Abstract
Leukocytosis contributes to exercise-induced immune modulation, which is a mechanism of cardiovascular protection. However, this process is poorly defined in children. We therefore measured leukocytes in 45 healthy, 18 overweight, 16 type 1 diabetic, and 8 asthmatic children at pre-, end-, and 30-min post-exercise (30-min intermittent or 6-min continuous). In all groups, total leukocytes, neutrophils, lymphocytes, and monocytes increased at end-exercise, but returned to baseline by 30-min post-exercise, including neutrophils, previously reported to remain elevated for at least some exercise formats. This highly preserved pattern indicates the importance of the adaptive response to physical stress across multiple health conditions.
INTRODUCTION
The many known beneficial health effects of exercise depend on the correct balance of numerous integrated adaptive responses (4). While many biochemical details of this adaptation are still unclear, several well-established molecular events are known to occur during exercise, mostly in an intensity-dependent fashion, such as increased oxygen consumption, carbon dioxide and lactate production (29), and increased systemic concentrations of glucoregulatory mediators (6), growth factors, cytokines (3), and oxidative stress markers (25). One major aspect of this complex adaptive network is the marked leukocytosis that is also induced by exercise; all major subtypes of white blood cells (WBCs) (neutrophils, lymphocytes, monocytes) are acutely mobilized and activated during physical activity (12), and their secretion of cytokines/chemokines, as well as the gene expression of a number of pro-/anti-inflammatory mediators and growth factors (2),(15), are acutely altered. The current paradigm is a 40–100% increase of all leukocyte subtypes for most exercise formats, with lymphocytes and monocytes rapidly returning to baseline after exercise cessation (in fact slightly dipping below baseline) (16) and neutrophils (PMNs) presenting a more variable post-exercise profile (returning close to baseline for less intense exercise, or tending to remain elevated for one to several hours after exercise with more intense physical challenges) (19). The physiological rationale for these strong immunologic responses is still incompletely understood, although it has been hypothesized this may be a priming mechanism for possible muscle injuries and impending open-wound infections (17),(22).
The optimization of exercise regimens is relevant to all children, as physical activity is now considered an essential component of proper physical and psychological growth and development (24). Exercise is especially relevant for children with dysmetabolic states that even include a chonic inflammatory component (obesity, metabolic syndrome, type 1 and 2 diabetes, asthma, etc.). In these conditions, an imbalance of inflammatory processes during physical activity may result in reduced exercise-related health effects, especially the prevention or delayed onset and progression of cardiovascular complications (23). Exercise-induced leukocytosis may play a crucial role in the inflammatory response to stress. As the overwhelming majority of our knowledge on leukocyte responses to exercise derives from studies in adults, which often markedly differ in many metabolic aspects from children, the physiological pediatric leukocyte responses to exercise is incompletely defined, especially concerning exercise formats reproducing real-life scenarios. More importantly, it is currently unknown whether this response differs across groups of children with chronic inflammatory conditions, in which only a direct assessment of the molecular adaptation to different exercise formats can provide the correct conceptual physiological basis for optimal utilization of exercise as a disease-management tool.
The present study therefore aims to define the characteristics of exercise-induced leukocytosis during and following two exercise formats that reproduce the intensity and duration of real-life physical challenges. Studies were performed in healthy children and in children with common chronic pediatric conditions—overweight (OW), type 1 diabetes mellitus (T1DM), and exercise-induced asthma (EIA)—whose known hyperactive immunological status may potentially alter their leukocyte mobilization and/or function in response to physiological stress.
MATERIALS AND METHODS
Study Design and Participants
All study procedures were authorized by the UC Irvine Institutional Review Board (UCI IRB) and conducted at the UCI Institute for Clinical Translational Science (UCI ICTS). 87 peri-pubertal children were enrolled, each performing one of two exercise protocols (either 30 or 6 min of cycling) as described below.
Forty-five participants were normal-weight (<85th percentile for age- and gender-adjusted BMI or BMI%), healthy children; of these 36 (CTRL-1) completed the 30-min exercise protocol (Ex1) and 9 (CTRL-2) the 6-min protocol (Ex2). Eighteen children (OW) were >90th BMI%, but were otherwise healthy, while 16 others had type 1 diabetes (T1DM); these two groups also performed the Ex1 exercise protocol. The last 8 participants had exercise-induced asthma (EIA); this group, similar to CTRL-2, performed Ex2.
Preliminary Visit
At least 2 days before the main study, all subjects and respective parents/guardian participated in a preliminary visit in which they were fully informed of all procedures and possible risks, and signed consent and assent forms. A medical history and physical examination ensured that the following enrollment criteria were fulfilled—CTRL-1 and CTRL-2: no prior diagnosis of chronic disease, recent common cold, or injury; not taking over-the-counter or prescription drugs; normal vital signs and BMI% at visit; OW group: same as above except for BMI% >90th; T1DM group: same as CTRL groups except for prior T1DM diagnosis and insulin as only medication; EIA group: same as CTRL groups except for prior diagnosis of exercise-induced asthma. In an attempt to balance maturational status across groups, pubertal status was determined in CTRL-1, OW, and T1DM via completion of a validated standard questionnaire, commonly used in our and other institutions conducting research on children (18). Mean Tanner stage for the three groups were 2.8±0.3 (1–5), 2.4±0.3 (1–5), and 3.0±0.3 (1–4), respectively. For the CTRL-2 and EIA groups, Tanner stage data were incomplete; however, the mean ages (14.1 and 14.2 years) and the gender composition (5/4 and 5/3 M/F ratios) of these two groups were so similar that effects due to differences in mean maturational status are highly unlikely.
All participants then completed a preliminary cycling test (Ergoline 800S, SensorMedics, Yorba Linda, CA) conducted by an exercise physiologist from the UCI ICTS. After 2 min of unloaded pedaling, the resistance on the ergometer was increased by 10–20 watts per min (~10% of individually predicted maximal workload/min) until subjects were unable to sustain pedaling (5). Breath-by-breath gas exchange was measured by a standard metabolic cart (SensorMedics, Yorba Linda, CA), and the anaerobic threshold (AT, a level beyond which cellular aerobic energy production must be accompanied by anaerobic processes) and maximal aerobic capacity (VO2max, the gold standard marker of overall fitness for adults and children) were obtained.
Study Day Procedures
All subjects reported to the UCI ICTS at ~7:30 a.m. in the morning of the study day. Normal vital signs were confirmed, and an intravenous catheter was started on the left median cubital vein for multiple blood draws. After 90 min of rest, baseline blood samples were obtained, and exercise protocols were started.
30-min Exercise Protocol (CTRL-1, OW, and T1DM Groups)
Subjects performed 10 repeats of 2 min cycling at 80% VO2max followed by 1 min rest; of the total 30 min, therefore, 20 min were actual exercise and 10 min rest (Ex1, Fig. 1). 80% VO2max was chosen as it corresponds to approximately midway between AT and VO2max, a work-rate that would induce a full systemic and molecular exercise response. The intermittent pattern renders the challenge more acceptable to children and more closely mimics spontaneous physical activity; it has therefore become a successful standard research protocol for thousands of children studies at the UCI ICTS. Due to possible fluctuations in blood glucose that may confound data interpretation in T1DMs, additional precautions were taken in that group, with multiple prior blood glucose samples and insulin adjustments, ensuring that for at least 90 min before the study, and throughout all study procedures, plasma glucose and insulin were in the physiologic range. The details of these pre-study procedures in T1DM have been previously described in greater detail (8).
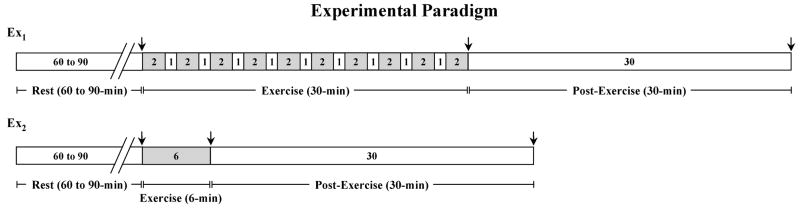
Fig. 1.
Schematic of exercise protocols. Grey areas represent actual exercise time. Arrows indicate time of blood sampling.
6-min Exercise Protocol (CTRL-2 and EIA Groups)
The second groups of healthy children (CTRL-2) and the asthmatic (EIA) performed a 6-min cycling exercise at a constant rate resulting in a heart rate of 170 beats per min, corresponding to ~70% VO2max (Ex2, Fig. 1); this is the standard exercise challenge test for diagnosis and monitoring of exercise-induced asthma, as per guidelines issued by the American Thoracic Society (1). While it would have been ideal to have all children from all study groups perform both exercise protocols, logistical (initial enrollment in separate research protocols) and ethical reasons (inability of EIA children to perform the longer protocol if an asthmatic episode occurred) prompted the choice of the reported number and type of exercise challenges.
Post-Exercise Procedures
All participants, irrespective of which exercise protocol they had performed, were moved to a reclining chair immediately after exercise cessation where they sat comfortably for 30 min. 2.0 cc whole blood samples were drawn at baseline, immediately after (within the first 15 sec after the end of cycling, while the subject is still sitting on the ergometer), and 30-min post-exercise. Only three time points could be used due to volume limitations relative to the young ages of the participants and to additional assays required by the original protocol design.
Samples were stored in a sterile B.D. Vacutainer tubes (Becton, Dickson and Company, Franklin Lakes, NJ; Lot 6178160) coated with 3.6 mg K2-EDTA. The tubes were then processed at the UCIMC Department of Pathology and Laboratory Medicine. In short, the samples were inverted 60 times, placed on a mechanical mixer for 2 min and run through a Beckman Coulter LH750 System (Beckman Coulter, Fullerton, CA) for WBC count with automated differential and hematocrit. Twelve tubes were loaded onto one hemogard-specific cassette, which was then inserted into a loading bay and afterwards transferred to the sampling station, where the stripper plate locks onto the tube. The tube cap is then pierced by sensing needle, and 300 μL of sample is aspirated into the blood sampling valve. 28 μL is delivered with 6 mL of diluent to the WBC bath. Count was determined when a cell in a conductive liquid goes through a small aperture and changes the electrical resistance between the pulse of the submerged electrodes. For WBC differential, an additional 31 μL of original blood was retracted into a mixing chamber in the presence of lytic reagent. The VCS technology was then employed: electrical impedance for cell volume, conductivity for internal cellular nuclear and granularity, and laser light scattering for cell shape and granularity. The signals were then amplified and analyzed.
Statistical Analysis
Data analysis was performed by the UCI ICTS biostatistics core. For each study group, the mixed model, a statistical model for analyzing longitudinal data, was applied to evaluate absolute cell count changes in total WBC, lymphocytes, neutrophils and monocytes, respectively, through the three exercise time-points. Data were first tested for presence of overall differences within the three time point sets, with an overall significance level set at p=0.05; paired comparisons between baseline, end-exercise, and 30-min post-exercise were then evaluated at p=0.0167 level, with and without adjusting for covariates (age, gender, BMI%, and VO2max). All procedures were performed with SAS 9 statistical software (Cary, NC).
Adjusting for Covariates
Application of the mixed model as the statistical analysis tool was used to account for the possible effects of a number of covariates (gender, age, BMI% and VO2max), since they have been previously reported as possible confounding characteristics (27). In no instance, however, this adjustment revealed a significant effect on the patterns of exercise-induced leukocyte changes, and therefore the final analyses were completed without adjusting for covariates. Interestingly, while in all groups, the 30-min post lymphocyte counts were slightly (albeit not significantly) below baseline, the only group in which, after adjustment for covariates, this reduction approached significance was the T1DM group, in which lymphocytes were 15% lower than baseline with a p-value of 0.035, not sufficient to reach significance after Bonferroni correction.
RESULTS
Leukocyte Responses to Ex1 (30-min Intermittent)
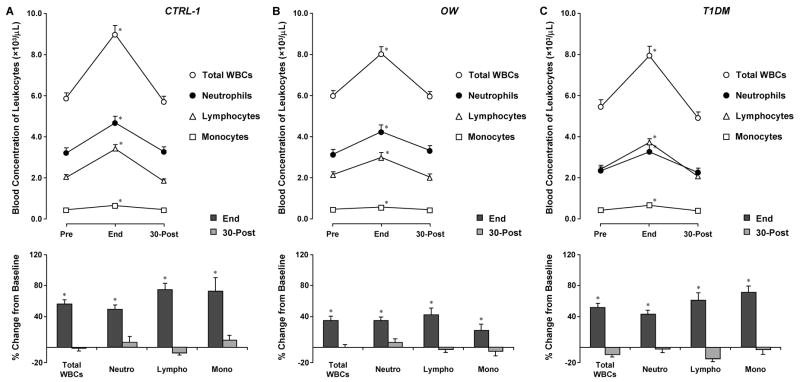
In CTRL-1, the mean baseline total WBC count was 5.9±0.3 (all total and subtype leukocyte counts results are in units: ×103/μL) (Fig. 2). As expected, leukocytosis occurred by end-exercise (9.0±0.3, +55%, p<0.001) and was corrected by 30-min post-exercise recovery (5.7±0.3). Lymphocytes and monocytes (baseline: 2.1±0.1; 0.42±0.03, respectively) followed a similar pattern, increasing at end-exercise (3.4±0.1, +73%; 0.65±0.03, +72%, respectively; p<0.001 for both) and returning to baseline levels at 30-min post (1.9±0.1; 0.44±0.03, respectively). Interestingly, and contrary to what has been reported in many exercise studies on adults, neutrophils also followed a similar pattern, with baseline levels of 3.2±0.3, an end-exercise increase to 4.7±0.3 (+48%, p<0.001), and a return to levels comparable to baseline by 30-min post-exercise (3.3±0.3).
Fig. 2.
Leukocyte responses to 30-min intermittent cycling at 80% VO2max (Ex1) in healthy (CTRL-1), overweight (OW) and type 1 diabetic (T1DM) children. Values are group means ± SE. * signify p<0.001, end-exercise versus both baseline and 30-min post-exercise.
In the other two groups utilizing the 30-min protocol (OW and T1DM), leukocyte responses to exercise were similar to CTRL-1, with comparable leukocytosis at end-exercise and return to baseline by 30-min of recovery. In the OW group, baseline levels of total WBCs, neutrophils, lymphocytes, and monocytes were 6.0±0.3, 3.2±0.3, 2.1±0.2, and 0.46±0.03, respectively; exercise-induced changes were 8.0±0.3 (+35%), 4.2±0.3 (+35%), 3.0±0.2 (42%), and 0.56±0.03 (+22%), respectively (Fig. 2) and p<0.001 for all changes; and at 30-min post, counts were 6.0±0.3, 3.3±0.3, 2.0±0.2, and 0.43±0.03, respectively. In the T1DM group, baseline levels of total WBCs, neutrophils, lymphocytes, and monocytes were 5.4±0.4, 2.3±0.3, 2.4±0.1, and 0.40±0.05, respectively; exercise-induced changes were 7.9±0.4 (+48%), 3.3±0.3 (+40%), 3.7±0.1 (+57%), and 0.65±0.05 (+66%), respectively, p<0.001 for all; and 30-min post counts were 4.9±0.4, 2.2±0.3, 2.1±0.1, and 0.38±0.05, respectively.
Leukocyte Responses to Ex2 (6-min Continuous)
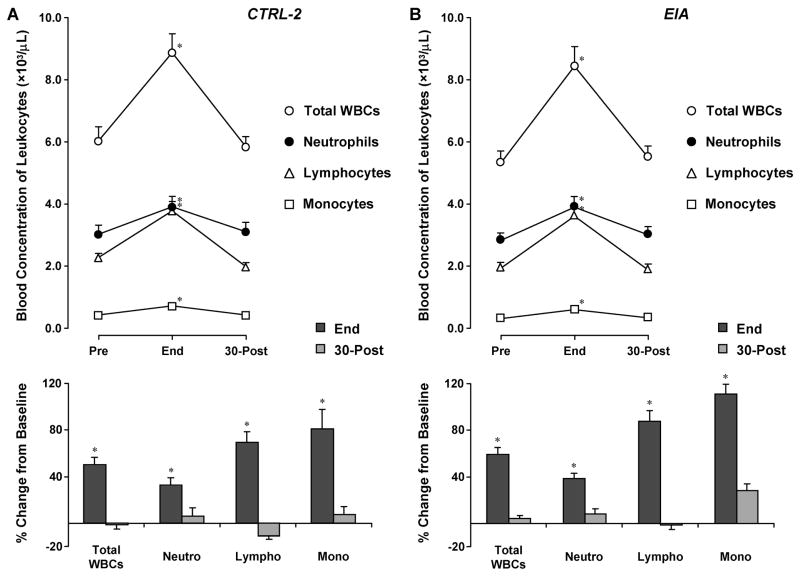
The remaining two groups of subjects (CTRL-2 and EIA) performed a constant load, 6-min exercise protocol at HR of 170 bpms (resulting in ~70% VO2max) (Fig. 1). Despite the marked differences between the two types of exercise, the general pattern of leukocyte changes was similar to that observed in the 30-min exercise protocol. In CTRL-2, significant increases were observed from baseline to end-exercise in total WBCs (from 6.0±0.5 to 8.9±0.5, +50%, again, all leukocyte counts are in units: ×103/μL), neutrophils (from 3.0±0.3 to 3.9±0.3, +33%), lymphocytes (from 2.3±0.2 to 3.8±0.2, +69%) and monocytes (from 0.42±0.05 to 0.72±0.05, +81%) (Fig. 2), p<0.001 for all changes. At 30-min post all counts returned to baseline levels (total WBCs, 5.8±0.5; neutrophils, 3.1±0.3; lymphocytes, 2.0±0.2; and monocytes, 0.43±0.05).
Similarly, in the EIA group, all leukocyte counts were significantly increased at end-exercise as compared to baseline: total WBCs, from 5.3±0.5 to 8.4±0.5, +60%; neutrophils, from 2.8±0.3 to 3.9±0.3, +39%; lymphocytes, from 1.9±0.3 to 3.6±0.3, +88%; and monocytes, from 0.32±0.06 to 0.61±0.06, +111%), p<0.001 for all. Again, by 30-min post, all leukocyte counts had returned to levels comparable to baseline (total WBCs: 5.5±0.5; neutrophils: 3.0±0.3; lymphocytes: 1.9±0.3; monocytes: 0.35±0.06). As eosinophils and basophils have been implicated in chronic anaphylactic conditions such as asthma, their levels were also examined between CTRL-2 and EIA. Values were similar between groups at baseline (0.31±0.04 and 0.22±0.05, respectively for eosinophils; 0.031±0.007 and 0.031±0.005, respectively, for basophils), and remained similar at end-exercise (0.42±0.03 and 0.28±0.02, respectively, for eosinophils; 0.044±0.007 and 0.029±0.005, respectively, for basophils) and at 30-min post (0.28±0.01 and 0.21±0.01, respectively, for eosinophils; 0.033±0.004 and 0.023±0.004, respectively, for basophils).
It is important to report that four out of the eight EIA subjects actually developed an asthmatic episode during the exercise challenge, while still managing to finish the 6 min bout. Interestingly, the WBC response to exercise was similar in these subjects when compared to the EIA subjects who did not experience an asthmatic attack as well as to control subjects. This consistency also included eosinophil and basophil responses to exercise.
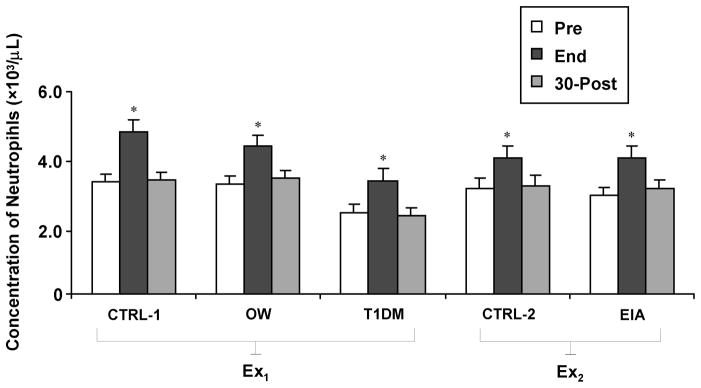
Individual Neutrophil Responses to Exercise
As post-exercise neutrophil counts have shown considerable variability in prior studies, possibly due to differences in exercise intensity, timing of sampling, and age of participants, individual neutrophil profiles were analyzed in depth for all children in this study (Fig. 5). The rapid correction of end-exercise neutrophilia, shown by the mean values of each experimental group, was indeed reflected in the large majority of individual profiles (83/87 subjects, or 95.5%). In the only 4 instances that neutrophilia was sustained at the 30-min post time-point, no systematic unifying characteristic across subjects was found, as they belonged to different experimental groups (one in CTRL-1, one in TIDM, and two in CTRL-2), and differed considerably by gender, BMI, fitness, and maturational status.
Fig. 5.
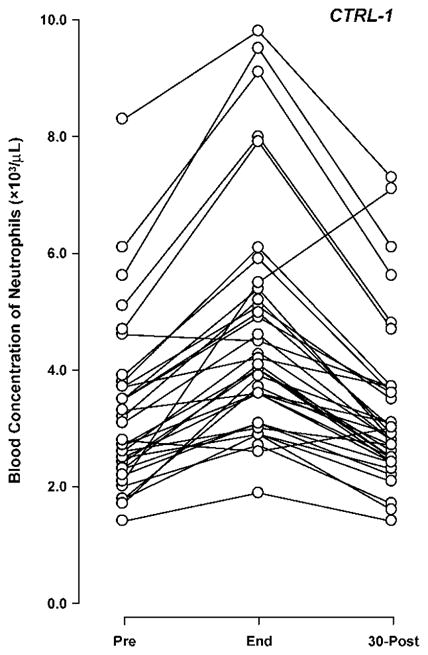
Individual neutrophil response to 30 min of intermittent cycling exercise (Ex1) in 36 healthy children.
DISCUSSION
The most interesting finding from this study is that healthy children as well as children with different common chronic inflammatory conditions (overweight, T1DM, EIA) undergoing different exercise formats (30-min intermittent at 80% VO2max and 6-min constant at ~70% VO2max) all experienced a highly consistent pattern of leukocyte changes in response to exercise. In all experimental groups, all leukocyte subtypes increased significantly at end-exercise, and counts returned to near-baseline levels within the following 30 min.
The fact that this pattern of leukocyte changes is so strictly maintained, independent of exercise type and pediatric subpopulation, suggests its importance as a crucial component of pediatric adaptation to stress. Furthermore, this response pattern was observed not only for the two exercise regimens used in our study (utilizing differing moderate intensities—80% and 70% VO2max—and durations—30-min intermittent and 6-min constant), but also in the more strenuous and prolonged protocol used in a prior study by Timmons et al (60-min constant load at 70% VO2max) in healthy children (26). More importantly, in our study, the same pattern was maintained in healthy children and children with commonly occurring chronic conditions involving immune dysfunctions (overweight, T1DM, EIA). This remarkable homogeneity was not only displayed through mean group results, but was confirmed at the individual level in >95% of participants. Indeed, only 4 out of 87 subjects displayed sustained post-exercise neutrophilia. These subjects were distributed across 3 different experimental groups, and varied broadly in a number of pertinent characteristics: one was a healthy post-pubertal female with somewhat high BMI% and low fitness; the second, a T1DM pre-pubertal female with low BMI% and high fitness; and the last two healthy pre-pubertal males, one with low BMI% and with high fitness and the other with medium BMI% and fitness. It should be noted that we did not make any measurement past the 30-min post time-point. Earlier studies have shown, at least for more intense and prolonged exercise regimens, that neutrophils (after returning to baseline 30 min following exercise cessation) may have a rebound increase after an additional 30 min (10),(27). Ascertaining whether this observation also occurs in response to moderate exercise challenges used in the present study will require additional work.
Despite the reported lack of differences across groups in the characteristics of post-exercise leukocytosis, prior evidence suggests that exaggerated inflammatory activation in response to exercise indeed occurs in dysmetabolic conditions; in type 1 diabetic children, for instance, elevated levels of IL-6 and accelerated kinetics of multiple pro-inflammatory mediators have been observed during exercise (7),(21), while in obese children elevated markers of both oxidative stress and inflammation have been reported (13). It is therefore likely that these changes, while significantly affecting the overall inflammatory status, did not occur as a consequence of apparent increases in leukocyte circulating numbers. Alternative mechanisms may include cytokine secretion from tissues other than leukocytes, and/or the enhancement of cytokine production from the same number of circulating leukocytes. The latter hypothesis is supported by recent observations that at least a fraction of circulating leukocytes display enhanced cytokines secretion and increased RNA expression of possibly hundreds of leukocyte genes in response to exercise for adult subjects (2),(15),(30). That this may also occur in children was recently confirmed in a study by our group, in which expression of ~140 genes was altered in healthy pre-pubertal boys challenged with the same exercise protocol used in the present study (20). Whether these changes reflect an activation of previously circulating leukocytes, a release of new leukocytes with varying activation levels into circulation, or a combination of those two processes, and the numbers of the originally circulating leukocytes that remain in the bloodstream after correction of exercise-induced leukocytosis, are all intriguing questions that the current study cannot elucidate but that are likely to be the objectives of future investigations.
A relatively well-established concept associated with post-exercise leukocyte status is that shortly after exercise cessation, a rapid decline of lymphocyte counts below pre-exercise levels is often observed; as this is also often associated with reduced NK cells function, a transiently increased risk of upper respiratory infections has been reported, leading to the formulation of the “open window” of susceptibility hypothesis (17). In our study, although the mean post-exercise lymphocyte counts of all five experimental groups indeed dropped slightly below baseline, this drop was quantitatively very small, never reaching statistical significance. Interesting, while the lymphocyte counts in the OW, EIA, and in the two CTRL groups were on average ~7% lower at 30-min post-exercise when compared to baseline, this reduction was ~15% in the T1DM; and even though this reduction was also not statistically significant, the observed process is consistent with the notion of increased susceptibility to infections in diabetic children (11),(28) and suggests that exercise modulation of immune responses may be a risk factor of this pathogenetic mechanism. It should also be noted that our study only reported overall lymphocyte counts; differing behaviour of lymphocyte subpopulations (B cells, T helpers and T suppressors, NK cells) in response to exercise can certainly represent an additional mechanism by which immunity is modulated through exercise and deserves attention in future research.
The practical importance of our findings rests on a current paradoxical situation of the increased need for specific exercise protocols tailored towards a variety of pediatric conditions and the unavailability of physiological basis to develop them. If properly utilized, regular physical activity retains major protective effects against cardiovascular morbidity and mortality (14). Maintaining an active lifestyle is therefore even more important for patients at increased risk for these outcomes, such as the obese, diabetic, and asthmatic pediatric subpopulations (9),(23). Obesity, diabetes, and asthma, on the other hand, are intrinsically associated with immunologic dysregulations that may alter molecular responses and possibly reduce the health benefits of exercise (for at least some types of activities) (4). While the extent and physiological significance of these potential alterations are still incompletely understood, the optimal use of exercise in preventing diseases in children must be based on a thorough understanding of all aspects of its underlying mechanism and various molecular pathways. In this context, the observation from the present study can help define a strongly preserved component of exercise adaptation in the pediatric population as a whole.
In conclusion, we report here that the pattern of acute exercise-induced leukocytosis is rapidly corrected in children within 30 min after exercise cessation. This process occurred similarly across different exercise formats, as well as in healthy children and in children with common chronic conditions, suggesting that this response is a strongly protected adaptive paradigm during the pediatric age.
Fig. 3.
Leukocyte responses to 6-min continuous cycling at ~70% VO2max (Ex2) in healthy children (CTRL-2) and children with exercise-induced asthma (EIA). Values are group means ± SE. * signify p<0.001, end-exercise versus both baseline and 30-min post-exercise.
Fig. 4.
Neutrophil responses to two exercise formats (30-min intermittent cycling at 80% VO2max, Ex1, and to 6-min continuous cycling at ~70% VO2max, Ex2) in healthy children (CTRL-1 and CTRL-2) and children with exercise-induced asthma (EIA), overweight (OW), and type 1 diabetes (T1DM). Values are group means ± SE. * signify p<0.001, end-exercise versus both baseline and 30-min post-exercise.
TABLE 1.
Demographic information for the five experimental groups.
| Age (Yr) | Gender (M/F) | Height (cm) | Weight (kg) | BMI% | VO2max (mL/min/kg) | n | |
|---|---|---|---|---|---|---|---|
| CTRL-1 | 13.0 ± 0.5 | 15/21 | 153.2 ± 2.6 | 47.0 ± 2.4 | 53.9 ± 3.9 | 35.9 ± 1.1 | 36 |
| OW | 11.3 ± 0.5 | 11/7 | 148.0 ± 2.6 | 59.8 ± 4.7 | 96.5 ± 0.6 | 28.5 ± 1.4 | 18 |
| T1DM | 13.5 ± 0.4 | 10/6 | 162.8 ± 3.8 | 50.8 ± 2.9 | 53.5 ± 6.0 | 40.5 ± 2.5 | 16 |
| CTRL-2 | 14.1 ± 0.8 | 5/4 | 164.7 ± 3.5 | 53.9 ± 3.9 | 52.9 ± 8.1 | 42.6 ± 2.8 | 9 |
| EIA | 14.2 ± 0.5 | 5/3 | 171.5 ± 3.1 | 61.3 ± 3.1 | 66.1 ± 3.6 | 38.0 ± 3.0 | 8 |
Data are means ± SE. BMI% is BMI percentile according to age in months and gender.
Acknowledgments
The authors would like to thank all of the UCI ICTS staff members for the hard work in making this research successful.
GRANTS
This research was funded by NIH (National Institute of Health) grants M01-RR00827-28 and K-23 RR018661-01, and by the JDRF (Juvenile Diabetes Research Foundation) grant #11-2003-332.
References
- 1.American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 2.Connolly PH, V, Caiozzo J, Zaldivar F, Nemet D, Larson J, Hung S, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol. 2004;97:1461–1469. doi: 10.1152/japplphysiol.00316.2004. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DM, Nemet D, Galassetti P. Exercise, stress, and inflammation in the growing child: from the bench to the playground. Curr Opin Pediatr. 2004;286 doi: 10.1097/01.mop.0000126601.29787.39. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DM, Radom-Aizik S, Schwindt C, Zaldivar F., Jr Dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol. 2007;103:700–709. doi: 10.1152/japplphysiol.00225.2007. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–634. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 6.Galassetti P, Mann S, Tate D, Neill RA, Wasserman DH, Davis SN. Effect of morning exercise on counterregulatory responses to subsequent, afternoon exercise. J Appl Physiol. 2001;91:91–99. doi: 10.1152/jappl.2001.91.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatric Diabetes. 2006;7:16–24. doi: 10.1111/j.1399-543X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 8.Galassetti PR, Iwanaga K, Pontello AM, Zaldivar FP, Flores RL, Larson JK. Effect of prior hyperglycemia on IL-6 responses to exercise in children with type 1 diabetes. American Journal of Physiology Endocrinology and Metabolism. 2006;290:E833–839. doi: 10.1152/ajpendo.00445.2005. [DOI] [PubMed] [Google Scholar]
- 9.Giannini C, Mohn A, Chiarelli F. Physical exercise and diabetes during childhood. Acta Biomed. 2006;77(Suppl 1):18–25. [PubMed] [Google Scholar]
- 10.Ladha AB, Courneya KS, Bell GJ, Field CJ, Grundy P. Effects of acute exercise on neutrophils in pediatric acute lymphoblastic leukemia survivors: a pilot study. J Pediatr Hematol Oncol. 2006;28:671–677. doi: 10.1097/01.mph.0000243644.20993.54. [DOI] [PubMed] [Google Scholar]
- 11.Lopez R, Fernandez O, Jara G, aelum VB. Epidemiology of necrotizing ulcerative gingival lesions in adolescents. J Periodontal Res. 2002;37:439–444. doi: 10.1034/j.1600-0765.2002.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. 1988;6:333–363. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- 13.McMurray RG, Zaldivar F, Galassetti P, Larson J, Eliakim A, Nemet D, Cooper DM. Cellular immunity and inflammatory mediator responses to intense exercise in overweight children and adolescents. J Investig Med. 2007;55:120–129. doi: 10.2310/6650.2007.06031. [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 15.Nieman DC, Henson DA, Davis JM, Dumke CL, Utter AC, Murphy EA, Pearce S, Gojanovich G, McAnulty SR, McAnulty LS. Blood leukocyte mRNA expression for IL-10, IL-1Ra, and IL-8, but not IL-6, increases after exercise. Journal of Interferon Cytokine Research. 2006;26:668–674. doi: 10.1089/jir.2006.26.668. [DOI] [PubMed] [Google Scholar]
- 16.Nieman DC, Henson DA, Johnson R, Lebeck L, Davis JM, Nehlsen-Cannarella SL. Effects of brief, heavy exertion on circulating lymphocyte subpopulations and proliferative response. Med Sci Sports Exerc. 1992;24:1339–1345. [PubMed] [Google Scholar]
- 17.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–251. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;V17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 19.Pyne DB. Regulation of neutrophil function during exercise. Sports Med. 1994;17:245–258. doi: 10.2165/00007256-199417040-00005. [DOI] [PubMed] [Google Scholar]
- 20.Radom-Aizik S, Zaldivar FP, Leu S, Wilson LD, Cooper DM. Exercise-induced differential gene expression in PBMCs of early and late pubertal boys. Med Sci Sports Exerc (Abstract, ACSM Conference on Integrative Physiology of Exercise) 2006;38(Suppl 1):S35. [Google Scholar]
- 21.Rosa JS, Oliver SR, Mitsuhashi M, Flores RL, Pontello AM, Zaldivar FP, Galassetti PR. Altered kinetics of IL-6 and other inflammatory mediators during exercise in children with type 1 diabetes. J Investig Med. 2008;56:701–713. doi: 10.2310/JIM.0b013e31816c0fba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon HB. The immunology of exercise. A brief review. JAMA. 1984;252:2735–2738. [PubMed] [Google Scholar]
- 23.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 24.Strong WB, Malina RM, Blimkie CJR, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, Rowland T, Trost S, Trudeau F. Evidence based physical activity for school-age youth. The Journal of Pediatrics. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 25.Tiidus PM, Pushkarenko J, Houston ME. Lack of antioxidant adaptation to short-term aerobic training in human muscle. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 1996;271:R832–836. doi: 10.1152/ajpregu.1996.271.4.R832. [DOI] [PubMed] [Google Scholar]
- 26.Timmons BW, Tarnopolsky MA, Bar-Or O. Immune responses to strenuous exercise and carbohydrate intake in boys and men. Pediatr Res. 2004;56:227–234. doi: 10.1203/01.PDR.0000132852.29770.C5. [DOI] [PubMed] [Google Scholar]
- 27.Timmons BW, Tarnopolsky MA, Snider DP, Bar-Or O. Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc. 2006;38:293–304. doi: 10.1249/01.mss.0000183479.90501.a0. [DOI] [PubMed] [Google Scholar]
- 28.Tuvemo T. The role of trace elements in juvenile diabetes mellitus. Pediatrician. 1983;12:213–219. [PubMed] [Google Scholar]
- 29.Wasserman K, Sietsema KE. Assessing cardiac function by gas exchange. Cardiology. 1988;75:307–310. doi: 10.1159/000174390. [DOI] [PubMed] [Google Scholar]
- 30.Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ, Wilson LD, Cooper DM. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100:1124–1133. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]