Abstract
A wealth of evidence supports the essential contributions of mast cells (MCs) to immune defense against bacteria and parasites; however, the role of MCs in viral infections has not been defined. We now report that rodent, monkey, and human MCs are able to detect dengue virus (DENV), a lymphotropic, enveloped, single-stranded, positive-sense RNA virus that results in MC activation and degranulation. We observe that the response of MCs to DENV also involves the activation of antiviral intracellular host response pathways, melanoma differentiation-associated gene 5 (MDA5) and retinoic acid inducible gene 1 (RIG-I), and the de novo transcription of cytokines, including TNF-α and IFN-α, and chemokines, such as CCL5, CXCL12, and CX3CL1. This multifaceted response of MCs to DENV is consequential to the containment of DENV in vivo because, after s.c. infection, MC-deficient mice show increased viral burden within draining lymph nodes, which are known to be targeted organs during DENV spread, compared with MC-sufficient mice. This containment of DENV is linked to the MC-driven recruitment of natural killer and natural killer T cells into the infected skin. These findings support expanding the defined role of immunosurveillance by MCs to include viral pathogens.
Mast cells (MCs) have been increasingly acknowledged for their roles in immunosurveillance for pathogens and for promoting innate immune responses. They are hematopoietic cells found in connective tissues and at the host–environment interface. First implicated in pathogen recognition in studies examining parasitic infections, the contribution of MCs to survival of infected hosts was subsequently illustrated, largely in various models of bacterial infection (1). In response to both of these subclasses of pathogens, MCs recognize and react with a biphasic response: nearly instantaneously beginning to release preformed mediators during the process of degranulation, followed by a second wave of release of de novo–produced cytokines. These immediate, innate responses of MCs to pathogens can contribute to pathogen clearance (1). For example, bacteria or their products have been shown to initiate MC-driven processes, including promoting the trafficking of neutrophils into sites of infection, enhancing bacterial clearance, and promoting the movement of nonresident dendritic cells into infected skin (1–3). In contrast to their well-defined contributions to surveillance for bacteria and parasites, MC responses to viruses have hardly been examined (1).
Whether and how MCs recognize viral pathogens is a key question; furthermore, the functional consequences of MC activation in response to viruses are largely unknown. The limited reports that have examined direct stimulation of MCs by viruses have done so mostly in vitro (1). In one, it was shown that MCs release histamine in response to Sendai virus (4), which could implicate the process of degranulation because histamine is prestored within granules (5). Since this observation, MC responses to viral products have mostly been studied with respect to cytokine responses. For example, the gp120 envelope protein of HIV promotes MC cytokine production (6). Despite this, HIV has also been shown to infect MCs, which can be a reservoir for persistent virus (7), illustrating the importance of investigating whether the role of MCs during viral infections is actually protective. Along these lines, very little in vivo data exist regarding the role of MCs in viral infections. A report examining a peritonitis model of Newcastle virus demonstrated that MCs can promote the recruitment of CD8+ T cells (8). These studies suggest that MCs may be involved in innate recognition of virus and highlight the need to further define the contribution of MCs to immune defense against viral pathogens.
One virus that appears to have some capacity to stimulate MCs is dengue virus (DENV) (9), a single-stranded RNA virus from the genus Flavivirus (10). As an arbovirus and human pathogen, DENV particles are injected into the skin by mosquitoes, after which they infect surrounding cells, including dendritic cells (11). During the progression of infection, DENV travels to draining lymph nodes (DLNs) and can subsequently establish viremia in the host (12). Cells that can kill DENV-infected cells, such as natural killer (NK) cells and T cells, have been implicated in DENV clearance in vivo (13–15), yet the cell types involved in initial surveillance for DENV are less defined. MCs are prevalent in the skin and, therefore, are likely to be among the first immune cells to encounter s.c.-injected DENV. DENV has been shown to promote cytokine production by human MC lines (9), but whether MCs are also able to degranulate in response to DENV has been unclear, as has whether they can contribute to a consequential functional antiviral response in vivo.
Results
Degranulation of MCs in Response to DENV.
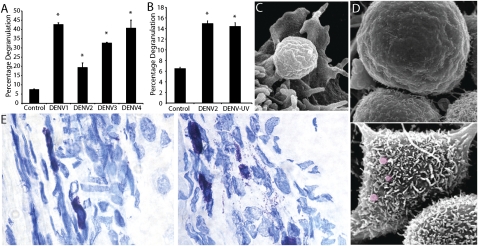
We first examined the ability of DENV to induce degranulation in the MC-like line rat basophilic leukemia-2H3 cells (RBLs). RBLs were treated with DENV, and an assay for degranulation—measurement of the release of the product, β-hexosaminidase—was used to quantify granule exocytosis. Because DENV can be divided into four serotypes that infect humans globally (16), we examined the ability of a representative from each antigenic group to promote degranulation, and we observed that all serotypes induced significant degranulation after 1 h (Fig. 1A). Although earlier time points showed modest increases in degranulation, we did not observe statistically significant degranulation before 1 h with these conditions, in contrast to the responses of RBLs to pharmacologic stimuli such as ionomycin, which directly promote calcium flux and result in significant degranulation within 15 min in this assay. Interestingly, the extent of degranulation did not differ at 1 h between RBLs exposed to live virus and to virus that had been inactivated with UV light (Fig. 1B), suggesting that DENV particles do not need to be infectious to prompt MC degranulation.
Fig. 1.
MCs degranulate in response to DENV. (A) DENV1–4 induced degranulation of RBLs. (B) Comparable RBL degranulation to live and UV-killed DENV2. Percentage degranulation in A and B was compared with spontaneous release from unstimulated MCs. *P < 0.05. (C and D) SEM of RBLs. (C) A single granule emerging from the ruffled surface of a RBL after DENV2 treatment. (D) Cell surfaces are visualized without (Upper) and with (Lower) exposure to DENV. Some extracellular granules are false-colored red. (E) Images of mouse footpad sections of control (Left) and DENV2-injected (2 × 105 pfu of DENV were injected s.c.; Right) footpads. MCs in Left are fully granulated and stain metachromatically (purple), whereas MCs in Right are partially degranulated with free purple granules visible in the surrounding tissue. (Magnification: 20×.)
Because direct degranulation of MCs in response to DENV has not been previously reported, we undertook microscopy studies to visualize the responses of MCs to DENV. By scanning electron microscopy (SEM), we observed extracellular granules as early as 30 min after exposure (although shown in Fig. 1C at 3 h after exposure) to DENV2. In these images, most extracellular granules were washed away in the process of preparing the cells for SEM; yet, several granules appear on the cell surfaces, entering the extracellular environment (Fig. 1D). Morphological changes also occurred in DENV2-activated RBLs, continuing beyond 12 h in culture (Fig. 1D). These DENV2-activated RBLs displayed morphology consistent with the extension of filopodia or with membrane ruffling (Fig. 1D), as has been observed in reports of MC activation by degranulating stimuli, such as IgE (17). Imaging of these cells by light microscopy supported the presence of extracellular granules around RBLs after DENV2 exposure (Fig. S1). These results indicate that that the RBLs are capable of degranulating as a result of direct stimulation by DENV.
We next sought to determine whether MCs could detect DENV and degranulate in vivo. Because mosquitoes usually inject DENV s.c. (16), we chose this route of injection to examine MC responses to DENV. Footpads from mice that had been injected with 2 × 105 pfu of DENV2 were harvested at 3 h postinoculation and sectioned, followed by staining with the MC-specific stain, toluidine blue. Although control-injected tissue showed little degranulation, with the vast majority of MCs staining densely for granules, DENV2-injected footpad tissue contained regions of MC degranulation where many extracellular granules could be visualized in the tissue (Fig. 1E). To quantify DENV-induced MC degranulation in vivo, we also intracellularly stained single-cell suspensions from footpads with avidin–FITC (which binds granules with high specificity) and observed a drastic decrease in staining for granules within cells that express the MC marker ckit after DENV infection (Fig. S2).
Cumulatively, these results indicate that rodent MCs recognize DENV and degranulate in response to its presence (Fig. 1); however, because DENV is considered to be a human pathogen, we were curious whether primate MCs would have similar responses. Therefore, we assessed the ability of DENV to induce degranulation of MCs in monkey skin and in a human MC-like cell line (Fig. 2). To begin, we injected DENV2 into monkey skin explants, which were maintained in cell culture conditions. At 1 h after injection of 5 × 106 pfu of DENV2 into multiple locations of ∼1.5-cm2 skin explants, the skin was stained for MCs in whole mount. We observed that MCs remain largely intact in control-injected monkey skin explants (Fig. 2A Left), whereas extensive degranulation can be seen in tissues injected with DENV2 (Fig. 2A Right and Fig. S3).
Fig. 2.
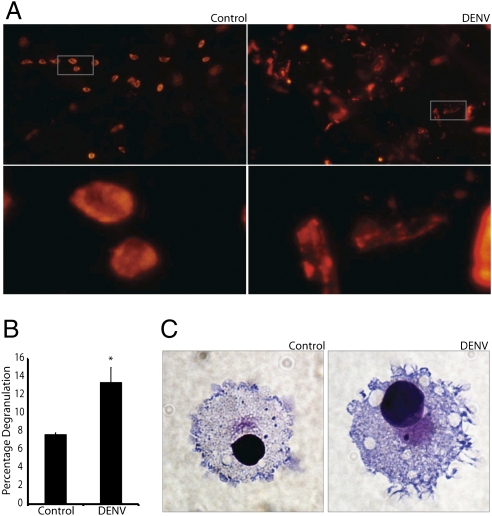
Primate MCs degranulate in response to DENV. (A) Images of whole-mounted monkey skin after ex vivo control (Left) or DENV2 injection (5 × 106 pfu; Right). After 1 h, explants were fixed and stained with the MC granule-specific probe, avidin–TRITC. Lower images reveal details of the boxed areas in the Upper images. Control tissue contains MCs staining densely for granules, whereas DENV-treated tissue contains free granules and cells that appear to be hypogranulated. (Magnification: 20×.) (B) Graph quantifies degranulation by human MCs (LAD2). Degranulation is significantly increased in DENV-treated, compared with control, cells. *P = 0.02. (C) Images of LAD2 cells without (Left) and with (Right) exposure to DENV2. Control cells contain several darkly staining granules. After DENV exposure, intracellular granules are not apparent and morphological changes consistent with granule release, e.g., membrane ruffling, are seen. (Magnification: 60×.)
The human tumor-derived MC line LAD2, when exposed to DENV2, also showed a degranulation response, as measured by β-hexosaminidase release after 1 h of stimulation (Fig. 2B). Additionally, we observed that LAD2 cells displayed morphological changes in response to DENV2 (Fig. 2C). These changes were consistent with the extension of filopodia by DENV2-activated RBLs that we previously had observed by SEM (Fig. 1 C and D). These data indicate that primate MCs are capable of detecting DENV and degranulating in response to its presence.
MC's Anti-DENV Transcriptional Program.
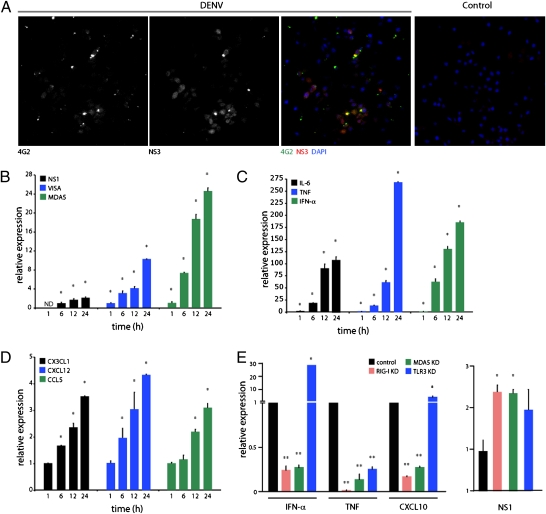
To further investigate the interaction between MCs and DENV, we investigated whether MCs could become infected by DENV, and, in parallel, we examined the expression of several host factors associated with MC activation or antiviral immunity. The intracellular production of dsRNA is a key intermediate step in the replication of DENV (10). Also important to replication are several viral proteins, such as nonstructural protein 3 (NS3) (18). To determine whether DENV could infect RBLs, we stained cells for NS3 at 24 h after exposure to DENV to reveal evidence of viral replication. Although virus could be observed in contact with many MCs (as determined by staining for the DENV envelope–specific antibody 4G2), only some of the cells in culture stained positive for NS3 (Fig. 3A). These observations, combined with a plaque-forming assay (Fig. S4A), support the ability of MCs to sustain some degree of DENV replication. The recognition of dsRNA within virally infected cells has been shown to be an important aspect of viral containment within infected hosts (19). Therefore, we also examined the expression of genes involved in dsRNA recognition within DENV-activated MCs. Melanoma differentiation-associated gene 5 (MDA5) is a virus sensor, expressed ubiquitously in the cytoplasm, that has been shown to be induced by intracellular dsRNA (20). Binding of MDA5 to dsRNA promotes the activation of a cascade of genes known to be responsible for antiviral responses. One such gene is virus-induced signaling adapter (VISA), which also functions downstream of retinoic acid inducible gene 1 (RIG-I), another intracellular dsRNA sensor, activating IFN-β signaling in a manner either dependent on or independent of Toll-like receptor 3 (TLR3) (19–22). These two genes, VISA and MDA5, have been reported to be induced by the presence of intracellular dsRNA (20), and we also observed that DENV2-treated RBLs increase their expression of MDA5 and VISA (Fig. 3B). Activation of these two pathways has previously been reported to occur in mouse fibroblasts in response to DENV, although, in their model, each pathway was individually shown to be dispensable for DENV activation of IFN-response genes (23). Despite our findings that MDA5 and VISA are induced in RBLs, we observed little increase in viral RNA copies (as measured by DENV gene NS1) (Fig. 3B). We also verified that RIG-I is directly activated by DENV in RBLs because we observed ubiquitination of RIG-I (Fig. S4B), which has been recently reported to be a key step in dsRNA-initiated RIG-I signaling (24). Together, these data support previous observations that MCs are remarkably resistant to direct infection by DENV (25) but also suggest that detection of intracellular dsRNA does occur and promotes innate “antiviral” responses.
Fig. 3.
MC induction of viral immunity genes after exposure to DENV. (A) RBLs stained with DAPI and for dengue envelope (4G2) and NS3 at 24 h after DENV infection [multiplicity of infection (MOI) = 5]. Control image depicts stained, uninfected cells. (Magnification: 20×.) (B) Enhanced expression of MDA5 and VISA over 24 h coincides with a twofold increase of DENV NS1. ND, not detected. (C and D) DENV enhances expression of cytokines IL-6, TNF, and IFN-α (C) and chemokines CXCL12, CCL5, and CX3CL1 (D). For B–D, expression for host genes was normalized to levels in control RBLs at 1 h. For NS1, values were normalized to the first detected levels, at 6 h. All values were determined by real-time PCR in RBLs with and without DENV treatment (MOI = 1). (E) IFN-α, TNF, and CXCL10 expression by RBLs (Left) or NS1 levels (Right) at 24 h after DENV treatment in cells with control siRNA or siRNA against RIG-I, MDA5, or TLR3. For B–D, * signifies a significant increase and ** indicates a significant decrease compared with control (P < 0.05).
We next investigated MC responses to DENV by identifying MC-derived factors, including cytokines, that are modulated by the presence of DENV and that might function within a site of infection. MCs can produce a wide range of cytokines after activation in response to varied stimuli, including pathogens or pathogen-associated products (1). TNF is a well-characterized product that MCs both prestore and produce de novo (26) and which is involved in varied innate as well as adaptive immune functions in the host, from promoting vascular changes to prompting swelling in DLNs (2, 27). We observed that, within 1 h, TNF expression is significantly up-regulated by RBLs with stimulation by DENV2 and continues to increase remarkably over a 24-h period, as does another potent proinflammatory cytokine, IL-6 (Fig. 3C). Additionally, enhanced expression of IFN-α occurred in DENV2-activated RBLs (Fig. 3C). Previously, in the context of TLR activation, IFN-α production by human MCs was suggested to be specific to viral stimuli, for example, being produced in response to TLR3 ligands (dsRNA) but not by TLR4, TLR2, or TLR5 stimulation (which respond to bacterial lipopolysaccharide, peptidoglycan, or flagellin, respectively) (28). With exposure to DENV2, RBLs also increased their production of IFN-α by 1 h and maintained this increased production over the course of 24 h (Fig. 3C). Because genes stimulated by type I interferons can have broad influence on both innate and adaptive immune responses (29), activation of IFN-response genes in MCs is likely to promote viral containment in the host.
Another response that appears to be activated in MCs in the presence of DENV is chemokine production. Production of the chemokines CCL2, CCL3, and CCL5 has previously been reported to occur in human MC lines exposed to DENV (9), and another model of MC stimulation by Newcastle virus or TLR3 ligands also promoted CXCL10 and CCL5 production (9). In the latter study, MCs were shown to be able to promote the recruitment of CD8+ T cells into the peritoneal cavity of mice (9). Therefore, we designed experiments to identify chemokines produced by DENV-activated RBLs. In addition to up-regulation of CCL5, RBLs enhanced their production of the chemokines CXCL12 and CX3CL1 (Fig. 3D). Interestingly, each of these chemokines has been shown to promote the recruitment of various subsets of T cells and NK cells (30, 31), cell types that are particularly important in the context of viral immunity for recognizing intracellular pathogens, including DENV (32, 33). These findings raise the possibility that MCs are consequential for immune surveillance against DENV through the recruitment of cytotoxic cells.
Therefore, we questioned whether any of the cellular sensors for viral RNA contribute to the observed transcriptional response. For this experiment, RBLs were treated with control siRNA or siRNA against RIG-I, MDA5, or TLR3 followed by treatment with DENV. With knockdown (KD) of RIG-I and MDA5, both IFN-α and CXCL10 expression were greatly reduced in response to DENV, whereas, in contrast, TLR3 KD did not reduce levels of these cytokines in response to DENV and even allowed enhanced levels (Fig. 3E). For TNF, all three viral sensors contributed to the magnitude of expression in response to DENV (Fig. 3E). In contrast, none of these KDs appeared to influence the ability of DENV-activated RBLs to degranulate. Interestingly, RIG-I KD or MDA5 KD, resulted in enhanced viral burden within MCs, whereas TLR3 KD did not achieve statistically significant increases under these conditions (Fig. 3E). These results demonstrate differential regulation of the host inflammatory response genes by MCs depending on the activation of viral pattern-recognition machinery and show that these transcriptional responses also appear to be uncoupled from MC degranulation.
MCs Promote a Functional Innate Response to DENV.
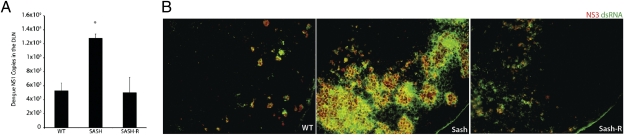
Based on the observation that DENV activates MCs, we undertook studies to assess whether MCs contribute to DENV clearance in vivo. Here, we used MC-deficient (Sash) mice and Sash mice that had been reconstituted with MCs (Sash-R), and we compared these groups to WT mice with respect to DENV burden in tissues. Because DENV is a lymphotropic virus, known to progress from the initial stage of cutaneous infection to infection of the DLNs (12), we measured DENV NS1 in the popliteal DLN at 24 h after injection of 2 × 105 pfu of DENV2 in the rear footpads. In our study, Sash mice had significantly higher DENV copies than WT mice did in their DLNs, yet Sash-R mice experienced similar viral burdens as WT mice did (Fig. 4A). These results were supported visually by staining of infected DLNs for signs of DENV replication (dsRNA and DENV NS3 protein) (Fig. 4B). These data reveal a striking increase in the extent of viral replication within the DLNs of MC-deficient compared with MC-sufficient mice, specifically implicating MCs in inhibiting viral spread from the footpad to the DLN.
Fig. 4.
Impaired clearance of DENV with MC deficiency. (A) Viral burden in the DLNs of WT, Sash, and Sash-R mice as determined by real-time PCR for DENV NS1, assessed at 24 h after footpad injection of 2 × 105 pfu of DENV2. * signifies a significant increase compared with WT (P = 0.0002; n = 6–8 for each group). (B) Viral replication detected in DLN sections from WT, Sash, and Sash-R mice by staining for NS3 (red) and dsRNA (green). For isotype and uninfected control images and channel-series staining, see Fig. S5.
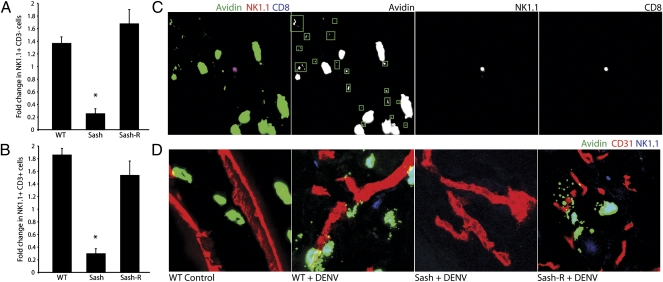
Because MCs are involved in many aspects of innate immune responses, there are multiple potential mechanisms for their contribution to host defense during the initial hours of DENV infection. However, based on the catalytic function that MCs have on the recruitment of innate immune cells, including neutrophils and dendritic cells, during bacterial infections (1) as well as our observations that several chemokines involved in NK and T-cell recruitment were up-regulated in RBLs (Fig. 3D), we hypothesized that various subsets of NK and/or T cells might be recruited to sites of infection in an MC-dependent manner. NK cells are rare lymphocytes, most of which have cytotoxic activity. In various models, they have been shown to infiltrate into inflamed peripheral tissues where only a few are needed to search for infected or stressed host cells (31). NK cell markers are also expressed by an assortment of T cells (NKT cells), some of which express T-cell receptors specific for the nonclassical MHC class 1 molecule (CD1d) and have been shown to recognize antigens such as glycosylated lipids of microbial origin (34). To determine whether MCs promote NK or NKT cell recruitment to sites of viral infection, we examined DENV2-infected footpads to determine whether any of these cells had entered into the tissue. As determined by flow cytometry, NK cells (NK1.1+, CD3−) as well as presumed NKT cells (NK1.1+, CD3+) both increased in numbers in an MC-dependent fashion within DENV-infected tissue by 24 h (Fig. 5 A and B and Fig. S6 A and B). Interestingly, although both of these groups of cells were augmented in infected tissues or the associated vasculature by 24 h, the percentage of NK1.1+ cells that also expressed CD3 was enriched compared with NK1.1+CD3− cells after DENV infection, relative to the proportions of these two subsets in saline-injected controls (Fig. S6 C and D). Although we observed a significant enhancement of NK1.1+ cells in infected footpads, our flow cytometry data do not distinguish between the cells that are in the tissue versus the cells that are within the local vasculature. For this reason, we also examined tissue sections to determine whether NK1.1+ cells could be located visually in the tissue. In mice that had been infected with DENV2 for 24 h, NK1.1+ cells were present in locations of MC activation, where extracellular granules could be seen (Fig. 5D and Fig. S7). We even observed NK1.1+ cells directly interacting with MCs (Fig. S8). Interestingly, a subset of NK1.1+CD8+ cells has been shown to infiltrate influenza-infected lungs (35), and we also observed that many of the NK1.1+ cells within the DENV-infected footpads expressed CD8 (Fig. 5C). To verify that NK1.1+ cells had entered the skin and exited the vasculature, we stained tissues for blood vessels and observed that NK1.1+ cells were localized proximal to but independent from the blood vessels during DENV infection (Fig. 5D). When DENV-infected tissues from Sash mice were examined, neither MCs nor NK1.1+ cells were found in infected footpads (Fig. 5D). Similarly, uninfected tissues did not reveal MC activation, and NK1.1+ cells could not be found within the tissues (Fig. 5D). As expected, MC activation in Sash-R mice also recapitulated this NK1.1+ cell recruitment into infected tissues. To verify that NK and NKT cells are consequential to anti-DENV immunity in our model, we depleted these cells by injection of an antibody against NK1.1. This study supported the involvement of NK1.1+ cells in early DENV containment by the host because depletion of these cells also allowed augmented titers of DENV within DLNs at 24 h (Fig. S9). Cumulatively, these results demonstrate that recruitment of NK cells and T cells bearing NK cell markers is likely to be one mechanism of MC-mediated containment of DENV infection.
Fig. 5.
MC-dependent recruitment of NK and NKT cells to site of DENV injection. Relative numbers of NK1.1+ CD3− (A) and NK1.1+CD3+ (B) cells after infection are increased over saline control tissues in WT and Sash-R mice but decreased in Sash mice, resulting in a significantly reduced fold increase for Sash mice compared with MC-sufficient mice (*P < 0.01; n = 4–6). (C) Microscopy of a footpad section 24 h after injection of 2 × 105 pfu of DENV2 shows that MCs (green) are activated in the vicinity of a recruited NK1.1+ (red) and CD8+ (blue) cell, based on the presence of proximal extracellular granules (boxed in green in C). A larger magnification of the surrounding tissue is included as Fig. S7. (D) Staining for CD31 (blood vessels; red), MC granules (green), and NK1.1+ cells (blue) demonstrates that NK1.1+ cells are found within the tissue in areas of MC activation in MC-sufficient mice at 24 h after injection of 1 × 105 pfu of DENV2 but not in uninfected (WT control) or DENV-infected Sash mice. (Magnification in C and D: 20× by confocal microscopy.)
Discussion
Decades of research have solidified the role of the MC in immune surveillance and innate immunity against pathogens (1). This role has been particularly well defined in the cases of MC protection against parasitic and bacterial challenges but only suggested for viral pathogens. We sought to address how MCs respond to a primary DENV infection and whether they contribute to host defense for this viral pathogen. Several lines of evidence suggest that MCs recognize viral products, mostly in the context of in vitro culture systems (1), with DENV being one virus reported to promote cytokine production (but not, previously, to induce degranulation of MCs cultured and matured from cord blood progenitors) (9). In the case of viral respiratory infections, it has also been shown that MCs increase in numbers after infection (36, 37). However, whether MCs can promote the clearance of a viral pathogen has not been investigated. Here, we provide evidence that MCs contribute to immunosurveillance for DENV, just after s.c. injection into the skin, by undergoing degranulation to release preformed mediators. This release appears to be in response to viral structural proteins because UV-inactivated virus also prompts degranulation. In vivo degranulation has not previously been observed in response to viral stimulation. Our results show a functional role for MCs in limiting the spread of DENV through DLNs, the normal route of infection progression (12). We also observed that MCs can become infected by DENV, yet they appear relatively resistant to infection, as has been suggested by others (25). Still, the possibility that MC interaction with virus could also potentially be detrimental for the host (7) emphasizes the need to individually investigate the responses of MCs to unique viral challenges.
Beyond degranulation, MCs respond to DENV by shifting their transcriptional program and up-regulating cytokines and chemokines, including CCL5 and CX3CL1, as well as genes for the detection of cytoplasmic dsRNA. Our data suggest that, in the case of CXCL10, chemokine up-regulation at least partially depends on DENV detection by the cytosolic sensors RIG-I and MDA5 rather than by TLR3. These chemokines are well characterized to recruit immune effector cells, including cytotoxic lymphocytes, to sites of peripheral inflammation (30, 31). In our DENV model, we see MC-dependent infiltration of NK1.1+ cells, subsets of which also expressed T-cell markers. Although NKT cells have long been associated with antiviral immunity and were reported to increase in the blood of DENV-infected human patients (15), their role in DENV infection has not been adequately explored. For example, a large percentage of virus-specific CD8+ and CD4+ T cells in a lymphocytic choriomeningitis virus model had an NKT phenotype (38), and NKT cells have been shown to be protective in models of herpes, respiratory syncytial, and hepatitis B viruses (39–41). NK cells are critical innate effector lymphocytes, also important for the early containment of several viral infections, including herpes simplex 1 and others (31, 42). They represent a small fraction of lymphocytes in the peripheral blood but exhibit potent antiviral activity and have also been implicated in DENV infection (14, 31). Remarkably, we saw MC-dependent infiltrations of NK1.1+ cells in DENV-infected footpads, suggesting that recruitment of NK and NKT cells by MCs may be one mechanism by which MCs control infection within tissues and limit viral spread to DLNs. Previously, MC recruitment of NK cells has been suggested, based on their capacity to promote the chemotaxis of these cells in vitro (43), yet the evidence presented herein supports the idea that NK cells may reach sites of viral challenge in vivo in an MC-promoted fashion. Future questions remain regarding any direct effects MCs may have on these cells, for example, through their potential to act as antigen-presenting cells, in light of our observations that NK1.1+ cells can closely associate with MCs during infection.
Our data suggest a model in which mosquito injection of DENV in the skin is quickly met with an MC sentinel response. MCs not only resist DENV infection themselves but also appear to enhance the recruitment of NK and NKT cells to facilitate viral clearance. We previously reported TNF as mediating drastic changes to the host vasculature during bacterial infection, including promoting the expression of E-selectin on the vascular endothelium to facilitate leukocyte rolling in the vicinity of infection (2). Therefore, we expect that the MC-dependent cellular recruitment response that we observed during DENV infection is likely to involve chemotaxis of cells directed by MC-derived chemokines as well as the affects of other prestored and de novo–produced MC-derived factors, such as TNF. Here, we see that MCs not only act as a first line of defense against parasitic and bacterial challenges but also can serve this role for certain viral challenges, through the specific induction of intra- and extracellular programs tailored for antiviral defense.
Methods
Animal studies were performed using C57BL/6 mice and cynomolgus macaques. In vitro degranulation was assessed by β-hexosaminidase assay. Detailed methods are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank D. Sergio, E. E. Ooi, P. Paradkar, C. Chan, and W. X. G. Ang. This work was supported by start-up funds from Duke-NUS GMS; US National Institutes of Health Grants R01DK077159, R01AI50021, and R37DK5081; and the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105079108/-/DCSupplemental.
References
- 1.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shelburne CP, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977;270:614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- 5.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 6.Patella V, Florio G, Petraroli A, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human FcεRI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000;164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom JB, et al. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109:5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orinska Z, et al. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood. 2005;106:978–987. doi: 10.1182/blood-2004-07-2656. [DOI] [PubMed] [Google Scholar]
- 9.King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–8419. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knipe DM, Howley PM. Fields Virology. 4th Ed. Philadelphia: Lippincott-Raven; 2001. [Google Scholar]
- 11.Palucka AK. Dengue virus and dendritic cells. Nat Med. 2000;6:748–749. doi: 10.1038/77470. [DOI] [PubMed] [Google Scholar]
- 12.Marchette NJ, Halstead SB, Falkler WA, Jr, Stenhouse A, Nash D. Studies on the pathogenesis of dengue infection in monkeys. 3. Sequential distribution of virus in primary and heterologous infections. J Infect Dis. 1973;128:23–30. doi: 10.1093/infdis/128.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Yauch LE, et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shresta S, Kyle JL, Robert Beatty P, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004;319:262–273. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Azeredo EL, et al. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol. 2006;143:345–356. doi: 10.1111/j.1365-2249.2006.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 17.Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004;172:4048–4058. doi: 10.4049/jimmunol.172.7.4048. [DOI] [PubMed] [Google Scholar]
- 18.Bollati M, et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antiviral Res. 2010;87:125–148. doi: 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu LG, et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CL, Gale M., Jr CARD games between virus and host get a new player. Trends Immunol. 2006;27:1–4. doi: 10.1016/j.it.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Loo YM, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 25.Brown MG, et al. Dramatic caspase-dependent apoptosis in antibody-enhanced dengue virus infection of human mast cells. J Leukoc Biol. 2009;85:71–80. doi: 10.1189/jlb.0308167. [DOI] [PubMed] [Google Scholar]
- 26.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan JB, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 28.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: Evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Katze MG, He Y, Gale M., Jr Viruses and interferon: A fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 30.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 31.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 32.Kurane I, Hebblewaite D, Brandt WE, Ennis FA. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984;52:223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dejnirattisai W, et al. A complex interplay among virus, dendritic cells, T cells, and cytokines in dengue virus infections. J Immunol. 2008;181:5865–5874. doi: 10.4049/jimmunol.181.9.5865. [DOI] [PubMed] [Google Scholar]
- 34.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 35.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castleman WL, Sorkness RL, Lemanske RF, Jr, McAllister PK. Viral bronchiolitis during early life induces increased numbers of bronchiolar mast cells and airway hyperresponsiveness. Am J Pathol. 1990;137:821–831. [PMC free article] [PubMed] [Google Scholar]
- 37.Sorden SD, Castleman WL. Virus-induced increases in bronchiolar mast cells in Brown Norway rats are associated with both local mast cell proliferation and increases in blood mast cell precursors. Lab Invest. 1995;73:197–204. [PubMed] [Google Scholar]
- 38.Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 39.Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα14-Jα281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- 40.Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–4303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke SM, et al. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.