Abstract
Methylation of cytosine in DNA plays a crucial role in development through inheritable gene silencing. The DNA methyltransferase Dnmt1 is responsible for the propagation of methylation patterns to the next generation via its preferential methylation of hemimethylated CpG sites in the genome; however, how Dnmt1 maintains methylation patterns is not fully understood. Here we report the crystal structure of the large fragment (291–1620) of mouse Dnmt1 and its complexes with cofactor S-adenosyl-L-methionine and its product S-adenosyl-L-homocystein. Notably, in the absence of DNA, the N-terminal domain responsible for targeting Dnmt1 to replication foci is inserted into the DNA-binding pocket, indicating that this domain must be removed for methylation to occur. Upon binding of S-adenosyl-L-methionine, the catalytic cysteine residue undergoes a conformation transition to a catalytically competent position. For the recognition of hemimethylated DNA, Dnmt1 is expected to utilize a target recognition domain that overhangs the putative DNA-binding pocket. Taking into considerations the recent report of a shorter fragment structure of Dnmt1 that the CXXC motif positions itself in the catalytic pocket and prevents aberrant de novo methylation, we propose that maintenance methylation is a multistep process accompanied by structural changes.
Keywords: maintenance DNA methylation, X-ray crystallography, multidomain structure
In mammals, genomic DNA is often methylated at the fifth position of the cytosine base in CpG sequences (1). This DNA methylation, which is one of the major epigenetic modifications, plays a crucial role in development, genome stability, X-chromosome inactivation, and silencing of retrotransposons (2–4). DNA methyltransferases (Dnmts) catalyze the transfer of a methyl group to the fifth position of cytosine bases in DNA. Mammalian genomes carry three distinct active Dnmt genes. Two of the three genes, Dnm3a and Dnmt3b, encode enzymes that show activity toward unmethylated DNA (5, 6) and are responsible for creating global DNA methylation patterns during embryogenesis and gametogenesis (7, 8). Once the DNA methylation patterns are established, they are maintained by Dnmt1 encoded by the Dnmt1 gene, which ensures the transmission of lineage-specific DNA methylation patterns during replication (9). Dnmt1 preferentially methylates the hemimethylated state of DNA that appears just after replication or repair. To do this, Dnmt1 interacts both with proliferating cell nuclear antigen (PCNA), a factor that is a prerequisite for replication (10), and Np95/Uhrf1, a factor that is necessary for the maintenance of DNA methylation and that binds hemimethylated DNA at replication foci (11–14). Knockout of Dnmt1 in mice leads to embryonic lethality at a stage corresponding to global methylation in the genome (9).
All of the DNA methyltransferases identified to date utilize S-adenosyl-L-methionine (AdoMet) as the methyl group donor. The reaction mechanism of cytosine-C5 methylation has been analyzed for the prokaryotic DNA (cytosine-C5)-methyltransferase M.HhaI (15). A key catalytic process is the nucleophilic attack of the enzyme on the sixth carbon of the target cytosine. This attack is made by the thiol group of the cysteine residue in the conserved PCQ motif (motif IV) of the methyltransferase (Fig. S1). The reaction is catalyzed by protonation of the N3 position of the cytosine by the glutamate in the ENV motif (motif VI).
Dnmt1 is a large single polypeptide, and its primary sequence is highly conserved among various species. Mouse Dnmt1 comprises 1620 amino acid residues with a size of 180 kD. The C-terminal catalytic domain (residues 1125–1620) and the remaining N-terminal regulatory domain (residues 1–1111) are connected by a flexible KG-repeat (residues 1112–1124) (Fig. 1A). In the N-terminal region, the N-terminal 243 residues form an independent domain that serves as a platform (16) for the binding of many proteins or DNA including PCNA (10, 16–20).
Fig. 1.
Multidomain structure of mouse Dnmt1(291–1620). (A) Replication foci targeting sequence (RFTS), zincfinger-like motif (CXXC), bromo-associated homology domain 1 (BAH1) and 2 (BAH2), and catalytic domain are schematically illustrated. The catalytic domain comprising 10 conserved motifs (I ∼ X), and target recognition domain (TRD) between the motifs VIII and IX. The numbers of the amino acid residues are indicated. (B) Ribbon model of mouse Dnmt1(291–1620). Around the C-terminal catalytic domain (blue), the other domains including the RFTS (magenta), CXXC motif (cyan), and two BAH domains BAH1 (green) and BAH2 (orange) are shown. Four zinc ions are shown in red spheres. All of the zinc ions are in a motif similar to Zn-finger motif (Fig. S10). The KG-repeat (1112–1124) linker connecting the N-terminal region and the C-terminal catalytic domain is in a flexible structure as the density map showed disorder.
In the present study, we crystallized a large fragment of Dnmt1 lacking only the N-terminal 1–290 residues (Dnmt1(291–1620)) (Fig. 1B) that selectively methylates hemimethylated DNA in vitro (21). Recently, the crystal structure of a Dnmt1 fragment encompassing amino acids 550–1602 in complex with unmethylated DNA has been reported (22). Based on both structures, we propose that multiple changes occur during maintenance methylation.
Results and Discussion
RFTS Is Inserted into the Putative DNA-Binding Pocket of Dnmt1.
We have succeeded in crystallizing a Dnmt1 fragment comprising the amino acid sequence 291–1620 and determined the following three crystal structures: the substrate unbound free form, and the S-adenosyl-L-homocystein-(AdoH)bound and AdoMet-bound forms (Table S1). The structures were successfully modeled as electron density maps (Fig. S2A), and the amino acid assignment was confirmed by methionine sites (Fig. S2B). The whole crystal structure of Dnmt1(291–1620) showed a distinct multidomain structure comprising the replication foci targeting sequence (RFTS), a zinc-finger-like (CXXC) motif, two tandemly connected bromo-associated homology (BAH) domains, and the catalytic domain (Fig. 1B). The multiple domains in the N-terminal region surround and make contact with the C-terminal catalytic domain.
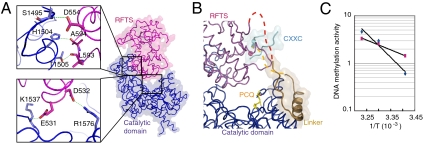
Among the N-terminal domains, the position of RFTS is unique. It is apparently inserted deeply into the DNA-binding pocket, where it forms several hydrogen bonds with the catalytic domain (Fig. 2A). The electrostatic potential of the RFTS and the catalytic domains at the interface is a negative and positive net charge, respectively (Fig. S3). Because the RFTS domain interacts via limited numbers of contacts with the next molecules in the crystal structure, it is unlikely that the crystallization affected the orientation of the RFTS domain. It has been reported that the RFTS domain is required for recruitment of Dnmt1 to replication foci (23). Interestingly, however, based on our present structure, DNA cannot bind to the catalytic pocket owing to steric hindrance from RFTS, which sits in the pocket.
Fig. 2.
The RFTS domain plugs into the DNA-binding pocket. (A) The RFTS domain is positioned in the DNA-binding pocket of Dnmt1 and stabilized by several hydrogen bonds in the catalytic domain. (B) The linker (brown) between the RFTS domain (deep red) and the CXXC motif (light blue) interacts with the catalytic domain (dark blue) by hydrophobic forces to maintain the position of the PCQ loop (see Fig. S7). C. Fitting to Arrhenius equation of the DNA methylation activity of Dnmt1(291–1620) (cyan diamonds) containing the RFTS domain and Dnmt1(602–1620) (magenta squares) lacking the RFTS domain. The activation energies of Dnmt1(291–1620) and Dnmt1(602–1620) were calculated to be 110 and 30 kJ/mol, respectively.
It is puzzling that, with the RFTS domain inserted deeply into the DNA-binding pocket where hemimethylated DNA is expected to fit, Dnmt1(291–1620) still shows methylation activity toward hemimethylated DNA (21). It is reasonable to speculate that unless the inserted RFTS domain is released, DNA cannot access the catalytic center. The linker connecting the RFTS domain and CXXC motif is interacting with the PCQ loop at the catalytic center by the hydrophobic interaction of the linker amino acid residues (F631, F634, and F635) with the ones (Y1243 and F1246) following the PCQ loop (Fig. 2B and Fig. S4). The interaction between the linker and the PCQ loop contributes to narrowing the entrance of the DNA-binding pocket and to anchoring the RFTS domain to the DNA-binding pocket, and consequently the catalytic center is completely masked.
It is reasonable to expect that significant activation energy is necessary to change the positions of the RFTS domain and the CXXC motif to allow DNA binding and subsequent DNA methylation activity. Recombinant Dnmt1(291–1620) demonstrates higher DNA methylation activity toward hemimethylated DNA than unmethylated ones (21). In addition, Dnmt1(602–1620), in which the RFTS coding sequence is deleted, also showed a specificity toward hemimethylated DNA similar to that of Dnmt1(291–1620) containing RFTS (Fig. S5). Intriguingly, the activation energy of Dnmt1(291–1620) for the DNA methylation reaction was about threefold larger than that of Dnmt1(602–1621) (Fig. 2C).
Recently, the crystal structure of a Dnmt1 fragment comprising residues 650–1602 in complex with unmethylated DNA has been reported (22). The reported Dnmt1 fragment lacks RFTS domain. In our structure, different from the structure by Song et al. (22), the CXXC motif sits where unmethylated DNA binds. One of the two zinc ions and the interacting peptide chain in the CXXC motif are not seen in our present structure. Superimposition of our structure and that by Song et al. is shown in Fig. 3. For the binding of substrate DNA, the RFTS domain must change its position. In addition, the CXXC motif is pushed toward the catalytic center in the complex with unmethylated DNA, in which position the CXXC motif cannot bind the hemimethylated form of CpG (22). The sequence following the CXXC motif forms an α-helix in our structure but forms a loop in Song’s (22). The interaction of the CXXC motif with DNA may affect the linker between RFTS and CXXC and possibly induces a conformational change in Dnmt1. Because almost no significant structural difference in the catalytic domain including PCQ loop is found between our structure and that by Song’s (see Fig. 3), the binding of unmethylated DNA may thereby affect exclusively the release of the RFTS domain from the DNA-binding pocket. For DNA methylation, displacement of RFTS from the catalytic center and movement of the CXXC motif must require energy, which suggests that there is a mechanism to regulate the insertion of RFTS into the catalytic domain. The interaction with the target DNA via the CXXC motif with its net positive charge (Fig. S6) can be a crucial step for the initiation of the release of the RFTS domain from the DNA-binding pocket for the DNA methylation activity.
Fig. 3.
Model of conformational changes that are predicted to be induced by DNA binding. Superimposition of the free-form structure including the RFTS domain (blue, PBD ID code 3av4) and the complex with unmethylated DNA structure (yellow, PDB ID code 3pt6). The CXXC motifs (dark blue) and the linker connecting the RFTS domain and CXXC motif (light blue) are indicated in deep colors, and the rest sequences are in pale colors. The RFTS domain (pale gray) and unmethylated DNA (pale yellow) are shown in surface model. The sequences involved in the interaction of the linker connecting the RFTS domain and CXXC motif with the PCQ loop at the catalytic center are magnified.
AdoMet Induces a Position Change of the Cysteine Residue in the PCQ Loop at the Catalytic Center.
The 10 motifs characteristic to DNA (cytosine-C5)-methyltransferases (24) are conserved in the catalytic domain of Dnmt1 (Fig. S1). The three-dimensional structure of Dnmt1 shows that the spatial arrangement of the motifs is similar to the reported structure of M.HhaI (PDB ID code 5mht) (Fig. S7A). It is reasonable to assume that the DNA methylation mechanism of Dnmt1 is similar to that of M.HhaI (15), and thus the C1229 in the PCQ loop is expected to form a covalent bond with the sixth carbon of the target cytosine base. In M.HhaI, the side chain conformation of C81 in the PCQ loop at the catalytic center is facing away from the double-stranded DNA, in the presence of AdoMet (25). The C81 turns to approach the cytosine base to be methylated only when it binds to DNA (26). From this, O’Gara et al. proposed an ordered mechanism in which DNA binding precedes the binding of AdoMet (27). Similar to M.HhaI, the side chain of C1229 in the PCQ loop of Dnmt1(291–1620) faces away from the putative DNA-binding site in the absence and presence of AdoH (Fig. 4A and Fig. S7B). Notably, the side chain of C1229 in the PCQ loop turns to face the putative DNA-binding pocket, which is the catalytically competent position corresponding to the side chain of C81 of M.HhaI, when it binds to DNA (Fig. 4B). The phenyl group of F1232 is apparently pushing the side chain of C1229 into its position (Fig. S8). Distinct from M.HhaI, the present study clearly shows that prebinding of AdoMet to Dnmt1 shifts the position of the side chain of C1229 in the PCQ loop close to the expected position of the flipped-out cytosine. Thus, we propose that binding of AdoMet shifts the Dnmt1 structure to a state that is ready to receive hemimethylated DNA from Np95/Uhrf1, a factor that specifically binds to hemimethylated DNA for the maintenance methylation in vivo (11). As soon as the methyl group is transferred from AdoMet to the cytosine base to produce AdoH, the side chain of C1229 immediately faces away. The binding of AdoMet does not change the overall structure of Dnmt1 including the catalytic domain other than the cysteine residue in the PCQ loop. A possible reason for the AdoMet-induced change in the position of the side chain of C1229 might be that the DNA-binding pocket of Dnmt1 is slightly narrower than that of M.HhaI when it is not binding DNA (Fig. S7B). As discussed above, the amino acid residues following the PCQ loop are interacting with the linker between the RFTS domain and the CXXC motif (Fig. S4). These forces that pull the PCQ loop toward the DNA-binding pocket might affect the position change of C1229 induced by AdoMet binding in Dnmt1.
Fig. 4.
AdoMet turns the side chain of the cysteine in the PCQ loop toward the target cytosine. (A) Superimposition of the PCQ loop of Dnmt1 in its free form (green), with AdoH (orange), and with AdoMet (blue). (B) Binding of AdoMet to the catalytic site induces the side chain of C1229 to turn toward the target cytosine (Left). C1229 is expected to bind to C6 and the methyl-group of AdoMet binds to C5, of the cytosine (red arrows). After transfer of the methyl-group to the fifth position of cytosine, C1229 turns back to the inactive AdoH-binding form (Right). The position of cytosine was taken from PDB ID code 5mht, the structure of M.HhaI complex with methylated DNA and AdoH.
Recognition of Hemimethylated DNA.
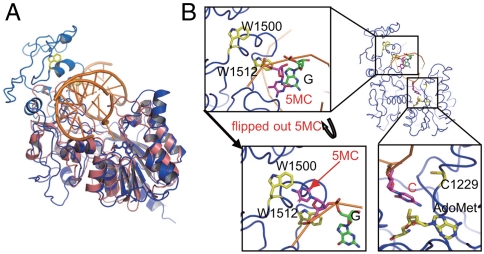
The substrate specificity of Dnmt1, which preferentially methylates hemimethylated DNA, is unique among DNA (cytosine-C5)-methyltransferases (28). In bacterial DNA (cytosine-C5)-methyltransferases, the target recognition domain (TRD), which recognizes the sequence to be methylated, is located between motifs VIII and IX (29–31) (Fig. S1). Intriguingly, the TRD of Dnmt1 is unusually large as compared with those of bacterial enzymes (24). This large TRD overhangs the putative DNA-binding pocket where double-stranded DNA fits for the methylation reaction (Fig. 5A). Because the 10 motifs comprising the catalytic center access the target cytosine to be methylated from one side, it seems reasonable that the TRD that overhangs the DNA-binding pocket from the other side is responsible for recognizing the methylated cytosine base in the hemimethylated CpG sequence. By mimicking the three-dimensional structure of the M.HhaI complex with DNA, which is the active form in complex with DNA and in which the target cytosine base is flipped outside the double-stranded DNA, we fitted DNA into the catalytic pocket of Dnmt1. According to the resultant model, W1512 is close to the methylated cytosine that is opposite the target cytosine of the hemimethylated CpG (Fig. 5B). In addition, W1500 becomes close to this methylated cytosine when the base is flipped outside the double-stranded DNA (Fig. 5B). In accordance with this observation, we replaced W1500 and W1512 with A or L and determined the DNA methylation activity of the mutants. All the mutants of Dnmt1 showed almost complete abolition of DNA methylation activity toward both hemimethylated and unmethylated DNA (Fig. S9). Because we expected that the position of the methylated CpG would be fixed, either facing the antisense CpG or in a flipped-out configuration, it was surprising that the mutations at W1500 and W1512 both inhibited the DNA methylation activity. This observation may suggest that both residues are involved in recognition of the target hemimethylated DNA for methylation activity.
Fig. 5.
The TRD of Dnmt1 overhangs the putative DNA-binding pocket. (A) Superimposition of the catalytic domain of Dnmt1 with M.HhaI. The positions of the TRD of Dnmt1 (light blue) and the hemimethylated double-stranded DNA fitted form of the 10 motifs (blue) of the catalytic domain of Dnmt1(291–1620) superimposed with M.HhaI (PDB ID code 5mht) (pink) are shown. (B) DNA-fitted model of Dnmt1(291–1620) showing that W1512 is close to the methylated cytosine base of the hemimethylated DNA when the methylated cytosine (5MC) stays inside the double-stranded DNA, whereas W1500 is close to when the 5MC is flipped out.
Maintenance Methylation Is Accompanied by Structural Changes.
The structure reported by Song et al. (22) showed the Dnmt1(550–1602) complex with DNA, which is positioned away from the catalytic center, leading them to propose that the deduced structure represents the auto-inhibition form of Dnmt1 protecting from de novo DNA methylation. In contrast, our present crystal structure, although not containing DNA, but including the RFTS and the methyl group donor AdoMet, shows three striking features. First, the RFTS is inserted into the catalytic pocket such that DNA cannot access the catalytic center. Second, the CXXC motif is in a position where the DNA binds in the structure reported by Song et al. (22). Last, the complex with AdoMet causes C1229 to flip toward the target cytosine. This residue is expected to form a covalent bond with the sixth position of the target cytosine base. The results clearly indicate that multiple structural changes must take place for faithful maintenance DNA methylation.
Materials and Methods
Expression and Purification of Dnmt1(291–1620) and Dnmt1(602–1620).
Mouse Dnmt1 was cloned into baculovirus in accordance with the manufacturer’s instructions and was used to infect to Sf9 cells. The expressed Dnmt1 protein was purified as described elsewhere (21) and then further purified by size-exclusion chromatography in 0.35 M NaCl, 20 mM HEPES-Na (pH 7.0), concentrated to 9 mg/mL, and then used for crystallization. To prepare seleno-methionine (Se-Met) labeled Dnmt1, Sf9 cells were collected 18 h after infection, washed with sterilized PBS, and then transferred into Sf900II medium depleted of methionine and cysteine (Invitrogen), and supplemented with 100 mg/L Se-Met and 150 mg/L L-cysteine. The cells were collected after 48 h, and Se-Met-labeled Dnmt1 was purified by the identical protocol used for native protein.
Crystallization
Crystals were obtained by vapor diffusion by mixing equal volumes of protein solution (6.5 mg/mL) and reservoir solution containing 0.35 M NaCl, 2% Tascimate (Hampton Research) 20 mM TCEP-HCl (Hampton Research), 18–20% PEG3350, and 100 mM Tris-HCl (pH 9.0). The incubation temperature was changed stepwise from 28 to 23 °C. In brief, the solution was initially incubated at 28 °C for 6 to 12 h, and then transferred to 23 °C for crystal growth. The crystals that formed were transferred to a solution of PEG3350 2% higher than the reservoir solution to stabilize the crystal. Crystals were treated by the gradual addition of PEG 200 to a final concentration of 20% and flash frozen in liquid nitrogen. To determine the initial phase of diffraction, the crystals were soaked with 5 mM Ta6Br14 derivative for 2 h.
X-ray Data Collection
Data sets were collected on BL44XU at SPring-8 with a DIP6040 imaging-plate detector (Bruker AXS) and a MX225-HE charge coupled device detector (Raynonix). The native data was collected at 2.75-Å resolution. Isomorphous derivative datasets of tantalum cluster derivative crystal was acquired by tuning X-rays at 1.2454 Å (Derivative I: Ta6Br14). Diffraction data of Se-Met derivative crystals were collected with X-rays of 0.90000 Å (remote), 0.97894 Å (peak), and 0.97954 Å (edge) (Derivative II: Se-Met). Anomalous difference Fourier maps of all datasets (Native and Derivative I, II) showed clear peaks at the same positions and suggested the existence of anomalous scatterers that specifically bound to the molecule. These peaks were assumed to be zinc ions, because it is predicted that zinc binding motifs (CXXC motif) exist in the primary structure of Dnmt1. Next three datasets of native crystals were collected at 0.9000 Å (remote), 1.28220 Å (peak), and 1.28309 Å (edge) for phase determination by the anomalous effect of zinc ions (Derivative III: Zinc dataset). The complex crystal with AdoMet or SAH was prepared by soaking with crystallization buffer and cryoprotectant including cofactor ligand, of which the final concentration was 100 μM. Two complex datasets for AdoMet (Native AdoMet) and SAH (Native SAH) were collected at 3.10 Å and 3.25 Å resolution, respectively. All X-ray experiments were performed at 90 K. These SPring-8 diffraction data were processed and scaled with HKL2000 (32). The native crystals and derivative crystals belonged to the space group P21212. The experimental conditions and statistics of intensity data acquisition are given in Table S1.
Structure Determination
For the initial phase determination, the heavy atom sites of the Ta6Br14 clusters were determined by using the difference Patterson map calculated with Native data and Derivative I data at 6-Å resolution, and the SIRAS method was applied. The anomalous effects of selenium atoms in the Se-Met derivative and the intrinsic five zinc ions were used for phase determination by the MAD method with SHARP (33). Twenty-three out of 28 methionine sites in Dnmt1(291–1620) were identified by selenium peaks higher than 3.5σ in the electron density distribution in the anomalous difference Fourier map. Five zinc sites were identified from the anomalous difference Fourier map. Model building was performed by using the program Coot (34), and structural refinement was carried out with the programs REFMAC (35) and BUSTER (36). Crystallographic R and Rfree for 5% of the reflections excluded from the refinement were calculated to monitor the structural refinement procedures. The results of the structural analysis are summarized in Table S1. The free form structure of the final R and Rfree values were 23.5% and 26.9%, respectively. The main-chain dihedral angles for 90.6% were in the favored and 1.6% were in outlier of the Ramachandran plot as defined in MolProbity (37). These refinement statistics of the AdoH and AdoMet complexes are given in Table S1. The refined structure was validated by using the program MolProbity (37). All molecular graphics were created with Pymol (DeLano Scientific, http://www.pymol.org).
Determination of DNA Methylation Activity
The DNA methylation activity was determined as described elsewhere (20). For determination of the activation energy, Dnmt1(291–1620) and Dnmt1(602–1620) were incubated at 20, 30, and 37 °C for 1 h.
Supplementary Material
Acknowledgments.
We wish to thank Ms. Yumiko Yamagami for constructing the baculoviruses coding Dnmt1 and expression in sf9 cells, and Ms. Keiko Shinohara for purification of Dnmt1. This work was partly supported by Grants-in-Aid for Scientific Research B by the Japan Society for the Promotion of Science (S.T.), by the Japan Aerospace Exploration Agency–Granada Crystallization Facility High Quality Protein Crystallization Project on the Protein Structure and Function Analysis for Application (A.N.), and by the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology, Japan (A.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Atomic coordinates and structure factors for the reported crystal structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3av4, 3av5, and 3av6).
See Commentary on page 8919.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019629108/-/DCSupplemental.
References
- 1.Ehrlich M, et al. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982;10:2709–2721. doi: 10.1093/nar/10.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 3.Reik W, Lewis A. Co-evolution of X-chromosome inactivation and imprinting in mammals. Nat Rev Genet. 2005;6:403–410. doi: 10.1038/nrg1602. [DOI] [PubMed] [Google Scholar]
- 4.Goll M-G, Bestor T-H. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 5.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 6.Aoki A, et al. Enzymatic properties of de novo-type mouse DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 2001;29:3506–3512. doi: 10.1093/nar/29.17.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okano M, Bell D-W, Haber D-A, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 8.Kaneda M, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 9.Li E, Bestor T-H, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 10.Chuang L-S, et al. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science. 1997;277:1996–2000. doi: 10.1126/science.277.5334.1996. [DOI] [PubMed] [Google Scholar]
- 11.Sharif J, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 12.Arita K, Ariyoshi M, Tochio H, Nakamura Y, Shirakawa M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature. 2008;455:818–821. doi: 10.1038/nature07249. [DOI] [PubMed] [Google Scholar]
- 13.Avvakumov G-V, et al. Structural basis for recognition of hemi-methylated DNA by the SRA domain of human UHRF1. Nature. 2008;455:822–825. doi: 10.1038/nature07273. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto H, et al. The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. Nature. 2008;455:826–829. doi: 10.1038/nature07280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J-C, Santi D-V. Kinetic and catalytic mechanism of HhaI methyltransferase. J Biol Chem. 1987;262:4778–4786. [PubMed] [Google Scholar]
- 16.Suetake I, Hayata D, Tajima S. The amino-terminus of mouse DNA methyltransferase 1 forms an independent domain and binds to DNA with the sequence involving PCNA binding motif. J Biochem. 2006;140:763–776. doi: 10.1093/jb/mvj210. [DOI] [PubMed] [Google Scholar]
- 17.Rountree M-R, Bachman K-E, Baylin S-B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet. 2000;25:269–277. doi: 10.1038/77023. [DOI] [PubMed] [Google Scholar]
- 18.Kim G-D, Ni J, Kelesoglu N, Roberts R-J, Pradhan S. Co-operation and communication between the human maintenance and de novo DNA (cytosine-5) methyltransferases. EMBO J. 2002;21:4183–4195. doi: 10.1093/emboj/cdf401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameshita I, et al. Cyclin-dependent kinase-like 5 binds and phosphorylates DNA methyltransferase 1. Biochem Biophys Res Commun. 2008;377:1162–1167. doi: 10.1016/j.bbrc.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama Y, et al. The DNA-binding activity of mouse DNA methyltransferase 1 is regulated by phosphorylation with casein kinase 1δ/ε. Biochem J. 2010;427:489–497. doi: 10.1042/BJ20091856. [DOI] [PubMed] [Google Scholar]
- 21.Vilkaitis G, Suetake I, Klimašauskas S, Tajima S. Processive methylation of hemimethylated CpG sites by mouse Dnmt1 DNA methyltransferase. J Biol Chem. 2005;280:64–72. doi: 10.1074/jbc.M411126200. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Rechkoblit O, Bestor T-H, Patel D-J. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science. 2011;331:1036–1040. doi: 10.1126/science.1195380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonhardt H, Page A-W, Weier H-U, Bestor T-H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992;71:865–873. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, et al. The DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 1994;22:1–10. doi: 10.1093/nar/22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng X, Kumar S, Posfai J, Pflugrath J-W, Roberts R-J. Crystal structure of the HhaI DNA methyltransferase complex with S-adnosyl-L-methhionine. Cell. 1993;74:299–307. doi: 10.1016/0092-8674(93)90421-l. [DOI] [PubMed] [Google Scholar]
- 26.Klimasauskas S, Kumar S, Roberts R-J, Cheng X. HhaI methyltransferase flips its target base out of the DNA helix. Cell. 1994;76:357–369. doi: 10.1016/0092-8674(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 27.O’Gara M, Zhang X, Roberts R-J, Cheng X. Structure of a binary complex of HhaI methyltransferase with S-adenosyl-L-methionine formed in the presence of a short non-specific DNA oligonucleotide. J Mol Biol. 1999;287:201–209. doi: 10.1006/jmbi.1999.2608. [DOI] [PubMed] [Google Scholar]
- 28.Gruenbaum Y, Cedar H, Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982;295:620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- 29.Trautner T-A, Balganesh T-S, Pawlek B. Chimeric multispecific DNA methyltransferases with novel combinations of target recognition. Nucleic Acids Res. 1988;16:6649–6658. doi: 10.1093/nar/16.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilke K, et al. Sequential order of target-recognizing domains in multi specific DNA-methyltransferases. EMBO J. 1988;7:2601–2609. doi: 10.1002/j.1460-2075.1988.tb03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange C, Jugel A, Walter J, Noyer-Weidner M, Trautner T-A. ‘Pseudo’ domains in phage-encoded DNA methyltransferases. Nature. 1991;352:645–648. doi: 10.1038/352645a0. [DOI] [PubMed] [Google Scholar]
- 32.Otwinowski Z, Minor W, editors. Proceedings of X-ray Diffraction Data Collected in Oscillation Mode. New York: Academic; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 33.Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Generation, representation and flow of phase information in structure determination: Recent developments in and around SHARP 2.0. Acta Crystallogr D Biol Crystallogr. 2003;59:2023–2030. doi: 10.1107/s0907444903017694. [DOI] [PubMed] [Google Scholar]
- 34.Emsley P, Lohkamp B, Scott W-G, Cowtan K. Features and development of coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murshudov G-N, Vagin A-A, Dodson E-J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 36.Bricogne G, et al. Cambridge: Global Phasing Ltd; 2009. BUSTER, version 2.8.0. [Google Scholar]
- 37.Chen V-B, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.