Abstract
Although it is well established that neural cells are ectodermal derivatives in bilaterian animals, here we report the surprising discovery that some of the pharyngeal neurons of sea urchin embryos develop de novo from the endoderm. The appearance of these neurons is independent of mouth formation, in which the stomodeal ectoderm joins the foregut. The neurons do not derive from migration of ectoderm cells to the foregut, as shown by lineage tracing with the photoactivatable protein KikGR. Their specification and development depend on expression of Nkx3-2, which in turn depends on Six3, both of which are expressed in the foregut lineage. SoxB1, which is closely related to the vertebrate Sox factors that support a neural precursor state, is also expressed in the foregut throughout gastrulation, suggesting that this region of the fully formed archenteron retains an unexpected pluripotency. Together, these results lead to the unexpected conclusion that, within a cell lineage already specified to be endoderm by a well-established gene regulatory network [Peter IS, Davidson EH (2010) Dev Biol 340:188–199], there also operates a Six3/Nkx3-2–dependent pathway required for the de novo specification of some of the neurons in the pharynx. As a result, neuroendoderm precursors form in the foregut aided by retention of a SoxB1-dependent pluripotent state.
Keywords: cell fate specification, embryonic development, neurogenesis, enteric nerve
The nervous system of the sea urchin consists of an highly ordered array of neurons that develop sequentially in the anterior neuroectoderm, within or near the ciliary band, and in the pharyngeal region (1, 2) (Fig. 1). During embryogenesis, the anterior neuroectoderm is localized to the animal pole by controlled canonical Wnt-dependent processes (3, 4), and the ciliary band is positioned by TGF-β signaling through nodal and bone morphogenetic protein pathways (5–8). However, the mechanisms regulating development of pharyngeal neurons have not been explored. In other bilaterian animals, the nerves that innervate the gut are thought to develop from cells that migrate from either the ventral or dorsal ectoderm. In arthropods, individual cells and cells in epithelial vesicles arising from the ventral stomodeal ectoderm and presumptive nerve cord migrate to give rise to the stomatogastric nervous system (9), whereas in chordates, neural crest cells exit the neural tube and populate the enteric nervous system (10). Currently there is no evidence of a comparable ectodermal cell population in embryos of echinoderms or other invertebrate deuterostomes (11).
Fig. 1.
The structure of the sea urchin embryonic nervous system. (A and B) A 4-d embryo immunostained for serotonin (green), SynB (red), and nuclei (DAPI; blue). (C) Schematic indicating the three parts of the nervous system from an oral-vegetal view (OVV). These regions are the anterior neuroectoderm, located at the animal pole (AP; dark blue) and containing serotonergic neurons (green) and nonserotonergic neurons (purple in C and red between the dashed arrows in B); the ciliary band (CB; medium blue), which forms between oral (white) and aboral (light blue) ectoderm and contains nonserotonergic neurons (orange ovals); and the pharyngeal neurons close to the lower lip of the mouth (M in A and C) (curved white arrow in A; red ovals in C). (Scale bar in B: 20 μm.)
The pharnyx is derived from both oral (stomodeal) ectoderm and foregut endoderm. Although all of the pharyngeal neurons are expected to come from the ectoderm, unexpectedly we found that some cells in the foregut expressed the neural marker synaptotagmin B (SynB) in embryos in which the foregut was separated from the stomodeal ectoderm. Here we present these observations as well as the results of further tests that strongly support the surprising conclusion that some pharyngeal neurons develop directly from tissue specified as endoderm. Using an ectodermal cell-tracking approach with the photoactivatable protein KikGR (12), we eliminate the possibility that these neurons are derived from ectodermal precursors that migrated to the foregut. We demonstrate that the transcription factor Six3, which is required for these foregut neurons and all embryonic neurons, is transiently expressed in the foregut lineage. We also show that a Six3-dependent gene, Nkx3-2, which is expressed only in the oral anterior ectoderm and the foregut, is also essential for the development of pharyngeal neurons. Importantly, the Six3/Nkx3-2 pathway operates in foregut precursors well after deployment of the endoderm gene regulatory network (13), but foregut cells also express SoxB1, a transcription factor present in cells whose fates are not fully committed. These findings demonstrate that neurons develop directly from cells in endodermal tissue.
Results
Neurons Develop in the Foregut of Exogastrulae and in Embryos Lacking a Mouth.
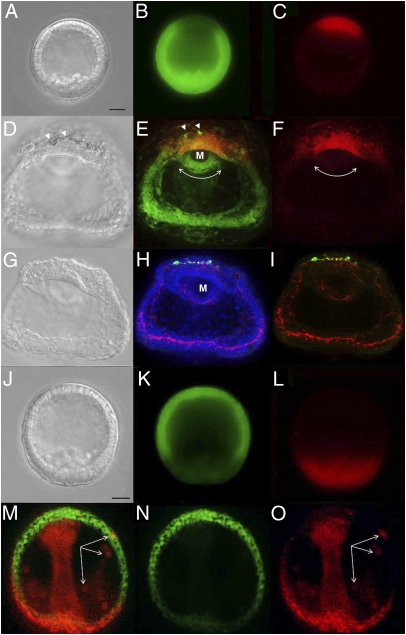
Some foregut neurons were observed in two kinds of embryos that develop at very low frequencies in populations of normal embryos. In 4-d embryos in which the gut had evaginated at the beginning of gastrulation, SynB-expressing cells were observed in the foregut region (Fig. 2A). The same was observed in embryos in which the foregut and stomodeal ectoderm failed to join and form the mouth opening (Fig. 2B). Furthermore, as in the animal pole and ciliary band neurogenic fields, cells in the foregut are subject to Notch-mediated lateral inhibition. Treatment with the γ-secretase inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester), which often causes exogastrulation, results in significantly more neurons not only in animal pole and ciliary band ectoderm, but also in the foregut (Fig. 2D), compared with normal larvae (Fig. 2 A and C). Close examination reveals that processes extend from these cells throughout the foregut (Fig. S1). Long processes connecting foregut neurons to other pharyngeal neurons derived from the ectoderm can be seen in embryos in which the mouth has formed normally (Fig. 2 F–H). Together, these data indicate that neurons are present in the foregut.
Fig. 2.
Pharyngeal neurons develop in the foregut. Embryos are labeled as in Fig. 1A. (A) Development of pharyngeal neurons is independent of mouth formation. Neurons are present in the foregut of an exogastrulated 4-d embryo (red arrow; lateral view). (B) A 4-d embryo in which the foregut did not contact the oral ectoderm (red arrows; lateral view). FG, foregut; MG, midgut; HG, hindgut. (C) Same embryo as in A, rotated 90 degrees to facilitate comparison with D. (D) DAPT-treated 4-d pluteus (oral views). Hindguts in A, C, and D are out of the plane of focus. (E–H) Normal 6-d pluteus. (E) Differential interference contrast images. M, mouth; MG, midgut; brackets, coelomic pouches adjacent to foregut; dashed trapezoid, foregut. (F) confocal stack. (G and H) Higher-magnification confocal sections of pharyngeal region (dotted rectangle in F) illustrating connections of neural cells (white arrows) on the left (G) and right (H) sides of the foregut to other nerves around the mouth (M). Cells to the left (G) or right (H) of the arrows are coelomic pouch cells adjacent to the foregut. (Scale bars in A, F, and G: 20 μm.)
Neural Precursors Do Not Migrate to the Foregut from Ectoderm.
To test whether the foregut neurons are derived from neural precursors that migrated from the ectoderm to the foregut, we tracked ectoderm cells using the photoactivatable protein KikGR (12), introduced by mRNA injection at the single-cell stage. Because these neurons depend on Six3 (4), and because Six3 is expressed predominantly in animal pole ectoderm, we believed that such precursors likely would be derived from this region of the embryo. Consequently, we created embryos in which animal pole ectoderm was labeled red by KikGR photoactivation at early mesenchyme blastula stage, while the rest of the embryo remained green (Fig. 3 A–C). At 4 d, after the time when neurons appear in the pharyngeal region, we observed no red cells there, although many migrating green mesenchyme cells were evident (Fig. 3 D–F; see Fig. S2 for a series of 2-μm optical sections through the gut region). The absence of migratory (red) ectoderm cells is not related to deleterious effects of photoactivation of KikGR, as demonstrated by the normal development of pharyngeal neurons, as well as the rest of the embryo (Fig. 3 G–I).
Fig. 3.
Ectodermal cells do not migrate to the pharynx. (A–C) Animal pole cells of early mesenchyme blastulae were labeled by photoactivating KikGR (from green to red in C) synthesized from mRNA injected at the one-cell stage. (D–F) The same embryo as in A–C at 4 d lacks red cells in the mouth region (M; curved arrows in E and F). Arrowheads in E indicate migratory mesenchyme cells. (G–I) This same embryo developed normally, and differentiated neurons in the mouth and ciliary band and at the animal pole (SynB, red; serotonin, green; DAPI, blue; cf. Fig. 1 A and B). (J–L) Presumptive mesenchyme and endoderm cells in an early mesenchyme blastula were labeled by photoactivating KikGR (red; L), whereas ectoderm cells remained green. (M–O) In this embryo, no green ectoderm progeny were visible in the foregut 1 d later; however, red mesenchyme cells that migrated from the vegetal plate were detected (white arrows in M and O). One of these, a pigment cell that intercalates into the ectoderm, is visible in M (yellow). (Scale bars in A and J: 20 μm.) A, D, G, and J are differential interference contrast images.
To explore whether the foregut neurons come from other ectodermal regions, we produced embryos in which the endomesoderm cells were labeled red by photoactivation and the remaining cells that give rise to ectoderm were green (Fig. 3 J–L). In this experiment, we performed assays at an earlier stage when the foregut is easier to analyze, but after the time at which neurons begin to differentiate there, as determined by SynB in situ hybridization (ISH) (Fig. S3A). Again, no cells of ectodermal origin were detected in the gut (Fig. 3 M–O). In contrast, red migratory skeletogenic mesenchyme and nonskeletogenic cells were readily detected (Fig. 3M, arrows). These results provide strong evidence that foregut neurons do not arise from the presumptive ciliated band, the stomodeal ectoderm, or indeed from any ectodermal source, but instead develop in situ in the gut.
Six3 and Nkx3-2 Are Expressed in Foregut and Required for Pharyngeal Neuron Development.
Previous studies clearly showed that Six3 is required for development of pharyngeal neurons at 3 d (4); this is also the case a day later, long after these neurons normally develop (Fig. S3 B and C). Although Six3 transcripts are not detectable in the foregut endoderm at gastrula stages (4, 14), they are present at mesenchyme blastula stage in the precursors to this part of the gut (Fig. 4 A and B, red), which are adjacent to nonskeletogenic mesenchyme expressing gcm (15) (Fig. 4 A and B, green), the same criterion used to show that members of the endoderm gene regulatory network are expressed in the presumptive foregut (13).
Fig. 4.
A Six3 > Nkx3-2 > SynB neural pathway operates in the foregut. (A) Double FISH shows that in a 24-h mesenchyme blastula, Six3 (red) is expressed in foregut precursors adjacent to those expressing gcm (green) in nonskeletogenic mesenchyme; vegetal view (vv). (B) Schematic of the foregut lineage (red). AP, animal pole; LV, lateral view; SM, skeletogenic mesenchyme; NSM, nonskeletogenic mesenchyme; FgEn, foregut endoderm. (C and D) ISH shows that Nkx3-2 is expressed in oral animal pole ectoderm and foregut endoderm during gastrulation (C), but not in Six3 morphants (D). (E) Nkx3-2 morphants lack neurons in the foregut (curved arrow), as well as nonserotonergic neurons (red; anti-SynB) that develop on the oral side of animal pole ectoderm (dashed arrows; compare with regions between dashed arrows in Fig. 1B); serotonergic neurons (green). (F–H) Double FISH shows that Nkx3-2 (red) and SynB (green) mRNAs are coexpressed in the foregut (white arrows). (H) Higher-magnification image of the mouth shown in F. (Scale bar in C: 20 μm.)
The expression of Six3 in foregut precursors and the appearance of the first SynB-expressing neurons in the foregut (Fig. S2A) are separated by at least a day of development, implying intermediate steps in this process. Thus, we examined the expression of Six3-dependent regulatory genes (4) and found that one, Nkx3-2, is expressed in the animal pole ectoderm starting at the mesenchyme blastula stage (Fig. S3E) and then in the oral animal pole ectoderm and foregut of gastrulating embryos (Fig. 4C and Fig. 3 F and G), that is, the same regions in which pharyngeal neurons develop (Fig. 1 A and B). Nkx3-2 expression is detectable only in these territories, and in each territory it depends on Six3 (Fig. 4D). It is required for the development of all pharyngeal neurons, as shown in two different Nkx3-2 morphants (Fig. 4E and Fig. S3 H and I; compare with normal embryos shown in Fig. 1 A and B). These results suggest that Nkx3-2 has a cell-autonomous role in promoting neurogenesis in the foregut, a conclusion strongly reinforced by the finding of that Nkx3-2 and SynB are coexpressed in the gut (Fig. 4 F–H, white arrows). Although the connection between Nkx3-2 and SynB transcription may be direct, that between Six3 and Nkx3-2 transcription likely is not direct, given that expression of Six3 precedes that of Nkx3-2 by at least 12 h and accumulation of their mRNAs does not overlap during gastrula stages (4) (Fig. 4C). If there were a direct connection, then the appearance of Six3 and Nkx3-2 transcripts likely would be separated by only several hours in this system (16). Taken together, these results indicate that Six3 supports expression of Nkx3-2, which is required for differentiation of pharyngeal neurons not only in the oral animal pole ectoderm, but also in the foregut.
Foregut Endoderm Expresses SoxB1.
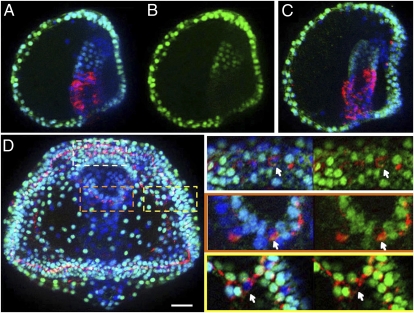
At the time when Nkx3-2 and SynB are expressed in the foregut, SoxB1 also is expressed in this region, as demonstrated by comparison with Endo1, a marker of midgut and hindgut (Fig. 5 A and B). Interestingly, SoxB1 is most closely related to Sox1, Sox2, and Sox3, which have been shown to maintain a neural precursor state in mouse embryos (17, 18). Just as these factors disappear when neurons differentiate in mouse embryos, so also is SoxB1 eliminated from differentiating neurons in ectoderm and foregut endoderm in sea urchin embryos. These cells contain SynB (red), but they are devoid of SoxB1 (green) (Fig. 5D, white arrows). As expected, if SoxB1 supports an uncommitted neural precursor state within foregut endoderm, then its expression should not depend on Six3, and in fact this is the case (Fig. 5C). Because the loss of SoxB1 results in gastrulation defects (19), we cannot determine whether it is required for foregut neural development or Nkx3-2 expression.
Fig. 5.
SoxB1 mRNA accumulates in the foregut, disappears from differentiating neurons and does not depend on Six3. (A–C) Immunostaining of a normal 2-d embryo (A and B) or a Six3 morphant (C), lateral views, for SoxB1 (green) and Endo1 (A and C, red), which labels midgut and hindgut, and DAPI (A and C, nuclei). (D) SoxB1 protein disappears from differentiated nerve cells: SoxB1- (green), SynB- (red), and DAPI-labeled (blue) 4-d embryo, oral view. Optical sections of regions outlined in white (animal pole ectoderm), orange (mouth region), and yellow (ciliary band) rectangles are shown in three (left) or two (right) combined color channels. Arrows indicate that differentiated nerve cells (red cytoplasm) lack SoxB1 (green nuclei). (Scale bar in E: 20 μm.)
Discussion
Here we demonstrate that, in bilaterian embryos, unexpectedly, neurons develop de novo in cells already specified as endoderm, challenging the dogma that they always originate from ectoderm. Endodermal neurogenesis is mediated by Six3 and Nkx3-2, the same factors required for neurogenesis in the oral animal pole ectoderm. However, the Six3/Nkx3-2 pathway is able to operate in the context of a fully functional endomesoderm regulatory network that has driven cells far down the endoderm specification pathway (13). We propose that endodermal neurogenesis in the sea urchin embryo uses the initial underlying neural potential of early blastomeres (3, 4) that may be preserved in foregut endoderm by selective SoxB1 perdurance.
The early endoderm gene regulatory network is directly activated by canonical Wnt signaling, which is required for endoderm development (20). Expression of all components of this network becomes restricted to endoderm precursors by the eighth cleavage, when endoderm and nonskeletogenic mesoderm segregate (13). At this time, specification of endoderm is well underway because, in addition to positive inputs directly from nuclear β-catenin, cross-regulatory interactions among the early endoderm network genes have been established. Importantly, expression of these genes is uniform in the ring of foregut precursors (13), indicating that at the hatching blastula stage, there is no evidence of separate populations of endodermal and neural cells. It is not until ninth cleavage, several hours later, that Six3 expression is activated in presumptive foregut cells by an as-yet undefined mechanism. In previous work, we established that Six3 functions near the top of the neurogenic regulatory hierarchy in the anterior neuroectoderm at the animal pole (4) and likely has a similar role in the endoderm. Consequently, we propose that Six3 also is necessary to generate neuroendodermal precursor cells, several of which give rise to neural progeny well after morphogenesis of the endoderm has begun.
The Six3-dependent foregut neural specification pathway initially operates in more cells than will give rise to neurons. The Six3-dependent gene Nkx3-2 is expressed initially throughout a significant fraction of the foregut endoderm but later in only a subset of these cells. How restriction of Nkx3-2 expression and neural capacity occurs between gastrula and pluteus larval stages is unclear, but Notch-mediated lateral inhibition is at least partly involved.
The finding that SoxB1 is expressed exclusively in the foregut region of the archenteron may provide an important clue to understanding how neurons can develop there. We propose that SoxB1 function supports retention of pluripotency in this region, at least in part by antagonizing canonical Wnt signaling that drives endomesoderm development. Previously, we showed that SoxB1 suppresses β-catenin activity in normal embryos during the period when early endoderm is specified and Six3 expression begins, and that misexpression of SoxB1 can completely block endomesoderm development (19). Thus, persistent SoxB1 expression specifically in the foregut could delay progression to a stable endodermal fate, which precludes neurogenesis. Subsequently, the combination of reduced endoderm network function via SoxB1 and expression of Six3 and Nkx3-2 could specify the foregut as neuroendoderm. If SoxB1 functions to maintain pluripotency, then the transition to a terminally differentiated state should require elimination of SoxB1. This appears to be the case, given that SoxB1 is successively down-regulated in all endomesoderm cell lineages except the foregut as they commit to specific fates: first skeletogenic mesenchyme (21), then nonskeletogenic mesoderm (21–23), and then midgut and hindgut (this study). This pattern of early ubiquitous SoxB1 expression followed by down-regulation through cell signaling is similar to that of the apparent SoxB1 ortholog Sox2 (19, 21, 24, 25), whose role in maintaining pluripotency in mouse embryonic and induced pluripotent stem cells is now well established (26, 27). The other SoxB1 class factors, Sox1 and Sox3, also maintain neural progenitors in an undifferentiated state, and their removal is also required for neural differentiation (17, 18). Similarly, SoxB1 is present throughout the ectoderm and foregut endoderm, but disappears from differentiating neurons in each of these regions. Our data suggest that SoxB1 executes at least some of the same functions in sea urchin embryos as the SoxB class factors do in vertebrate embryos.
In the absence of signaling, the initial regulatory state of early blastomeres supports neural fate specification in all embryos in which it has been examined (28), as well as in embryonic stem cells (29). Our work suggests that this basal neural regulatory state is not lost in some endoderm lineages of sea urchin embryos, just as it is not lost in some mesoderm lineages of mouse embryos (30). Whether persistence of neural capacity in endoderm lineages is a property of other metazoans or is a derived character in echinoderms is not yet known. However, intriguingly, in mouse adult gut mucosa, cryptic neural stem cells have been detected after treatment of mice with the neural-inducing agent 5-HT4 (31). If vertebrate neuroendoderm precursor cells do exist, they likely are rare and escape detection because of the large contributions of the neural crest to the enteric nervous system.
In sea urchin embryos, the challenges now are to understand the gene regulatory mechanism that maintains latent neurogenic capacity in cells already running nonectodermal developmental programs and that which triggers progression of the neural gene regulatory program in a few of these cells, leading them to become neurons.
Materials and Methods
Embryo Culture.
Adult sea urchins, Strongylocentrotus purpuratus, were maintained in seawater at 10 °C. Embryos were cultured by standard methods in artificial seawater at 15 °C. In some experiments, embryos were cultured in 5 μM DAPT.
Microinjection of Morpholino Antisense Oligonucleotides.
Eggs were prepared as described previously (4). Approximately 2 pL of solution containing 22.5% glycerol and morpholinos (MOs; Gene Tools) were injected. For Nkx3-2MO1 (5′CGTTCATGTTGGTCTGAAATGATGC3′) and MO2 (5′GCGATCCAAATGGATTCCAATTTCG), the concentrations were 0.4 mM. For Six3MO (4), the concentration was 0.75 mM.
Green-to-Red KikGR Fluorescence Protein Photoactivation.
The pKikGR plasmid was a gift from Dr. Atsushi Miyawaki (RIKEN Brain Institute, Saitama, Japan). Synthesized mRNA (mMessageMachine, 0.5 μg/μL; Ambion) was injected into fertilized eggs. At 24 h postfertilization, embryos were immobilized in hypertonic artificial seawater (32 mg/mL NaCl) for 2 min and photoactivated by illuminating with 360-nm UV light for one 3-s pulse at the desired region. Embryos continued development to 2–4 d in normal artificial seawater, at which point they were checked for migration of labeled cells. Photoactivation and photography were carried out with a Zeiss Axiovert 200M microscope. Optical sections were obtained with an ApoTome unit (Zeiss), and stacked images were prepared using Adobe Photoshop. This experiment was carried out on 30 different embryos derived from three different matings.
Whole-Mount in Situ Hybridization.
Embryos were fixed, hybridized, and stained as described previously (4). Two-color FISH was performed as described previously (32) at probe concentrations of 0.1 ng/μL.
Immunohistochemistry.
Embryos were fixed in 2% formaldehyde. Primary antibodies were incubated overnight at 4 °C using the following dilutions: serotonin, 1:1,000 (Sigma-Aldrich); Endo1, 1:250 (33); SynB (1e11), 1:1,000 (34); and SoxB1, 1:1,000 (21). Bound primary antibodies were detected by incubation with Alexa-coupled secondary antibodies for 1 h.
Supplementary Material
Acknowledgments
We thank Dr. Sarah Knox for assistance with confocal microscopy and Dr. Robert Burke for the SynB antibody. This work was supported by the Intramural Research Program, National Institute of Dental and Craniofacial Research.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018513108/-/DCSupplemental.
References
- 1.Nakajima Y, Kaneko H, Murray G, Burke RD. Divergent patterns of neural development in larval echinoids and asteroids. Evol Dev. 2004;6:95–104. doi: 10.1111/j.1525-142x.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 2.Burke RD, et al. A genomic view of the sea urchin nervous system. Dev Biol. 2006;300:434–460. doi: 10.1016/j.ydbio.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaguchi S, Yaguchi J, Burke RD. Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development. 2006;133:2337–2346. doi: 10.1242/dev.02396. [DOI] [PubMed] [Google Scholar]
- 4.Wei Z, Yaguchi J, Yaguchi S, Angerer RC, Angerer LM. The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development. 2009;136:1179–1189. doi: 10.1242/dev.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradham CA, et al. Chordin is required for neural but not axial development in sea urchin embryos. Dev Biol. 2009;328:221–233. doi: 10.1016/j.ydbio.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapraz F, Besnardeau L, Lepage T. Patterning of the dorsal-ventral axis in echinoderms: Insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 2009;7:e1000248. doi: 10.1371/journal.pbio.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaguchi S, Yaguchi J, Angerer RC, Angerer LM, Burke RD. TGFβ signaling positions the ciliary band and patterns neurons in the sea urchin embryo. Dev Biol. 2010;347:71–81. doi: 10.1016/j.ydbio.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saudemont A, et al. Gene regulatory network analysis in an echinoderm reveals ancestral regulatory circuits regulating ectoderm patterning and morphogenesis downstream of nodal and BMP2/4. PLoS Genet. 2010;6:e1001259. doi: 10.1371/journal.pgen.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartenstein V. Development of the insect stomatogastric nervous system. Trends Neurosci. 1997;20:421–427. doi: 10.1016/s0166-2236(97)01066-7. [DOI] [PubMed] [Google Scholar]
- 10.Burns AJ, Pachnis V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol Motil. 2009;21:100–102. doi: 10.1111/j.1365-2982.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 11.Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Chapter 1: Gene regulatory networks in neural crest development and evolution. Curr Top Dev Biol. 2009;86:1–14. doi: 10.1016/S0070-2153(09)01001-1. [DOI] [PubMed] [Google Scholar]
- 12.Poustka AJ, et al. A global view of gene expression in lithium- and zinc- treated sea urchin embryos: New components of gene regulatory networks. Genome Biol. 2007;8:R85.1–18. doi: 10.1186/gb-2007-8-5-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 2005;6:233–238. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter IS, Davidson EH. The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev Biol. 2010;340:188–199. doi: 10.1016/j.ydbio.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransick A, Rast JP, Minokawa T, Calestani C, Davidson EH. New early zygotic regulators expressed in endomesoderm of sea urchin embryos discovered by differential array hybridization. Dev Biol. 2002;246:132–147. doi: 10.1006/dbio.2002.0607. [DOI] [PubMed] [Google Scholar]
- 16.Bolouri H, Davidson EH. Transcriptional regulatory cascades in development: Initial rates, not steady state, determine network kinetics. Proc Natl Acad Sci USA. 2003;100:9371–9376. doi: 10.1073/pnas.1533293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 18.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 19.Kenny AP, Oleksyn DW, Newman LA, Angerer RC, Angerer LM. Tight regulation of SpSoxB factors is required for patterning and morphogenesis in sea urchin embryos. Dev Biol. 2003;261:412–425. doi: 10.1016/s0012-1606(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 20.Davidson EH, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 21.Kenny AP, Kozlowski D, Oleksyn DW, Angerer LM, Angerer RC. SpSoxB1, a maternally encoded transcription factor asymmetrically distributed among early sea urchin blastomeres. Development. 1999;126:5473–5483. doi: 10.1242/dev.126.23.5473. [DOI] [PubMed] [Google Scholar]
- 22.Oliveri P, Davidson EH, McClay DR. Activation of pmar1 controls specification of micromeres in the sea urchin embryo. Dev Biol. 2003;258:32–43. doi: 10.1016/s0012-1606(03)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Sethi AJ, Angerer RC, Angerer LM. Gene regulatory network interactions in sea urchin endomesoderm induction. PLoS Biol. 2009;7:e1000029. doi: 10.1371/journal.pbio.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 25.Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masui S, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka S, et al. Pluripotency of embryonic stem cells. Cell Tissue Res. 2008;331:5–22. doi: 10.1007/s00441-007-0520-5. [DOI] [PubMed] [Google Scholar]
- 28.Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tropepe V, et al. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 30.Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaguchi S, Yaguchi J, Angerer RC, Angerer LM. A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell. 2008;14:97–107. doi: 10.1016/j.devcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Wessel GM, McClay DR. Two embryonic, tissue-specific molecules identified by a double-label immunofluorescence technique for monoclonal antibodies. J Histochem Cytochem. 1986;34:703–706. doi: 10.1177/34.6.3084626. [DOI] [PubMed] [Google Scholar]
- 34.Burke RD, et al. Neuron-specific expression of a synaptotagmin gene in the sea urchin Strongylocentrotus purpuratus. J Comp Neurol. 2006;496:244–251. doi: 10.1002/cne.20939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.