Abstract
Pediatric high-grade gliomas (HGGs) of the thalamic region account for up to 13% of pediatric HGGs and usually result in only anecdotal long-term survival. Because very little is known about these tumors, we aimed to further characterize them. In our series of 99 pediatric thalamic HGGs, there were no significant differences in survival between patients with tumors affecting the thalamus alone (including bithalamic lesions) and patients with tumors affecting the thalamus plus adjacent structures. Tumor resection (event-free survival/overall survival) and an early treatment response to radiotherapy/chemotherapy (event-free survival) had independent prognostic significance, as shown by Kaplan-Meier and multivariate Cox regression analyses. When we compared clinical characteristics and outcomes of pediatric thalamic HGG with those of pediatric (nonthalamic) supratentorial (n = 177) as well as pediatric pontine HGG (including diffuse intrinsic pontine gliomas; n = 234), we found that thalamic HGG shared more similarities with pontine than with supratentorial HGG, but overall, it appeared to represent a clinically distinct subgroup of pediatric HGG. The varying extent of tumor resection in the different tumor localizations may play some role in the observed clinical differences, as shown by multivariate Cox regression analyses, but the tumor site itself was also identified as an independent prognostic parameter. Thus, an additional location-specific effect on survival and/or tumor biology, despite different neurosurgical accessibility, has to be considered. Therefore, future investigations should try to further characterize the obviously site-specific heterogeneity of pediatric HGG on a molecular genetic basis.
Keywords: children, high-grade glioma, pontine, supratentorial, thalamic
Approximately 5% of pediatric brain tumors arise in the thalamic region.1,2 Usually, these brain tumors are of glial origin, with up to 50% showing a high-grade histology.2–6 Thalamic gliomas are not easily accessible for subtotal or total tumor resection, thereby suggesting a rather unfavorable prognosis. However, even with significant residual tumor after neurosurgery, children with low-grade thalamic gliomas of childhood can have a 5-year overall survival (OS) rate of >80% after additional radiotherapy and/or chemotherapy.7,8 Unfortunately, similar outcomes do not apply to children with high-grade gliomas (HGGs) in the thalamic region. Although reports of survival among children with these tumors are scarce and often difficult to interpret because the studies mix data for adults and children with low-grade and high-grade gliomas and with tumors of other locations and non-glial histology, the overall prognosis appears to be extremely poor, with only anecdotal long-term survival.1,2,5,6,9,10
Thus, in the current study, we further characterized thalamic HGGs in children and adolescents, with special emphasis on potential prognostic factors, thereby investigating whether these tumors represent a clinically distinct subset of pediatric HGGs clearly differing from pontine gliomas on the one hand and (non-thalamic) supratentorial HGG on the other.
Patients and Methods
Patient Characteristics and Inclusion/Exclusion Criteria
Patient data were obtained from the HIT-GBM data base of the Brain Tumor Study Group of the Society of Pediatric Oncology and Hematology in Germany, Austria, and Switzerland (Gesellschaft für Paediatrische Haematologie und Onkologie [GPOH]). The HIT-GBM database contains clinical data for all patients enrolled in the various HIT-GBM trials during the period 1994–2007.11–14
Our current study used the following inclusion criteria: (1) histopathological diagnosis of a HGG (ie, World Health Organization [WHO] grade III anaplastic pilocytic astrocytoma, WHO grade III anaplastic ganglioglioma, pleomorphic xanthoastrocytoma with anaplasia, WHO grade III anaplastic oligodendroglioma/oligoastrocytoma, WHO grade III anaplastic astrocytoma, WHO grade IV gliosarcoma, WHO grade IV giant cell glioblastoma, and WHO grade IV glioblastoma multiforme),15 confirmed by central neuropathological review (The German Brain Tumor Reference Center, Department of Neuropathology, University of Bonn, Bonn, Germany); (2) neuroradiological diagnosis of a diffuse intrinsic pontine glioma (DIPG) confirmed by central neuroradiological review (Department of Neuroradiology, University Hospital Wuerzburg, Wuerzburg, Germany) and typical clinical symptoms of brainstem affection;16 and (3) patient age of 0–17 years at the time of initial diagnosis.
In addition, thalamic HGGs were defined as HGGs affecting either the thalamus alone (including bithalamic lesions), thalamus plus adjacent diencephalic/mesencephalic structures, or thalamus plus cortical and/or cerebellar and/or pontine structures. Pontine gliomas were defined either as DIPG confirmed by central neuroradiological review (thus, also including pontine tumors without histology or with WHO grade II histology) or as any pontine lesion with an underlying histopathological diagnosis of HGG confirmed by central neuropathological review. Non-thalamic supratentorial HGGs (hereafter referred to as supratentorial HGGs) were defined as any pediatric supratentorial HGG without thalamic affection. Any HGGs with a diencephalic or mesencephalic location otherwise not specified as thalamic or pontine tumor were excluded from this group.
On the basis of these criteria, we identified 510 pediatric patients; 99 had thalamic HGG, 234 had pontine gliomas, and 177 had supratentorial HGG. The clinical and treatment characteristics of these patients are listed in Tables 1 and 2. Additional information on the composition of the different groups of pediatric HGGs is given in Table 3. Informed consent for statistical analyses was provided by all patients and/or their parents at the time of enrolment in the various HIT-GBM trials, in accordance with the Declaration of Helsinki.
Table 1.
Clinical characteristics of pediatric high-grade glioma patients with thalamic, pontine, and supratentorial tumors
| Characteristic | Thalamic high-grade gliomas | Pontine gliomas | Supratentorial high-grade gliomas |
|---|---|---|---|
| No. of pediatric patients | 99 | 234 | 177 |
| Age at diagnosis, years | |||
| Median | 11.5 | 8.0 | 11.7 |
| Mean | 10.8 | 8.6 | 10.7 |
| Range | 0–17.7 | 1.3–17.9 | 0–17.9 |
| <11 years | 47 (47.5) | 170 (72.6) | 81 (45.8) |
| ≥11 years | 52 (52.5) | 64 (27.4) | 96 (54.2) |
| Sex | |||
| Female | 45 (45.5) | 104 (44.4) | 76 (42.9) |
| Male | 54 (54.5) | 130 (55.6) | 101 (57.1) |
| Histological tumor grading | |||
| WHO II | NA | 9 (8.8) | NA |
| WHO III | 67 (67.7) | 55 (53.9) | 64 (36.2) |
| WHO IV | 32 (32.3) | 38 (37.3) | 113 (63.8) |
| No histology | NA | 132 (56.4% of total)a | NA |
| Histological diagnosis | |||
| Astrocytoma II | NA | 9 (8.8) | NA |
| Anaplastic ganglioglioma III | 0 | 1 (1) | 4 (2.3) |
| Pleomorphic xanthoastrocytoma with anaplasia | 0 | 0 | 4 (2.3) |
| Anaplastic pilocytic astrocytoma III | 3 (3) | 0 | 7 (4) |
| Anaplastic oligodendroglioma/oligoastrocytoma III | 3 (3) | 0 | 8 (4.5) |
| Anaplastic astrocytoma III | 61 (61.6) | 54 (52.9) | 41 (23.1) |
| Gliosarcoma IV | 1 (1) | 0 | 4 (2.3) |
| Giant cell glioblastoma IV | 1 (1) | 1 (1) | 14 (7.8) |
| Glioblastoma multiforme IV | 30 (30.3) | 37 (36.3) | 95 (53.7) |
| No histology | NA | 132 (56.4% of total)a | NA |
| Secondary high-grade glioma | |||
| Yes | 5 (5.6) | 2 (0.9) | 13 (7.9) |
| No | 84 (94.4) | 211 (99.1) | 151 (92.1) |
| Unknown | 5 (5.1% of total)a | 21 (9% of total)a | 13 (7.3% of total)a |
| Tumor-predisposing syndromes | |||
| Yes | 2 (2.1) | 2 (0.9) | 7 (4.1) |
| No | 95 (97.9) | 226 (99.1) | 164 (95.9) |
| Unknown | 2 (2% of total)a | 6 (2.6% of total)a | 6 (3.4% of total)a |
| Syndrome | NF1 (n = 2) | NF1 (n = 1) | NF1 (n = 5) |
| Li Fraumeni (n = 1) | Li Fraumeni (n = 1) | ||
| Turcot (n = 1) | |||
| Primary metastases | |||
| Yes | 6 (6.4) | 3 (1.4) | 13 (7.7) |
| No | 88(93.6) | 217 (98.6) | 155 (92.3) |
| Unknown | 5 (5.1% of total)a | 14 (6% of total)a | 9 (5.1 of total)a |
Data are no. (%) of patients, unless otherwise indicated. NA indicates not applicable; NF1 indicates neurofibromatosis type 1; WHO indicates World Health Organization.
aEach percentage of unknown data sets always refers for comparison reasons to the total patient numbers of each group (100%).
Table 2.
Treatment characteristics of pediatric patients with thalamic, pontine, and supratentorial high-grade gliomas
| Characteristic | No. (%) of patients |
||
|---|---|---|---|
| Thalamic high-grade glioma | Pontine glioma | Supratentorial high-grade glioma | |
| Extent of surgical resection | |||
| Gross total tumor resection | 0 | 0 | 68 (38.4) |
| Subtotal tumor resection | 19 (19.2) | 10 (4.2) | 36 (20.3) |
| Partial tumor resection | 31 (31.3) | 21 (9) | 44 (24.9) |
| Biopsy/no tumor surgery | 49 (49.5) | 203 (86.8) | 29 (16.6) |
| Radiotherapy | |||
| Yes | 95 (96) | 218 (93.2) | 157 (88.7) |
| No | 4 (4) | 16 (6.8) | 20 (11.3) |
| Chemotherapy | |||
| No | 1 (1) | 22 (9.4) | 10 (5.6) |
| HIT-GBM-A | 1 (1) | 14 (6) | 11 (6.2) |
| HIT-GBM-B | 7 (7.1) | 26 (11.1) | 13 (7.3) |
| HIT-GBM-C | 26 (26.3) | 54 (23.1) | 37 (20.9) |
| HIT-GBM-D | 53 (53.5) | 83 (35.5) | 71 (40.1) |
| Other chemotherapy | 11 (11.1) | 29 (12.4) | 35 (19.9) |
Table 3.
Composition of the different pediatric high-grade glioma groups with regard to tumor extension (thalamic group), diagnostic verification (pontine group), or affected sites (supratentorial group)
| Group | No. (%) of patients |
|---|---|
| Thalamic high-grade gliomas (n = 99) | |
| Thalamus alone (including bithalamic lesions) | 31 (31.3) |
| Thalamus plus adjacent diencephalic/mesencephalic structures | 38 (38.4) |
| Thalamus plus cortical and/or cerebellar and/or pontine structures | 30 (30.3) |
| Pontine gliomas (n = 234) | |
| Diffuse intrinsic pontine glioma confirmed by neuroradiological review | 168 (71.8) |
| Brainstem high-grade glioma confirmed by neuropathological review | 66 (28.2) |
| Supratentorial high-grade gliomas (n = 177) | |
| Predominantly cortical lesions | 156 (88.1) |
| Non-cortical supratentorial lesions | 21 (11.9) |
HIT-GBM Treatment Protocols
Eligible pediatric patients aged >3 years but <18 years with HGG and/or DIPG had been enrolled in the various HIT-GBM trials during the period 1994–2007. In all of these trials, the best feasible tumor resection was recommended before the commencement of chemotherapy and/or radiotherapy. Standard fractionated radiotherapy (54–59.4 Gy total dose; daily fractions of 1.8 Gy over 6–7 weeks) was also common for all HIT-GBM trials. In HIT-GBM-A, oral etoposide and trofosfamide were given during radiotherapy and continued for 1 additional year after radiotherapy.11 In HIT-GBM-B, cisplatin, etoposide, and ifosfamide were given in 2 cycles concomitantly with radiotherapy, followed by intravenous low-dose cyclophosphamide and subcutaneous interferon-γ.12 In HIT-GBM-C, weekly vincristine injections were added to the concomitant HIT-GBM-B radiochemotherapy. Chemotherapy with cisplatin, etoposide, ifosfamide, and vincristine was continued during maintenance treatment.13 The concomitant radiochemotherapy of HIT-GBM-C was also adopted for HIT-GBM-D, followed by maintenance therapy with prednisolone, vincristine, and lomustine (CCNU). The therapeutic efficiency of 2 courses of high-dose methotrexate before radiotherapy was studied for feasibility in a pilot study (HIT-GBM pilot D)14 and then as a randomized question in HIT-GBM-D. Children aged <3 years were treated primarily with surgery and chemotherapy alone, in accordance with the HIT-SKK chemotherapy protocol for infant patients with brain tumors.17
Central neuropathological and central neuroradiological reviews were highly recommended in HIT-GBM-A and HIT-GBM-B and became mandatory for the HIT-GBM-C and HIT-GBM-D trials.
The extent of tumor resection was determined on the basis of early postsurgical imaging and/or the neurosurgical report. Gross total tumor resection was defined as 100% macroscopic removal of the tumor mass. Subtotal tumor resection was defined as removal of <100% but ≥90% of the tumor mass, and partial tumor resection was defined as <90% resection. Biopsies were performed as either open or stereotactic biopsies without tumor debulking.
Definition of Treatment Response
Complete response (CR) was defined as no radiological evidence of tumor on contrast-enhanced CT or MRI scan, with disappearance of multifocal lesions and tumor cells from the cerebrospinal fluid in cases of disseminated disease.
Partial response (PR) was defined as a radiologically determined (by CT or MRI) decrease in the size of solid tumor parts by >50%, as determined by the product of the 2 largest perpendicular diameters and/or tumor volume, as calculated by using the formula axial × coronal × sagittal/2 (referring to the largest diameters). If the tumor consisted of solid and cystic parts, both parts were evaluated separately. In disseminated disease, the distant tumor lesions showed either a reduction in size or a stable appearance, and no new tumor lesions were detectable.
Stable disease was defined as a radiologically determined decrease in the size of solid tumor parts by <50% or an increase of ≤20%, determined as described above for PR. In disseminated disease, the distant tumor lesions showed no progression of >20%, and no new tumor lesions were detectable.
Progressive disease was defined as a radiologically determined increase in the size of solid tumor parts by >20% (determined as described above for PR) and/or the development of new tumor lesions.
Statistical Analysis
Statistical analysis was retrospectively performed using the SPSS statistical package (SPSS Inc). OS and event-free (EFS) survival were determined by Kaplan-Meier analysis and log-rank testing. An event was defined as tumor relapse or progression, occurrence of a secondary malignancy, or death due to any cause. Thalamic and supratentorial HGG and pontine gliomas were all defined by the following parameters: histological tumor grading (WHO grade III or WHO grade IV), sex (male vs female), age at diagnosis (<11 vs ≥11 years), and extent of tumor resection (total resection, subtotal resection, partial resection, or biopsy and no resection). Furthermore, thalamic HGGs were also characterized by initial/postsurgical and week 8 tumor size (largest tumor diameter and/or tumor volume), as well as by evaluation of treatment response at week 8 and patterns of relapsed or progressive disease (only local relapse or progression and/or secondary metastases).
The prognostic relevance regarding EFS and OS of the different pediatric HGG groups was compared for the subgroups of age, grading, sex, tumor location, and extent of tumor resection, as defined above. The survival analysis was completed for each histological subtype by a Cox regression analysis for the same subgroups, with the exception that “extent of tumor resection” was characterized by the parameters “tumor resection” and “no tumor resection.” To compare the epidemiological distribution of disease and treatment characteristics between the different HGG groups, we performed the 2-sided chi-square test to determine significant differences regarding sex ratio, distribution of grade III and IV tumors, age <11 versus ≥11 years, tumor resection, primary metastases, predisposing tumor syndromes, and secondary HGG.
For all statistical analyses, the significance level was set at P < .05.
Results
Clinical Characteristics and Prognostic Factors of Pediatric Thalamic HGG
Clinical and histological characteristics of the pediatric thalamic HGGs in this study are listed in Tables 1 and 3. Common initial symptoms of patients involved signs of increased intracranial pressure, motor weakness (including hemiparesis), and gait disturbance. The median duration of symptoms until tumor diagnosis was only 1.3 months.
Gross total tumor resection could not be achieved in any patient. Subtotal and partial tumor resection were performed in only ∼50% of patients but implied significantly better 5-year EFS and OS than did tumor biopsy (Tables 2 and 4). There were no significant differences in frequencies of subtotal or partial tumor resection between the different subgroups of pediatric thalamic HGGs affecting either thalamus alone or extending into the different adjacent structures (Table 5).
Table 4.
Clinical characteristics of pediatric thalamic high-grade gliomas
| Characteristic | Value | Event-free survival |
Overall survival |
||
|---|---|---|---|---|---|
| 5-year EFS %±SD | Pa | 5-year OS %±SD | Pa | ||
| Tumor extension | NS | NS | |||
| Thalamus alone (including bithalamic lesions) | n =31 | 7.3±5 | 7.4±5.1 | ||
| Thalamus plus adjacent diencephalic/mesencephalic structures | n =38 | 5.4±3.7 | 6.9±5.9 | ||
| Thalamus plus cortical and/or cerebellar and/or pontine structures | n =30 | 10.3±5.7 | 15.9±7.7 | ||
| Extent of surgical resection | .0479 | .0272 | |||
| Tumor resection | n =50 | 10.4±4.6 | 13.6±5.9 | ||
| No tumor resection | n =49 | 4.2±2.9 | 9.2±4.6 | ||
| Grading | NS | .0462 | |||
| WHO III | n =67 | 8.8±3.7 | 14.6±5.6 | ||
| WHO IV | n =32 | 3.2±3.2 | 3.8±3.8 | ||
| Sex | NS | NS | |||
| Male | n =45 | 2.5±2.4 | 6±4 | ||
| Female | n =54 | 11.6±4.9 | 17.3±6.3 | ||
| Age at diagnosis | NS | NS | |||
| <11 years | n =47 | 6.8±3.8 | 6.8±3.8 | ||
| ≥11 years | n =52 | 6.5±3.9 | 6.5±3.9 | ||
| Initial/postsurgical tumor size | |||||
| Maximum tumor diameter | n =84 | ||||
| Range | 0.6–8.9 cm | ||||
| Mean | 4.3 cm | ||||
| <4.3 cm (mean) | n =42 | 8.1±4.5 | NS | 8.7±5.2 | NS |
| ≥4.3 cm (mean) | n =42 | 9.5±4.5 | 8.0±5.2 | ||
| Tumor volume | n =51 | ||||
| Range | 0.3–224.3 cm3 | ||||
| Mean | 36.3 cm3 | ||||
| <36.3 cm3 (mean) | n =25 | 12.2±7.7 | NS | 9.7±8.3 | NS |
| ≥36.3 cm3 (mean) | n =26 | 3.9±3.8 | 0 | ||
| Tumor volume at week 8 (after radiotherapy) | |||||
| Maximum tumor diameter | n =53 | ||||
| Range | 0.3–8.0 cm | ||||
| Mean | 4.0 cm | ||||
| <4.0 cm (mean) | n =25 | 6.3±5.6 | NS | 0 | NS |
| ≥4.0 cm (mean) | n =28 | 3.6±3.5 | 4.3±4.2 | ||
| Tumor volume | n =41 | ||||
| Range | 0–195.4 cm3 | ||||
| Mean | 22.1 cm3 | ||||
| <22.1 cm3 (mean) | n =20 | 14.2±8.5 | .0263 | 14.2±11.5 | NS |
| ≥22.1 cm3 (mean) | n =21 | 0 | 0 | ||
| Response at week 8 (after radiotherapy) | < .0001 | NS | |||
| CR | n =7 | 42.9±18.7 | 50.0±20.4 | ||
| PD | n =10 | 0 | 0 | ||
| PR | n =30 | 10.0±4.5 | 20.0±12.6 | ||
| SD | n =43 | 7.0±3.9 | 15.3±6.7 | ||
| Unknown | n =9 | 0 | 0 | ||
| Pattern of relapsed/progressive disease | NS | NS | |||
| Only local relapse/progression | n =72 | 8.8±3.5 | 14.7±5.1 | ||
| With or without secondary metastases | n =27 | 3.7±3.6 | 0 | ||
| Cerebrospinal fluid/leptomeningeal metastases | n =18 | ||||
| Íntracerebral metastases | n =6 | ||||
| Cerebrospinal fluid/leptomeningeal and intracerebral metastases | n =3 | ||||
| Cox regression analysis | NA | NA | NA | ||
| Tumor extension | NS | NS | |||
| Tumor resection | .015 | .002 | |||
| Grading | .013 | .009 | |||
| Sex | NS | NS | |||
| Age | NS | NS | |||
| Response at week 8 | .001 | NS | |||
CR indicates complete response; EFS indicates event-free survival; NA indicates not applicable; NS indicates not significant; OS indicates overall survival; PD indicates progressive disease; PR indicates partial response; SD indicates stable disease.
aDetermined by log-rank test.
Table 5.
Tumor surgery in relation to extension of pediatric thalamic high-grade gliomas
| Characteristic | No. (%) of patients |
||
|---|---|---|---|
| Subtotal resection | Partial resection | Biopsy | |
| Thalamus alone | 5 (16) | 11 (36) | 15 (48) |
| Thalamus plus adjacent diencephalic/mesencephalic structures | 10 (26) | 14 (37) | 14 (37) |
| Thalamus plus cortical and/or cerebellar and/or pontine structures | 4 (13) | 6 (20) | 20 (67) |
There were no significant differences between the different extents of surgery as determined by the chi-square test.
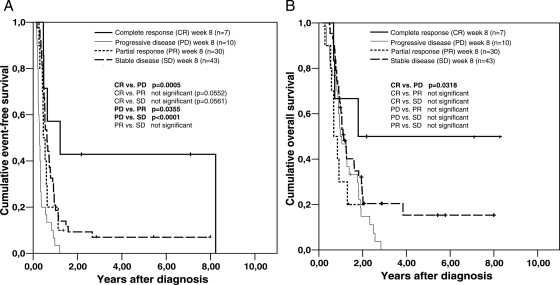
Treatment response evaluation at week 8 showed significantly better OS and EFS for patients with CR after radiotherapy (Fig. 1 and Table 4). This was in concordance with the observation that a lower residual tumor volume at week 8 resulted in a significantly prolonged EFS (Table 4).
Fig. 1.
Kaplan-Meier analysis of event-free survival (A) and overall survival (B) in pediatric patients with thalamic high-grade gliomas, with respect to their treatment response evaluation at week 8. Differences between the various subgroups were significant for the comparison of event-free survival between the patient group with complete response (CR) versus the one with progressive disease (PD), the one with PD versus the one with partial response (PR), and the one with PD versus the one with stable disease (SD). There was also a significant difference for the comparison of overall survival between the patient group with CR versus the one with PD.
Patients with WHO grade III thalamic HGG also demonstrated a significantly prolonged OS but not EFS (Table 4).
Other parameters such as tumor extension, initial/postsurgical tumor size (largest tumor diameter and/or tumor volume), patterns of relapsed/progressive disease (only local relapse/progression and/or secondary metastases), age, and sex did not result in any significant differences in EFS or OS in univariate analyses (Table 4).
By multivariate Cox regression analysis, histological grading and tumor resection were both identified as independent prognostic factors for EFS and OS, with tumor response at week 8 being a factor only for EFS (Table 4).
Comparison of Thalamic HGGs with Supratentorial HGGs or Pontine Gliomas
Considering the distribution of clinical parameters and tumor characteristics, it appeared that thalamic and supratentorial HGGs share a similar mean/median patient age at diagnosis, percentage of secondary HGG, presence of a tumor predisposing syndrome, and primary metastases at time of diagnosis (Tables 1 and 6). However, significant differences were found regarding the frequency of total tumor resection and biopsy or no surgery (no total tumor resection, but 49.5% tumor biopsy only in thalamic HGG versus 38.4% total tumor resection and 16.6% tumor biopsy in supratentorial HGG; Tables 2 and 6). Furthermore, the distribution of WHO grade III and IV gliomas was reversed between thalamic and supratentorial HGGs in our pediatric series: although approximately two-thirds of thalamic HGGs showed a grade III histology and one-third showed a grade IV histology, for supratentorial HGGs, nearly two-thirds were grade IV, and slightly more than one-third were grade III tumors (Tables 1 and 6).
Table 6.
Comparison of clinical characteristics of thalamic high-grade gliomas (HGGs) with supratentorial HGGs or pontine gliomas in pediatric patients
| Distribution |
Event-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| Thalamic vs pontine | Thalamic vs supratentorial | Thalamic vs pontine | Thalamic vs supratentorial | Thalamic vs. pontine | Thalamic vs supratentorial | |
| Total population | NA | NA | NS | <.0001 | .0079 | <.0001 |
| Extent of surgery | NA | |||||
| Total | <.001a | NA | NA | NA | NA | |
| Subtotal | NSa | NS | NS | NS | NS | |
| Partial | NSa | NS | .0131 | NS | .0499 | |
| Biopsy/no surgery | <.001a | NS | .0357 | NS | NS | |
| Grading | NSa | <.001a | ||||
| WHO III | NS | <.0001 | .0133 | <.0001 | ||
| WHO IV | NS | .0031 | NS | .0035 | ||
| Sex | NSa | NSa | ||||
| Male | NS | <.0001 | NS | .0003 | ||
| Female | NS | .0002 | .0141 | .0028 | ||
| Age at diagnosis | ||||||
| Mean | <.001b | NSb | NA | NA | NA | NA |
| <11 years | NSa | NSa | NS | .0001 | NS | .0016 |
| ≥11 years | NS | .0045 | NS | .006 | ||
| Secondary HGG | .014a | NSa | NA | NA | NA | NA |
| Tumor syndrome | NSa | NSa | NA | NA | NA | NA |
| Primary metastasis | .015a | NSa | NA | NA | NA | NA |
| Cox regression analysis | NA | NA | ||||
| Tumor site | NS | <.001 | NS | <.001 | ||
| Tumor resection | .016 | <.001 | .007 | <.001 | ||
| Grading | NS | <.001 | NS | <.001 | ||
| Sex | NS | NS | NS | .035 | ||
| Age | NS | NS | NS | NS | ||
NA indicates not applicable; NS indicates not significant; pontine indicates pediatric pontine gliomas; supratentorial indicates pediatric supratentorial HGGs; thalamic indicates pediatric HGG.
Statistical significance was determined by the two-sided chi-square test (a) or by Student's t-test (b). All other testing for statistical significance was performed by using the log rank test.
When we compared thalamic HGGs and pontine gliomas, we found significant differences in the mean patient age at diagnosis and the percentage of secondary HGGs and primary metastases (Tables 1 and 6). However, the distributions of grade III and IV tumors were similar in the 2 subpopulations.
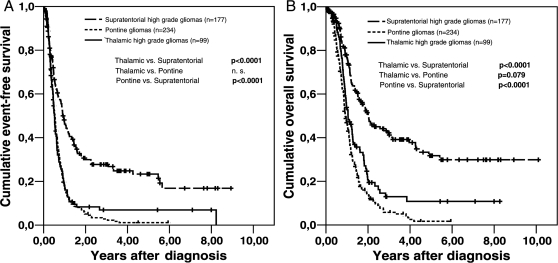
Regarding survival, thalamic and supratentorial HGGs showed significantly different EFS and OS; thalamic HGG and pontine gliomas differed significantly with regard to OS but not EFS (Fig. 2 and Table 6).
Fig. 2.
Kaplan-Meier analysis of event-free survival (A) and overall survival (B) in pediatric patients with thalamic, pontine, and (nonthalamic) supratentorial high-grade gliomas (HGGs). Differences between the various subgroups were all significant, except for the comparison of event-free survival between the thalamic and pontine HGGs.
The mean OS rates (± standard deviation) at 1, 2, and 5 years differed between the 3 patient subgroups. The 1-year OS rates were 53.8% ± 5.2% for thalamic, 46.9% ± 3.4% for pontine, and 76.6% ± 3.3% for supratentorial HGGs. The 2-year OS rates were 20.7% ± 4.4% for thalamic, 13.8% ± 2.5% for pontine, and 51.5% ± 4.1% for supratentorial HGGs. The 5-year OS rates were 10.8% ± 3.8% for thalamic, 1.7% ± 1.1% for pontine, and 31.8% ± 4.6% for supratentorial HGGs.
In contrast, EFS rates between thalamic HGG and pontine gliomas seemed quite similar for the first year but also appeared to differ more over time; EFS in supratentorial HGG differed at all time points from EFS in thalamic HGG or pontine gliomas. The 1-year EFS rates were 18.2% ± 4% for thalamic, 17.3% ± 2.6% for pontine, and 46.8% ± 3.9% for supratentorial HGGs. The 2-year EFS rates were 8.3% ± 2.9% for thalamic, 5.0% ± 1.5% for pontine, and 29.9% ± 3.6% for supratentorial HGGs. The 5-year EFS rates were 6.9% ± 2.7% for thalamic, 1.1% ± 0.8% for pontine, and 23.3% ± 3.7% for supratentorial HGGs.
Subgroup analysis of EFS revealed significant differences between thalamic and supratentorial HGGs in nearly all subgroups investigated (“partial tumor resection,” “biopsy only/no tumor surgery,” “WHO III tumors,” “WHO IV tumors,” “male patients,” “female patients,” “age at diagnosis <11 years,” “age at diagnosis ≥11 years”); only the subgroup of patients who underwent subtotal tumor resection showed a similar EFS (Table 6).
In contrast, there were no significant differences in EFS between the different subgroups of thalamic HGGs and pontine gliomas.
Subgroup analysis of OS showed a more complex pattern: although thalamic and supratentorial HGG had almost no significant differences in OS between the various neurosurgical subgroups with a different extent of tumor resection (with the exception of partial resection), the subgroups “WHO III tumors,” “WHO IV tumors,” “male patients,” “female patients,” “age at diagnosis <11 years,” and “age at diagnosis ≥11 years” had a significantly longer OS among patients with supratentorial HGGs than those with thalamic HGG (Table 6). When we compared OS in thalamic HGG and pontine gliomas, we found that female patients and patients with a grade III tumor had a longer duration of survival in the thalamic group than in the pontine group; all other subgroups had similar OS (Table 6).
Multivariate Cox regression analysis revealed that tumor resection was the only identifiable independent prognostic factor for EFS and OS between thalamic HGG and pontine gliomas, whereas “tumor site” (EFS and OS), “tumor resection” (EFS and OS), “tumor grading” (EFS and OS), and “sex” (only OS) were all independent prognostic factors for survival between thalamic and supratentorial HGGs; “age” was not a prognostic factor in any of the analyses.
Discussion
HGGs in children represent a nonhomogenous group of glial tumors characterized by WHO grade III or IV histology and/or an unfavorable clinical outcome. Pediatric HGGs of the thalamic region account for up to 13% of pediatric HGGs18 and are associated with only anecdotal long-term survival, supposedly owing to the fact that these tumors are not completely resectable.1,2,5,6,9,10 However, pediatric thalamic HGGs are not very well characterized in general, because reports are very scarce and often unspecific. Thus, the current study aimed at further characterizing pediatric thalamic HGGs and also investigated whether thalamic HGG may represent a distinct clinical subset in comparison with supratentorial and pontine HGGs in children.
The HIT-GBM database18,19 was screened for pediatric patients with thalamic, supratentorial, and pontine HGGs. Definitions of the different subsets of pediatric HGGs are given in Table 3. To compare clearly defined clinical subsets, we excluded HGGs that affected only basal ganglia and/or midbrain without thalamic involvement and that may also imply a poorer prognosis.20 This was in concordance with previous studies of thalamic tumors that even excluded all tumors that may have arisen from other structures and only subsequently invaded the thalamus.2,7 Because, in many of our cases, it was difficult to determine whether the main tumor bulk had arisen from the thalamus or adjacent structures, all tumors that affected the thalamus were included in the present study, with the exception of confirmed DIPG obviously secondarily affecting the thalamus.
In our study, we found no significant differences in survival rates between pediatric patients with HGGs affecting the thalamus alone, thalamus plus adjacent diencephalic/mesencephalic structures, or thalamus plus cortical and/or cerebellar and/or pontine structures (Table 4). These findings suggest that all pediatric HGGs affecting either thalamus alone or thalamus and adjacent structures do not represent different clinical entities or imply a different clinical outcome.
Although gross total tumor resection could not be achieved in any patient with thalamic HGG, tumor resection had an independent prognostic significance regarding EFS and OS, as shown by Kaplan-Meier and multivariate Cox regression analyses. This finding is in contrast to that of a previous study in which the extent of surgical resection did not affect progression-free survival of pediatric patients with thalamic HGG.2 Interestingly, the positive effect of tumor resection on survival seems to represent a more complex treatment situation than just tumor debulking (ie, reduction of tumor size alone), because initial/postsurgical tumor size could not be shown as a prognostic factor itself (Table 4). In addition, there was also no significant correlation between the observed frequencies of tumor resection and affection of either thalamus alone and/or adjacent structures (Table 5).
Regarding the possible role of additional treatment strategies for pediatric thalamic HGG, the statistical evaluation of radiological treatment response at week 8 (ie, after radiotherapy and, in most cases, concomitant with chemotherapy) produced interesting results. There was a significantly prolonged EFS in all patients with some treatment response (CR, PR, or stable disease) (Fig. 1). This finding was in concordance with the observation that a lower residual tumor volume at week 8 resulted in a significantly prolonged EFS (Table 4). Furthermore, the small number of patients (n = 7) who were in CR at week 8 (Fig. 1) showed an impressive 5-year OS (mean±SD 42.9% ± 18.7%). Because no patient had started radiotherapy and/or chemotherapy in CR (no gross total tumor resection), these patients appeared to have an additional benefit from radiotherapy and/or chemotherapy. Future studies will have to confirm this interesting finding. Nevertheless, in our patient series, treatment response at week 8 was an independent prognostic factor for EFS, as shown by multivariate Cox regression analysis (Table 4).
Comparing the clinical characteristics and potential prognostic parameters of pediatric thalamic HGGs with pediatric supratentorial HGGs and pediatric pontine gliomas, the current study showed that thalamic and pontine HGGs do share more similarities than thalamic and supratentorial HGGs, as shown schematically in Table 6. However, there were also still significant clinical differences between thalamic and pontine HGG—for example, the different mean age at diagnosis (11.5 vs 8.0 years) and the different long-term OS (mean 5-year OS rate±SD, 10.8% ± 3.8% vs 1.7% ± 1.1%). Thus, all results strongly suggest that thalamic HGG may represent a clinically distinct subgroup of pediatric HGG, with defined clinical differences from both supratentorial and pontine pediatric HGG.
The varying extent of tumor resection in the different tumor localizations may play some role in explaining the different clinical outcomes between thalamic, pontine, and supratentorial HGGs in pediatric patients. By multivariate Cox regression analyses, the effect of tumor resection showed an independent prognostic value between thalamic and pontine HGGs and between thalamic and supratentorial HGGs.
Because the tumor site itself was also identified as an independent prognostic parameter, at least between thalamic and supratentorial HGGs, any detailed explanation for the observed clinical differences might have to go beyond the pure differences in neurosurgical accessibility in the different tumor localizations. Varying tumor biology may also explain clinical differences between the investigated tumor sites. This hypothesis is supported by growing evidence that pediatric DIPG and pediatric supratentorial HGGs have clearly distinct molecular genetic signatures, suggesting that the clinical differences between these HGG populations might indeed be based on different tumor biologies. Furthermore, limited data from adult patients with glioblastoma multiforme (GBM) suggest that thalamic GBM might show a distinct molecular genetic profile, with TP53 mutations and lack of EGFR amplifications otherwise found predominantly in tumor subsets of secondary and younger adult GBM patients.21
Unfortunately, similar findings are not yet available for a representative number of clearly defined pediatric thalamic HGGs. Thus, no comparison with other clinical subsets of pediatric HGGs is yet possible. Future investigations should therefore focus on this interesting issue to further characterize the obviously site-specific heterogeneity of pediatric HGG on a molecular genetic basis.
Funding
The present research is based on clinical data from the HIT-GBM trials, which were all funded by the Deutsche Kinderkrebsstiftung, Bonn, Germany.
Acknowledgments
We gratefully acknowledge the ongoing support of the Deutsche Kinderkrebsstiftung (Bonn, Germany). Without this support, performance of clinical trials as well as quality control measures, such as central neuropathological and central neuroradiological review and a central review of radiotherapy planning, would not be possible in the GPOH (Gesellschaft für Paediatrische Haematologie und Onkologie) brain tumor (HIT) network. We also thank all colleagues who contributed patients and their data to the HIT-GBM trials.
The presented work is part of the MD thesis of Sandra Butenhoff.
Conflict of interest statement. None declared.
References
- 1.Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997;13:514–520. doi: 10.1007/s003810050128. doi:10.1007/s003810050128. [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Gajjar A, Sanford RA, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29:29–35. doi: 10.1159/000028681. doi:10.1159/000028681. [DOI] [PubMed] [Google Scholar]
- 3.Hirose G, Lombroso CT, Eisenberg H. Thalamic tumors in childhood. Clinical, laboratory, and therapeutic considerations. Arch Neurol. 1975;32:740–744. doi: 10.1001/archneur.1975.00490530062005. [DOI] [PubMed] [Google Scholar]
- 4.Mayer M, Ponsot G, Kalifa C, et al. Thalamic tumors in children. A study of 38 cases. Arch Fr Pediatr. 1982;39:91–95. [PubMed] [Google Scholar]
- 5.Bernstein M, Hoffman HJ, Halliday WC, et al. Thalamic tumors in children: long-term follow-up and treatment guidelines. J Neurosurg. 1984;61:649–656. doi: 10.3171/jns.1984.61.4.0649. doi:10.3171/jns.1984.61.4.0649. [DOI] [PubMed] [Google Scholar]
- 6.Albright AL. Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg. 2004;100(suppl.):468–472. doi: 10.3171/ped.2004.100.5.0468. [DOI] [PubMed] [Google Scholar]
- 7.Puget S, Crimmins DW, Garnett MR, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007;106(suppl.):354–362. doi: 10.3171/ped.2007.106.5.354. [DOI] [PubMed] [Google Scholar]
- 8.Thieme B, Von Hornstein S, Pietsch T, et al. Thalamic low-grade glioma (LGG) in children—analysis of prognostic factors. Report of the multicenter trial HIT-LGG 1996 [abstract] Neuro Oncol. 2008;10:453. [Google Scholar]
- 9.Fernandez C, Maues de Paula A, Colin C, et al. Thalamic gliomas in children: an extensive clinical, neuroradiological and pathological study of 14 cases. Childs Nerv Syst. 2006;22:1603–1610. doi: 10.1007/s00381-006-0184-6. doi:10.1007/s00381-006-0184-6. [DOI] [PubMed] [Google Scholar]
- 10.Broniscer A, Chintagumpala M, Fouladi M, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavourable low-grade glioma in children. J Neurooncol. 2006;76:313–319. doi: 10.1007/s11060-005-7409-5. doi:10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 11.Wolff JE, Mölenkamp G, Westphal S, et al. Oral trofosfamide and etoposide in pediatric patients with glioblastoma multiforme. Cancer. 2000;89:2131–2137. doi: 10.1002/1097-0142(20001115)89:10<2131::aid-cncr14>3.0.co;2-j. doi:10.1002/1097-0142(20001115)89:10<2131::AID-CNCR14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Wolff JE, Wagner S, Reinert C, et al. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J Neurooncol. 2006;79:315–321. doi: 10.1007/s11060-006-9147-8. doi:10.1007/s11060-006-9147-8. [DOI] [PubMed] [Google Scholar]
- 13.Wolff JE, Driever PH, Erdlenbruch B, et al. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer. 2010;116:705–712. doi: 10.1002/cncr.24730. doi:10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 14.Wolff JE, Kortmann RD, Wolff B, et al. High dose methotrexate for pediatric high grade glioma: results of the HIT-GBM-D Pilot study. J Neurooncol. 2011;102:433–442. doi: 10.1007/s11060-010-0334-2. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: IARC, Lyon; 2007. [Google Scholar]
- 16.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. doi:10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. doi:10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 18.Kramm C, Rausche U, Butenhoff S, et al. Hochmaligne Gliome im Kindes- und Jugendalter. Monatsschr Kinderheilk. 2008;156:1201–1207. doi:10.1007/s00112-008-1799-3. [Google Scholar]
- 19.Wolff JE, Classen CF, Wagner S, et al. Subpopulations of malignant gliomas in pediatric patients: analysis of the HIT-GBM database. J Neurooncol. 2008;87:155–164. doi: 10.1007/s11060-007-9495-z. doi:10.1007/s11060-007-9495-z. [DOI] [PubMed] [Google Scholar]
- 20.Fujii O, Soejima T, Kuwatsuka Y, et al. Supratentorial glioblastoma treated with radiotherapy: use of the Radiation Therapy Oncology Group recursive partitioning analysis grouping for predicting survival. Jpn J Clin Oncol. 2010;40:726–731. doi: 10.1093/jjco/hyq051. doi:10.1093/jjco/hyq051. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Yamashita J, Watanabe T. Molecular genetic analysis of deep-seated glioblastomas. Cancer Genet Cytogenet. 2004;153:64–68. doi: 10.1016/j.cancergencyto.2003.12.010. doi:10.1016/j.cancergencyto.2003.12.010. [DOI] [PubMed] [Google Scholar]