpression of the PTEN tumor suppressor and the EGFR deletion mutant variant III (EGFRvIII;odds ratio, 51; 95% confidence interval [CI], 4–669; P < .001).55 However, this has been suggested to be a prognostic phenomenon.31 Ongoing clinical trials of erlotinib and other EGFR-directed drugs are summarized in Table 3.
PI3K and Related Pathways
PI3K plays a role in intracellular signaling pathways regulating cell survival, growth, and proliferation. Activated PI3K is recruited to the cell membrane where it mediates signaling after receptor activation. Downstream signaling proteins include AKT, a promoter of growth, proliferation, and survival; glycogen synthase kinase-3 (GSK-3), a regulator of c-MYC and cyclin degradation; and mammalian target of rapamycin (mTOR), a regulator of protein synthesis and negative regulator of PI3K.56
Regulators of PI3K signaling are frequently mutated in glioblastomas, and preclinical studies suggest that inhibiting the PI3K pathway may have therapeutic potential.12 NVP-BEZ235, an orally available kinase inhibitor of PDK1, mTOR, and PI3K, induced G1 arrest of a glioblastoma cell line in vitro and enhanced TMZ efficacy in vivo.57 Glioblastoma cells treated with LY294002, a specific PI3K inhibitor, became sensitized to chemotherapy-induced apoptosis.58 These preclinical studies suggest that PI3K inhibitors have the potential to overcome TMZ resistance in recurrent glioblastoma. NVP-BEZ235 treatment is currently in phase I trials involving patients with solid tumors (Table 3).
Enzastaurin, a PKC/PI3K/AKT inhibitor, suppressed proliferation and induced apoptosis via a caspase-dependent mechanism in glioblastoma cells in vitro59 and inhibited growth of human glioblastoma xenografts, which was accompanied by decreased phosphorylation of downstream signaling molecules, including GSK-3β.60 In vivo models showed that enzastaurin combined with radiotherapy synergistically reduced tumor volume, radiation-induced satellite tumor formation, upregulation of VEGF expression, neovascularization, and GSK-3β phosphorylation.61 In a phase II study of enzastaurin in patients with recurrent heavily pretreated glioblastoma, an interim analysis showed that objective radiographic responses occurred in ∼20% of patients.62 The subsequent phase III trial comparing lomustine and enzastaurin at first or second recurrence was the first phase III trial to evaluate a targeted therapy for recurrent glioblastoma. However, a planned interim analysis found that enzastaurin treatment did not significantly increase PFS, leading to enrolment being halted. The final analysis confirmed the absence of any significant difference across all efficacy end points (Table 2).29
In addition to being ineffective for glioblastoma, enzastaurin monotherapy appears to have moderate tolerability (eg, it was associated with thrombocytopenia and prolonged QTc as dose-limiting toxicities) and limited efficacy in patients with malignant glioma.63 In a phase I/II trial, enzastaurin had limited efficacy in patients with anaplastic glioma (6-month PFS, 16%) and negligible efficacy in patients with glioblastoma (6-month PFS, 7%).28
SRC and SRC-Family Kinases
SRC and SFKs are frequently activated in glioblastoma cell lines and patient samples,17 and SFK overexpression has also been reported,19 although it was not reported in the Cancer Genome Atlas study.12 SRC and SFKs are promiscuous regulators of multiple signaling pathways regulating cell growth, proliferation, adhesion, migration, and invasion, which are important processes in tumor invasion and metastasis. In particular, SFKs mediate signaling from growth factor receptors that are commonly overexpressed in glioblastomas, providing a potential mechanism for SFK activation. Recently, SRC and FYN (an SFK) were shown to mediate oncogenic EGFR and EGFRvIII signaling in a rodent glioblastoma model.19 SRC inhibition also reduced glioblastoma cell viability and migration in vitro and decreased growth in vivo.17 Transgenic mice expressing v-SRC, a viral oncogenic homolog of cellular SRC, develop brain tumors that rapidly progress to mimic the morphological and molecular characteristics of human glioblastoma, providing additional strong evidence that SFKs may be a promising target for glioblastoma therapy.64
Dasatinib is a potent inhibitor of SRC and SFK tyrosine kinase activity and has been approved for the treatment of certain types of leukemia on the basis of activity against BCR-ABL.65 Dasatinib also has inhibitory activity against c-KIT and PDGFR.66 In glioblastoma cells, dasatinib inhibited migration and induced autophagic cell death, and autophagy was increased by combining dasatinib with TMZ.19,67 In vivo, dasatinib inhibited invasion, promoted tumor regression, induced apoptosis in EGFRvIII-expressing glioblastomas, and enhanced the activity of anti-EGFR antibodies.19
Trials of dasatinib are ongoing in several solid tumors, including glioblastoma (Table 3). A phase I/II trial involving patients with newly diagnosed glioblastoma is assessing dasatinib combined with radiotherapy and concomitant TMZ, followed by adjuvant dasatinib plus TMZ. Trials of dasatinib for treatment of recurrent glioblastoma include a phase II trial of dasatinib monotherapy, a phase I trial of dasatinib in combination with erlotinib, and a randomized phase I/II trial of dasatinib in combination with CCNU that has started its phase I component with patients who have recurrent glioblastoma as part of an EORTC initiative (Table 3).
PDGFR
PDGFR is a receptor tyrosine kinase with α and β isoforms. Overexpression of PDGFR-α has been demonstrated in all grades of astrocytoma, including in 1 in 6 glioblastomas,49 indicating a potential role in tumor development.68 Several PDGFR-targeting agents have been developed that may have therapeutic potential against tumors with elevated PDGFR expression.
Sorafenib is an orally available antiangiogenic agent that inhibits tumor cell growth and proliferation by blocking the action of intracellular and receptor kinases, including PDGFR, RAF kinase, VEGFR2, and c-KIT.69 In human glioblastoma cell lines, sorafenib inhibited proliferation synergistically in combination with bortezomib, a proteosome inhibitor,70 and rottlerin, an experimental inhibitor of protein kinase C.71 A phase II trial found that first-line TMZ and radiotherapy followed by TMZ plus sorafenib was tolerated by patients with glioblastoma, although preliminary efficacy data for this regimen (median PFS duration, 6 months; 12-month PFS rate, 16%) were similar to data for standard therapy (Table 2).24 The results of clinical trials of sorafenib are summarized in Tables 2 and 3.
Preclinical trials of imatinib, a small-molecule inhibitor of PDGFR, ABL, and c-KIT, have shown growth inhibition in a subpopulation of CXCL12-expressing glioblastoma cells72 and radiosensitizing activity.73 However, in phase II trials involving recurrent glioblastoma, imatinib alone or combined with hydroxyurea had limited antitumor activity (Table 2).37–41 The combination of imatinib, hydroxyurea, and vatalanib, a VEGFR inhibitor, was well tolerated in a phase I trial and has been suggested as a possible multitargeted regimen for glioblastoma.36 Ongoing trials include a trial of imatinib and TMZ in patients with either newly diagnosed or recurrent glioblastoma and 6 trials involving treatment of recurrent glioblastoma with imatinib monotherapy or imatinib combined with TMZ or hydroxyurea (Table 3).
Tandutinib is an orally active inhibitor of PDGFR, FLT3, and c-KIT tyrosine kinase activity. Although no preclinical data have been reported for tandutinib in glioblastoma, 2 early-phase trials are assessing tandutinib in recurrent or progressive glioblastoma as monotherapy or combined with bevacizumab (Table 3).
Although gene expression and preclinical data suggest that PDGFR may be a promising target for treating glioblastoma, the available clinical data suggest otherwise. Trial data are awaited from novel combination regimens involving PDGFR inhibitors.
Integrins
Integrins play key roles regulating cellular adhesion, migration, and invasion. In addition to a structural role, integrins also activate intracellular signaling proteins, including SRC. In various tumors, integrins have an established role in metastasis and angiogenesis.74 Therefore, targeting integrin function may have potential for treating glioblastoma.
Cilengitide is a specific αV integrin inhibitor in clinical development. In vitro, cilengitide blocked glioma cell adhesion without effecting tumor radiosensitivity, despite increasing radiation-induced vascular endothelial cell death. However, cilengitide combined with radiotherapy in vivo more than doubled the median duration of survival time to >110 days, compared with radiotherapy alone (duration of survival, 50 days).75 A second study showed inconsistent effects of cilengitide on cell migration or invasiveness across several glioma cell lines, although additive effects were observed for cilengitide combined with TMZ.76
Cilengitide has been assessed in clinical trials (Table 2). In a phase I/IIa trial, cilengitide combined with the current standard of therapy in patients with newly diagnosed glioblastoma was well tolerated, with an encouraging 6-month PFS rate of 69%. Tumor O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation predicted a higher likelihood of achieving 6-month PFS, as shown by increases in the durations of PFS and OS to 13.4 months and 23.2 months, respectively, compared with 3.4 and 13.1 months for patients without MGMT promoter methylation.21 On the basis of these findings, a similar regimen is being compared with radiotherapy/TMZ alone in the phase III CENTRIC trial in patients with newly diagnosed glioblastoma whose tumors have a hypermethylated MGMT promoter (Table 3). In a phase IIa study of recurrent glioblastoma, cilengitide monotherapy was well tolerated but was largely inactive (6-month PFS rate, 15%); long-term disease stabilization was seen in a small subset of patients: 10% were progression free for >12 months, and 5% were progression free for >24 months.27
A recent preclinical study has suggested that integrin inhibitors may paradoxically stimulate tumor growth and angiogenesis if doses are missed.77 However, because of the artificial dosing schedule and nonglioma models used for preclinical investigations, this may not represent an issue for ongoing trials in glioblastoma.78
c-MET Inhibitors
Aberrant signaling by the MET receptors and its ligand, hepatocyte growth factor (HGF), has been observed in various tumors, including glioblastoma, and potential involvement in tumorigenesis and metastasis has been reported.79 In a recent study, c-MET overexpression was detected in 18 (29%) of 62 glioblastoma samples, and patients with c-MET overexpression had shorter median survival durations than did those with little or no c-MET expression (median durations of survival, 11.7 vs 14.3 months).80
Inhibitors of HGF or c-MET have shown preclinical activity against glioblastoma cell lines.79 The anti-HGF antibody AMG102 enhanced TMZ-induced inhibition of glioblastoma cell line growth in vitro and in xenografts,81 and in an ongoing phase II trial in patients with recurrent glioblastoma, AMG102 was well tolerated, with initial evidence of response seen in a small proportion of patients (Table 2).26 PF02341066, an orally available ATP-competitive small-molecule inhibitor of c-MET that inhibited glioblastoma growth and cMET phosphorylation in preclinical studies,82 is under clinical investigation in patients with advanced cancers.
Glutamate Receptor Inhibition
Alpha-amino-3-hydroxy-5 methyl-4-isoxazolepropionate (AMPA) glutamate receptor antagonists have been used to prevent neurotoxicity in several nontumor neurologic disorders. Because glioblastomas secrete glutamate, and because preclinical evidence suggests a role of the glutamate/AMPA system in proliferation and migration, talampanel, an orally available BBB-permeable AMPA inhibitor, has been assessed in clinical trials. Initial phase I/II data for first-line talampanel combined with the standard of care have suggested improved efficacy compared with recent historical controls; the median OS duration was 18.3 months (95% CI, 14.6–22.5 months).25 However, a phase II trial of talampanel monotherapy in patients with recurrent disease found no significant activity (6-month PFS rate, 4.6%; median PFS duration, 5.9 weeks; median OS duration, 13 weeks) (Table 2).44
Histone Deacetylase Inhibition
Histone deacetylases (HDACs) are involved in multiple processes shaping the malignant phenotype of glioma, including maintanance of stemness, angiogenesis, and resistance to DNA damage. Vorinostat is an orally available inhibitor of class I and II HDAC approved for advance cutaneous T cell lymphoma. In a phase II study of recurrent glioblastoma, vorinostat monotherapy was well tolerated and had modest clinical activity (6-month PFS rate, 15.2%; median OS duration, 5.7 months).45 Vorinostat is currently being evaluated for use in newly diagnosed and recurrent glioblastoma as a combination therapy.
Death-receptor targeting has been an experimental approach for malignant glioma for >1 decade.83 Death-receptor ligand activation can also have nonapoptotic effects, as demonstrated using anti-CD95 antibody treatment of mouse glioblastoma models.84 APG101 is an inhibitor of CD95 ligand consisting of the CD95 receptor extracellular domain fused to the Fc domain of IgG. A randomized phase II trial of APG101 plus radiotherapy versus radiotherapy has recently been initiated in patients with recurrent glioblastoma. Future research will determine whether inducing apoptosis or relying on the nonapoptotic properties of death ligands will be advantageous for glioblastoma treatment.
Poly [ADP-ribose] polymerase (PARP) is a DNA repair enzyme implicated in the resistance of tumors to DNA damaging anticancer agents and radiotherapy.85 Iniparib (BSI-201), which has recently demonstrated clinical efficacy in triple-negative breast cancer,86 is currently being explored in a phase I/II study in patients with newly diagnosed glioblastoma.
Discussion
Targeted therapies have revolutionized oncology, causing a shift from systemic and/or slow-release implants of cytotoxic drugs towards highly specific agents that are more selective at targeting tumor cells. Clinical studies of EGFR and PDGFR inhibitors as monotherapy, however, have thus far failed to demonstrate any efficacy for glioblastoma. New data indicate that subtypes of glioblastoma exist with distinct molecular characteristics, suggesting that to fully evaluate targeted agents, patient selection based on tumor subtype may be needed. Because of the progressive nature of glioblastoma and the accumulation of genomic and proteomic changes, it is also possible that a recurrent tumor may have characteristics different from those of the primary tumor, suggesting that additional biopsy specimens should be obtained from tumors at recurrence to ensure that an appropriate therapy is selected. Translation of promising preclinical data into a clinically useful therapy remains challenging, with data frequently generating new questions or hypotheses that need to be addressed in the laboratory.
Targeted agents are likely to have the greatest potential when used in combination to increase the activity of standard chemotherapies, broadening the range of pathways inhibited by treatment and/or counteracting mechanisms of resistance. In addition, as for classic cytotoxic agents, an intact BBB may represent an important impediment limiting the efficacy of targeted therapies. Numerous trials are ongoing to investigate combinations of targeted therapies with other agents, potentially accompanied by novel methods of patient monitoring or assessment based on the mechanism of action, allowing for more individualized patient therapy. To enhance the clinical relevance and cover the epidemiology of the disease, these trials have to include older patients. Older patients (eg, those aged >65 years) represent almost 40% of all patients with glioblastoma, and their tumors may have different biological features. Thus, targeted agents may act differentially, as shown for bevacizumab,87,88 and careful analysis of older patients in these trials—or even in separate trials for patients aged >65 years—will most likely be rewarding. Dialog between preclinical and clinical research will allow us to address the questions or hypotheses arising from the use of novel therapies, leading to the fine-tuning of clinical trial regimens and a better understanding of which patients may benefit from a particular therapy. Coupled with advances in tumor screening and outcome assessment, this will hopefully result in new treatment options and meaningful patient benefits.
Funding
StemScientific, funded by Bristol-Myers Squibb, provided writing and editorial support. Bristol-Myers Squibb did not influence the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Acknowledgments
We take full responsibility for the content of this publication and confirm that it reflects our viewpoints and medical expertise.
Conflict of interest statement. W.W. has received research grants from Apogenix and Eli Lily for projects unrelated to the current manuscript; has acted as an advisor for Roche/Genentech, Eli Lilly, Schering-Plough/MSD, BMS, Antisense Pharma, and Noxxon Pharmaceutics; and has received honoraria from the speakers' bureaus of Roche/Genentech, Schering-Plough/MSD and Wyeth/Pfizer. M. Weller has served on advisory boards for Astra Zeneca, Bayer Schering, BMS, MSD, Merck Serono, Miltenyi Biotech, Roche/Genentech, and Schering-Plough; has received speakers honoraria from Merck Serono and Schering-Plough; and has received unrestricted research funding from Merck Serono and MSD for projects unrelated to the current manuscript. T.B. has served as a paid consultant for Schering-Plough/MSD and Genentech/Roche and has received grant or research support from Millenium, Astra Zeneca, and Schering-Plough. A.W.K.Y. has served as a paid consultant for Eden; has received honoraria from the speakers' bureaus of Schering-Plough/MSD, Merck, and Genentech; has played an advisory role for Genentech, Merck, Novartis, Eli Lilly, and Antisense Pharma; and has received research support from Novartis for projects unrelated to the topic of the manuscript. M.P.: has served on the advisory board for Miltenyi Biotech; has received speakers honoraria from Merck Serono, Sigma Tau Pharmaceuticals, and Miltenyi Biotech; and has received unrestricted research funding from Merck Serono and Nuon Therapeutics for projects unrelated to the current manuscript. M. Weiler has no conflict of interest.
References
- 1.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–2299. doi: 10.1002/cncr.20621. doi:10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Wick A, Pascher C, Wick W, et al. Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol. 2009;256:734–741. doi: 10.1007/s00415-009-5006-9. doi:10.1007/s00415-009-5006-9. [DOI] [PubMed] [Google Scholar]
- 5.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. doi: 10.1200/JCO.2009.26.5520. doi:10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 6.Chi AS, Sorensen AG, Jain RK, et al. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist. 2009;14:621–636. doi: 10.1634/theoncologist.2008-0272. doi:10.1634/theoncologist.2008-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. doi:10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Pulfer S, Li S, et al. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res. 2001;61:5491–5498. [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. doi:10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. doi:10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wick A, Dorner N, Hofer S, et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol. 2011;69:586–592. doi: 10.1002/ana.22336. [DOI] [PubMed] [Google Scholar]
- 12.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. doi:10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. doi:10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kil IS, Kim SY, Lee SJ, et al. Small interfering RNA-mediated silencing of mitochondrial NADP+-dependent isocitrate dehydrogenase enhances the sensitivity of HeLa cells toward tumor necrosis factor-alpha and anticancer drugs. Free Radic Biol Med. 2007;43:1197–1207. doi: 10.1016/j.freeradbiomed.2007.07.009. doi:10.1016/j.freeradbiomed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. doi:10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 16.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. doi:10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Bernasconi P, Clauser KR, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. doi:10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. doi:10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Lu KV, Zhu S, Cvrljevic A, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. doi:10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combs SE, Hartmann C, Welzel J, et al. Influence of expression of EGFR and PTEN on outcome in patients with primary glioblastoma treated with standard radiochemotherapy and cetuximab: Interim analysis from the GERT-Protocol. J Clin Oncol. 2009;27(Suppl. 15s):99s. (abstract 2050) [Google Scholar]
- 21.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. doi:10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 22.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. doi:10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. doi:10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. doi:10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 25.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27:4155–4161. doi: 10.1200/JCO.2008.21.6895. doi:10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reardon D, Cloughesy T, Raizer J, et al. Phase II study of AMG 102, a fully human neutralizing antibody against hepatocyte growth factor/scatter factor, in patients with recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(Suppl. 15s):101s. (abstract 2051) [Google Scholar]
- 27.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. doi:10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 28.Kreisl TN, Kotliarova S, Butman JA, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12:181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. doi:10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SM, Kuhn J, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas (MG) (NABTC 04-02) J Clin Oncol. 2009;27(Suppl. 15s):88s. doi: 10.1093/neuonc/not247. (abstract 2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. doi:10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II study of bevacizumab plus erlotinib for recurrent malignant gliomas. J Clin Oncol. 2009;27(Suppl. 15s):98s. (abstract 2045) [Google Scholar]
- 33.de Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89–97. doi: 10.1007/s11060-008-9637-y. doi:10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96:219–230. doi: 10.1007/s11060-009-9950-0. doi:10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. doi:10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reardon DA, Egorin MJ, Desjardins A, et al. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115:2188–2198. doi: 10.1002/cncr.24213. doi:10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desjardins A, Quinn JA, Vredenburgh JJ, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83:53–60. doi: 10.1007/s11060-006-9302-2. doi:10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 38.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. doi:10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 39.Raymond E, Brandes AA, Dittrich C, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–4665. doi: 10.1200/JCO.2008.16.9235. doi:10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reardon DA, Dresemann G, Taillibert S, et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101:1995–2004. doi: 10.1038/sj.bjc.6605411. doi:10.1038/sj.bjc.6605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dresemann G, Weller M, Rosenthal MA, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96:393–402. doi: 10.1007/s11060-009-9976-3. doi:10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]
- 42.Thiessen B, Stewart C, Tsao M, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 43.Prados MD, Gilbert M, Kuhn J, et al. Phase I/II study of sorafenib and erlotinib for patients with recurrent glioblastoma (GBM) (NABTC 05-02) J Clin Oncol. 2009;27(Suppl. 15s):88s. (abstract 2005) [Google Scholar]
- 44.Iwamoto FM, Kreisl TN, Kim L, et al. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer. 2010;116:1776–1782. doi: 10.1002/cncr.24957. doi:10.1002/cncr.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group study. J Clin Oncol. 2010;27:2052–2058. doi: 10.1200/JCO.2008.19.0694. doi:10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. doi:10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 47.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. doi:10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–2552. doi: 10.1200/JCO.2008.19.9356. doi:10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batchelor T, Mulholland P, Neyns B, et al. A phase III randomized study comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, with lomustine alone in recurrent glioblastoma patients. Ann Oncol. 2010;21(Suppl 8):viii4. doi: 10.1200/JCO.2012.47.2464. (LBA7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 51.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raizer JJ. HER1/EGFR tyrosine kinase inhibitors for the treatment of glioblastoma multiforme. J Neurooncol. 2005;74:77–86. doi: 10.1007/s11060-005-0603-7. doi:10.1007/s11060-005-0603-7. [DOI] [PubMed] [Google Scholar]
- 53.Steinbach JP, Klumpp A, Wolburg H, et al. Inhibition of epidermal growth factor receptor signaling protects human malignant glioma cells from hypoxia-induced cell death. Cancer Res. 2004;64:1575–1578. doi: 10.1158/0008-5472.can-03-3775. doi:10.1158/0008-5472.CAN-03-3775. [DOI] [PubMed] [Google Scholar]
- 54.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. doi:10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 55.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. doi:10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 56.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma–animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. doi:10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. doi:10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 58.Opel D, Westhoff MA, Bender A, et al. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. doi:10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 59.Rieger J, Lemke D, Maurer G, et al. Enzastaurin-induced apoptosis in glioma cells is caspase-dependent and inhibited by BCL-XL. J Neurochem. 2008;106:2436–2448. doi: 10.1111/j.1471-4159.2008.05586.x. doi:10.1111/j.1471-4159.2008.05586.x. [DOI] [PubMed] [Google Scholar]
- 60.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cβ-selective inhibitor, enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. doi:10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 61.Tabatabai G, Frank B, Wick A, et al. Synergistic antiglioma activity of radiotherapy and enzastaurin. Ann Neurol. 2007;61:153–161. doi: 10.1002/ana.21057. doi:10.1002/ana.21057. [DOI] [PubMed] [Google Scholar]
- 62.Fine HA, Kim L, Royce C, et al. Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas. J Clin Oncol. 2005;23(Suppl 16s):115s. (abstract 1504) [Google Scholar]
- 63.Kreisl TN, Kim L, Moore K, et al. A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res. 2009;15:3617–3623. doi: 10.1158/1078-0432.CCR-08-3071. doi:10.1158/1078-0432.CCR-08-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weissenberger J, Steinbach JP, Malin G, et al. Development and malignant progression of astrocytomas in GFAP-v-src transgenic mice. Oncogene. 1997;14:2005–2013. doi: 10.1038/sj.onc.1201168. doi:10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- 65.Brave M, Goodman V, Kaminskas E, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–359. doi: 10.1158/1078-0432.CCR-07-4175. doi:10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 66.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. doi:10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 67.Milano V, Piao Y, LaFortune T, et al. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. doi:10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 68.Ozawa T, Brennan CW, Wang L, et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24:2205–2218. doi: 10.1101/gad.1972310. doi:10.1101/gad.1972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. doi:10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 70.Yu C, Friday BB, Lai JP, et al. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5:2378–2387. doi: 10.1158/1535-7163.MCT-06-0235. doi:10.1158/1535-7163.MCT-06-0235. [DOI] [PubMed] [Google Scholar]
- 71.Jane EP, Premkumar DR, Pollack IF. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther. 2006;319:1070–1080. doi: 10.1124/jpet.106.108621. doi:10.1124/jpet.106.108621. [DOI] [PubMed] [Google Scholar]
- 72.Hagerstrand D, Hesselager G, Achterberg S, et al. Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene. 2006;25:4913–4922. doi: 10.1038/sj.onc.1209497. doi:10.1038/sj.onc.1209497. [DOI] [PubMed] [Google Scholar]
- 73.Holdhoff M, Kreuzer KA, Appelt C, et al. Imatinib mesylate radiosensitizes human glioblastoma cells through inhibition of platelet-derived growth factor receptor. Blood Cells Mol Dis. 2005;34:181–185. doi: 10.1016/j.bcmd.2004.11.006. doi:10.1016/j.bcmd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. doi:10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mikkelsen T, Brodie C, Finniss S, et al. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int J Cancer. 2009;124:2719–2727. doi: 10.1002/ijc.24240. doi:10.1002/ijc.24240. [DOI] [PubMed] [Google Scholar]
- 76.Maurer GD, Tritschler I, Adams B, et al. Cilengitide modulates attachment and viability of human glioma cells, but not sensitivity to irradiation or temozolomide in vitro. Neuro Oncol. 2009;11:747–756. doi: 10.1215/15228517-2009-012. doi:10.1215/15228517-2009-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds AR, Hart IR, Watson AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. doi:10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 78.Weller M, Reardon D, Nabors B, et al. Will integrin inhibitors have proangiogenic effects in the clinic? Nat Med. 2009;15:726. doi: 10.1038/nm0709-726. doi:10.1038/nm0709-726. [DOI] [PubMed] [Google Scholar]
- 79.Toschi L, Janne PA. Single-agent and combination therapeutic strategies to inhibit hepatocyte growth factor/MET signaling in cancer. Clin Cancer Res. 2008;14:5941–5946. doi: 10.1158/1078-0432.CCR-08-0071. doi:10.1158/1078-0432.CCR-08-0071. [DOI] [PubMed] [Google Scholar]
- 80.Kong DS, Song SY, Kim DH, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–148. doi: 10.1002/cncr.23972. doi:10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 81.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. doi:10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 82.Yamazaki S, Skaptason J, Romero D, et al. Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug Metab Dispos. 2008;36:1267–1274. doi: 10.1124/dmd.107.019711. doi:10.1124/dmd.107.019711. [DOI] [PubMed] [Google Scholar]
- 83.Fulda S, Wick W, Weller M, et al. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 84.Kleber S, Sancho-Martinez I, Wiestler B, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. doi:10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Powell C, Mikropoulos C, Kaye SB, et al. Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitizers. Cancer Treat Rev. 2010;36:566–575. doi: 10.1016/j.ctrv.2010.03.003. doi:10.1016/j.ctrv.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 86.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. New Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 87.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. doi:10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]