Abstract
FGF signaling is one of the few cell–cell signaling pathways conserved among all metazoans. The diversity of FGF gene content among different phyla suggests that evolution of FGF signaling may have participated in generating the current variety of animal forms. Vertebrates possess the greatest number of FGF genes, the functional evolution of which may have been implicated in the acquisition of vertebrate-specific morphological traits. In this study, we have investigated the roles of the FGF signal during embryogenesis of the cephalochordate amphioxus, the best proxy for the chordate ancestor. We first isolate the full FGF gene complement and determine the evolutionary relationships between amphioxus and vertebrate FGFs via phylogenetic and synteny conservation analysis. Using pharmacological treatments, we inhibit the FGF signaling pathway in amphioxus embryos in different time windows. Our results show that the requirement for FGF signaling during gastrulation is a conserved character among chordates, whereas this signal is not necessary for neural induction in amphioxus, in contrast to what is known in vertebrates. We also show that FGF signal, acting through the MAPK pathway, is necessary for the formation of the most anterior somites in amphioxus, whereas more posterior somite formation is not FGF-dependent. This result leads us to propose that modification of the FGF signal function in the anterior paraxial mesoderm in an amphioxus-like vertebrate ancestor might have contributed to the loss of segmentation in the preotic paraxial mesoderm of the vertebrate head.
Keywords: evo-devo, somitogenesis, gene family
Only a few cell–cell signaling molecules are known to play a major role during metazoan embryonic development. Among them, the FGFs were discovered in the mid-1970s in vertebrates. FGFs are small proteins, generally secreted, characterized by a conserved functional domain and acting through binding to their transmembrane receptors [FGF receptors (FGFRs)], causing them to homodimerize. This leads to intracellular autophosphorylation and further activation of cytoplasmic signaling cascades, such as the MAPK and the PI3K pathways (1).
Sequencing of several complete metazoan genomes has shown that FGF and FGFR genes were already present in the common ancestor of diploblastic and bilaterian animals (2). In vertebrates, at least 22 genes coding for FGFs and 4 coding for their FGFRs are known. In contrast, only 3 genes coding for FGFs and 2 coding for FGFRs have been described in Drosophila (2). In the urochordate Ciona intestinalis, a member of the sister group of vertebrates, only 6 FGF genes are present, for which some orthology relationships with vertebrate FGFs are still not resolved (3). This large number of FGFs and FGFRs in vertebrates raises the question of their implication in the evolution of vertebrate-specific morphological characteristics.
In vertebrates, FGF signaling is involved in different developmental processes, among which are neural and mesoderm induction in the early embryo, and somitogenesis and limb bud formation at later stages (4). To investigate the evolution of FGF signaling pathway function during embryogenesis at the invertebrate chordate-to-vertebrate transition, we undertook a study of the embryonic role of this pathway in the cephalochordate amphioxus (Branchiostoma lanceolatum). Amphioxus is an amenable model system to address this question because of its basal position in the chordate tree. Moreover, its genomic, morphological, and developmental characteristics are probably highly similar to those of the chordate ancestor (5).
We describe here the content of the amphioxus FGF genes, as well as their evolutionary relationships with vertebrate FGFs, using phylogeny and deep synteny conservation analyses. Detailed study of the expression patterns of all amphioxus FGFs indicates that FGF-dependent developmental processes in amphioxus are as varied as those of vertebrates. Moreover, our functional analysis shows that (i) during early development, FGF signal in amphioxus controls cellular movements through a MAPK-independent pathway, similar to some vertebrates; (ii) in contrast to what is described in vertebrates, neural and mesoderm induction are not FGF-dependent in amphioxus; and (iii) a MAPK-dependent FGF signal is necessary during gastrulation for the formation of the rostral but not the posterior somites, where it is instead required for their normal maturation. Overall, our work shows that the role of the FGF signaling pathway during neural and mesoderm induction is a specific vertebrate acquisition. In addition, changes in the requirement for the FGF signal during somitogenesis, particularly at the rostral level, could explain the appearance of the “new head” in vertebrates (6).
Results
Amphioxus FGF Gene Content.
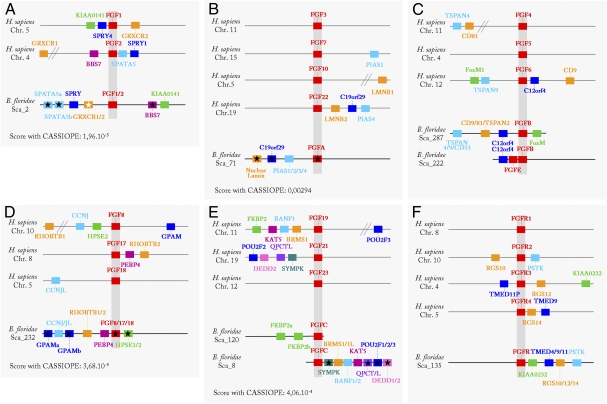
BLAST searches on the Branchiostoma floridae genome for FGF and FGFR genes resulted in the identification of eight putative genes containing an FGF domain and one FGFR coding gene. The corresponding full-length cDNAs were cloned in B. lanceolatum. All the predicted FGF proteins contain a highly conserved FGF domain, and five contain a putative signal peptide in their N-terminal region (Table S1). The unique FGFR gene was previously described as the ortholog of the four vertebrate FGFRs (7). We used phylogenetic reconstructions based on the alignment of the FGF domains to assign the orthology relationships of the amphioxus FGF genes. No matter the method used (maximum likelihood or Bayesian inference), only three FGF genes have a well-supported position in the phylogenetic tree (Fig. S1), namely, FGF1/2, FGF8/17/18, and FGF9/16/20, which are placed at the base of the vertebrate FGF1/2, FGF8/17/18, and FGF9/16/20 paralogy groups, respectively. The other five genes were named FGFA, FGFB, FGFC, FGFD, and FGFE. To go further, we looked into synteny conservation of the FGF and FGFR gene loci between amphioxus and vertebrates (Fig. 1). For FGF1/2 (Fig. 1A), FGF8/17/18 (Fig. 1D), and FGFR (Fig. 1F), synteny conservation with vertebrates reinforces their orthology relationships. We also found amphioxus/vertebrate synteny conservation for FGFA, FGFB, and FGFC genes (Fig. 1 B, C, and E), indicating that they are orthologs of the FGF3/7/10/22, FGF4/5/6, and FGF19/21/23 vertebrate paralogy groups, respectively.
Fig. 1.
Synteny conservation among vertebrate and amphioxus FGF chromosomal regions. For clarity, only synteny conservation with human FGF1/2 (A), FGF3/7/10/22 (B), FGF4/5/6 (C), FGF8/17/18 (D), FGF19/21/23 (E), and FGFR1/2/3/4 (F) paralogy groups is represented. Names of the human genes are according to the Ensembl database, and amphioxus names are according to our phylogenetic analyses. Genes for which synteny conservation was found using the CASSIOPE system are marked by a black star. A white star marks amphioxus genes for which phylogenetic signal is insufficient; in this case, the name is according to the best reciprocal BLAST hit result.
FGFs Show a Dynamic Expression Pattern During Embryogenesis.
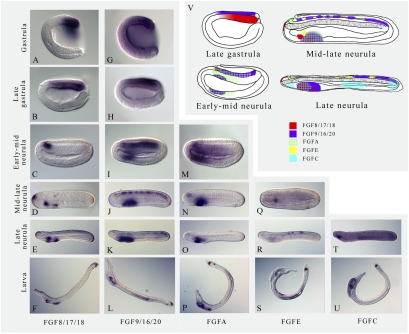
We analyzed the expression pattern of FGFR and the eight FGF genes from the eight-cell stage to the larval stage. The FGFR gene is ubiquitously expressed at all the studied stages, except in the epidermis, with a higher expression level in the mesoderm (Fig. S2). Expression of two of the FGF genes (FGFB and FGFD) could not be detected by whole-mount in situ hybridization, and FGF1/2 shows ubiquitous expression until the larval stage, except in the epidermis. The remaining five FGFs show a restricted expression pattern (Fig. 2, more detailed expression patterns are shown in Figs. S3–S7) (8, 9). Interestingly, only FGF8/17/18, FGFA, and FGFE show transient expression in the presumptive mesoderm. Indeed, FGF8/17/18 is expressed in the posterior dorsal mesendoderm in the gastrula stage embryo (Fig. 2 A and B), but this expression fades rapidly and is no longer visible in the mesoderm at the midneurula stage (Fig. 2D). FGFA expression in the anterior dorsal mesendoderm is only observed at the early midneurula stage (Fig. 2M). FGFE is also expressed in a very restricted mesodermal domain, the first left somite, and only very transiently in midlate neurula embryos (Fig. 2Q). Four amphioxus FGFs, including FGF8/17/18, FGF9/16/20, FGFA, and FGFE, are expressed in the central nervous system, although the expression is very dynamic. The first three are detected in the anterior neural plate and cerebral vesicle but at different developmental stages (Fig. 2V). FGF9/16/20 is the earliest neurally expressed gene, with restricted expression in the presumptive neural plate of the gastrulating embryo (Fig. 2 G and H). It is thereafter expressed segmentally in the neural tube until the late neurula stage (Fig. 2 J and K); at that time, FGFE expression starts to be observed in some neurons up until the larval stage (Fig. 2 R and S). With respect to the endoderm, specific expression in the gut is first observed for FGFC in the late neurula stage embryo (Fig. 2T). Subsequently, in the larva, FGF9/16/20, FGFA, and FGFE are expressed in the midgut region, with the first two also being expressed in the anus (Fig. 2 L and P). The pharyngeal endoderm is the embryonic region showing the most complex FGF expression combination (Fig. 2V). Indeed, in the larva, all the FGF genes having a restricted expression pattern are expressed in the pharynx. FGFA and FGF8/17/18 are expressed around the mouth; FGF8/17/18, FGF9/16/20, and FGFA in the first gill slit; FGF8/17/18 and FGFC in the preoral pit; FGF9/16/20, FGFA, FGFE, and FGFC in the club-shaped gland; and FGFC in the most anterior part of the pharyngeal endoderm (Fig. 2 F, L, P, S, and U). The expression in the larva seems to reflect the expression in different regions of the pharyngeal endoderm at the late neurula stage (Fig. 2V).
Fig. 2.
Amphioxus FGF genes show very dynamic embryonic expression patterns. Expression of FGF8/17/18 (A–F), FGF9/16/20 (G–L), FGFA (M–P), FGFE (Q–S), and FGFC (T and U) from gastrula to larva. (V) Schematic representation of the expression of FGF8/17/18, FGF9/16/20, FGFA, FGFE, and FGFC from the late gastrula stage to the late neurula stage. Little overlap of expression is observed in the pharyngeal region. Only stages for which a restricted expression pattern was observed are presented. Anterior is to the left and dorsal is to the top. For detailed descriptions of the expression patterns see Figs. S3–S7.
Early FGF Signaling Controls Gastrulation via a MAPK-Independent Pathway.
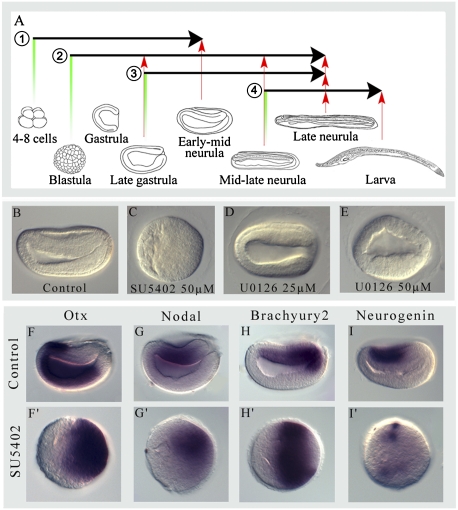
To understand the role of FGF signaling during gastrulation, we treated embryos with SU5402, an FGFR inhibitor, and U0126, a MAPK pathway inhibitor. Treatments were performed at two different developmental stages, the four- to eight-cell stage and the blastula stage (treatments 1 and 2 in Fig. 3A). When FGF signaling is inhibited with SU5402 at the earlier stage, the embryos are not arrested in their development (Fig. S8) but fail to gastrulate and form a sphere of cells with two distinct regions (Fig. 3C). In these embryos, Otx and Nodal are expressed in one-half of the sphere, possibly corresponding to the presumptive mesendoderm cells that failed to invaginate (Fig. 3 F, F′and G, G′). Brachyury2 is expressed as a ring that suggests the presumptive blastoporal region (Fig. 3 H, H′), and Neurogenin, a proneural marker, is expressed in patches in one-half of the embryo (Fig. 3 I, I′). Altogether, these results show that mesodermal and neural induction is not abolished. On the other hand, treatment with U0126, even at toxic concentrations (50 μM), leads to milder developmental defects without abolishing gastrulation, suggesting that FGF signaling is not acting through the MAPK pathway to control these cell movements (Fig. 3 B, D, and E). Interestingly, when embryos are treated with either SU5402 or U0126 at the blastula stage (treatment 2 in Fig. 3A), corresponding to the maternal-zygotic transition, gastrulation occurs normally. These results suggest that gastrulation movements are controlled by a maternally inherited FGF signal acting before the blastula stage.
Fig. 3.
Inhibition of FGF and MAPK signaling pathways after fertilization produces different phenotypes. (A) Scheme of the pharmacological treatments performed in this study with SU5402 and U0126. Black arrows represent the time window for each continuous treatment, and red arrows represent the time points at which embryos where fixed. (B–E) Side views of embryos fixed at the early midneurula stage after treatment 1. Expression of Otx (F and F′), Nodal (G and G′), Brachyury2 (H and H′), and Neurogenin (I and I′) in early midneurula embryos after treatment 1. In the side views, anterior is to the left and dorsal is to the top.
FGF Signaling Controls Anterior Somitogenesis but Not Posterior Elongation of the Embryo.
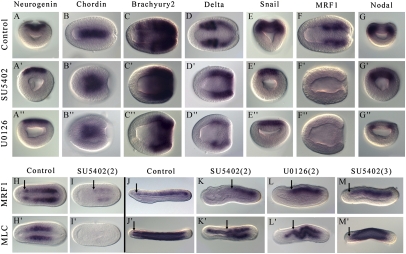
Several treatments were performed using both SU5402 and U0126, as shown in Fig. 3A. First, we investigated the expression pattern of several marker genes in late gastrula embryos after treatment 2. With either treatment, the proneural gene Neurogenin shows normal expression restricted to the neural plate (Fig. 4 A–A′′). Chordin, an axial dorsal mesendoderm marker (presumptive notochord domain), also shows normal expression, although the expression domain is enlarged in treated embryos (Fig. 4 B–B′′). In contrast, the expression of paraxial dorsal mesendoderm marker genes is highly sensitive to both treatments. Indeed, the expression of Brachyury2, Delta, Snail, and MRF1 is completely abolished in the most anterior presumptive somitic region after FGF or MAPK pathway inhibition, whereas their expression in other regions of the embryo is not altered (Fig. 4 C–C′′, D–D′′, E–E′′, and F–F′′). Interestingly, Nodal expression is not abolished in the presumptive anterior somites, suggesting that it is not under the control of the FGF signaling pathway (Fig. 4 G–G′′). We then investigated the expression of MRF1 as well as that of MLC (Myosin Light Chain) at later stages. In midlate neurula SU5402-treated embryos, MRF1 starts to be expressed in the paraxial mesoderm but only in the posterior half of the embryo, whereas the anterior half shows no expression (Fig. 4 H and I). No labeling is observed for MLC in midlate neurula SU5402-treated embryos (Fig. 4 H′ and I′). The same result is obtained for MRF1 just before the mouth opens; there is an anterior region of the paraxial mesoderm free of MRF1 expression (Fig. 4 J and K). Moreover, although expression of MLC in the notochord of SU5402-treated embryos persists all along the body axis, its expression in the paraxial mesoderm is only detected more posteriorly than in control embryos (Fig. 4 J′ and K′). These results indicate a total absence of anterior somite formation as confirmed by embryo sections (Fig. S9). Similar results are observed in U0126-treated embryos (compare Fig. 4 K, K′ with L, L′). To ensure that the difference in sensitivity to FGF signaling inhibition between the anterior and posterior somites was not attributable to degradation of SU5402, we treated the embryos at the late gastrula stage, before the formation of the first somites (treatment 3 in Fig. 3A). In this case, as shown by the expression of MRF1 and MLC, both anterior and posterior somites form, although somite and notochord morphology are abnormal (compare Fig. 4 J, J′ with M, M′). These results indicate that FGF signaling, through activation of the MAPK pathway, is specifically required between the blastula and the late gastrula stages for the formation of the most anterior somites.
Fig. 4.
FGF and MAPK signaling inhibition induces loss of the most anterior somites. Expression patterns by whole-mount in situ hybridization of Neurogenin (A–A′′), Chordin (B–B′′), Brachyury2 (C–C′′), Delta (D–D′′), Snail (E–E′′), MRF1 (F–F′′ and H–M), Nodal (G–G′′), and MLC (H′–M′) after treatments with SU5402 (50 μM) or with U0126 (25 μM). Embryos after treatment 2 were fixed at the late gastrula stage (A–G′′), at the midneurula stage (H, H′ and I, I′), or at the premouth stage (J–L and J′–L′). Embryos after treatment 3 were fixed at the premouth stage (M, M′). A–A′′, E–E′′, and G–G′′ are blastopore views. B–B′′; C–C′′; D–D′′; F–F′′; H, H′; I, I′; and J′–M′ are dorsal views. J–M are lateral views. The most anterior limit of MRF1 and MLC is marked by a black arrow. Anterior is to the left in dorsal and lateral views, and dorsal is to the top in side and blastopore views.
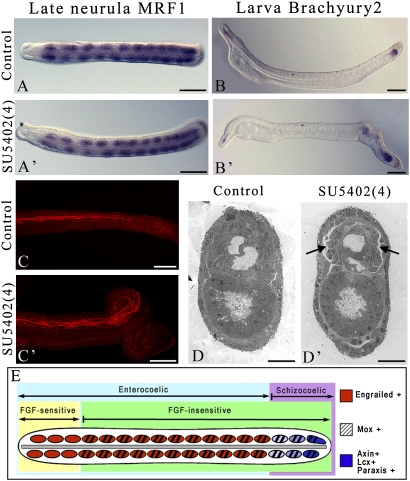
To deepen our understanding of the implications of FGF signaling during somitogenesis, we treated embryos with SU5402 at a later stage, just before the somites start to form directly from the tailbud by schizocoely and not by enterocoely from the presomitic mesoderm (treatment 4 in Fig. 3A). As shown by the expression of MRF1 just before the mouth opens, posterior somites still form following treatment (Fig. 5 A, A′). Later on, at the larval stage, somite budding remains normal. However, the larvae show a strong posterior phenotype. Morphology and maintenance of Brachyury2 expression indicate that maturation of the posterior notochord is altered (Fig. 5 B, B′). Moreover, although the somites formed from the tailbud during treatment develop muscle fibers, these are not correctly aligned (Fig. 5 C, C′). Our transmission electron microscopy (TEM) analysis shows that there are no major differences between control and treated embryos, except maybe some intercellular spaces that appear between somitic cells and the epidermis (Fig. 5 D, D′).
Fig. 5.
Posterior somite budding is not dependent on FGF signaling. Expression of MRF1 at the late neurula stage in control embryos (A) and SU5402-treated embryos (treatment 4) (A′). (Scale bars = 50 μm.) Expression of Brachyury2 in control embryos (B) and in embryos fixed at the larva stage after treatment 4 with SU5402 (B′). In the control embryos, Brachyury2 is expressed in the most anterior notochord and in the tailbud, whereas in the treated embryos, Brachyury2 is still expressed in the posterior notochord anterior to the tailbud. (Scale bars = 50 μm.) (C and C′) F-Actin staining of larval posterior striated muscle fibers in control embryos and in embryos after treatment 4 with SU5402. (Scale bars = 50 μm.) TEM pictures of transverse sections at the larva stage of the posterior region of control embryos (D) and of embryos after treatment 4 with SU5402 (D′). Arrows indicate the space between the somitic cells and the epidermis.(Scale bars = 10 μm.) (E) Schematic representation of the different somitic regions in amphioxus. Dorsal view; anterior is to the left.
Discussion
Evolution of the FGF Gene Family in Chordates.
An explosion of the FGF family diversity occurred at the base of the vertebrate lineage. Indeed, at least 22 FGF genes are known in vertebrates, whereas only 6 genes were found in C. intestinalis (3). Phylogenetic studies have thus far failed to decipher the orthology relationships between all urochordate and vertebrate FGFs, such that the evolutionary history of the FGF family in chordates remains unresolved. In this study, using both phylogenetic reconstructions and precise synteny conservation analysis, we show that all vertebrate FGF paralogy groups have an amphioxus pro-ortholog, with the exception of FGF homologous factors (FHF) (FGF11/12/13/14). Interestingly, FGFE, one of the two amphioxus FGFs for which we find no clear orthology, lacks a putative signal peptide, as do all the vertebrate FHFs (Table S1). Moreover, FGFE gene expression at the late neurula stage, in a subpopulation of neurons in the neural tube (Fig. 2R), resembles that of zebrafish and Xenopus FGF13 (10, 11). These arguments suggest that FGFE may be the amphioxus ortholog of the FHF group. Altogether, these data indicate that the chordate ancestor already possessed one FGF ortholog of each vertebrate paralogy group. This implies that the high number of FGF genes in vertebrates is the result of high gene retention after the two rounds of whole-genome duplication that occurred at the base of this lineage (12). The origin of the amphioxus FGFD is still unknown, and we currently have no clue as to whether it appeared specifically in the cephalochordate lineage or if an ortholog was present in the chordate ancestor and was then lost before the urochordate/vertebrate divergence.
Our study shows that amphioxus FGF genes have very dynamic and diverse expression patterns, even though some overlap is observed (Fig. 2V). These results clearly suggest that, as in vertebrates, FGF signaling is implicated in many developmental processes in cephalochordates (4). It is nevertheless difficult to compare the detailed expression of amphioxus FGFs with their vertebrate orthologs. On the one hand, many data are available showing that FGFs sometimes have different expression patterns in different vertebrate species; on the other hand, we often only have expression data for a single vertebrate species. Nevertheless, some useful comparisons can be made. Indeed, FGF8/17/18 is the earliest gene showing a restricted expression pattern, which was first observed in the presumptive mesoderm. In zebrafish as in Xenopus, FGF8 is one of the FGFs showing the earliest restricted expression, which is also observed in the future mesoderm (10, 13), suggesting a conserved role of at least this paralogue between amphioxus and vertebrates. Another noticeable conserved expression domain is the one described above for FGFE in some specific neurons (10, 11). In contrast, FGF9/16/20 is characterized by early expression, first restricted to the whole neural plate in amphioxus, whereas no comparable expression has been described for any of the vertebrate FGFs belonging to the FGF9/16/20 paralogy group (10), suggesting nonconserved functions between amphioxus and vertebrates. If we compare our data at the level of the whole FGF family with what is known in vertebrates, it appears that expression of FGF ligands in the central nervous system and the pharyngeal region is a conserved feature among chordates. However, vertebrate FGFs are also expressed in many structures that are absent in amphioxus, such as neural crest cell derivatives, placodes, and brain-specific regions, suggesting functional acquisitions during evolution. The most striking difference between amphioxus and vertebrates is the absence of specific FGF expression in amphioxus somites and notochord, except the early FGF8/17/18 expression in the presumptive mesoderm at the gastrula stage. This might be associated with the differences in the requirement for the FGF signal during somitogenesis that we functionally demonstrate in this study.
FGF Function During Chordate Gastrulation.
In vertebrates, FGFs are known to be critical for normal gastrulation movements (14–16). Here, we show that inhibition of the FGF signaling pathway during early development in amphioxus leads to gastrulation failure with absence of blastoderm invagination, as previously suggested by Yu and Zhang (17). Although cell movements during amphioxus gastrulation differ considerably from those seen in vertebrates, the role of the FGF pathway in shaping the gastrula during these early events is an ancestral characteristic of chordates. In sea urchin, FGF signaling is also important for normal invagination during gastrulation and for migration of the primary mesenchyme cells. This implies that FGF may be implicated in early embryonic cellular movements in all deuterosomes (18).
Interestingly, SU5402 and U0126 treatments at the four to eight-cell stage in amphioxus do not produce the same phenotype. In effect, U0126-treated embryos do gastrulate. Hence, the intracellular signal activated by FGFs during early developmental stages in amphioxus, and which is necessary for normal cell movements, is not the MAPK pathway. It is interesting to note that the same conclusions were drawn in Xenopus even though the time at which this MAPK-independent FGF signal controls morphogenesis is different (19, 20).
In vertebrates, FGF signaling during early development is not only important for normal gastrulation movements but for two essential processes, mesoderm and neural induction. On FGF signal inhibition, vertebrate embryos fail to form mesoderm as demonstrated by the absence of expression of early mesodermal marker genes like Brachyury (4). The “default model” for neural induction, proposed more than 10 y ago, postulated that the ectoderm will acquire a neural fate in the absence of any signal and that bone morphogenic protein (BMP) acts as the master signal for epidermal fate induction in vertebrates (21). However, it has been shown that this model is incomplete, because FGF is also required for neural induction (21). In this study, we have shown that early inhibition of FGF signaling in amphioxus abrogates normal gastrulation but that the spherical embryo still contains cells with presumptive neural, mesodermal, and endodermal fates, as shown by marker gene expression. This suggests that the role of the FGF signal in mesoderm and neural induction was acquired in the chordate lineage after cephalochordate divergence. These results lead us to propose that the “default model” could be applied to amphioxus, supported by the fact that activation of the BMP signaling pathway in amphioxus is sufficient to abolish neural induction (22, 23).
FGF Control of Somitogenesis.
Amphioxus somites form in an anterior-to-posterior sequence, with somitogenesis classically divided into two phases. The most anterior early-arising somites form as bilateral pairs by means of enterocoelic evagination of the wall of the archenteron. Later on, the remaining posterior somites form from the tailbud by schizocoely, alternately on the left and right sides of the embryo (Fig. 5E). Beyond this ontological division, expression of several genes clearly functionally divides anterior from posterior somites. Engrailed, OligA, and Pbx (24) are specific gene markers of the anterior enterocoelic somites, and Axin, Lcx, and Paraxis specifically label the tailbud during posterior somitogenesis (24). However, several data suggest an additional division of the amphioxus somites, particularly within the anterior enterocoelic ones. Ontogenetically, the most anterior of these enterocoelic somites form simultaneously, whereas the most posterior form sequentially. Moreover, gene expression also differentiates these two somitic regions. Indeed, Mox is never expressed in the most anterior simultaneously formed somites, suggesting a functional difference from those in the posterior (25). Our data show that inhibition of FGF or MAPK pathways at the blastula stage induces a complete loss of the most anterior enterocoelic somites, whereas formation of all the most posterior somites is independent of these two pathways. Therefore, we clearly establish the presence of three different somitic populations in amphioxus: (i) the most anterior enterocoelic, FGF-sensitive, Mox-negative, and Engrailed-positive somites; (ii) the posterior enterocoelic, FGF-insensitive, Mox- and Engrailed-positive somites; and (iii) the posterior schizocoelic, FGF-insensitive, Engrailed-negative, and Mox-, Axin-, Lcx-, and Paraxis-positive somites (Fig. 5E). This implies that regulation of the formation of tailbud-derived somites by a posterior FGF signal, controlling the position of the “wavefront” as proposed in the “clock and wavefront” model (26), would have been acquired specifically in vertebrates, although we cannot prove that it is not a secondary loss in the cephalochordate lineage.
FGF and the Vertebrate Head.
Since it was proposed by Goethe 200 y ago, the question of whether the vertebrate head is segmented or not has been debated (27). Some anatomical studies supported the idea that vertebrates were primitively segmented from one end to the other, with anterior paraxial mesoderm claimed to form somite-like units or “somitomeres” in the head (28). A segmented vertebrate head upheld the argument for evolution by simple elaboration of the anterior part of an amphioxus-like ancestral animal. However, the bulk of morphological comparative data among different vertebrate species (29), as well as the absence of any periodical pseudosegmental structures in lampreys (30) and of comparable expression patterns of genes known to function in somitogenesis, present major obstacles to this hypothesis and instead support the absence of cephalic somitomeres in vertebrates.
Accepting that the vertebrate head is unsegmented, two major hypotheses may explain how the vertebrate body plan was achieved from a completely segmented hypothetical amphioxus-like ancestor. First, as Gans and Northcutt (6) proposed, a “new head” appeared in vertebrates, mostly derived from the neural crest and epidermal placodes. This “new head” was added to the rostral part of the body. The second hypothesis is that the head would have been acquired by losing the epithelial segmentation of the most anterior paraxial mesoderm. This implies that the anterior end of amphioxus would be homologous to the anterior end of the vertebrate head.
Of these two hypotheses, the second is the more plausible because different expression patterns and anatomical studies support the homology between the rostral parts of amphioxus and vertebrate embryos (31). In fact, the evolution of the vertebrate head was probably possible not only through the appearance of the neural crest and placodes but also via loss of mesoderm segmentation in the anterior region, thereby relaxing the developmental constraints imposed by the somites. Our results provide a mechanistic explanation as to how this evolution might have occurred; namely, through loss of FGF function in the development of the paraxial mesoderm during early gastrulation in a hypothetical vertebrate ancestor. Indeed, simple inhibition of the FGF signal in amphioxus specifically induces loss of the most anterior somites, creating an anterior region with unsegmented paraxial mesoderm, as in the vertebrate head. In support of this, data obtained in Xenopus suggest that inhibition of the FGF signal in the anterior region of vertebrates is necessary for head formation. Indeed, Shisa, which antagonizes FGF function by inducing FGFR retention in the endoplasmic reticulum, is expressed in the anterior part of the vertebrate gastrula, and its knock-down in Xenopus leads to an anterior truncation of the head (32). It is tempting to propose that FGF signal inhibition in the anterior region of vertebrates could have been partly achieved by the recruitment of Shisa.
Conclusions
In this work, we present data suggesting that both loss and gain of FGF function have been instrumental in the evolution of the vertebrate body plan. Modification of the FGF requirement for somitogenesis during gastrulation could have been a major event in the appearance of the vertebrate head. Likewise, gain of FGF function during posterior somitogenesis probably contributed to the plasticity evident in the posterior elongation process of vertebrates.
Materials and Methods
Synteny Conservation Analysis.
Artificial contigs for each FGF human paralogy group and for the FGFR group were constructed by assembling the chromosomic regions containing the human FGF or FGFR genes and 50 genes upstream and downstream. Conserved regions in vertebrates and amphioxus were found using CASSIOPE (33) (SI Materials and Methods).
Embryo Methods.
Gametes were obtained as previously described (34), and embryos were fixed and processed for whole-mount in situ hybridization as described (35). The accession numbers of the sequences used for probe synthesis are provided in SI Materials and Methods.
SU5402 and U0126 (Calbiochem), dissolved in DMSO at 10−2 M, were added to cultures of embryos (SI Materials and Methods and Fig. S10). The maximum final DMSO concentration was 0.5%. Control embryos were raised with 0.5% DMSO in sea water.
For F-Actin staining, embryos were washed in PBS + 0.1% Tween 20 after fixation and then in PBS + 0.1% Triton X100. They were subsequently incubated with Texas Red X-Phalloidin (Invitrogen) dissolved at 5 U/mL in PBS for 1 h. After washes in PBS, embryos were transferred to Mowiol (Calbiochem) for confocal imaging.
TEM was performed according to Lacalli and West (36) with some minor modifications: Postfixation was performed with 1% OsO4, and aqueous uranyl acetate was avoided before embedding in Epon resin.
Supplementary Material
Acknowledgments
We thank Laurent Kodjabachian for providing the SU5402 and Laeticia Bariat for technical assistance. The laboratory of H.E. is supported by the Agence Nationale de la Recherche BLAN 1716 01. S.B. holds a fellowship from the Association pour la Recherche sur le Cancer.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU606032.1, EU606035.1, EU606036.1, EU606033.1, EU606034.1, EU606038.1, HM854710, EU606037.1, HM854709, HM359129, HM359124, HM359125, and HM359126).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014235108/-/DCSupplemental.
References
- 1.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Satou Y, Imai KS, Satoh N. Fgf genes in the basal chordate Ciona intestinalis. Dev Genes Evol. 2002;212:432–438. doi: 10.1007/s00427-002-0266-8. [DOI] [PubMed] [Google Scholar]
- 4.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Schubert M, Escriva H, Xavier-Neto J, Laudet V. Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol. 2006;21:269–277. doi: 10.1016/j.tree.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: A new head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- 7.D'Aniello S, et al. Gene expansion and retention leads to a diverse tyrosine kinase superfamily in amphioxus. Mol Biol Evol. 2008;25:1841–1854. doi: 10.1093/molbev/msn132. [DOI] [PubMed] [Google Scholar]
- 8.Meulemans D, Bronner-Fraser M. Insights from amphioxus into the evolution of vertebrate cartilage. PLoS ONE. 2007;2:e787. doi: 10.1371/journal.pone.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JK, Meulemans D, McKeown SJ, Bronner-Fraser M. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 2008;18:1127–1132. doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Dev Dyn. 2009;238:1467–1479. doi: 10.1002/dvdy.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thisse B, Thisse C. Fast release clones: A high throughput expression analysis. ZFIN Direct Data Submission. 2004. Available at http://zfin.org.
- 12.Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thisse B, et al. Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission. 2001. Available at http://zfin.org.
- 14.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 15.Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: A role for FGFR1 in morphogenetic movement through the primitive streak. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, Zhang PJ. Mechanisms of amphioxus gastrulating cell movements: Forces that drive the most primitive form of chordate gastrulation. Orig Life Evol Biosph. 2006;36:337. [Google Scholar]
- 18.Röttinger E, et al. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development. 2008;135:353–365. doi: 10.1242/dev.014282. [DOI] [PubMed] [Google Scholar]
- 19.Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Stern CD. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol. 2006;18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Yu JK, et al. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 23.Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev Biol. 2010;344:377–389. doi: 10.1016/j.ydbio.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaster-Jones L, et al. Expression of somite segmentation genes in amphioxus: A clock without a wavefront? Dev Genes Evol. 2008;218:599–611. doi: 10.1007/s00427-008-0257-5. [DOI] [PubMed] [Google Scholar]
- 25.Minguillón C, Garcia-Fernàndez J. The single amphioxus Mox gene: Insights into the functional evolution of Mox genes, somites, and the asymmetry of amphioxus somitogenesis. Dev Biol. 2002;246:455–465. doi: 10.1006/dbio.2002.0660. [DOI] [PubMed] [Google Scholar]
- 26.Pourquié O. Vertebrate somitogenesis: A novel paradigm for animal segmentation? Int J Dev Biol. 2003;47:597–603. [PubMed] [Google Scholar]
- 27.de Beer GR. The Development of the Vertebrate Skull. London: Oxford Univ Press; 1937. [Google Scholar]
- 28.Meier S. Development of the chick embryo mesoblast: Morphogenesis of the prechordal plate and cranial segments. Dev Biol. 1981;83:49–61. doi: 10.1016/s0012-1606(81)80007-3. [DOI] [PubMed] [Google Scholar]
- 29.Freund R, Dörfler D, Popp W, Wachtler F. The metameric pattern of the head mesoderm—Does it exist? Anat Embryol (Berl) 1996;193:73–80. doi: 10.1007/BF00186835. [DOI] [PubMed] [Google Scholar]
- 30.Kuratani S, Horigome N, Hirano S. Developmental morphology of the head mesoderm and reevaluation of segmental theories of the vertebrate head: Evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev Biol. 1999;210:381–400. doi: 10.1006/dbio.1999.9266. [DOI] [PubMed] [Google Scholar]
- 31.Holland LZ. Chordate roots of the vertebrate nervous system: Expanding the molecular toolkit. Nat Rev Neurosci. 2009;10:736–746. doi: 10.1038/nrn2703. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 33.Rascol VL, et al. CASSIOPE: An expert system for conserved regions searches. BMC Bioinformatics. 2009;10:284. doi: 10.1186/1471-2105-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuentes M, et al. Insights into spawning behavior and development of the European amphioxus (Branchiostoma lanceolatum) J Exp Zoolog B Mol Dev Evol. 2007;308:484–493. doi: 10.1002/jez.b.21179. [DOI] [PubMed] [Google Scholar]
- 35.Somorjai I, Bertrand S, Camasses A, Haguenauer A, Escriva H. Evidence for stasis and not genetic piracy in developmental expression patterns of Branchiostoma lanceolatum and Branchiostoma floridae, two amphioxus species that have evolved independently over the course of 200 Myr. Dev Genes Evol. 2008;218:703–713. doi: 10.1007/s00427-008-0256-6. [DOI] [PubMed] [Google Scholar]
- 36.Lacalli TC, West JE. Ciliary band formation in the doliolaria larva of Florometra. I. The development of normal epithelial pattern. J Embryol Exp Morphol. 1986;96:303–323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.