Abstract
Arterial tissue-engineering techniques that have been reported previously typically involve long waiting times of several months while cells from the recipient are cultured to create the engineered vessel. In this study, we developed a different approach to arterial tissue engineering that can substantially reduce the waiting time for a graft. Tissue-engineered vessels (TEVs) were grown from banked porcine smooth muscle cells that were allogeneic to the intended recipient, using a biomimetic perfusion system. The engineered vessels were then decellularized, leaving behind the mechanically robust extracellular matrix of the graft wall. The acellular grafts were then seeded with cells that were derived from the intended recipient—either endothelial progenitor cells (EPC) or endothelial cell (EC)—on the graft lumen. TEV were then implanted as end-to-side grafts in the porcine carotid artery, which is a rigorous testbed due to its tendency for graft occlusion. The EPC- and EC-seeded TEV all remained patent for 30 d in this study, whereas the contralateral control vein grafts were patent in only 3/8 implants. Going along with the improved patency, the cell-seeded TEV demonstrated less neointimal hyperplasia and fewer proliferating cells than did the vein grafts. Proteins in the mammalian target of rapamycin signaling pathway tended to be decreased in TEV compared with vein grafts, implicating this pathway in the TEV's resistance to occlusion from intimal hyperplasia. These results indicate that a readily available, decellularized tissue-engineered vessel can be seeded with autologous endothelial progenitor cells to provide a biological vascular graft that resists both clotting and intimal hyperplasia. In addition, these results show that engineered connective tissues can be grown from banked cells, rendered acellular, and then used for tissue regeneration in vivo.
Keywords: bypass graft, collagen, mechanical conditioning
Arterial bypass graft implantation remains the primary therapy for patients with advanced cardiovascular disease. The ongoing need for arterial conduits is due to the poor clinical efficacy of existing synthetic grafts in small diameter artery applications (<6 mm) (1). In addition, many patients with arterial disease lack adequate saphenous vein or other conduit for bypass procedures (2, 3). Tissue-engineering approaches can be used to generate biologically based conduits to address the need for vascular grafts (4, 5). In one approach, native vessels can be decellularized and/or cross-linked, and can serve as conduits for small diameter arterial grafting (6, 7). However, procuring native allogeneic vessels for decellularization is a significant practical hurdle, which has heretofore limited the extensive application of such an approach. We report here the results of a large animal study of a tissue-engineered graft that is engineered from allogeneic cells, and is decellularized to provide a suitable matrix for endothelialization.
A viable endothelium is generally required to prevent thrombosis of small caliber vascular grafts in animal models, especially for decellularized vessels that are mostly comprised of collagen (8, 9). The source of endothelium to coat the vascular graft could be obtained from a minimally invasive peripheral blood draw to harvest endothelial progenitor cells, as opposed to isolating endothelial cells from vein by a surgical procedure (10–12). The approach of integrating a tissue-engineered vessel, decellularization, and an endothelial progenitor cell population has not been reported previously to the best of our knowledge. Decellularization of a completely tissue-engineered vessel could provide a designer vessel that could be stored like synthetic (e.g., Teflon or Dacron) materials and would be available when needed as a bypass graft (13). The decellularization of a tissue-engineered vessel is more efficient than engineering a vessel for an individual patient and eliminates the extended lead time for autologous vessel culture that may limit the widespread use completely autologous grafts (14). In addition, demonstrating utility of an acellular, engineered connective tissue should point the way to producing other acellular tissues that may involve no culture, or only a short culture period for treating patients with a variety of connective tissue defects.
Results
Tissue-Engineered Vessel and Mechanical Properties.
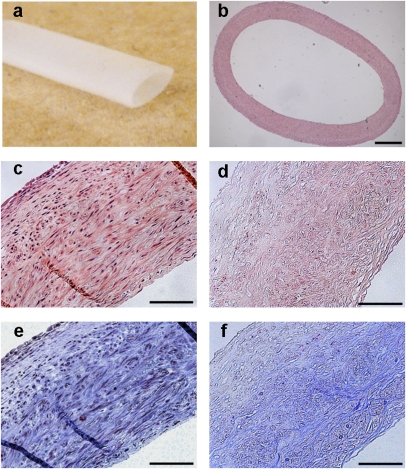
A biomimetic perfusion system was used to culture small diameter vessels, as reported (Fig. 1A) (15, 16). Aortic smooth muscle cells were cultured on a degradable polyglycolic acid mesh scaffold in bioreactors for 10 wk. At the conclusion of culture, the fresh tissue-engineered vessel had a robust, dense layer of smooth muscle cells with minimal residual scaffold polyglycolic acid (PGA) fibers (Fig. 1 B and C). The vessel wall thickness of the engineered vessels (442 ± 13 μm; n = 8) was not significantly different after decellularization (459 ± 17 μm; n = 8). Before decellularization, engineered tissues had 15–20 layers of smooth muscle cells interposed with layers of collagenous extracellular matrix. Few PGA polymer residuals were observed near the luminal aspects of the engineered vessels. After decellularization, nuclei were removed from the engineered vessels (Fig. 1D), whereas collagen was found throughout the engineered and decellularized vessels (Fig. 1 E and F). The histological findings were confirmed by assays for DNA and collagen content. The DNA of the fresh vessel (5.7 ± 0.4% of dry weight; n = 8) was significantly reduced after decellularization (0.8 ± 0.05% dry weight; n = 8; P < 0.001). In contrast, the collagen content increased as a percentage of the dry weight after decellularization (69 ± 2.0; n = 8) compared with the fresh vessels (51 ± 2.4; n = 8; P < 0.001). Because the collagen matrix remains after decellularization and other cellular proteins are removed, the amount of collagen tends to increase as a percentage of dry weight after decellularization. The loss of cellular proteins was also evaluated by immunohistochemistry and Western blot analysis (Fig. S1). On immunohistochemistry, major histocompatibility complex I (MHC-I) was removed after decellularization (Fig. S1 A and B). By Western blot analysis, the smooth muscle protein calponin, as well as β-actin and GAPDH, were removed after decellularization (Fig. S1C). Hence, the decellularization method resulted in a loss of cellular components but retention of the collagenous extracellular matrix.
Fig. 1.
Tissue-engineered vessel from porcine SMC. (A) Porcine tissue-engineered vessel after 10 wk in culture. (B) Histology of the tissue-engineered vessel by H&E staining. H&E of the tissue-engineered vessel before (C) and after (D) decellularization with a loss of nuclei. Masson's Trichrome stain before (E) and after (F) decellularization demonstrating preservation of collagen (blue) throughout the matrix. (Scale bars: B, 500 μm; C–F, 100 μm.)
Porcine tissue-engineered vessels had mechanical properties that were comparable to human saphenous vein. The burst pressure of the fresh (1,337 ± 103 mm Hg; n = 4) and decellularized tissue-engineered vessels (1,300 ± 59; n = 6) were similar, and near the burst pressure of a human saphenous vein at 1,680 ± 307 (17). The mechanical properties of the fresh and decellularized vessels were also compared by obtaining stress–strain curves (Fig. S2). Stress–strain curves for engineered and decellularized vessels were similar, although decellularized vessels had a somewhat lower ultimate tensile strength [fresh TEV, 1.44 ± 0.068 MPa (n = 2) and decellularized TEV, 1.03 ± 0.208 MPa (n = 4)]. Therefore, the mechanical properties, collagen content, and wall thickness of the decellularized engineered vessels were similar to engineered vessels and human saphenous vein.
Endothelial Progenitor Cells (EPCs) Isolated from Peripheral Blood.
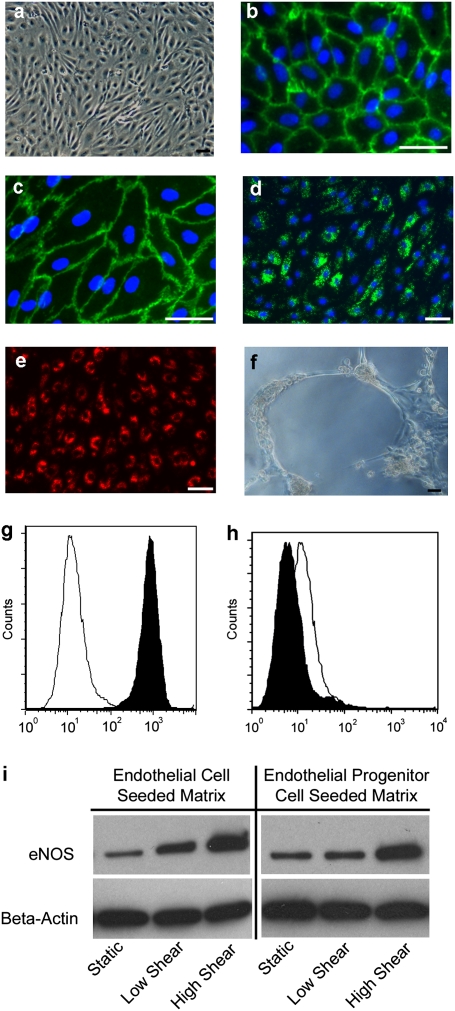
Peripheral blood EPCs were isolated from pigs that were destined to received engineered grafts. EPCs exhibited characteristic endothelial properties by morphology, phenotype, and function. As shown by phase contrast microscopy, EPCs had a typical cobblestone morphology (Fig. 2A) that was similar to that of differentiated porcine endothelium (Fig. S3A), which was also obtained from pigs destined to receive grafts. The EPCs uniformly expressed CD31 (platelet endothelial cell adhesion molecule-1; Fig. 2B) and CD144 (vascular endothelial cadherin; Fig. 2C). EPCs were also positive for von Willibrand factor (vWF) that was present as granules in the cytoplasm (Fig. 2D). The functional characteristics of the EPCs were shown by the uptake of acetylated LDL (Fig. 2E) and the formation of capillary-like tubes when cultured on Matrigel (Fig. 2F). Flow cytometry verified the immunofluorescent staining with expression of CD31 (Fig. 2G), and absence of hematopoietic marker CD45 (Fig. 2H). After exposure to laminar shear stress of 15 dyne/cm2 for 24 h, EPCs and differentiated endothelial cells (ECs) both showed an increase in eNOS protein (Fig. 2I), which is expected for functional EC (18, 19). Hence, the characteristics of porcine peripheral blood-derived EPC were similar in all ways assessed to differentiated porcine EC (Fig. S3) (20, 21).
Fig. 2.
Characterization of porcine EPCs from peripheral blood. (A) EPCs exhibit typical cobblestone endothelial cell morphology. Immunofluorescent detection of CD31 (B), CD144 (C), vWF (D) demonstrate endothelial cell phenotype. Functional properties of the EPCs included incorporation of aLDL (E) and formation of capillary-like tubes on Matrigel (F). (Scale bars: 50 μm.) Flow cytometry is positive for CD31 (G) and negative for CD45 (H) (black peak is antibody stained). (I) EPC-derived EC and differentiated aortic EC response to shear stress on a decellularized porcine artery by immunoblotting for eNOS protein: low shear = 1 dyne/cm2; high shear = 15 dynes/cm2.
Porcine Implantation.
A porcine implantation model was used to assess the efficacy of the EC- or EPC-seeded decellularized engineered vessels as small diameter arterial grafts. As shown in the study outline (Fig. S4), the preparation of the TEV for implantation included forming an engineered vessel, then decellularizing the tissue, harvesting ECs or EPCs from graft recipients, retroviral cell labeling with GFP, seeding the lumen of the decellularized tissue with labeled EC (or EPC), and then implanting the cell-seeded graft into the recipient carotid. The stability of the GFP-labeled EPC was evaluated in vitro over a 45-d period and 3–4 passages using flow cytometry, which showed that >99% of EPC were GFP+ after 45 d (Fig. S5). EC (or EPC) seeding onto the graft lumen resulted in an average percent coverage of 64 ± 9% (n = 5). A total of five cell-seeded vessels were implanted, including n = 3 for EPC-seeded engineered grafts and n = 2 for EC-seeded grafts. The control grafts for the study were nonseeded, decellularized matrices (n = 3) and internal jugular vein (n = 8). Aspirin and clopidogrel were given 1 d before surgery and continued for the duration of the study.
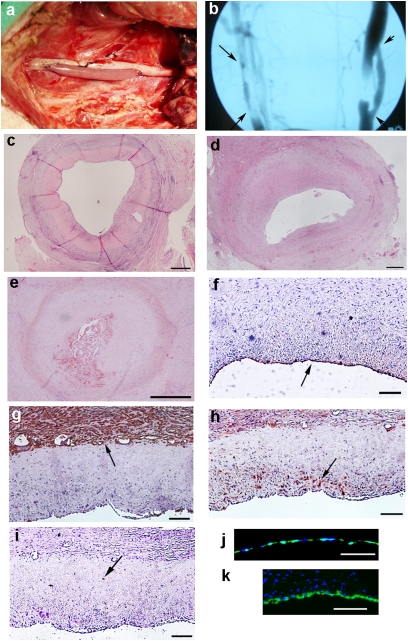
All grafts were implanted in the common carotid as end-to-side grafts, to mimic clinical vascular bypass, for a 30-day period (Fig. 3A). In vivo imaging by angiography or MRI was performed at 30 d, which was combined with histological analysis to assess patency (Fig. 3B). The patency rate of the cell-seeded TEV vessels was 5/5 (EC- and EPC-seeded; Table 1), whereas the nonseeded TEV had a patency rate of 0/3, with histology revealing luminal clot likely due to exposure of host platelets to the collagenous TEV wall (Fig. S6B). Explants of cell-seeded TEV demonstrated migration of host cells into the residual engineered matrix and minimal inflammatory response (Fig. 3C). In contrast to cell-seeded TEV, vein grafts demonstrated substantial intimal hyperplasia in the 3/8 patent grafts (Fig. 3D and Fig. S6 C and D) and in most of the 5/8 occluded grafts. In 3/5 occluded vein grafts, there was significant intimal hyperplasia with a thrombosis in the remnant lumen (Fig. 5E). The other two occluded vein grafts appeared to have failed by a thrombotic event that occurred before substantial intimal hyperplasia could develop.
Fig. 3.
Implantation of vessels. (A) TEV anastomosed as an end-to-side bypass in the porcine carotid artery. (B) Angiogram of a vein graft (left, arrows at anastomoses) and an EPC-seeded TEV (right, arrowheads at anastomoses) 30 d after implantation. (C) Low magnification cross-section of an explanted EPC-seeded engineered vessel with cellular repopulation of the matrix. Patent vein graft (D) and an occluded vein graft (E), both demonstrating intimal hyperplasia. Immunohistochemical staining of explanted cell-seeded grafts for vWF on the luminal surface (arrow) (F), SMC α-actin expressing cells (arrow) on the periphery of the residual engineered matrix (G), and vimentin-positive cells (arrow) populating the residual graft matrix (H). (I) CD45 (arrow) stain for leukocytes. (J) The preimplant engineered matrix seeded with GFP+-labeled EPCs. After 30 d of implantation, GFP+-labeled EPCs were detected on the lumen of the engineered vessel (K). (Scale bars: C–E, 500 μm; F–K, 100 μm.)
Table 1.
Graft patency rates
| Graft | Patency |
| Cell-seeded tissue-engineered vessel | 5/5 |
| EPC-seeded TEV | 3/3 |
| EC-seeded TEV | 2/2 |
| Non-seeded tissue engineered Vessel | 0/3 |
| Vein graft (internal jugular vein) | 3/8 |
An endothelial layer was found on all of the explanted cell-seeded TEV, as indicated by vWF staining (Fig. 3F). All of the cell-seeded TEV had α-actin–positive staining on the outside of the residual engineered vessel (Fig. 3G), and two of the cell-seeded engineered vessels also had α-actin positive staining in the neointima. In the two samples with α-actin–positive staining in the neointima, the inner and outer α-actin layers were separated by an α-actin negative region that was the residual TEV (Fig. S6A). However, a vimentin stain for fibroblasts was positive in the residual TEV at 30 d (Fig. 3H), indicating that although α-actin cells did not extensively repopulate the matrix by 30 d, there were notable numbers of fibroblasts in the matrix at that time. There was not a significant inflammatory response in the residual engineered vessels, as shown by sparse CD45 positive cells (Fig. 3I).
The lumens of the cell-seeded engineered vessels stained positively for GFP-labeled EC or EPC before implantation (Fig. 3J). At the 30-d explant point, GFP+ cells were found in 4/5 cell-seeded grafts and the luminal coverage ranged from 5% to 60%, with an average of 35% (Fig. 3K). The non-GFP+ endothelial cells on the TEVs (average 65%) were likely host-derived, because in vitro studies showed GFP retention by EC for at least 45 d. In addition, the GFP+ cells were found only at the luminal surface and did not appear to migrate into the neointima or residual vessel matrix. Thus, the GFP+ ECs and EPCs did not appear to contribute directly to any neointima in cell-seeded TEV.
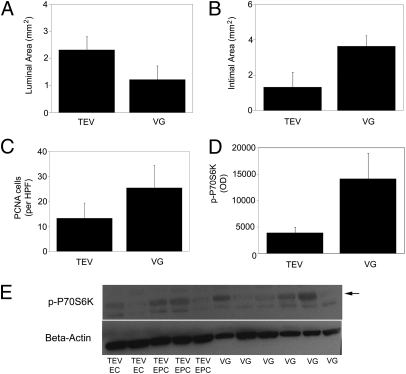
Morphometric analysis quantified the graft differences of the explanted cell-seeded engineered grafts compared with vein grafts. The luminal area (Fig. 4A) of the cell-seeded tissue-engineered vessels (2.31 ± 0.49 mm2; n = 5) tended to be larger than those of the vein grafts (1.22 ± 0.51 mm2; n = 6), and the intimal area (Fig. 4B) of the cell-seeded TEV (1.32 ± 0.84 mm2; n = 5) tended to be lower than that of the vein grafts (3.63 ± 0.59 mm2; n = 6) (Fig. S7). Although intima-to-media ratios are frequently reported when analyzing vein grafts, this approach does not directly apply to tissue-engineered vessels because there is no defined media. In the cell-seeded tissue-engineered vessels, the neointima-to-residual engineered vessel ratio was 0.55 ± 0.34 (n = 5) compared with vein grafts with an intima-to-media ratio of 1.34 ± 0.34 (n = 6). All of the vein grafts with intimal hyperplasia (6/8) were included to quantify intimal hyperplasia and luminal (or residual luminal) area. Cellular proliferation in the neointima and the graft wall were evaluated by PCNA staining, which showed that vein grafts (25.4 ± 9.1; n = 6) appeared to have a greater number of proliferating cells per high power field in comparison with the cell-seeded TEV (13.3 ± 6.1) (Fig. 4C and Fig. S8). Although the proliferation rates (i.e., PCNA+ cells as a fraction of total cells) in the vein grafts (19.1 ± 5.3; n = 6) were similar to the cell-seeded tissue-engineered vessels (18.6 ± 7.6; n = 6), there were overall greater number of cells in the vein grafts. Hence, the cell-seeded tissue-engineered vessels tended to have greater luminal area, less neointimal area, and fewer proliferating cells than did the vein grafts, all of which likely contributed to improved patency of the cell-seeded TEV. Although these observations did not reach statistical significance, the trends were entirely consistent.
Fig. 4.
Characterization of explanted grafts. The luminal area (A) and intimal area (B) were measured for the cell-seeded tissue-engineered vessels (TEV) (n = 5) and the vein grafts (VG) [n = 6, patent vein grafts (n = 3) and occluded by intimal hyperplasia (n = 3)]. (C) Number of PNCA+ nuclei per high power field of the engineered graft neointima and vessel wall compared with vein graft intima and media. Immunoblots of engineered cell-seeded vessels (n = 5) or vein grafts (n = 6) for phosphorylated p70S6K and quantified and normalized by β-actin expression (D and E). TEV, 3,920 ± 1,096 OD; VG, 14,124 ± 4,795 OD. Data represents mean ± SEM.
The mammalian target of rapamycin (mTOR) signaling pathway has been implicated in cell proliferation of smooth muscle cells in vein grafts, and inhibitors of the mTORC1 complex have been shown to ameliorate intimal hyperplasia in vascular stents (22, 23). To evaluate elements of the mTOR pathway in explanted specimens, midgraft segments of the cell-seeded TEV and vein grafts were analyzed for the expression of the downstream effector phosphorylated p70S6K and the upstream activator phosphorylated Akt. By immunoblotting, the protein expression of p-p70S6K tended to be higher in the vein grafts than in the cell-seeded TEV, although this difference did not achieve significance {vein graft 3.6-fold greater than TEV [TEV 3,920 ± 1,096 optical density (OD), n = 5 and vein graft 14,124 ± 4,795 OD, n = 6]; Fig. 4D}. The expression of phosphorylated Akt was also somewhat higher in vein grafts than in engineered grafts [vein graft 1.8 fold greater than TEV (TEV 6,361 ± 468 OD, n = 5 and vein graft 11,602 ± 5,122 OD, n = 6); Fig. S9]. An increased expression of p-Akt tends to occur earlier than 4 wk in the development of hyperplasia (24), and so the difference in p-Akt may have been higher at 2 wk than at our 4-wk time point. Hence, decreased activation of the mTOR pathway in the TEVs compared with the vein grafts may have contributed to decreased neointimal hyperplasia and improved patency of the cell-seeded TEV.
Discussion
In the pig carotid model, the EPC- and EC-seeded TEV outperformed the vein graft by demonstrating a higher patency rate (100% vs. 38%) and a trend toward a lower neointimal response. The end-to-side graft implantation in the pig model is an accelerated intimal hyperplasia model, which typically results in lower patency rates than porcine interpositional vein grafts (25). Although both the cell-seeded TEV and the vein grafts had an endothelial layer, there was a substantial difference in graft patency that correlated to the degree of intimal hyperplasia. Obviously, the tissue-engineered matrix is acellular, whereas the vein graft has a living smooth muscle layer that can contribute to neointimal thickening and luminal occlusion. In contrast, host cells appear to migrate slowly into the decellularized engineered matrix, perhaps retarding the formation of neointima. Activation of mTOR pathway proteins was less in engineered grafts compared with veins, and because the immunoblot data were normalized to β-actin expression, this observation means that p-Akt and p-p70S6k were lower for cells in engineered grafts compared with veins. The explant morphometric data, the cellular proliferation results, and the protein expression levels of the mTOR pathway all provide supportive data regarding the resistance of the cell-seeded tissue-engineered vessel to both intimal hyperplasia and to thrombotic occlusion.
Endothelial progenitor cells have been used to coat vascular grafts to produce an antithrombotic surface and prevent thrombosis (7, 8). In vitro studies have shown that EPCs, even when harvested from patients with cardiovascular disease, support a similar antithrombotic phenotype to differentiated ECs when exposed to laminar shear stress (26). The GFP+ ECs and EPCs that survived over the 30-d implant period could have played a direct role in maintaining an antithrombotic surface, possibly through the local release of nitric oxide because the preconditioning to shear was shown to increase eNOS (27, 28). The non-GFP+ endothelial cells on the explanted cell-seeded TEV likely originated from adjacent ECs or circulating EPCs in the host. The EC labeling here demonstrated that the EC or EPC can be less than 100% confluent on the lumen at the time of implant and still maintain patency. However, the decellularized TEV occluded in this animal model if ECs or EPCs were not seeded onto the lumen.
The allogenic decellularized tissue-engineered matrix did not induce a substantial inflammatory response, which is important because an inflammatory response can contribute to stenosis (29, 30). Although smooth muscle cells were not found in the residual matrix, there was a smooth muscle cell layer outside all of the engineered vessels at explant. Only two of the five cell-seeded engineered vessels had any neointima that was positive for smooth muscle cells, but even in those tissues, there was a separation of the neointima from the outer layer of smooth muscle cells. Hence, it is unlikely that smooth muscle cells in the neointima migrated from outside the graft, but instead may have been derived from adjacent host tissue at the anastomosis. In addition, a circulating bone marrow progenitor may have also contributed to the development of the neointima (31, 32).
Intimal hyperplasia as a result of cellular proliferation is associated with the mTORC1 pathway and its downstream effector, p70S6K (33, 34). The pharmacological inhibitor rapamycin has been shown to have an antiproliferative effect on vascular smooth muscle cells in vitro and in vivo and has been used to great clinical advantage with drug-eluting stents (23, 35). In this study, phosphorylated p70S6K and Akt tended to have higher expression levels in the vein grafts than in the cell-seeded TEV. The higher expression level of p-p70S6K correlated with an increased number of proliferative cells in the vein graft compared with the cell-seeded TEV. This observation implies that differences in mTORC1 pathway activation may underlie the improvements in patency of the engineered grafts.
In summary, a tissue-engineered vessel can be decellularized to create a readily available engineered connective tissue. Because the engineered connective tissues (vessels) can be produced from allogeneic cells, the months-long process required to culture a collagen-rich and mechanically robust tissue is moved “off-line,” not requiring cells from the actual recipient. EPC that are obtained by using a minimally invasive blood draw can be seeded onto such a graft, to result in a functional arterial prosthesis within only a few weeks. This decellularized tissue functions well as an arterial graft and is gradually remodeled in vivo by host cells. The paradigm of using allogeneic cells to engineer acellular connective tissues may find applicability in other areas of tissue engineering, thereby shortening the wait time for engineered replacements of other connective tissues.
Methods
Culture and Decellularization of Engineered Vessel.
The bioreactor systems, cell seeding, and medium replenishment proceeded as described (15, 16, 36). Briefly, the scaffold for the tissue-engineered vessel consisted of a PGA mesh (Concordia) that was sewn by using Dexon suture (Syneture) around a silicone tube (Saint Gobain) with an outer diameter of 4 mm and a length of 12 cm. A suspension of passage three aortic smooth muscle cells at a density of 12 × 106 cells were used to seed the PGA mesh. After 10 wk under pulsatile culture conditions in the bioreactor, the vessels were decellularized by using a two solution detergent process as described (13, 37). The tissue-engineered vessel was placed into the first decellularization solution containing 8 mM CHAPS (Sigma), 1 M NaCl (Sigma), and 25 mM EDTA (Boston Bioproducts) in PBS. The vessel was stirred for 1 h on a stirplate in an incubator at 37 °C. The vessel was rinsed in PBS three times for 10 min each. The vessel was placed in a second decellularization solution containing 1.8 mM SDS (Sigma), 1 M NaCl (Sigma), and 25 mM EDTA (Boston Bioproducts) in PBS. Again, it was stirred for 1 h in an incubator at 37 °C and rinsed in PBS three times for 10 min each. The vessel was rinsed in EBM-2 medium (Lonza, without hydrocortisone) with 10% FBS (HyClone) for 24 h in an incubator at 37 °C and rinsed in PBS three times for 10 min each.
Isolation of ECs from Artery and EPCs from Peripheral Blood.
ECs were isolated from the saphenous artery of 6- to 8-wk-old Yorkshire pigs by scraping the cells off the artery and plating on fibronectin-coated plates (BD Science), and culturing in EGM2 (Lonza, without hydrocortisone) (8, 11). The EPCs were collected from the blood of 6- to 8-wk-old Yorkshire pigs. After the initial 5 mL of blood was discarded, 30 mL was collected and 1,000 U heparin was added. The mononuclear fragment was obtained by a Histopaque density gradient (Sigma) centrifugation at 400 × g for 30 min with the brake turned off. The cell pellet was washed three times in PBS and resuspended in EGM2 supplemented with 20% FBS (HyClone). The cell seeding density was 1 to 3 × 107 cells per well, and the wells were precoated with fibronectin. The medium was changed at 48 h, and was changed every 2 d thereafter until cell colonies appeared.
EC and EPC Labeling.
ECs and EPCs were labeled with retroviral vectors expressing GFP (38). Cells were infected at 20–30% confluence and again at 70–80% confluence. The infection was repeated for a total of four rounds. The cells were then sorted by flow cytometry for GFP expression, and then seeded onto the TEV.
Preconditioning of ECs or EPCs on the TEV.
Vessels were placed in a flow chamber and seeded at a density of 2 × 105 EC or EPC cells per cm2 by rotating the vessel 90° every 30 min. After 360° rotation (2 h), medium was replaced with fresh medium and remained static for another 2 h. Shear stress was calculated by using Poiseuille's equation: τ = 4 μQ/πr3, where τ is shear stress, μ is fluid viscosity, Q is medium flow rate, and r is radius of the vessel. Shear stress was started at 1 dyne/cm2 for 2 d and gradually increased to 15 dynes/cm2 over 36 h. The vessels were maintained at 15 dynes/cm2 for 24 h. The edge of each graft was analyzed for confluency by examining the luminal surface by staining for DAPI (Vector Labs) and F-actin (Invitrogen) and using ImageJ to quantify the coverage of the luminal area.
Porcine Implant Study.
The implanation protocol was approved by the Institutional Animal Care and Use Committee of Yale and Duke universities in compliance with animal care and handling with the Guide for the Care and Use of Laboratory Animals published by National Institute of Health. One day before surgery, aspirin (5 mg/kg) and clopidogrel (1 mg/kg) were given. Aspirin and clopidogrel were continued daily over the 30-d study. At the time of implantation, heparin (100 IU/kg) was administered i.v. before arterial clamping. TEV (with or without cell seeding) were implanted by using 6-0 prolene as an end-to-side anastomosis to the common carotid artery. Vein grafts were autologous internal jugular vein and were implanted in the contralateral common carotid artery.
Explant Characterization.
Standard histological stains of hematoxylin & eosin (H&E), and Masson's trichrome stain for collagen, were used. Immunohistochemical staining for SMC α-actin (Dako), vimentin (Abcam), and vWF (Dako) used a streptavidin-biotinylated peroxidase kit (Vector 6101 or 6102). Immunofluorescent staining with anti-GFP antibody (Abcam) used a FITC secondary (Abcam). Morphometric analysis was performed by using ImageJ software to obtain luminal area and intimal area using elastic-Van Gieson stain.
Western Blot Analysis.
Cell lysates were prepared as described and separated by SDS/PAGE and transferred to polyvinylidene (PVDF) membrane (39). The following antibodies were used for Western blot analysis: eNOS (BD transduction laboratories), calponin (DAKO), GAPDH (Millipore), β-actin (Sigma), p-p70S6K (Cell Signaling), and p-AKT (Cell Signaling).
Vessel Characterization.
Mechanical testing included burst pressure and suture retention and was described (15). Stress–strain curves were obtained by placing hooks through a 4-mm ringlet and using an Instron (5800) with the cross-head speed set at 1 mm/sec. Collagen and DNA quantification was determined as described (37).
Statistics.
The properties (vessel thickness, collagen content, DNA quantification, and burst pressure) of the fresh and decellularized engineered vessels are expressed as mean ± SEM. Statistical significance was evaluated by using the unpaired Student t test. Statistical significance was evaluated by using the nonparametric Mann–Whitney test for the explanted vessel properties luminal area, intimal area, and protein expression).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL-083895, EB-008366 (to L.E.N.), and 1F32 HL083662 (to C.Q.).
Footnotes
Conflict of interest statement: L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019506108/-/DCSupplemental.
References
- 1.Yeager RA, et al. Differential patency and limb salvage for polytetrafluoroethylene and autogenous saphenous vein in severe lower extremity ischemia. Surgery. 1982;91:99–103. [PubMed] [Google Scholar]
- 2.Veith FJ, Moss CM, Sprayregen S, Montefusco C. Preoperative saphenous venography in arterial reconstructive surgery of the lower extremity. Surgery. 1979;85:253–256. [PubMed] [Google Scholar]
- 3.Giannoukas AD, et al. Pre-bypass quality assessment of the long saphenous vein wall with ultrasound and histology. Eur J Vasc Endovasc Surg. 1997;14:37–40. doi: 10.1016/s1078-5884(97)80223-7. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 5.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: The Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt CE, Baier JM. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 7.Amiel GE, et al. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12:2355–2365. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal S, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SW, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2, Unit 2C 1. [DOI] [PubMed] [Google Scholar]
- 12.Kalka C, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 14.McAllister TN, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 15.Niklason LE, et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 16.Niklason LE, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 17.L'Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575–3587. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Case J, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Timmermans F, et al. Endothelial progenitor cells: Identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun-Dullaeus RC, et al. Cell cycle protein expression in vascular smooth muscle cells in vitro and in vivo is regulated through phosphatidylinositol 3-kinase and mammalian target of rapamycin. Arterioscler Thromb Vasc Biol. 2001;21:1152–1158. doi: 10.1161/hq0701.092104. [DOI] [PubMed] [Google Scholar]
- 23.Morice MC, et al. RAVEL Study Group. Randomized Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 24.Stabile E, et al. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi H, Miyawaki F, Arakawa H, Tsuji T, Tanigawa M. Experience of vein grafting in Göttingen minipigs. Exp Anim. 2001;50:191–195. doi: 10.1538/expanim.50.191. [DOI] [PubMed] [Google Scholar]
- 26.Stroncek JD, et al. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473–3486. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kibbe MR, et al. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 28.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45(Suppl A):A64–A73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Davis C, Fischer J, Ley K, Sarembock IJ. The role of inflammation in vascular injury and repair. J Thromb Haemost. 2003;1:1699–1709. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 30.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. J Vasc Surg. 2010;51:736–746. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu K, et al. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 32.Diao Y, et al. Long-term engraftment of bone marrow-derived cells in the intimal hyperplasia lesion of autologous vein grafts. Am J Pathol. 2008;172:839–848. doi: 10.2353/ajpath.2008.070840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin KA, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 35.Sousa JE, et al. Four-year angiographic and intravascular ultrasound follow-up of patients treated with sirolimus-eluting stents. Circulation. 2005;111:2326–2329. doi: 10.1161/01.CIR.0000164271.01172.1A. [DOI] [PubMed] [Google Scholar]
- 36.Dahl SL, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001426. 68ra9. [DOI] [PubMed] [Google Scholar]
- 37.Gui L, Chan SA, Breuer CK, Niklason LE. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods. 2010;16:173–184. doi: 10.1089/ten.tec.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng L, Ben LH, Pober JS, Bothwell AL. Porcine endothelial cells, unlike human endothelial cells, can be killed by human CTL via Fas ligand and cannot be protected by Bcl-2. J Immunol. 2002;169:6850–6855. doi: 10.4049/jimmunol.169.12.6850. [DOI] [PubMed] [Google Scholar]
- 39.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.