Abstract
Background
The Zimbabwean national prevention of mother-to-child HIV transmission (PMTCT) program provided primarily single-dose nevirapine (sdNVP) from 2002–2009 and is currently replacing sdNVP with more effective antiretroviral (ARV) regimens.
Methods
Published HIV and PMTCT models, with local trial and programmatic data, were used to simulate a cohort of HIV-infected, pregnant/breastfeeding women in Zimbabwe (mean age 24.0 years, mean CD4 451 cells/µL). We compared five PMTCT regimens at a fixed level of PMTCT medication uptake: 1) no antenatal ARVs (comparator); 2) sdNVP; 3) WHO 2010 guidelines using “Option A” (zidovudine during pregnancy/infant NVP during breastfeeding for women without advanced HIV disease; lifelong 3-drug antiretroviral therapy (ART) for women with advanced disease); 4) WHO “Option B” (ART during pregnancy/breastfeeding without advanced disease; lifelong ART with advanced disease); and 5) “Option B+:” lifelong ART for all pregnant/breastfeeding, HIV-infected women. Pediatric (4–6 week and 18-month infection risk, 2-year survival) and maternal (2- and 5-year survival, life expectancy from delivery) outcomes were projected.
Results
Eighteen-month pediatric infection risks ranged from 25.8% (no antenatal ARVs) to 10.9% (Options B/B+). Although maternal short-term outcomes (2- and 5-year survival) varied only slightly by regimen, maternal life expectancy was reduced after receipt of sdNVP (13.8 years) or Option B (13.9 years) compared to no antenatal ARVs (14.0 years), Option A (14.0 years), or Option B+ (14.5 years).
Conclusions
Replacement of sdNVP with currently recommended regimens for PMTCT (WHO Options A, B, or B+) is necessary to reduce infant HIV infection risk in Zimbabwe. The planned transition to Option A may also improve both pediatric and maternal outcomes.
Introduction
Antiretroviral drugs (ARVs) are highly effective for the prevention of mother-to-child HIV transmission (PMTCT). Without ARV prophylaxis, the risk of transmission by 18 months of age ranges from 25–40% in breastfeeding populations [1]–[3]. However, recent trials have demonstrated that several ARV regimens, administered to mothers and/or infants during pregnancy and breastfeeding, can reduce transmission to 1–8% at 6–12 months of age [4]–[10]. The World Health Organization (WHO) now recommends three-drug antiretroviral therapy (ART) for all pregnant women with CD4 cells <350/µL or clinical Stage 3–4 disease. For those with less advanced disease, two options are recommended. “Option A” includes short-course zidovudine during pregnancy and extended infant nevirapine (NVP) prophylaxis throughout breastfeeding. “Option B” includes maternal 3-drug ART during pregnancy and breastfeeding, with cessation after weaning [11]. Select PMTCT programs in sub-Saharan Africa are implementing Option B, and an “Option B+” has also been proposed (lifelong ART for all pregnant, HIV-infected women, regardless of CD4 cell count or disease stage) [12]. However, most programs in sub-Saharan Africa plan to implement the WHO 2010 guidelines by selecting Option A [12].
In 2009, only 53% of pregnant women identified as HIV-infected worldwide received any ARVs for PMTCT, resulting in approximately 370,000 new infant infections [13]. Many of these women received the ARV regimens previously recommended as a “minimum” intervention by WHO: a single dose of nevirapine (sdNVP) to a pregnant woman in labor and her infant after birth [13], [14]. Although inexpensive and relatively easy to administer, sdNVP is less effective than currently recommended regimens (18-month transmission risks: 15–25%) [15]–[17] and can lead to drug-resistant virus that complicates later therapy for both mothers and infected infants [18], [19].
Zimbabwe is a low-income country where prolonged breastfeeding is the norm [20], [21]. The Zimbabwean Ministry of Health and Child Welfare (MOHCW) has provided sdNVP through the national PMTCT program since 2002, as one of the earliest PMTCT programs in Africa [22]. In 2006–2007, the MOHCW, with the Elizabeth Glaser Pediatric AIDS Foundation and the Organization of Public Health Interventions and Development Trust, demonstrated the feasibility of a pilot program providing the antenatal/intrapartum component of the WHO Option A regimen [23]. Our objective was to use simulation modeling to evaluate the potential benefits to both infants and mothers of replacing sdNVP with WHO 2010-recommended regimens on a national scale in Zimbabwe.
Methods
Analytic overview
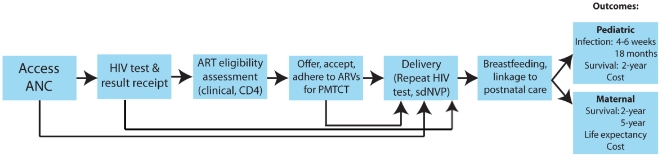
We linked two published computer simulation models to project clinical outcomes of five PMTCT strategies in Zimbabwe. First, a model of mother-to-child transmission (MTCT) during pregnancy and delivery [24] was modified to incorporate each step of the “cascade” of PMTCT-related care, from first presentation at antenatal care (ANC) through 18 months postpartum (Figure 1). Second, the Cost-effectiveness of Preventing AIDS Complications (CEPAC)-International model of adult HIV infection [25], [26] was used to project clinical outcomes for women following pregnancy, and was expanded to simulate infant outcomes from birth through the first two years of life. The models were linked by using CEPAC results as MTCT model inputs (Text S1). Outcomes of the linked models included risk of infant HIV infection at 4–6 weeks and 18 months of age and 2-year pediatric survival, as well as maternal 2-year survival, maternal 5-year survival, and maternal life expectancy after delivery. Additional details of model structure, data inputs, sensitivity analyses, and results are presented in the Appendix (Text S1).
Figure 1. “Cascade” of PMTCT and postnatal HIV care.
Opportunities to maximize the effectiveness of PMTCT interventions may be lost at each step in the pathway. ANC: antenatal care, ARVs: antiretroviral drugs, ART: antiretroviral therapy, sdNVP: single-dose nevirapine.
Population and PMTCT strategies evaluated
We simulated a population of pregnant and breastfeeding women in Zimbabwe who were HIV-infected at the time of conception [27]. For women identified as HIV-infected during ANC, five PMTCT strategies were evaluated (Text S1): 1) A “no antenatal ARVs” strategy, for reference comparison; 2) a single-dose of nevirapine (sdNVP) administered to laboring mothers and infants within 3 days of birth, reflecting the 2002–2009 national Zimbabwe PMTCT program and 2006 WHO “minimum” guidelines for resource-constrained settings [14]; 3) WHO 2010 “Option A:” short-course zidovudine during pregnancy and extended infant nevirapine during breastfeeding for women with CD4 >350/µL and no evidence of WHO stage 3–4 disease, with lifelong ART for women with advanced disease [11]; 4) WHO 2010 “Option B:” ART through pregnancy and breastfeeding regardless of CD4 or disease stage, with continuation after weaning for women with advanced disease [11]; and 5) the “Option B+” under consideration in select locations: lifelong ART for all pregnant, HIV-infected women, for comparison [12], [28]. ART-eligible women who linked to HIV-related healthcare after delivery were assumed to receive ART for their own health in all strategies (Text S1). Mothers were assumed to breastfeed their infants for a median duration of 18 months, based on Zimbabwean data [21]. Throughout the manuscript, the term “ARV” refers to any single or dual antiretroviral drug regimen used for PMTCT, while “ART” refers only to three-drug combination therapy (regardless if used for PMTCT or for therapy of maternal disease).
Model structure
MTCT model
The MTCT model is a previously-published decision-analytic simulation of a cohort of pregnant women from conception through delivery [24] (TreeAgePro 2010 software, Williamstown, MA). The model structure was expanded to include key steps in antenatal care, as well as linkage to postnatal maternal and pediatric care (Figure 1 and Text S1). Probabilities of HIV transmission and maternal and infant death before and during delivery were stratified by the severity of maternal HIV infection (ART “eligible,” defined as WHO stage 3–4 disease or CD4 350/<µL [11]; “not eligible;” or deceased) and by maternal receipt of postnatal HIV care and ART.
CEPAC adult model
The CEPAC-International model is a first-order Monte Carlo simulation of HIV infection, in which patients are simulated individually from model entry through death. Details of model structure and validation have been published previously [25], [26], [29] and are further described in the Appendix (Text S1). In brief, disease progression is characterized by a sequence of monthly transitions between health states; these include acute opportunistic and other infections prevalent in southern Africa, chronic HIV infection, and death. The model records all clinical events during each patient's lifetime. A cohort of ten million women is simulated to produce stable estimates of outcomes (Text S1).
In the CEPAC adult model, current CD4 count, opportunistic infection prophylaxis, and history or absence of previous opportunistic infections determine the monthly risk of opportunistic infections and HIV-related death (Table 1). HIV RNA suppression with effective ART leads CD4 counts to increase, reducing the risks for opportunistic infections and death. Before initiation of ART or after virologic failure on ART, CD4 counts decline at a rate determined by current RNA level; this is accompanied by increased risks of opportunistic infections and death. After planned interruption of suppressive ART at the time of weaning (Option B only), CD4 counts decline more rapidly based on data from ART interruption and PMTCT trials [30]–[32].
Table 1. Selected model input parameters for a simulation model of strategies to prevent mother-to-child transmission of HIV in Zimbabwe: Maternal data.
| Variable | Base Case Value | Range for sensitivity analyses | Data sources |
| Baseline maternal cohort characteristics | |||
| Age (mean (SD), in years) | 24.0 (5) | 20–30 | MOHCW [27] |
| HIV prevalence | 16% | MOCHW [27] | |
| HIV incidence | 0.96%/year | MOHCW [39] | |
| Mortality during pregnancy | 0.7% | 0–2% | MOHCW [27] |
| Live birth | 95.7–98% | 95–99% | MOHCW [27] |
| Among HIV-infected women at first ANC visit: | |||
| CD4 count (mean (SD), cells/µL) | 451 (50) | ZVITAMBO trial [21] (SD: assumption) | |
| ART eligible | 275 (50) | ZVITAMBO trial [21] | |
| Not ART eligible | 550 (50) | ZVITAMBO trial [21] | |
| Incident infection in pregnancy | 664 (50) | MACS [40] | |
| Initial HIV RNA (mean copies/ml) | 72,349 | CTAC [41] | |
| Access to PMTCT “cascade” | |||
| Access to ANC | 91% | 80–100% | MOHCW [27] |
| HIV testing in ANC | 87% | 58–95% | MOHCW [23], [39] |
| Receipt of HIV test result in ANC | 99% | 71–99% | MOHCW [23], [39] |
| Sensitivity of clinical assessment of ART eligibility | 36% | 20–50% | MTCT-Plus Cohort [43] |
| Receipt of CD4 test result (Option A) | 67% | [79] | |
| Delivery in health care facility | 69% | 50–100% | MOHCW [23], [39] |
| If HIV status negative/unknown, test in labor | 36% | MOHCW [23] | |
| If HIV diagnosed in labor, receive sdNVP | 80% | MOHCW [23] | |
| Postnatal HIV Care | |||
| Linkage to postnatal maternal care by 6 weeks postpartum | 79% | 51–100% | Average and range: [36], [44]–[48] |
| Loss to follow-up from postnatal maternal care | 16% (year 1); 6%/year(years 2+) | [37], [80], [81] | |
| Maternal antiretroviral therapy | |||
| Efficacy (% HIV RNA suppression at 24 weeks) | |||
| 1st-line NVP/ZDV/3TC initiated in pregnancy | 90% | [50] | |
| 1st-line NVP/d4T/3TC initiated post-partum | |||
| With sdNVP exposure | 85% | Difference between sdNVP exposed and unexposed: 0%–16% | OCTANE trial [49]Difference: [18], [69], [71] |
| Without sdNVP exposure** | 90% | Difference assumed = 5% [50] | |
| 2nd-line LPV/r/TDF/FTC | 72% | [82] | |
| CD4 decline (6 months after ART interruption) | 139 cells | 75–139 cells/µL | [30]–[32] |
CEPAC infant model
A first-order, Monte Carlo simulation model of infant HIV infection and survival was added to the CEPAC model. Modeled infants enter the postnatal model at birth. Based on events occurring before and during delivery in the MTCT model, infants are assigned one of three HIV categories (HIV-unexposed; HIV-exposed but uninfected; or HIV-infected) and three maternal disease categories (HIV-uninfected; HIV-infected and “ART eligible;” or HIV-infected and “ART non-eligible”). Over a two-year horizon, infants face a monthly probability of four key clinical events: 1) maternal HIV infection, if mother was previously uninfected, causing infants to transition from “unexposed” to “exposed-uninfected;” 2) maternal death, with risks derived from the adult CEPAC model as described above, after which infants are no longer at risk for HIV infection but are at higher risk of death due to orphanhood [33], [34]; 3) infant HIV infection through breastfeeding, if infant was previously uninfected; and 4) infant death from any cause. Risks of maternal death and of postnatal HIV transmission are stratified by maternal disease stage and ARV regimen, and risks of infant death are stratified by infant HIV exposure/infection status and receipt of ART if infected.
HIV/AIDS care and ART
For HIV-infected infants, a one-time probability of HIV diagnosis, linkage to HIV care, and ART initiation was modeled [13]. For mothers, care and ART were modeled in greater detail. A one-time probability of linkage to maternal HIV care was incorporated; this parameter reflects the probability that a post-partum mother will present to an HIV clinic for her own healthcare by six weeks postpartum. Women not linking to care within this period were assumed to present to HIV care upon later development of a severe opportunistic infection. Once in postnatal care for her own health, ART eligibility was assumed to be assessed through both CD4 testing and clinical evaluation. For women identified as ART-eligible, both ART and trimethoprim/sulfamethoxazole prophylaxis were initiated [13], [35]. For women identified as not yet ART-eligible in postnatal HIV care, medications were administered depending on the modeled breastfeeding prophylaxis regimen: no medications were dispensed for the “no antenatal ARVs” and “sdNVP” regimens; infant nevirapine syrup was dispensed for the “Option A” regimen; and maternal ART was dispensed for the “Option B” and “Option B+” regimens. Specific components of 3-drug ART regimens were simulated to reflect 2009 Zimbabwean guidelines and common current practice in Zimbabwe (Text S1 and Table 1) [35]. ART monitoring and switching strategies are also detailed in the Appendix (Text S1).
Loss to follow-up
During modeled ANC, women could be lost to follow-up (LTFU) at any stage between first presentation (booking) and delivery; if LTFU, no antenatal ARVs were received, but the opportunity to access HIV testing and sdNVP in labor remained. Women could also be LTFU between delivery and six weeks postpartum [36], or after linkage to postnatal HIV care [37], [38]. In the absence of specific maternal or pediatric data regarding monthly risks of LTFU and cessation of prophylactic ARVs during breastfeeding, the impact of such events was incorporated in sensitivity analyses via the highest published postnatal transmission estimates for Options A, B and B+. For HIV-infected infants, the impacts of loss to follow-up after established pediatric HIV care were included in cohort-based pediatric survival estimates.
Model input parameters
Maternal cohort characteristics and natural history
Baseline maternal characteristics reflected cohorts of pregnant women in Zimbabwe (Table 1 and Text S1). At first ANC visit, mean age was 24.0 [27] and HIV prevalence was 16% [39]; HIV incidence during pregnancy and breastfeeding was 0.96%/year [39]. Among HIV-infected women, mean CD4 was 275 cells/µL if ART-eligible [21], 550 cells/µL if not ART-eligible [21], and 664 cells/µL if incidently infected during pregnancy [40]. Because detailed clinical data to inform HIV disease progression in the absence of ART were not available from Zimbabwe, these natural history model inputs to the CEPAC model were derived from a clinical cohort in South Africa (Text S1) [41]. For women remaining HIV-negative, life expectancy was projected using UNAIDS cause-deleted mortality rates [42].
Maternal access to care and ART
Modeled rates of access to ANC, HIV and CD4 tests in ANC, ARV drugs for PMTCT, and postnatal care reflected Zimbabwean national estimates whenever available (Table 1). In the sdNVP strategy, women were modeled to undergo clinical ART-eligibility assessment, but not CD4 testing, and to initiate lifelong ART if WHO stage 3–4 disease was identified [11]. The sensitivity of clinical assessment for ART eligibility was 36% [43]. In the base case analysis, to isolate the benefits of each regimen, ARV medications were assumed to be available for, accepted by, and adhered to by all women diagnosed as HIV-infected in ANC, and CD4 testing (but not result return) was assumed for all women under Option A. In addition, sdNVP was modeled to be provided to 80% of women newly HIV-diagnosed in labor [23], [39].
The base-case rate of linkage to postnatal maternal HIV care reflected the median of published values; in sensitivity analyses, linkage rates ranged from 51–100%, depending on antenatal care and PMTCT regimen received [36], [44]–[48]. For women in HIV care and receiving ART, the 24-week “efficacy” of first-line, NNRTI-based maternal ART in suppressing HIV RNA to <400 copies/ml was 85% for women with previous sdNVP exposure [49] and 90% (assumed difference of 5%) for women without exposure to sdNVP [50]. In the six months following ART interruption at weaning (Option B), CD4 count was modeled to decline by 139 cells [30], [31]. Other modeled ART effects are described in Table 1.
Mother-to-child transmission risks
Mother-to-child HIV transmission risks were derived from randomized clinical trials in several African settings (Table 2) [1], [4], [5], [8]–[10], [15], [17], [21], [51]–[61]. MTCT risks during the intrauterine and intrapartum period (by 4–6 weeks of age) and postpartum period (6 weeks-18 months) were stratified by maternal HIV stage and by PMTCT regimen received. Postpartum transmission risks were additionally stratified by whether breastfeeding during the first six months of life was exclusive (EBF) or mixed (MBF, including any non-breastmilk liquid or solid) [62].
Table 2. Selected model input parameters for a simulation model of strategies to prevent mother-to-child transmission of HIV in Zimbabwe: Pediatric data.
| II. Mother-to-child transmission risks | ||||
| Base Case Value (Range for sensitivity analysis) | ||||
| Maternal HIV status | PMTCT regimen received | |||
| Intrauterine/intrapartum period (one-time risks) | ||||
| No antenatal ARVs | sdNVP* | scZDV | ART | |
| Mother ART eligible at conception | 0.273 [52], [83](0.199–0.322) [54], [83], [84] | 0.176 [21], [85](0.082–0.264) [15], [17], [86] | 0.136 [5](0.091–0.157) [87], [88] | 0.033 [89](0.011–0.041) [10], [58] |
| Mother not ART-eligible at conception | 0.175 [52], [83](0.127–0.206) [54], [83], [84] | 0.073 [21], [85](0.033–0.109) [15], [17], [86] | 0.036 [5](0.024–0.041) [8], [88] | 0.01 [10](0.004–0.028) [4], [10] |
| Mother with incident infection during pregnancy | 0.30(assumption) | 0.20 (assumption) | 0.16(assumption) | 0.033(assumed = eligible) |
SD: Standard deviation; MOHCW: Zimbabwe Ministry of Health and Child Welfare; MACS: Multicenter AIDS Cohort Study; CTAC: Cape Town AIDS Cohort; ANC: antenatal care; ART: 3-drug, combination antiretroviral therapy; (sd)NVP: (single-dose) nevirapine; scZDV: short-course zidovudine; WHO: World Health Organization; ZDV: zidovudine; 3TC: lamivudine; d4T: stavudine; LPV/r: lopinavir/ritonavir; TDF: tenofovir; FTC: emtricabine; EBF/MBF: exclusive/mixed breastfeeding.
*“Without sdNVP exposure” refers to situations in which sdNVP was not received, or was received in combination with short-course zidovudine or other antenatal/intrapartum medications which may decrease risk of NVP-associated NNRTI-resistant HIV [19].
Pediatric outcomes
Mortality estimates for HIV-unexposed children were derived from UNAIDS HIV-deleted mortality estimates [42] and mortality rates for exposed-uninfected infants were from the ZVITAMBO study in Zimbabwe [63] (Table 2). Mortality rates for untreated HIV-infected children, stratified by timing of HIV infection, were derived from a patient-level analysis of 1,930 children from 12 African PMTCT trials, after removing non-HIV-related causes of death [6]. ART was assumed available for 36% of HIV-infected children [64]. Mortality rates for children treated with ART were from a systematic review of pediatric ART in Africa [65] and a pooled patient-level analysis from 16 African cohorts [66]. For infants of any HIV infection or exposure status, maternal death was assumed to increase mortality 2-fold [33].
Model validation and sensitivity analyses
Model results were validated by comparison to published values (Text S1). Sensitivity analyses, described in detail in the Appendix (Text S1), examined the impact of variations in key maternal clinical and demographic characteristics, highest and lowest published MTCT risks for each regimen, access to ANC and HIV testing in ANC, linkage to postnatal HIV care, ART efficacy and survival following sdNVP exposure, CD4 cell decline following ART interruption, and pediatric and maternal non-HIV-related mortality rates. Parameters leading to substantial changes in model results were identified; a substantial change was defined as 1) a change in the relative order of the outcomes of the PMTCT regimens, or 2) a >10% relative change in the difference between projected outcomes for each ARV regimen (example provided in Text S1). In addition, we projected the impact of each PMTCT regimen on infant infection risk for an annual cohort of women becoming pregnant in Zimbabwe, including both HIV-infected and HIV-uninfected women at conception (Text S1).
Role of the funders and ethics approval
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This analysis was deemed “not human subjects research” by the Partners Healthcare IRB, and was approved as an exempt analysis.
Results
Base-case analyses (fixed levels of ARV uptake for PMTCT)
Pediatric outcomes
Among women HIV-infected at first ANC visit, the risks of infant infection at 4–6 weeks of age were projected to be 20.1% with no antenatal ARV prophylaxis, 10.8% with the 2002–2009 sdNVP-based national program, 7.2% with Option A, and 5.4% with Option B and B+ (Table 3, top). At 18 months of age, cumulative risk of infant HIV infection ranged from 25.8% (no antenatal ARVs) to 10.9% (Option B/B+). Two-year pediatric survival similarly ranged from 78.4% (no antenatal ARVs) to 84.9% (Option B/B+).
Table 3. Outcomes of strategies to prevent mother-to-child HIV transmission in Zimbabwe: Base-case model results.
| Model results | |||
| Pediatric outcomes (Infants born to women who are HIV-infected at conception) | |||
| Risk of HIV infection at 4–6 weeks of age (%) | Risk of HIV infection at 18 months of age (%) | 2-yearsurvival (%) | |
| No antenatal ARVs* | 20.1 | 25.8 | 78.4 |
| Single-dose NVP | 10.8 | 17.2 | 81.9 |
| Option A | 7.2 | 12.8 | 83.5 |
| Option B | 5.4 | 10.9 | 84.9 |
| Option B+ | 5.4 | 10.9 | 84.9 |
“No antenatal ARVs” refers to the receipt of no medications for HIV therapy or for prevention of mother-to-child transmission during the antenatal period. Women who link to postnatal HIV care are assumed to start ART after delivery if CD4 cell count is ≤350/µL or following development of a severe opportunistic infection.
Maternal outcomes
Postpartum survival among HIV-infected women was projected to range from 92.4% (no antenatal ARVs, sdNVP) to 94.0% (Option B+) at two years, and 79.8% (no antenatal ARVs, sdNVP) to 82.2% (Option B+) at five years after delivery (Table 3, bottom). The impacts of sdNVP-associated resistance and ART interruption manifested primarily after five years postpartum: projected life expectancy was slightly lower following sdNVP (13.9 years) or Option B (13.9 years) than following receipt of no antenatal ARVs or Option A (14.0 years). Projected maternal life expectancy was greatest (14.4 years) with lifelong universal ART (Option B+).
Sensitivity analyses
Sensitivity analyses were performed on all key model input parameters and assumptions (Figures 2 and 3, Text S1). The model input parameters with the greatest influence on pediatric and maternal outcomes are shown in Figure 2 (Figure 2a: 18-month infant infection risk; Figure 2b: maternal life expectancy). Improvements in HIV testing during pregnancy (2a and 2b: scenario 2), linkage to postnatal care (2a and 2b: scenario 3), and reduction in the proportion of women with CD4<350 (2a and 2b: scenario 4) as might result through universal HIV screening acceptance and early ART initiation prior to pregnancy) led to the greatest improvements in both maternal and pediatric outcomes. Lowest published MTCT risks for each regimen (2a: scenario 5) did not change the relative order of the infant outcomes of the PMTCT regimens, but markedly reduced overall transmission risks; lowest projected 18-month infection risks ranged from 8.1% (sdNVP) to 4.5% (Option B/B+).
Figure 2. Key sensitivity analyses, identifying selected parameters producing substantial changes in model results.
As detailed in the Methods section and Appendix (Text S1), a substantial change in results was defined as: 1) a change in the relative order of the outcomes of the PMTCT regimens, or 2) a >10% relative change in the difference between projected outcomes for each regimen. Panel 2a depicts parameters influencing 18-month mother-to-child HIV transmission risk, and Panel 2b depicts parameters influencing maternal life expectancy from delivery; these outcomes are shown on the vertical axes. Along the horizontal axes, each group of vertical bars represents a single scenario (numbered 1–6 in 2a and 1–5 in 2b), and each vertical bar represents a PMTCT regimen, as indicated.
Figure 3. Sensitivity analysis demonstrating the impact on maternal life expectancy of rates of linkage to postnatal HIV care.
The vertical axis represents maternal life expectancy, in years from delivery. The horizontal axis depicts the probability of linkage to postnatal HIV care for women who receive the PMTCT regimens shown. This probability of linkage to care is varied from 79% (the base case value) to 85%. In the base case, maternal life expectancy following the sdNVP and Option B regimens is lower than if no antenatal ARVs were received for PMTCT (triangles). This occurs as a result of modeled negative impacts of sdNVP-associated resistance (sdNVP regimen) and ART interruption (Option B regimen). When the probability of linkage to care following Option B is ≥81.8%, as indicated by the open arrow (2.8% more than if no antenatal ARVs are received), the negative impact of ART interruption is overcome. Similarly, when the probability of linkage to care following sdNVP is ≥82.8%, as indicated by the solid arrow (3.8% more than if no ARVs are received), the negative impact of sdNVP-associated resistance is overcome.
In the base case (Table 3), maternal life expectancy was slightly shorter following receipt of sdNVP (13.8 years) than if no antenatal ARVs were received for PMTCT (14.0 years). The maternal survival and life expectancy differences between no antenatal ARVs and sdNVP also depended on the degree to which sdNVP exposure was assumed to reduce subsequent 1st-line ART efficacy; at 0% difference, the regimens led to equal maternal outcomes (Appendix); at 16% difference, maternal outcomes associated with each strategy differed substantially (Figure 2b, scenario 5). Base-case maternal life expectancy was also shorter following receipt of Option B than if no antenatal ARVs were received (Table 3). This result did not change when a wide range of reported rates of CD4 decline after ART interruption were incorporated (Appendix) [30]–[32].
Figure 3 depicts the impact of linkage to postnatal HIV care on maternal life expectancy. In the base case, rates of linkage to postnatal care were assumed to be equal following receipt of any PMTCT regimen. As noted above, resulting maternal life expectancies were slightly lower following the sdNVP and Option B regimens than if no antenatal ARVs were received for PMTCT. However, if the receipt of sdNVP or Option B improved rates of linkage to postnatal care even slightly, compared to ANC without receipt of antenatal ARVs for PMTCT (2.8% increase in linkage for Option B, 3.8% increase for sdNVP), the negative impact of these regimens on life expectancy was overcome.
All other parameters varied in sensitivity analyses are detailed in the Appendix (Text S1). Notably, policy conclusions were insensitive to variations in maternal age, risk of maternal mortality due to pregnancy, probability of live infant birth, delivery location, impact of sdNVP exposure on short-term infant outcomes, and relative risk of infant mortality following maternal death.
Discussion
We linked two validated computer simulation models to project clinical outcomes of improved PMTCT regimens in Zimbabwe. These analyses simultaneously evaluate pediatric and maternal outcomes, a novel approach for model-based investigations of HIV care [67], [68], and are likely applicable to other sub-Saharan Africa settings where prolonged breastfeeding is common. Altogether, pediatric outcomes, including HIV infection risk and survival, would be markedly improved by replacing sdNVP-based programs with 2010 WHO guideline-concordant regimens at fixed levels of ARV uptake for PMTCT. However, it is worth noting that the largest improvements in both pediatric and maternal survival may result from a PMTCT program that offers lifelong ART to all pregnant, HIV-infected women regardless of CD4 count or disease stage (Option B+), as is currently being investigated [28] and already being considered for implementation in Malawi [12].
The choice of ARV regimen for PMTCT may have important effects on maternal health, important not only in its own right, but also for its impact on pediatric survival [33], [34]. First, this analysis underscores the potential long-term impact of sdNVP exposure. The modeled impacts of ARV regimens that are received only briefly during pregnancy or breastfeeding are small in comparison to the impact of lifelong ART for women who link to postnatal HIV care. Nonetheless, the sdNVP-associated life expectancy reduction of 0.1 years is comparable in magnitude to the modeled benefit of trimethoprim/sulfamethoxazole prophylaxis [25]. This life expectancy reduction results from reduced virologic suppression on NNRTI-based ART due to sdNVP-associated NNRTI-resistant virus. The difference in suppression among women with and without sdNVP exposure determines the degree to which sdNVP-based programs create a tradeoff between improved pediatric outcomes and reduced maternal life expectancy. The impact of sdNVP exposure may be negligible if sdNVP is received >12–24 months prior to ART initiation [49], [69]–[71]; in such cases, scale-up of sdNVP-based PMTCT services will improve both maternal and pediatric outcomes. However, absolute reductions in 24-week virologic suppression rates of 16–42% and 12–14% have been reported for women initiating ART <6 months and 6–12 months after sdNVP exposure, respectively [18], [49], [69] (base-case value for this analysis: 5%). Because protease inhibitor-based first-line ART is not widely available in many settings, the current analysis lends strong support to efforts to expand non-sdNVP-based PMTCT programs to improve both maternal and child health.
In addition to the impact of sdNVP exposure, the ART initiation and discontinuation strategies required by Option B may also impact maternal health. Because ART is recommended for all pregnant, HIV-infected in women in Option B, there may be a health benefit from initiating ART at higher CD4 counts than would prompt therapy in non-pregnant patients. However, it is not yet known whether the adverse effects of ART discontinuation after weaning will outweigh these benefits. When ART is interrupted in men and non-postpartum women, marked increases in viral load, inflammatory markers, and risk for both AIDS- and non-AIDS-related events have been reported [30], [72]. The current analysis models rapid CD4 declines, ranging from 75–139 cells/µL, in the 6 months following ART interruption at weaning in Option B [30], [32], [72]; this results in a decrease in maternal life expectancy of 0.6 years for women who interrupt ART at weaning (then resume when later required for their own health), compared to women who continue ART. In the absence of randomized data from postpartum women, we do not specifically simulate additional increased rates of non-AIDS-related events due to interruption; if additional non-AIDS-related risks exist, the negative impact of Option B will be greater than shown here. Data on AIDS- and non-AIDS-related events among postpartum women continuing or interrupting ART are anticipated in the next several years [28], and will more accurately inform the risks and benefits of Option B versus Option B+.
These analyses also highlight that linkage to postnatal maternal HIV care, and thus initiation of ART when needed, is the step in the PMTCT “cascade” that most dramatically influences maternal life expectancy. The impact of improved linkage to care on maternal life expectancy is greater than that of improved access to ANC or HIV testing (holding linkage rates constant for those in care and diagnosed), or any specific PMTCT regimen during pregnancy. Because published rates of linkage to postnatal care range widely and are rarely stratified by PMTCT regimen received [46], [47], model-based analyses can highlight areas for future research. For example, if women who receive sdNVP- or Option B-based PMTCT interventions are even slightly more likely to register in postnatal HIV care than are women who receive ANC but no medications for PMTCT (3–4% absolute increases in linkage rates), the life expectancy benefits of this improvement in linkage outweigh the negative effects of sdNVP-associated resistance and ART interruption. However, if receipt of these regimens does not improve linkage to postnatal care compared to ANC services alone, there may be an important tradeoff between long-term maternal and pediatric health with expansion of sdNVP- or Option B- based programs.
Although linkage to postnatal care was the access-to-care parameter with the greatest individual influence on maternal life expectancy, improved uptake at each step in a “cascade” of PMTCT care (including access to ANC, HIV testing and result receipt, and availability and acceptance of ARVs [73]; Figure 1) confers substantial benefits for both infant and maternal health. In fact, improvements in two dimensions are critical: both replacement of sdNVP with more effective regimens and improved uptake of any given PMTCT regimen. While the current analysis addresses the first dimension in greatest detail, further analyses examining the clinical impacts, costs, and cost-effectiveness of interventions to improve uptake are also needed.
This analysis has two main limitations. First, when detailed clinical data to inform modeled maternal and pediatric HIV disease progression were not available from Zimbabwe, data from surrounding southern African countries were used instead. Base-case clinical risks for HIV-infected mothers, such as risks of opportunistic infections and tuberculosis, were derived from South Africa, and might reasonably be expected to be similar in neighboring Zimbabwe. Similarly, risks of MTCT, after stratification by maternal CD4, PMTCT regimen received, and breastfeeding duration, might also be anticipated to be similar across Southern African settings. The impacts of non-Zimbabwean data were tested in extensive sensitivity analyses, as detailed in the Appendix (Text S1), and had little impact on model results. Second, life expectancy projections are subject to uncertainty about events occurring over long time horizons, as healthcare systems and HIV therapy may change substantially in the distant future. Because of the uncertainty inherent in long-term projections, we also report similar findings based on the short-term outcomes of MTCT risks, 2-year pediatric survival, and 2-year and 5-year maternal survival.
Model-projected clinical outcomes are not intended to be interpreted in isolation, but rather as one component of a decision-making framework that incorporates many factors, including feasibility, affordability, and equity. Implementation of more effective PMTCT regimens will be resource-intensive; costs will include not only drug costs, but also infrastructure, personnel, and training costs for clinics and laboratories. However, the 2010-recommended PMTCT regimens may also save money in the future by averting not only costly care for pediatric HIV infection [74], but also the costly morbidity and mortality associated with delayed maternal ART initiation [26], [75] or sdNVP-associated resistance [29]. Model-based analyses of pediatric outcomes have suggested that ART for PMTCT is very cost-effective in many settings, compared to sdNVP or to no ARVs [76]–[78]. Additional budgetary impact and cost-effectiveness analyses, comparing Option A to Option B and incorporating short-term and long-term benefits and costs for both mothers and infants, will comprise an important area of future research. In the interim, model-based analyses can assist policymakers to understand the clinical tradeoffs anticipated to result from the replacement of sdNVP with more effective PMTCT regimens.
Conclusions
Replacing sdNVP with currently WHO-recommended PMTCT regimens at a fixed level of ARV uptake for PMTCT will improve both maternal and pediatric health outcomes in Zimbabwe. The best currently available data suggest that continued dependence on sdNVP-based programs would impact positively on pediatric health, but may worsen long-term maternal outcomes, unless also accompanied by expanded access to postnatal HIV care and ART for eligible women. To avoid an untenable tradeoff between maternal and child health, additional resources are needed to implement WHO guideline-concordant PMTCT programs (Options A, B, or possibly B+) in resource-constrained settings, including Zimbabwe.
Supporting Information
The Appendix reports additional details of technical model structure, input data parameters, and model results, including extensive model validation and sensitivity analyses.
(DOC)
Acknowledgments
The authors gratefully acknowledge Ji-Eun Park, BS, and Asinath Rusibamayila, BS, for assistance in model development, analyses, and manuscript preparation; Callie Scott, MPH, Jessica Becker, BS, and Rodrigo Cerda, MD, MPH, for assistance with analyses; Adam Stoler, MS and Erin Rhode, MS for computer programming assistance; and Scott Dryden-Peterson, MD, for comments on the analysis.
We are indebted to the entire CEPAC-International team and investigators for their contributions, including Christine Danel, Thérèse N'Dri-Yoman, Eugène Messou, Raoul Moh, Eric Ouattara, Catherine Seyler, and Siaka Touré (Programme PACCI, Abidjan, Côte d'Ivoire); Yazdan Yazdanpanah (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, EA 2694, Faculté de Médecine de Lille, and Laboratoire de Recherches Économiques et Sociales, Centre National de la Recherche Scientifique Unité de Recherche Associée 362, Lille, France); Xavier Anglaret, Delphine Gabillard, Hapsatou Touré (INSERM U897, ISPED, Université Bordeaux 2, Bordeaux, France); Nagalingeswaran Kumarasamy and A. K. Ganesh (Y.R. Gaitonde Centre for AIDS Research & Education, Chennai, India); Catherine Orrell and Robin Wood (University of Cape Town, Cape Town, South Africa); Neil Martinson and Lerato Mohapi (Perinatal HIV Research Unit, WITS Health Consortium, Johannesburg, South Africa); Kara Cotich, Sue J. Goldie, April D. Kimmel, Marc Lipsitch, Alethea McCormick, Chara Rydzak, George R. Seage III, and Milton C. Weinstein (Harvard School of Public Health, Boston, MA, USA); C. Robert Horsburgh (Boston University School of Public Health); Heather E. Hsu (Harvard Medical School, Boston, MA, USA); Timothy Flanigan and Kenneth Mayer (Miriam Hospital, Providence, RI, USA); A. David Paltiel (Yale University, New Haven, CT, USA); Aima Ahonkhai, Jason Andrews, Ingrid V. Bassett, Melissa A. Bender, Julie Levison, Benjamin P. Linas, Elena Losina, Zhigang Lu, Sarah Lorenzana, Bethany Morris, Mai Pho, Lynn Ramirez, Corina Rusu, Callie A. Scott, Caroline Sloan, Alexis Sypek, Lauren Uhler, Bingxia Wang, and Angela Wong (Massachusetts General Hospital, Boston, MA, USA).
We are also indebted to the CEPAC-International Scientific Advisory Board, including Richard Chaisson, Victor De Gruttola, Joseph Eron, R.R. Gangakhedkar, Jonathan Kaplan, Salim Karim, Thérèse N'Dri Yoman, Douglas Owens, and John Wong.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this work was provided by the Elizabeth Glaser Pediatric AIDS Foundation (all authors) and the National Institute of Allergy and Infectious Disease (K01 AI078754 (ALC), K24 AI062476 (KAF), and R01 AI058736 (KAF, RPW, RW, JC)). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 2.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 3.Leroy V, Sakarovitch C, Cortina-Borja M, McIntyre J, Coovadia H, et al. Is there a difference in the efficacy of peripartum antiretroviral regimens in reducing mother-to-child transmission of HIV in Africa? AIDS. 2005;19:1865–1875. doi: 10.1097/01.aids.0000188423.02786.55. [DOI] [PubMed] [Google Scholar]
- 4.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 5.Dabis F, Bequet L, Ekouevi DK, Viho I, Rouet F, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–318. [PMC free article] [PubMed] [Google Scholar]
- 6.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyq255. Epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marazzi MC, Liotta G, Haswell J, Zimba I, Nielsen-Saines K, et al. TUAC101: Extended use of highly active antiretroviral therapy (HAART) during pregnancy in Southern Africa is highly protective in HIV-1 prevention of mother-to-child-transmission (PMTCT) also in women with higher CD4 cell counts 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, South Africa, 2009. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=2013.
- 8.Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(10)70288-7. Epub ahead of press. [DOI] [PubMed] [Google Scholar]
- 9.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. Accessed February 21, 2011. [PubMed]
- 12.Schouten E and Department of HIV and AIDS Ministry of Health M. Making it Happen: Revising national policies to reflect changes in WHO recommendations for preventing vertical transmission of HIV –Malawi. International AIDS Society, Vienna, Austria, 2010. Available: http://www.pedaids.org/Press-Room/Events/2010/IAS-2010-WHO-Satellite-Session/5_Making-it-Happen_Revising-national-policies-to-r.
- 13.World Health Organization. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2010. Progress Report. Available: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed February 21, 2011.
- 14.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants in resource-limited settings: towards universal access - recommendations for a public health approach. 2006. Available: http://www.who.int/hiv/pub/guidelines/pmtct/en/index.html. Accessed March 1, 2009.
- 15.Mofenson L, Taha T, Li Q, Kumwenda J, Kafulafula G, et al. for the PEPI Malawi Study Group. TUPEC053: Infant extended antiretroviral (ARV) prophylaxis is effective in preventing postnatal mother-to-child HIV transmission (MTCT) at all maternal CD4 counts International AIDS Society, Cape Town, South Africa, 2009. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=1251.
- 16.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 18.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 19.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 20.World Bank. Data and statistics: Country classification. 2009. Available: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0, contentMDK:20420458~menuPK:64133156~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html. Accessed February 1, 2010.
- 21.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 22.Perez F, Orne-Gliemann J, Mukotekwa T, Miller A, Glenshaw M, et al. Prevention of mother to child transmission of HIV: evaluation of a pilot programme in a district hospital in rural Zimbabwe. BMJ. 2004;329:1147–1150. doi: 10.1136/bmj.329.7475.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelsmann B, Shumba M, Maruva M, Keatinge J, Miller A, Mahomva A, Mbizvo E, Mashumba S, Perez F, Wilfert C, Dabis F. MOPE0521: Enhancing the uptake of antiretroviral drugs for PMTCT through more complex regimens. International AIDS Society, Mexico City, 2008. Available: http://www.aids2008-abstracts.org/aids2008_book_vol1_web.pdf, page 174.
- 24.Ciaranello AL, Seage GR, 3rd, Freedberg KA, Weinstein MC, Lockman S, et al. Antiretroviral drugs for preventing mother-to-child transmission of HIV in sub-Saharan Africa: balancing efficacy and infant toxicity. AIDS. 2008;22:2359–2369. doi: 10.1097/QAD.0b013e3283189bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, et al. Cost-effectiveness of HIV treatment in resource-poor settings–the case of Côte d'Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 26.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimbabwe Ministry of Health and Child Welfare Zimbabwe. 2008. Maternal and Perinatal Mortality Study 2007.
- 28.National Institutes of Health: IMPAACT Trial Network. P1077: The PROMISE Study (Promoting Maternal and Infant Survival Everywhere): Synopsis. 2010. Available: http://www.impaactgroup.org/files/IMPAACT_P1077_Synopsis.doc. Accessed February 21, 2011.
- 29.Ciaranello A, Lockman S, Freedberg KA, Hughes M, Chu J, et al. First-line antiretroviral therapy after single-dose nevirapine exposure in South Africa: A cost-effectiveness analysis of the OCTANE trial. AIDS. 2011;25:479–492. doi: 10.1097/QAD.0b013e3283428cbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 31.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 32.Kesho Bora Study Group. ThLBB105: Impact of Triple-ARV prophylaxis during pregnancy and breastfeeding compared with short-ARV prophylaxis for MTCT prevention on maternal disease progression. International AIDS Society, Vienna, Austria, 2010. Available: http://pag.aids2010.org/Abstracts.aspx?SID=644&AID=17446.
- 33.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 34.Zaba B, Whitworth J, Marston M, Nakiyingi J, Ruberantwari A, et al. HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology. 2005;16:275–280. doi: 10.1097/01.ede.0000155507.47884.43. [DOI] [PubMed] [Google Scholar]
- 35.Zimbabwe Ministry of Health and Child Welfare. National Drug and Therapeutics Policy Advisory Committee (NDTPAC) & AIDS and TB Unit. Guidelines for Antiretroviral Therapy in Zimbabwe 2009 [Google Scholar]
- 36.Manzi M, Zachariah R, Teck R, Buhendwa L, Kazima J, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 37.Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkhof MWG, Dabis F, Myer L, Bangsberg DR, Boulle A, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimbabwe Ministry of Health. 2009. National HIV Estimates, 2009.
- 40.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 42.United Nations. World Population Prospects: The 2008 Revision. 2009. Available: http://esa.un.org/unpd/wpp2008/index.htm. Accessed February 21, 2011.
- 43.Carter RJ, Dugan K, El-Sadr WM, Myer L, Otieno J, et al. CD4+ Cell Count Testing More Effective Than HIV Disease Clinical Staging in Identifying Pregnant and Postpartum Women Eligible for Antiretroviral Therapy in Resource-Limited Settings. J Acquir Immune Defic Syndr. 2010;55:404–410. doi: 10.1097/QAI.0b013e3181e73f4b. [DOI] [PubMed] [Google Scholar]
- 44.Ahoua L, Ayikoru H, Gnauck K, Odaru G, Odar E, et al. Evaluation of a 5-year programme to prevent mother-to-child transmission of HIV infection in Northern Uganda. J Trop Pediatr. 2010;56:43–52. doi: 10.1093/tropej/fmp054. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22:1679–1681. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–2423. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15:825–832. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 48.Kumwenda J, Mataya R, Kumwenda N, Kafulafula G, Li Q, Taha T. WEPDD106: Coverage of highly active antiretroviral therapy (HAART) among postpartum women in Malawi International AIDS Society, Cape Town, South Africa, 2009. Available: http://www.ias2009.org/pag/Abstracts. aspx?AID=1938.
- 49.Lockman S, Hughes MD, McIntyre J, Zheng Y, Chipato T, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coetzee D, Hildrebrand K, Boulle A, Maartens G, Louis F, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 51.Fawzi W, Msamanga G, Spiegelman D, Renjifo B, Bang H, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–338. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Chigwedere P, Seage GR, Lee TH, Essex M. Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: a meta-analysis of published clinical trials. AIDS Res Hum Retroviruses. 2008;24:827–837. doi: 10.1089/aid.2007.0291. [DOI] [PubMed] [Google Scholar]
- 53.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, et al. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 54.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 55.Thistle P, Spitzer RF, Glazier RH, Pilon R, Arbess G, et al. A randomized, double-blind, placebo-controlled trial of combined nevirapine and zidovudine compared with nevirapine alone in the prevention of perinatal transmission of HIV in Zimbabwe. Clin Infect Dis. 2007;44:111–119. doi: 10.1086/508869. [DOI] [PubMed] [Google Scholar]
- 56.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 57.Thomas T, Masaba R, Ndivo R, Zeh C, Borkowf C, et al. for the Kisumu Breastfeeding Study Team. 45aLB: Prevention of mother-to-child transmission of HIV-1 among breastfeeding mothers using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003–2007. Conference on Retroviruses and Opportunistic Infections, Boston, 2008. Available: http://www.retroconference.org/2008/Abstracts/33397.htm.
- 58.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 59.Kuhn L, Sinkala M, Kankasa C, Semrau K, Kasonde P, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One. 2007;2:e1363. doi: 10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leroy V, Newell ML, Dabis F, Peckham C, Van de Perre P, et al. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- 61.Vyankandondera J, Luchters S, Hassink E. N°LB7: Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA-study). International AIDS Society, Paris, France 2003 [Google Scholar]
- 62.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 63.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, et al. Child Mortality According to Maternal and Infant HIV Status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 64.UNAIDS/UNICEF/WHO. Children and AIDS: Fourth stocktaking report, actions and progress. 2009. Available: http://www.unicef.org/publications/index_46585.html. Accessed February 21, 2011.
- 65.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 66.Kids' ART-LINC Collaboration. Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 67.Soorapanth S, Sansom S, Bulterys M, Besser M, Theron G, et al. Cost-effectiveness of HIV rescreening during late pregnancy to prevent mother-to-child HIV transmission in South Africa and other resource-limited settings. J Acquir Immune Defic Syndr. 2006;42:213–221. doi: 10.1097/01.qai.0000214812.72916.bc. [DOI] [PubMed] [Google Scholar]
- 68.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009;49:1784–1792. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stringer JS, McConnell MS, Kiarie J, Bolu O, Anekthananon T, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000233. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuhn L, Semrau K, Ramachandran S, Sinkala M, Scott N, et al. Mortality and virologic outcomes after access to antiretroviral therapy among a cohort of HIV-infected women who received single-dose nevirapine in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009;52:132–136. doi: 10.1097/QAI.0b013e3181ab6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coovadia A, Hunt G, Abrams EJ, Sherman G, Meyers T, et al. Persistent Minority K103N Mutations among Women Exposed to Single-Dose Nevirapine and Virologic Response to Nonnucleoside Reverse-Transcriptase Inhibitor-Based Therapy. Clin Infect Dis. 2009;48:462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 73.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: The impact of health systems' performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2010;56:e45–48. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 74.Meyer-Rath G, Violari A, Cotton M, Ndibongo B, Brenna A, Long L, Panchia R, Coovadia A, Gibb DM, Rosen S . THLBB103: The cost of early vs. deferred paediatric antiretroviral treatment in South Africa - a comparative economic analysis of the first year of the CHER trial. International AIDS Society, Vienna, Austria, 2010. Available: http://pag.aids2010.org/Abstracts.aspx?SID=644&AID=17823.
- 75.Cleary S, Chitha W, Jikwana S, Okorafor OA, Boulle A. Health Systems Trust: South African Health Review. 2005. Available: http://www.hst.org.za/generic/29. Accessed March 15, 2011.
- 76.Robberstad B, Evjen-Olsen B. Preventing mother to child transmission of HIV with highly active antiretroviral treatment in Tanzania–a prospective cost-effectiveness study. J Acquir Immune Defic Syndr. 2010;55:397–403. doi: 10.1097/QAI.0b013e3181eef4d3. [DOI] [PubMed] [Google Scholar]
- 77.Orlando S, Marazzi M, Mancinelli S, Liotta G, Ceffa S, et al. Cost-effectiveness of using HAART in prevention of mother-to-child transmission in the DREAM-Project Malawi. Journal of Acquired Immune Deficiency Syndromes. 2010;55:631–634. doi: 10.1097/QAI.0b013e3181f9f9f5. [DOI] [PubMed] [Google Scholar]
- 78.Johri M, Ako-Arrey D. The cost-effectiveness of preventing mother-to-child transmission of HIV in low- and middle-income countries: systematic review. Cost Eff Resour Alloc. 2011;9:3. doi: 10.1186/1478-7547-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandala J, Torpey K, Kasonde P, Kabaso M, Dirks R, et al. Prevention of mother-to-child transmission of HIV in Zambia: implementing efficacious ARV regimens in primary health centers. BMC Public Health. 2009;9:314. doi: 10.1186/1471-2458-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toro PL, Katyal M, Carter RJ, Myer L, El-Sadr WM, et al. Initiation of antiretroviral therapy among pregnant women in resource-limited countries: CD4+ cell count response and program retention. AIDS. 2010;24:515–524. doi: 10.1097/QAD.0b013e3283350ecd. [DOI] [PubMed] [Google Scholar]
- 82.Murphy RA, Sunpath H, Lu Z, Chelin N, Losina E, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24:1007–1012. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16:1935–1944. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 84.Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–641. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 85.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, et al. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–1377. additional data at http://www.hivpresentation.com/index.cfm?vId=1375BB1328A1344-1423A-F1376F1377-C1370DC1376EC1268CFF1357&cID=1375C1373B1824B-1423A-F1376F1377-C1372E1378CE1379BBF1379B1372D1392&show=slide. [PMC free article] [PubMed] [Google Scholar]
- 86.Thistle P, Gottesman M, Pilon R, Glazier RH, Arbess G, et al. A randomized control trial of an Ultra-Short zidovudine regimen in the prevention of perinatal HIV transmission in rural Zimbabwe. Cent Afr J Med. 2004;50:79–84. [PubMed] [Google Scholar]
- 87.Kesho Bora Study Group. Eighteen-month follow-up of HIV-1-infected mothers and their children enrolled in the Kesho Bora study observational cohorts. J Acquir Immune Defic Syndr. 2010;54:533–541. doi: 10.1097/QAI.0b013e3181e36634. [DOI] [PubMed] [Google Scholar]
- 88.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 89.de Vincenzi I Kesho Bora Study Group. LBPEC01: Triple-antiretroviral (ARV) prophylaxis during pregnancy and breastfeeding compared to short-ARV prophylaxis to prevent mother-to-child transmission of HIV-1 (MTCT): the Kesho Bora randomized controlled clinical trial in five sites in Burkina Faso, Kenya 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, South Africa. 2009. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=3631.
- 90.Fassinou P, Elenga N, Rouet F, Laguide R, Kouakoussui KA, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Côte d'Ivoire. AIDS. 2004;18:1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Appendix reports additional details of technical model structure, input data parameters, and model results, including extensive model validation and sensitivity analyses.
(DOC)