Recombinant glucuronic acid dehydrogenase from the halophilic bacterium Chromohalobacter salexigens has been crystallized and X-ray diffraction data collected to a maximum resolution of 2.1 Å.
Keywords: glucuronic acid dehydrogenase, seaweed, short-chain dehydrogenase/oxidoreductase family
Abstract
Glucuronic acid dehydrogenase (GluUADH), the product of the Csal-2474 gene from the halophilic bacterium Chromohalobacter salexigens DSM 3043, is an enzyme with potential use in the conversion of glucuronic acid in seaweed biomass to fuels and chemicals. GluUADH is an enzyme that catalyzes the oxidation of glucuronic acid (GluUA) and galacturonic acid (GalUA) and has a preference for NAD+ rather than NADP+ as a cofactor. Recombinant GluUADH was crystallized in the presence of 0.2 M calcium acetate, 0.1 M Tris–HCl pH 7.0 and 20% PEG 3000 at 295 K. X-ray diffraction data were collected to a maximum resolution of 2.1 Å. The GluUADH crystal belonged to space group P63, with unit-cell parameters a = b = 122.58, c = 150.49 Å, γ = 120°. With one molecule per asymmetric unit, the crystal volume per unit protein weight (V M) is 2.78 Å3 Da−1. The structure was solved by the single anomalous dispersion method and structure refinement is in progress.
1. Introduction
Seaweed biomass is regarded as a third-generation biomass that can be used as an alternative feedstock to produce fuels and chemicals. Compared with traditional biomasses such as land crops and wood, seaweeds can be grown more rapidly with lower cost. Nevertheless, their utilization as an industrial feedstock is not yet effective owing to the limited knowledge of methods of converting the unusual monomers of seaweed polysaccharides into useful chemicals. For instance, d-glucuronic acid (GluUA) and l-rhamnose are two major constituents of Ulvales, common green seaweeds that are distributed worldwide. However, GluUA has not yet been used industrially despite its great potential as a biomass feedstock (Oppermann et al., 2003 ▶). If effective ways of converting GluUA to value-added chemicals can be developed, green seaweeds could become an important biomass feedstock in the future.
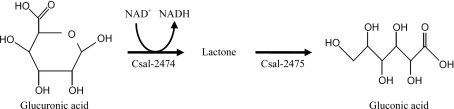
Recently, uronate dehydrogenases that can catalyze the oxidation of GluUA and d-galacturonic acid (GalUA) have been identified and characterized from Agrobacterium tumefaciens (Yoon et al., 2009 ▶; Boer et al., 2010 ▶). A. tumefaciens uronate dehydrogenase shows high sequence similarity to the glucuronic acid dehydrogenase (GluUADH) Csal-2474 from the halophilic bacterium Chromohalobacter salexigens. This may provide a novel method for the effective utilization of GluUA. With a preference for NAD+ rather than NADP+ as a cofactor, GluUADH catalyzes the oxidation of GluUA to yield d-glucuronic acid lactone, which can easily be converted to d-glucaric acid, a ‘top value-added chemical’ from biomass (Fig. 1 ▶). As an initial step towards determining the first crystal structure of glucuronic acid dehydrogenase, in this report we describe the cloning, expression, purification, crystallization and X-ray crystallographic analysis of GluUADH from the halophilic bacterium C. salexigens.
Figure 1.
Degradation pathway of glucuronic acid. Csal-2474, a glucuronic acid dehydrogenase, catalyzes the conversion of glucuronic acid to several forms of lactone, which are then converted to gluconic acid by Csal-2475.
2. Expression and purification of recombinant GluUADH
The forward and reverse primers were designed as 5′-CGGGATCCCTCAATCGTTTGTTGGTGAC-3′ and 5′-CCCAAGCTTTTACTCAGCGTCGTCGGGAT-3′ and contained BamHI and HindIII cleavage sequences. The Csal-2474 gene was amplified from chromosomal DNA of C. salexigens strain DSM 3043 by polymerase chain reaction (PCR; Saiki et al., 1988 ▶). The PCR product was then subcloned into pQE30 with His6 at the N-terminus. The resulting expression vector pQE80:Csal-2474 was transformed into Escherichia coli strain B834 (DE3) and the cells were grown in LB medium containing ampicillin at 310 K. After induction with 1.0 mM IPTG for a further 20 h at 295 K, the culture was harvested by centrifugation at 5000g and 277 K. The cell pellet was resuspended in ice-cold buffer A (50 mM Tris–HCl pH 8.0, 5 mM β-mercaptoethanol) and disrupted by ultrasonication. The cell debris was removed by centrifugation at 11 000g for 1 h and the lysate was bound to Ni–NTA agarose (Qiagen). After washing with buffer A containing 10 mM imidazole, the bound proteins were eluted with buffer A containing 300 mM imidazole. Further purification was performed using Superdex 200 (Amersham Biosciences) equilibrated with a final buffer consisting of 40 mM Tris–HCl pH 8.0 and 5 mM β-mercaptoethanol. The purity of the protein eluted from the column was checked by SDS–PAGE and was greater than 95% (Fig. 2 ▶). The protein was concentrated to a final concentration of 20 mg ml−1 for crystallization. SeMet-substituted protein was expressed in minimal medium supplemented with SeMet and was purified under the same conditions as the native protein.
Figure 2.
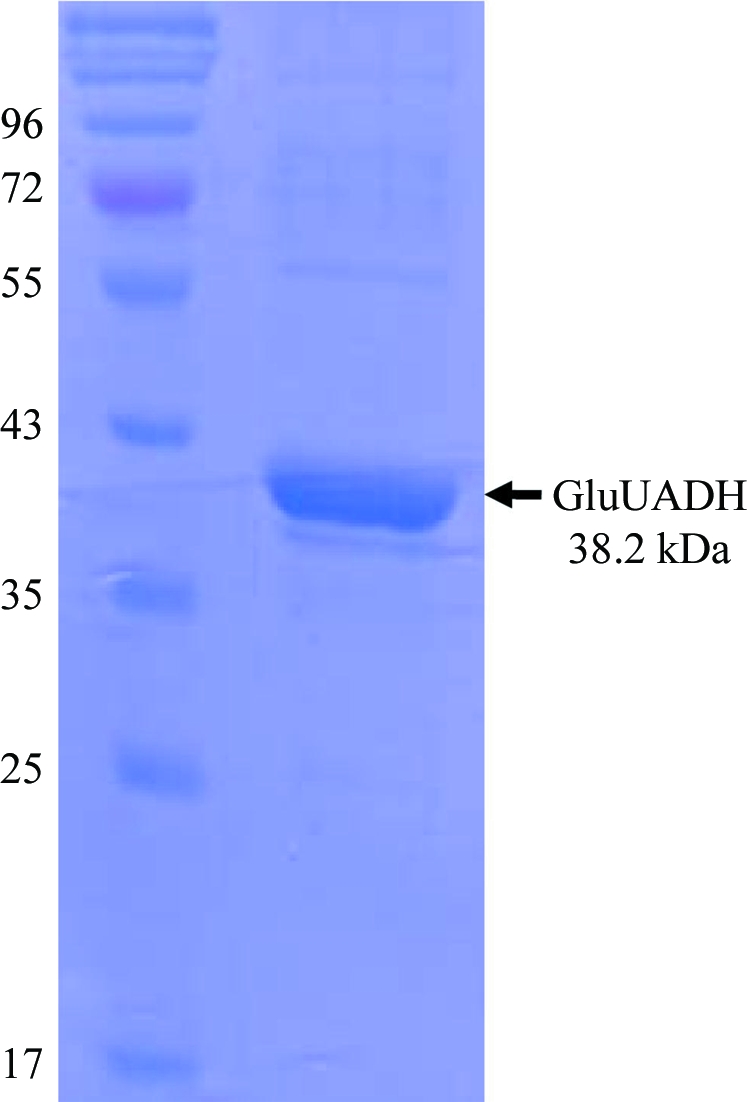
SDS–PAGE of the purified GluUADH Csal-2474 from the halophilic bacterium C. salexigens. Molecular-weight markers are labelled (in kDa) on the left of the gel and purified GluUADH protein with a molecular weight of 38.2 kDa is indicated on the right of the gel.
3. Crystallization
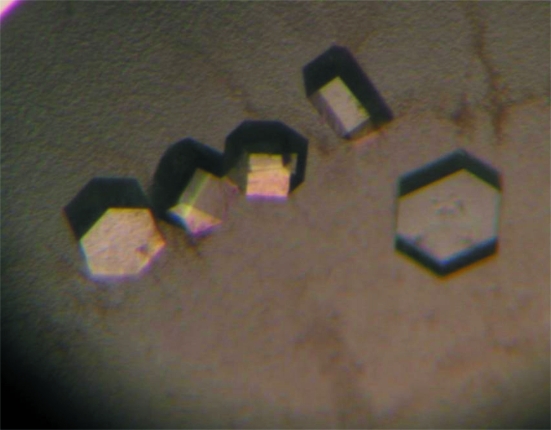
Purified GluUADH was initially screened using commercially available sparse-matrix screens from Hampton Research and Emerald BioSystems by the hanging-drop vapour-diffusion technique at 295 K. Each experiment consisted of mixing 1.5 µl protein solution (28 mg ml−1 in 40 mM Tris–HCl pH 8.0 and 5 mM β-mercaptoethanol) with 1.5 µl reservoir solution and equilibrating the drop against 0.3 ml reservoir solution. Crystals of GluUADH were observed in several crystallization screening conditions. After several steps that optimized the crystallization process using the sitting-drop vapour-diffusion method, the best-quality crystals were obtained in 2 d and reached maximum dimensions of approximately 0.05 × 0.2 × 0.2 mm using reservoir solution consisting of 0.1 M imidazole pH 8.0, 10% polyethylene glycol (PEG) 8K and 0.1 M calcium acetate (Fig. 3 ▶). The volumes of the sitting drop and the reservoir solution were the same as those used in the hanging-drop vapour-diffusion method for the initial crystallization screening. SeMet crystals of GluUADH were obtained using the same crystallization conditions as were used for the native protein crystals.
Figure 3.
Hexagonal crystals of GluUADH from C. salexigens. The best quality crystals were obtained using 0.1 M imidazole pH 8.0, 10% polyethylene glycol (PEG) 8K and 0.1 M calcium acetate.
4. X-ray analysis
The solution used to obtain the best crystals with 30% glycerol was used as the cryoprotectant and the crystals were immediately placed in a 100 K nitrogen-gas stream. X-ray diffraction data were collected from a native crystal to a resolution of 2.1 Å on the 6C1 beamline of Pohang Accelerator Laboratory (PAL, Republic of Korea) using a Quantum 210 CCD detector (ADSC, San Diego, California, USA). All data were indexed, integrated and scaled using the HKL-2000 software package (Otwinowski & Minor, 1997 ▶). The crystal of GluUADH belonged to space group P63, with unit-cell parameters a = b = 122.58, c = 150.49 Å, α = β = 90, γ = 120°. Assuming that the asymmetric unit contains four molecules of GluUADH, the crystal volume per unit of protein mass was 2.78 Å3 Da−1 (Matthews, 1968 ▶), corresponding to a solvent content of approximately 55.73%. A crystal of SeMet-substituted GluUADH was successfully obtained using the same solution as used for the native protein. SAD data were collected from an SeMet crystal on the 6C1 beamline (MXII) of PAL at a wavelength of 0.9795 Å. The crystal structure of GluUADH is being determined by the SAD method. The SOLVE and RESOLVE software (Terwilliger, 2003 ▶) was used to calculate the phase and find the selenium locations. Building of the structure model and refinement are in progress using WinCoot, REFMAC5 and CNS (Emsley & Cowtan, 2004 ▶; Murshudov et al., 2011 ▶; Brünger et al., 1998 ▶). The statistics of the diffraction data are summarized in Table 1 ▶.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Native | Se peak | |
|---|---|---|
| Beamline | 6C1 (MXII), PAL | 6C1 (MXII), PAL |
| Wavelength (Å) | 1.23985 | 0.97950 |
| Temperature (K) | 100 | 100 |
| Oscillation (°) | 1.0 | 1.0 |
| Total rotation range (°) | 90 | 360 |
| Space group | P63 | P63 |
| Unit-cell parameters (Å) | a = 56.74, b = 62.37, c = 103.61 | a = 56.45, b = 62.25, c = 103.43 |
| Resolution limits (Å) | 50.00–1.67 (1.73–1.67) | 50.00–2.06 (2.13–2.06) |
| Total reflections | 303666 | 1264119 |
| Unique reflections | 73189 | 157583 |
| Completeness (%) | 97.8 (88.4) | 97.8 (84.5) |
| Rmerge† (%) | 7.3 (34.4) | 7.6 (42.3) |
| 〈I/σ(I)〉 | 35.78 (3.09) | 32.55 (7.74) |
| Multiplicity | 4.1 (2.6) | 8.0 (5.2) |
R
merge = 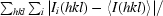
 , where Ii(hkl) and 〈I(hkl)〉 are the observed individual and mean intensities of a reflection, respectively.
, where Ii(hkl) and 〈I(hkl)〉 are the observed individual and mean intensities of a reflection, respectively. 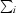 is the sum over the individual measurements of a reflection and
is the sum over the individual measurements of a reflection and  is the sum over all reflections.
is the sum over all reflections.
Acknowledgments
This work was supported by a grant from the Gyeongbuk Sea Grant Program funded by the Ministry of Land, Transport and Maritime Affairs of the Korean government. This work was also supported by a Korea Research Foundation grant funded by the Korean government (MEST; 313-2008-2-C00738) and by the National Research Foundation of Korea grant funded by the Korean Government (MEST; NRF-2009-C1AAA001-2009-0093483).
References
- Boer, H., Maaheimo, H., Koivula, A., Penttilä, M. & Richard, P. (2010). Appl. Microbiol. Biotechnol. 86, 901–909. [DOI] [PubMed]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Oppermann, U., Filling, C., Hult, M., Shafqat, N., Wu, X., Lindh, M., Shafqat, J., Nordling, E., Kallberg, Y., Persson, B. & Jörnvall, H. (2003). Chem. Biol. Interact. 143–144, 247–253. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K. B. & Erlich, H. A. (1988). Science, 239, 487–491. [DOI] [PubMed]
- Terwilliger, T. C. (2003). Methods Enzymol. 374, 22–37. [DOI] [PubMed]
- Yoon, S.-H., Moon, T. S., Iranpour, P., Lanza, A. M. & Prather, K. J. (2009). J. Bacteriol. 191, 1565–1573. [DOI] [PMC free article] [PubMed]