Abstract
To assess the accumulation of myeloid-derived suppressor cells (MDSCs) in the peripheral blood of patients with glioma and to define their heterogeneity and their immunosuppressive function.
Peripheral blood mononuclear cells (PBMCs) from healthy control subjects and from patients with newly diagnosed glioma were stimulated with anti-CD3/anti-CD28 and T cells assessed for intracellular expression of interferon (IFN)–γ. Antibody staining of PBMCs from glioma patients and healthy donors (CD33, HLADR, CD15, and CD14) followed by 4-color flow cytometry analysis–defined MDSC levels in the peripheral blood. To assess the role of MDSCs in suppressing T cell IFNγ production, PBMCs were depleted of MDSCs using anti-CD33 and anti-CD15 antibody-coated beads prior to T cell stimulation. Enzyme-linked immunosorbent assays were used to assess plasma arginase activity and the level of granulocyte colony-stimulating factor (G-CSF).
Patients with glioblastoma have increased MDSC counts (CD33+HLADR−) in their blood that are composed of neutrophilic (CD15+; >60%), lineage-negative (CD15−CD14−; 31%), and monocytic (CD14+; 6%) subsets. After stimulation, T cells from patients with glioblastoma had suppressed IFN-γ production when compared with healthy, age-matched donor T cells. Removal of MDSCs from the PBMCs with anti-CD33/CD15–coated beads significantly restored T cell function. Significant increases in arginase activity and G-CSF levels were observed in plasma specimens obtained from patients with glioblastoma.
The accumulation of MDSCs in peripheral blood in patients with glioma likely promotes T cell immune suppression that is observed in this patient population. Increased plasma levels of arginase and G-CSF may relate to MDSC suppressor function and MDSC expansion, respectively, in patients with glioma.
Keywords: Arginase, GBM, G-CSF, MDSC, neutrophil
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of myeloid cells that are known to accumulate in the tumor-bearing host, most likely in association with overproduction of select cytokines and growth factors, such as granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-2, and vascular endothelial growth factor (VEGF).1 MDSCs also play a role in other pathological conditions, such as inflammation, acute and chronic infection, trauma, and some autoimmune diseases.2–4 MDSCs express the common myeloid marker CD33 but lack the expression of mature myeloid marker HLADR.5 The major subpopulations of MDSCs in humans include neutrophilic (CD15+CD33+HLADR−), monocytic (CD14+CD33+HLADR−), and linage-negative (CD15−CD14−CD33+HLADR−) cells.6 Recent data from a number of groups demonstrate that these cells are responsible for tumor immune suppression in both mice and humans.7,8 MDSCs can suppress T cell function by several mechanisms, including the production of arginase that decreases the level of L-arginine, which is critical for normal T cell function.9 Reduced levels of arginine are known to reduce T cell receptor chain expression and to promote T cell dysfunction.9 These cells also secrete nitric oxide and reactive oxygen species, which are capable of inducing T cell suppression.10 Enhancement of anti-tumor immunity may be possible after eliminating MDSCs or enforcing their differentiation.4 Indeed, removing arginase-producing myeloid suppressor cells or blocking arginase activity improved T cell function in tumor-bearing mice models and in patients with renal cell carcinoma (RCC).8
Depressed T cell function with ineffective antitumor immunity is a hallmark of cancer, including glioblastoma (GBM), the highest-grade primary central nervous system cancer.11 The development of effect immunotherapy in patients with GBM is likely to depend on first identifying those immunosuppressive mechanisms that are involved in GBM-mediated immune suppression. Here we show that MDSCs are present in the blood of patients with GBM along with high arginase activity and that depletion of MDSCs in co-cultures resulted in improved T cell function. By demonstrating that MDSCs are present in patients with GBM, and that their removal can enhance the anti-tumor immune response, we provide the foundation for further clinical evaluation of this approach.
Materials and Methods
Patient Populations
Blood samples from patients with newly diagnosed GBM (n = 28) were included in the study. The mean age (± standard devation) of the patients was 46 ± 15 years, and 70% were male. All patients and healthy donors signed an institutional review board–approved, written informed consent form for collection of blood samples. Patients’ samples were collected prior to treatment. Healthy, age-matched donors were volunteers aged >50 years without any existing illness.
Reagents
RPMI cell line media, Hank's balanced salt solution without calcium, and magnesium and fetal bovine serum (FBS) were purchased from Invitrogen. Human immunoglobulin (Ig) G used for blocking of nonspecific antibody binding and dimethylsulfoxide (DMSO) were obtained from Sigma Aldrich. Ficoll-Hypaque was purchased from Amersham Pharmacia Biotech. T cell stimulation beads coated with anti-CD3 and anti-CD28 were purchased from Dynal Biotech/Invitrogen. Recombinant IL-2 was obtained from Chiron. Golgi Plug, Fix Perm, and Perm Wash were part of an intracellular cytokine staining kit from BD Biosciences. Unconjugated anti-human interferon (IFN)–γ, anti-human IFN-γ FITC, anti-human CD3, and anti-human CD4 were all from BD Biosciences. CD11b-PE was from e-Bioscience. Anti-human CD14-PerCP, anti-human CD15-PE, anti-human CD33-APC, and anti-human HLA-DR-FITC were from BD Pharmingen. The G-CSF enzyme-linked immunosorbent assay (ELISA) kit was from R&D Systems, and the Arginase kit was from Bio Assay Systems. 3H-Thymidine was obtained from Perkin Elmer.
Peripheral Blood Mononuclear Cell Isolation
Peripheral blood was drawn from patients with GBM and healthy donors in heparin-containing collection tubes and processed within 2 hours. Blood was diluted with an equal volume of plain RPMI and then layered over Ficoll. The solution was centrifuged at 1100 × g for 20 min by density centrifugation. Peripheral blood mononuclear cells (PBMCs) were isolated from the interface and washed in RPMI. Platelets were removed by an additional density centrifugation step over cold FBS. Cells were then frozen in freezing media containing 10% DMSO in FBS (10 × 106 PBMC/mL) for 2 days at −80°C and then in liquid nitrogen during the remaining time. For phenotypic and functional studies, all samples from a single patient were thawed together and used in the same experiment.
Fluorescence-activated Cell Sorting Analysis of Patient Peripheral Blood Mononuclear Cells
For the analysis of MDSCs in patient PBMCs, samples were thawed, washed, and stained in Hank's balanced salt solution without calcium or magnesium at room temperature. Nonspecific antibody binding was blocked by pretreatment of cells with human IgG (10 µg/mL) for 20 min at room temperature. Surface stains were added to cells for 30 min at 4°C. Cells were stained with antibodies to human CD14, CD15, CD33, and HLA-DR; were washed and fixed in 1% paraformaldehyde ; and were subjected to fluorescence-activated cell sorting (FACS) analysis. Data were acquired using Cellquest on a BD FACS Calibur and were analyzed using Cellquest software. At least 300, 000 live cell events were collected for each flow tube used in analysis. Results are expressed as the percentage of positive cells of the total, live-gated PBMCs.
Determination of Patient T Cell Function
Patient PBMC samples were thawed, washed, and left in complete RPMI 1640 media overnight in 6-well plates in the incubator at 37°C with 5% CO2. The following day, nonadherent cells were harvested, counted, and incubated in complete medium at a concentration of 1 × 106–1.5 × 106 cells/mL in 24-well tissue culture plates for 72 h after activation with anti-CD3– and anti-CD28–coated Dynalbeads (25 μL/mL) and 200 U/mL of IL-2. At the end of a 72-h period, Golgi plug (1 μ/mL; BD Bioscience) was added to cells for 6 h to block the secretion of cytokines and then cells were harvested and stained for FACS analysis. Surface staining of CD3 and CD4 was followed by intracellular staining for the cytokine IFN-γ. Nonstimulated PBMCs from each donor served as a negative control. In addition, specificity of IFN-γ staining was confirmed in each sample via subtraction of any nonspecific staining occurring in samples that had been pretreated with unlabeled anti-IFN-γ antibody before the addition of fluorochrome-labeled antibody.
To measure the proliferative capacity of T cells, PBMCs were stimulated with cross-linked anti-CD3/anti-CD28 (5 μL/mL; 1M TRIS; pH 8.0) in the presence of IL-2 (200 U/mL) for 72 h before pulsing the cultures with 3H-thymidine (1 µCi/well). PBMCs not stimulated but cultured for 3 days served as a negative control. The level of proliferation was expressed as counts per minute (cpm) of 3H –thymidine.
Myeloid-derived Suppressor Cells Depletion
Patient PBMCs were either incubated with anti-CD15 and anti-CD33 antibody-coated magnetic micro beads (Miltenyi; Biotech) or not. Cells (both tubes) were incubated at 4°C for 20 min, washed, and resuspended in PBE (ie, phosphate-buffered saline with BSA and EDTA). Cells incubated with anti-CD15/anti-CD33–coated beads were run on an LS magnetic column (Miltenyi; Biotech) for depletion of bead-labeled cells, in accordance with the manufacturer's instructions. The PBMCs, minus the CD33+CD15+ cells, were washed, and FACS analysis was performed on a small aliquot of cells to assure that the negatively selected population was depleted of MDSCs. Control PBMCs and the MDSC-depleted PBMCs were then resuspended in complete RPMI media, activated for 3 days (anti-CD3/anti-CD28 antibodies), and stained for intracellular IFN-γ, as described above.
Serum Cytokine Assay
Serum cytokine G-CSF was measured using a kit, in accordance with the protocol supplied by R&D System.
Serum Arginase Assay
Serum arginase was measured using a quantichrome arginase assay kit from Bioassay System. In brief, after thawing the serum sample, the sample was mixed with substrate buffer and manganese solution and incubated for 2 h at 37°C, followed by the addition of urea reagent; it was further incubated at room temperature for another 20 min. Optical density was determined at 530 nm in a Victor II spectrophotometer from Perkin Elmer. Arginase activity was calculated using the formula [OD Sample- OD Blank/OD Standard-OD water × 10.4.] Urea solution (1 M) was used as standard. Arginase activity was expressed as units per liter.
Statistical Analysis
Statistical comparisons between treatment groups were performed using 2-tailed t tests for 2 samples, assuming equal variances.
Results and Discussion
Patients with Glioblastoma have Elevated Levels of Myeloid-derived Suppressor Cells
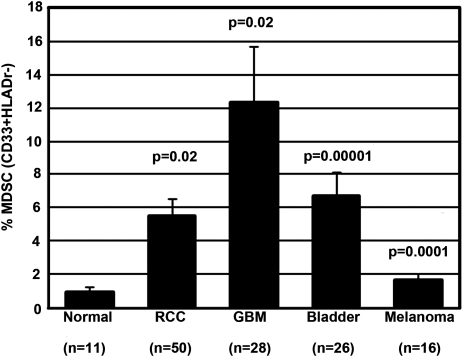
PBMCs from 28 patients with newly diagnosed GBM were analyzed for MDSCs and were compared with 11 healthy, age-matched donors. A highly significant increased number of circulating MDSCs was seen in patients with GBM (P = .02), compared with the levels detected in age-matched donors. Of note, patients with GBM, on average, had the highest number of MDSCs in their blood, compared with patients with other cancers, such as RCC, melanoma, and bladder carcinoma (Fig. 1). P values represent the increase in MDSC (percentage of total PBMCs) over the MDSC levels found in healthy donors.
Fig. 1.
Patients with glioblastoma (GBM) have elevated levels of myeloid-derived suppressor cells (MDSCs) compared with age-matched healthy donors and other cancer patients. Peripheral blood mononuclear cells (PBMCs) from patients with newly diagnosed GBM, healthy age-matched donors, and other patients with cancer were isolated and stored in liquid nitrogen. Cells were thawed, washed, and blocked with human immunoglobulin G and then immediately stained at 4°C with monoclonal antibodies to CD14, CD15, CD33, and HLA-DR. Cells were fixed with 1% paraformaldehyde, then acquired and analyzed by multicolor flow cytometry.
In patients with cancer, MDSCs have been defined as CD33+CD11b+ HLADR− cells, although this population is heterogeneous, on the basis of morphology and surface staining.12,13 In some cancers, such as RCC, the predominant MDSC subset is granulocytic, expressing CD157,14,15 along with CD66b, another marker co-expressed by neutrophils and neutrophilic-MDSCs16 (Ko et al; unpublished data). Similarly, MDSCs in advanced non–small cell lung cancer were also defined as CD15+CD33+CD14−.17 However, MDSCs were recently identified in the monocytic fraction of patients with melanoma who had the phenotype CD14+CD11b+HLADR−/low MDSC.18–20 MDSCs with a similar phenotype were also detected in PBMCs recovered from patients with prostate cancer and persons with head and neck cancer.21,22 In addition to the identification of human monocytic and neutrophilic MDSCs, prior studies identified an immature myeloid cell population composed of early-stage myeloid cells, as well as immature monocytes and dendritic cells with immunosuppressive properties.5 Thus, in patients with cancer, several populations of MDSCs have been defined, and it is likely that multiple populations are present in the PBMCs of patients with a single tumor type.
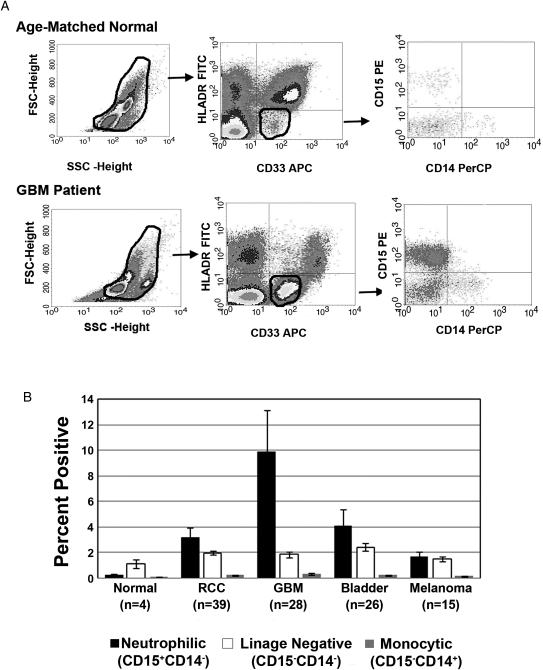
Given the presence of increased numbers of MDSCs (CD33+HLADR−) in patients with GBM, we examined the phenotypic heterogeneity in this population. On the basis of forward and side scatter flow analysis, a significant population of cells distinct from the lymphoid and monocyte gates were observed that were not detected in the analysis of PBMCs from healthy donors (Fig. 2A, top panel). Immunostaining (CD33, HLADR, CD14, and CD15) followed by flow analysis revealed that this population was composed of CD33+HLADR− cells. Gating on this CD33+HLADR− population, analysis of CD15 and CD14 staining revealed 3 populations, the largest being CD33+CD15+CD14−HLADR− (neutrophilic) subset, followed by the CD33+CD15−CD14−HLADR− (linage negative) population and, finally, the CD33+CD14+CD15−HLADR− (monocytic) population. The analysis of MDSCs obtained from 28 patients revealed that, in the CD33+HLADR− population, 82% of the MDSCs were neutrophilic, 15% were linage-negative MDSCs, and 3% were monocytic MDSCs (Fig. 2B). When comparing the percentage of all 3 MDSC subsets observed in the different tumor types we have examined, it appears that neutrophilic MDSCs represent the largest population in not only patients with GBM, but also in patients with RCC and bladder cancer; however, the highest percentage of this population occurred in the GBM population. With all 3 of these tumors, the linage-negative MDSC subset was the second-most dominant. Interestingly, for patients with melanoma, we observed that the neutrophilic MDSC and lineage-negative MDSC subsets were present in approximately equal percentages of subjects (Fig. 2B).
Fig. 2.
Neutrophilic subsets are prevalent in patients with glioblastoma (GBM). (A) The difference in FACS forward and side scatter profile shows that peripheral blood mononuclear cells (PBMCs) from the patients with GBM have a significant population of cells that is not detectable in PBMCs from healthy donors. Dot plots represent live gated events. The forward and side scatter gate was analyzed for CD3+HLADR− cells. Then the CD33+HLADR− gate was analyzed for cells expressing CD15 and CD14. (B) Columns represent mean percentages of myeloid-derived suppressor cells (MDSCs) in total PBMCs from healthy, age-matched donors, patients with GBM, and patients with other cancers. The majority of the MDSCs in patients with GBM are neutrophilic CD15+ CD14− (82%), followed by lineage-negative (lyn−15%) and monocytic (3%) MDSCs.
Our findings partially differ from those of a recent report, which showed that MDSCs observed in PBMC of patients with glioma were predominately linage negative (CD33+HLADR−).23 Although the percentage of lineage-negative MDSC cells in our study was not trivial, it was not as large as the neutrophilic MDSC population. The difference between our data and those of Rodrigues et al.23 regarding the neutrophilic MDSC population may be related to the fact that they gated on linage-negative cells and did not include granulocytes.
Because patients with GBM had significant levels of MDSCs and patients with GBM often are treated with glucocorticoids, we evaluated whether the increase in MDSCs is steroid dependent. Preoperative steroid doses in patients were compared with MDSC levels and with neutrophils, because the largest population of MDSCs phenotypically resembled neutrophils. We did not find a strong correlation between steroid dosing and neutrophil count in either the high- or low-MDSC groups. Moreover, there was no significant relationship between steroid dosing and high-versus-low MDSC levels (P = ns, Table 1). There may be a relationship between neutrophil count and high-versus-low MDSC levels (P < .05, on a single-arm t test; P > .05, on a 2-arm t test). Therefore, a tumor-induced increase in neutrophil counts may be associated with high MDSC levels, but this appears to be steroid independent.
Table 1.
Patients with glioblastoma, divided into 2 groups on the basis of relative expression of myeloid-derived suppressor cells (MDSCs) in the peripheral blood
| Total daily steroid dose at the time of surgery, mg | Neutrophil count, cells/μL | |
|---|---|---|
| High MDSCs | 16 | 13.81 |
| 16 | 10.91 | |
| 0 | 6.53 | |
| 16 | 13.15 | |
| 12 | 8.94 | |
| 0 | 21.67 | |
| 8 | 7.7 | |
| Average | 9.71 | 11.8 |
| Std. Dev | 7.25 | 5.1 |
| Median | 12 | 10.9 |
| Low MDSCs | 16 | 4.97 |
| 0 | 7.44 | |
| 8 | 8.56 | |
| 12 | 11.9 | |
| 16 | 9.02 | |
| 0 | 7.02 | |
| 0 | 8.29 | |
| 0 | 7.57 | |
| 0 | 4.17 | |
| 4 | 11.54 | |
| 16 | 12.24 | |
| 0 | 6.52 | |
| Average | 6 | 8.27 |
| Std. Dev | 7.13 | 2.58 |
| Median | 2 | 7.93 |
The low group was defined as those with MDSC percentages that were less than the mean (12.3) minus 2 times the standard error of the mean (6.6), and the high-MDSC group had values greater than that. Therefore, MDSC percentages >5.7% were considered high, and those ≤5.7% were considered low. The high- and low-MDSC groups were compared with regard to dosage of steroids at the time of surgery and to the neutrophil count in their blood.
P = .02 for neutrophil count and high-versus-low MDSC percentages.
P = not significant for steroid dosing and high-versus-low MDSC percentages.
In vitro Depletion of Patient Myeloid-derived Suppressors Cells Partially Restores Patient T Cell Production of IFN-γ and Proliferation
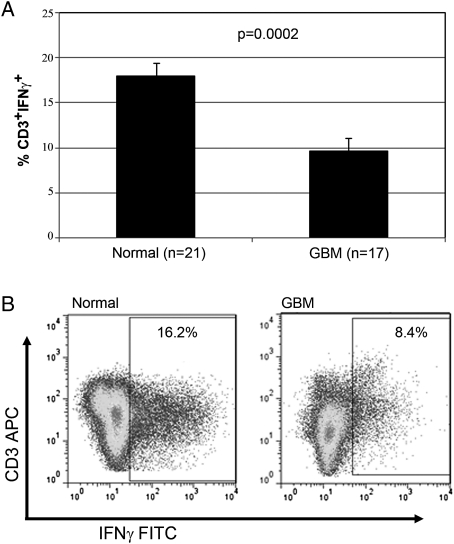
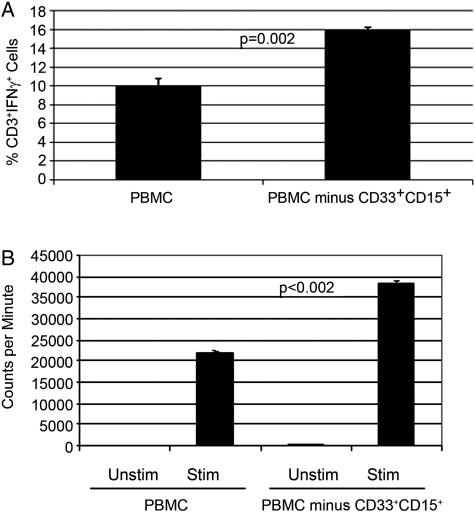
Because patients with GBM have increased numbers of MDSCs, compared with healthy donors, we examined the contribution that MDSCs play to T cell suppression that has been reported in this patient population.11 First, we evaluated CD3+ T cell production of IFN-γ from patients with GBM and compared that with the values for healthy, age-matched donors. Stimulation of PBMCs from healthy donors with anti-CD3/CD28 antibodies followed by intracellular staining of CD3+ T cells for IFN-γ production typically demonstrated that ∼17% of T cells produce IFN-γ. However, patient T cells after stimulation display significantly reduced amounts of IFN-γ expression (8%; P = .0002) (Fig. 3). These findings support the hypothesis that T cells from patients with GBM are suppressed in immune function. Next, we examined the role of MDSCs in producing the observed T cell suppression. We incubated PBMCs from patients with GBM with anti-CD33– and anti-CD15–coated magnetic beads or with control beads (no antibody) followed by exposure to a magnetic source. After immunodepletion, the remaining PBMCs were plated in complete media and then stimulated with anti-CD3/CD28–coated beads and exogenous IL-2 for 72 h. It was confirmed by FACS analysis that MDSCs were efficiently removed by this method. At the end of 72 h, CD3+ cells were stained for IFN-γ and analyzed by FACS. Figure 4A shows that IFN-γ production is increased after the MDSC depletion (P = .002) (Fig. 3A). Furthermore, the proliferation capacity of these cells also increased after the depletion of MDSCs (P ≤ .002) (Fig. 3B). Removal of CD33+CD15+ MDSCs increased the proliferation of PBMCs from 22, 000 cpm to 38, 000 cpm.
Fig. 3.
Patients with glioblastoma (GBM) are deficient in IFN-γ production. Peripheral blood mononuclear cells from patients with GBM or healthy donors were stored in liquid N2, thawed, and rested overnight in complete media, and equal numbers of nonadherent cells were stimulated the following day with anti-CD3/CD28 antibody–coated beads and interleukin-2 for 72 h. CD3+ gated cells were analyzed for intracellular cytokine (ICC) staining by FACS. Columns in top panel represent mean percentage of interferon (IFN)–γ-positive cells. Live gated events are shown in the bottom panel.
Fig. 4.
In vitro depletion of patient myeloid-derived suppressor cells (MDSCs) partially restores patient T cell production and proliferation of interferon (IFN)-γ. (A) PBMCs from patients with glioblastoma (GBM) (n = 3) were thawed and immediately stained for the expression of myeloid surface markers with or without the depletion of MDSC by anti-CD15 magnetic microbeads and magnetic column. Cells were then plated and stimulated with anti-CD3/CD28 antibodies, and after 72 h, IFN-γ was detected in CD3+ cells by FACS. Columns represent mean percentage of CD3+ cells staining positive for IFN-γ. (B) Patients’ PBMCs were left alone or CD33/CD15 magnetic beads were used to remove MDSCs. The cells were stimulated or not with anti-CD3/CD28 for 72 h. Then, 3H-thymidine was added 18 h before the harvest of the cells. Proliferation was measured by 3H incorporation in DNA.
To our knowledge, this is the first demonstration that MDSCs present in the peripheral blood of patients with GBM contribute to the immune suppression observed in patients' PBMCs. Because the neutrophilic MDSC subset was the largest population in the peripheral blood of patients with GBM in our study, it seems likely that these cells make a significant contribution to the observed immune suppression. Indeed, we and others have shown that depletion of CD15+ MDSC with anti-CD15–coated beads almost completely restored T cell IFN-γ production to PBMCs from patients with RCC.7,15 However, in our study, the precise suppressive activity of the neutrophilic MDSCs was not assessed, because we depleted MDSCs with anti-CD33 and anti-CD15 beads. Furthermore, on the basis of FACS analysis findings for blood from patients with GBM, we detected a linage-negative CD33+HLADR− subset along with a smaller monocytic subset (CD14+CD15−CD33+HLADR−). Thus, additional studies are needed to define the relative contribution that each of these MDSC subsets makes to T cell suppression in patients with GBM. The importance of MDSCs in suppressing T cell responses in various murine tumor models has been well documented, and reductions in the number of these cells are known to improve antitumor immunity.2,12,24 Likewise, in tumor-bearing patients, several strategies have been shown to reduce the number of MDSCs or to promote their differentiation into dendritic cells.4 We have shown that the tyrosine kinase inhibitor sunitinib has been shown to reduce the number of MDSCs in patients with RCC, and this loss of MDSCs coincided with a restoration of T cell function in the peripheral blood.7,25
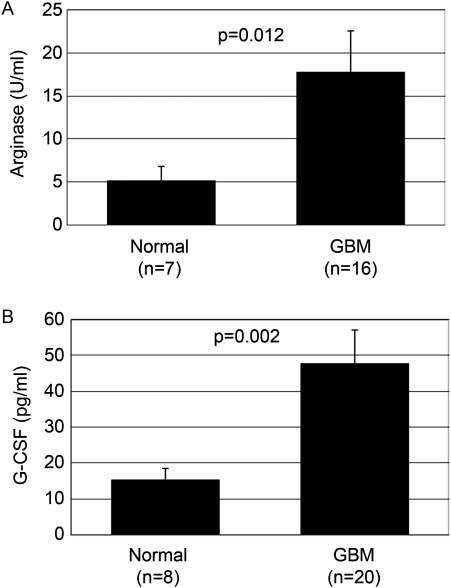
Patients with Glioblastoma have Elevated Plasma Arginase Activity
Although the importance of arginase in cancer has long been understood, the exact mechanism by which arginase influences tumor growth and immune escape was very recently elucidated.26 MDSCs are known to produce arginase, which depletes serum L-arginine. Arginase converts arginine to ornithine, which is needed for the synthesis of polyamines, which are associated with cellular proliferation.27 Most relevant, depletion of L-arginine from the microenvironment significantly inhibits T cell function.14 Elevated arginase activity in serum and the tumor microenvironment has been reported in several cancers, including breast, renal, prostate, and colorectal carcinomas and melanoma.15,16,28–30 High serum arginase activity has been proposed as a marker of disease status for some cancers.28 Here we show, for the first time in patients with GBM, that serum arginase activity is significantly elevated in these patients, compared with healthy donors (P = .012) (Fig. 5A). We propose that this increase in the serum arginase level in patients with GBM is due to the elevated numbers of MDSCs, as noted above and as seen in other cancers.28–30 However, additional studies are underway to test whether the increased levels of arginase in the serum account for the T cell dysfunction. This possibility seems likely, because other researchers have shown that increased levels of arginase in serum samples obtained from patients with RCC coincided with T cell suppression and that the addition of L-arginine to the PBMC cultures restored T cell function.15 It also appears that, in patients with RCC, the neutrophilic MDSC population contributes significantly to the increased arginase serum level, which is expected, because this was the major MDSC subset in this patient population. Because neutrophilic MDSCs are also highly expressed in the peripheral blood of patients with GBM, it is expected that this population of MDSCs will be a major producer of arginase in patients with GBM.
Fig. 5.
Patients with glioblastoma (GBM) have elevated levels of plasma G-CSF and arginase activity. (A) Serum arginase levels for healthy, age-matched donors (n = 7) and patients with GBM (n = 16) were measured using an arginase kit (Bio Assay Systems). Serum samples from patients had a significantly higher level of arginase than did control plasma samples (P = .012). (B) Serum samples from patients with GBM (n = 20) and healthy donors (n = 8) were thawed, and the G-CSF level was measured using an enzyme-linked immunosorbent assay (R&D Systems) in accordance with the instructions supplied by the vendor. Data represent mean ± standard error of the mean of 3 different experiments (P = .002).
Patients with Glioblastoma have Elevated Levels of Plasma Granulocyte Colony-stimulating Factor
Multiple cytokines and growth factors secreted in the tumor microenvironment promote MDSC expansion and activation, including GM-CSF, VEGF, IL-6, and G-CSF. G-CSF is important in mobilization of hematopoietic stem cells, progenitors, and mature cells (eg, neutrophils) into the blood circulation.31,32 G-CSF also appears to be important in regulating the accumulation/expansion of MDSCs, because in certain murine tumor models, treatment with anti-G-CSF antibody reduced MDSC counts, whereas administration of G-CSF increased these counts.33 G-CSF is secreted by several types of cancers, including GBM,34 and it is known to promote tumor growth and neo-vascularization. G-CSF can also skew T cell cytokine secretion to a Th2 phenotype T cell response.35 Given the roles that G-CSF plays in promoting MDSC accumulation, we sought to determine whether patients with GBM have increased serum G-CSF levels. Serum G-CSF levels in patients with GBM ranged from undetectable to 119.6 pg/mL, with a mean value (± standard error of the mean) of 47.6 ± 9.3 pg/mL (n = 20). However, G-CSF levels in healthy donors were distributed between undetectable and 28 pg/mL, with a mean value (± standard error of the mean) of 15.1 ± 3.5 pg/mL (n = 8). As shown in Fig. 5B, the level of serum G-CSF was increased in patients with GBM, compared with healthy donors (P = .002). It may be that the increase in G-CSF levels is partly responsible for MDSC accumulation in patients with GBM. This increase in G-CSF was somewhat select, because there was no significant increase in IL-6 or IL-8 levels in the plasma of patients with GBM.
It is also likely that tumor products produced by gliomas can activate/differentiate circulating mononuclear cells to acquire MDSC-like properties. In fact, a recent report23 demonstrated that incubation of normal human monocytes with glioma cell lines caused the monocytes to become immunosuppressive and display the phenotype of monocytic MDSCs. We have also shown that incubation of supernatants for GBM lines (eg, CCF52, a primary cell line derived from brain tumor) with whole-blood samples obtained from healthy donors will result in the accumulation of cells with the phenotype and suppressive properties of neutrophilic MDSCs (J. Ko; unpublished data. Although these studies suggest that GBM cells and tumor products can promote the accumulation of cells with the phenotype and function of MDSCs, the relationship of these cells to MDSCs isolated from patients with GBM requires additional investigation. Overall, our study suggests that MDSCs—particularly neutrophilic MDSCs and, to a lesser extent, linage-negative MDSCs—accumulate in patients with GBM and that CD33+HLADR+ cells from the blood are immunosuppressive, possibly involving the secretion of arginase.
Funding
This work was supported by the Rudy Fund, RO1CA116255, and the Wolf Family Foundation.
Acknowledgments
We acknowledge the assistance of Mary V. McGraw in coordinating patient blood samples.
Conflict of interest statement. None declared.
References
- 1.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. doi:10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. doi:10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24(6):431–446. doi: 10.1097/00002371-200111000-00001. doi:10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9(4):470–481. doi: 10.1016/j.coph.2009.06.014. doi:10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 6.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. doi:10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 7.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 8.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. doi:10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. doi:10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. Adv Exp Med Biol. 2007;601:213–223. doi: 10.1007/978-0-387-72005-0_22. doi:10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- 11.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21(1):31–42. doi: 10.1016/j.nec.2009.08.005. doi:10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–244. doi: 10.1016/j.coi.2010.01.021. doi:10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Su Z, Heiser A. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. doi:10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez PC, Ernstoff MS, Hernandez C, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 15.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. doi:10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CY, Wang YM, Wang CL, et al. Population alterations of L: -arginase- and inducible nitric oxide synthase-expressed CD11b(+)/CD14 (-)/CD15 (+)/CD33 (+) myeloid-derived suppressor cells and CD8 (+) T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. doi:10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 19.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. doi:10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 20.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. doi:10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 21.Vuk-Pavlovic S, Bulur PA, Lin Y, et al. Immunosuppressive CD14 + HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70(4):443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. doi:10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 25.Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158(3):638–651. doi: 10.1111/j.1476-5381.2009.00291.x. doi:10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Ann Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. doi:10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 28.Polat MF, Taysi S, Polat S, Boyuk A, Bakan E. Elevated serum arginase activity levels in patients with breast cancer. Surg Today. 2003;33(9):655–661. doi: 10.1007/s00595-002-2563-2. doi:10.1007/s00595-002-2563-2. [DOI] [PubMed] [Google Scholar]
- 29.Shukla VK, Tandon A, Ratha BK, et al. Arginase activity in carcinoma of the gallbladder: a pilot study. Eur J Cancer Prev. 2009;18(3):199–202. doi: 10.1097/CEJ.0b013e32832405eb. doi:10.1097/CEJ.0b013e32832405eb. [DOI] [PubMed] [Google Scholar]
- 30.Porembska Z, Zabek J, Grabon W, Rahden-Staron I, Baranczyk-Kuzma A. Arginase isoforms in human colorectal cancer. Clin Chim Acta. 2001;305(1–2):157–165. doi: 10.1016/s0009-8981(00)00432-0. doi:10.1016/S0009-8981(00)00432-0. [DOI] [PubMed] [Google Scholar]
- 31.Rapoport AP, Abboud CN, DiPersio JF. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF): receptor biology, signal transduction, and neutrophil activation. Blood Rev. 1992;6(1):43–57. doi: 10.1016/0268-960x(92)90007-d. doi:10.1016/0268-960X(92)90007-D. [DOI] [PubMed] [Google Scholar]
- 32.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 33.Shojaei F, Wu X, Qu X, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA. 2009;106(16):6742–6747. doi: 10.1073/pnas.0902280106. doi:10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hintzen RQ, Voormolen J, Sonneveld P, van Duinen SG. Glioblastoma causing granulocytosis by secretion of granulocyte-colony-stimulating factor. Neurology. 2000;54(1):259–261. doi: 10.1212/wnl.54.1.259. [DOI] [PubMed] [Google Scholar]
- 35.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. doi:10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]