Abstract
In this study, we document that Toxoplasma gondii differentiation and reactivation are mediated by systemic CD8 T-cell dysfunction during chronic infection. We demonstrate that CD8+ T-cell exhaustion occurs despite control of parasitemia during early-chronic toxoplasmosis. During later phases, these cells become exhausted, leading to parasite reactivation and mortality. Concomitant with increased CD8+ T-cell apoptosis and decreased effector response, this dysfunction is characterized by a graded elevation in expression of inhibitory receptor PD-1 on these cells in both lymphoid and nonlymphoid tissue. Blockade of the PD-1–PDL-1 pathway reinvigorates this suboptimal CD8+ T-cell response, resulting in control of parasite reactivation and prevention of mortality in chronically infected animals. To the best of our knowledge, this report is unique in showing that exposure to a persistent pathogen despite initial control of parasitemia can lead to CD8+ T-cell dysfunction and parasite reactivation.
Keywords: recrudescence, Toxoplasma gondii, adaptive immunity
Toxoplasmic encephalitis (TE) from reactivation of chronic Toxoplasma infection affects 24% to 47% of HIV-infected Toxoplasma-seropositive patients in the United States (1–3). Although the incidence of TE among AIDS patients declined threefold in the United States because of prophylactic treatment and highly active antiretroviral therapy, it still remains a significant problem in developing countries (4, 5). Hence, understanding the mechanisms responsible for parasite reactivation in chronically infected hosts is critical for evolving strategies for the development of immunotherapeutic agents against the parasite.
In the intermediate host, Toxoplasma undergoes stage conversion between the rapidly replicating tachyzoite that is thought to be responsible for acute toxoplasmosis and the slowly replicating, relatively quiescent, primarily encysted bradyzoite stage that can persist possibly for life (6). Effective CD8+ T-cell response, critical for control of both acute and chronic Toxoplasmosis in susceptible mice strains paradoxically do not ensure their long-term survival (7). Poor long-term survival of Toxoplasma-infected B10 mice is associated with reduced number of intracerebral T cells and TE development (8). Additionally, depletion of CD8+ T cells rather than CD4+ T cells had a greater effect on mortality of chronically infected animals (9). Taken together, these studies suggest that although critical for preventing parasite reactivation, CD8+ T-cell responses during late-chronic infection are incapable of preventing mortality in infected mice. However, the mechanism underlying this apparently ineffective CD8+ T-cell response during late-chronic infection has not been studied and it remains to be determined if this defect is responsible for loss of parasite control. We therefore tested whether CD8+ T cells during late-chronic toxoplasmosis become dysfunctional, as reported in some viral infections (10).
This article is unique in demonstrating that CD8+ T cells during later phases of chronic toxoplasmosis are unable to mount a potent anamnestic response, a hallmark of robust adaptive immunity. Moreover, CD8+ T cells from these mice exhibit a progressive increase in expression of inhibitory molecule PD-1 with concomitant parasite reactivation. In vivo blockade of PD-1 interaction with its receptor PDL-1 not only augmented polyfunctional CD8+ T-cell response, but it also inhibited parasite recrudescence and prevented the mortality of chronically infected mice in a CD8+ T-cell–dependent manner. To the best of our knowledge, this report of parasite reactivation caused by PD-1 mediated CD8+ T-cell dysfunction is unique.
Results
Toxoplasma-Infected Mice Show Increased Tachyzoite-Specific Gene Expression with Concomitant Decrease in CD8+ T-Cell Response During Chronic Toxoplasmosis.
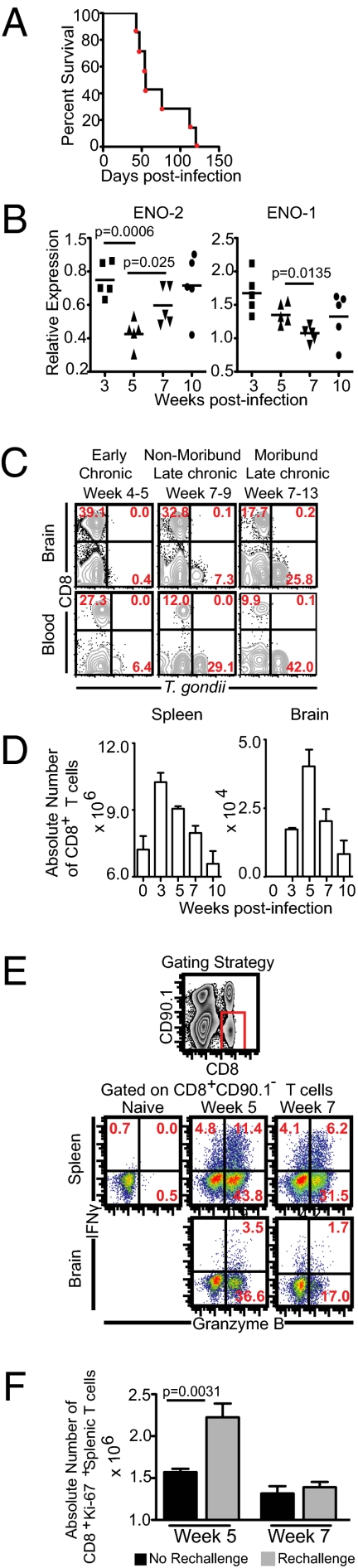
Humans are most commonly infected orally with Type II strains of Toxoplasma (11). To establish the loss of immune control during chronic infection, we first wanted to indentify the kinetics of long-term survival in B6 mice infected orally with type II strain (ME49) cysts. Most of these animals succumb to infection after 7 wk of challenge (Fig. 1A). Host innate and adaptive immunity are thought to cause differentiation of tachyzoites into chronic slow growing bradyzoites (12). Because encysted parasites can persist for life and are not believed to be associated with disease pathology, several studies have postulated that rather than an incidence of parasites, which remain primarily encysted in chronically infected brains, the degree of bradyzoite-tachyzoite interconversion is a better hallmark of disease reactivation (6, 13, 14). Therefore, we assayed parasite reactivation by changes in parasite stage-specific gene expression. We measured the transcription of the tachyzoite stage-specific Enolase-II (ENO-2) and the bradyzoite stage-specific Enolase-I (ENO-1), two genes that have been previously shown to change in very high levels (15–17). Concomitant with poor survival of Toxoplasma gondii-infected mice during the late-chronic phase, increased ENO-2 expression and decreased ENO-1 expression was noted at week 7 postinfection, suggesting parasite reactivation (Fig. 1B). Interestingly, we observed that expression of both ENO-2 and ENO-1 occurs at week 10, indicating that despite limited tachyzoite to bradyzoite differentiation, the parasites are still undergoing reactivation (i.e., a bradyzoite to tachyzoite conversion) (Fig. 1B). To verify that ENO-2 and ENO-1 corresponded to the tachyzoite and bradyzoite stage of T. gondii, the gene expression of the parasite were examined again in vivo using established models of acute and chronic infection. Mice were infected with Type I RH strain (lethal acute infection) or Type II ME49 strain (chronic) (18, 19). Some of the ME49-infected animals were treated with drugs known to control reactivation of chronic infection (18, 19). As expected, RH-infected mice exhibited high tachyzoite-specific gene expression (SAG-1, ENO-2) and minimal bradyzoite-specific gene expression (BAG-1, ENO-1) (Fig. S1). In contrast, drug-treated mice showed reduced SAG-1 and ENO-2 transcript levels and increased BAG-1 and ENO-1 expression vis-à-vis untreated ME49 infected mice. Next, we further confirmed the stage-specificity of the above markers using Type II parasites maintained in vitro under tachyzoite- or bradyzoite-inducing conditions (20). Immunofluorescent microscopic analysis showed that stage-specific protein expression corresponded with our stage-specific transcript profile (Fig. S1).
Fig. 1.
Parasite reactivation during late-chronic T. gondii infection correlates with poor CD8+ T-cell response. (A) C57BL/6 mice were infected with 10 ME49 cysts orally and survival was monitored on a daily basis. (B) Relative expression of ENO-1 and ENO-2 at weeks 3, 5, 7, and 10 postinfection in the brain was computed for each sample. Transcript levels at day 10 postinifection was taken as 1. (C) T. gondii-infected cells in the brain and blood leukocyte-gated samples were assayed by flow cytometry. (D) The absolute number of CD8+ T cells at weeks 3, 5, 7, and 10 postinfection. (E) IFN-γ and Granzyme B production by CD8+ T cells was evaluated in splenocytes or brain cells from infected mice in presence Toxoplasma lysate antigen. (F) At weeks 5 and 7 after primary infection, mice were rechallenged orally with 10 cysts and animals were killed 4 d later. The absolute number of cycling CD8+ T cells was evaluated in the spleen during early-chronic and late-chronic infection with or without rechallenge. The data represent two experiments with at least four mice per group. Error bars represent SD throughout.
Tissue cysts in brain occur almost exclusively in neurons and not in leukocytes during the chronic phase (21). Because a recent study has shown that leukocytes can be used for parasite dissemination during the acute phase (22), we examined these cells for parasitization as a further readout of reactivation. To characterize, whether parasite reactivation increased the frequency of Toxoplasma-infected leukocytes, mononuclear leukocyte-gated blood and brain cells were examined for parasites by flow cytometry. Although minimal infection of blood and brain leukocytes was noted during the early-chronic phase, high infection rates were observed during the late-chronic period, particularly in moribund mice (Fig. 1C). Similar to observations noted by Courret et al. (22), the level of parasite infection was much higher in circulating leukocytes vis-à-vis brain leukocytes assayed at the same time. It has been speculated that this may be because of multiple populations of nonleukocytic brain lineages that are differentially permissive to T. gondii in the brain (22). Next, we assessed if reactivated parasites preferentially infected specific leukocyte subsets in a site-dependent manner. Irrespective of tissue, the majority of parasitized leukocytes corresponded to a myeloid phenotype (CD3CD19NK1.1−CD11bhiF4/80+GR1+) (Fig. S2). Taken together, our data suggest that during the late-chronic phase, parasite-stage conversion results in reactivation of disease.
As Toxoplasma infection induces a robust CD8+ T-cell immunity that is known to be critical for long-term protection, it is enigmatic that this potent immune response failed to prevent recrudescence of infection (9). By measuring the kinetics of CD8+ T-cell immunity we demonstrate that CD8+ T-cell response peaked at weeks 3 to 5 postinfection followed by gradual contraction (Fig. 1D). Concomitant with this process, reduced frequency of splenic and brain IFN-γ+Granzyme B+ and IFN-γ−Granzyme B+ CD8+ T cells was noted (Fig. 1E).
One of the quintessential features of robust long-term CD8+ T-cell immunity is the generation of a rapid response upon secondary challenge (23). To verify the potency of secondary response, Toxoplasma-infected animals were rechallenged during the early-chronic and late-chronic phases and assessed for cycling CD8+ T cells (Fig. 1F). Mice rechallenged during the early-chronic phase demonstrated a significant increase in absolute number of proliferating splenic CD8+ T cells over unchallenged mice (Fig. 1F). In contrast, no significant change in cycling CD8+ T cells was noted in rechallenged animals during the late-chronic phase. These data clearly demonstrate that a potent recall response, a hallmark of robust CD8+ T-cell immunity, is compromised during later stages of chronic toxoplasmosis.
CD8+ T Cells Up-Regulate PD-1 During Chronic Toxoplasmosis.
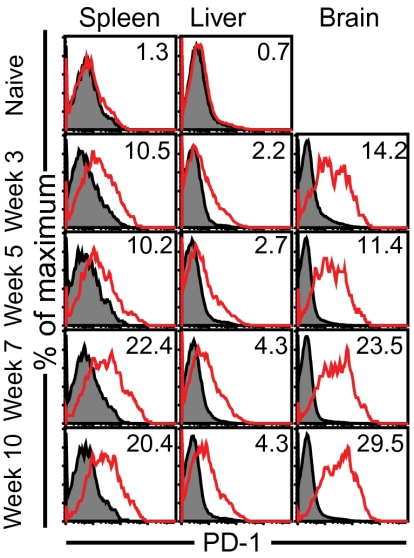
The role of PD-1 in mediating dysfunction in chronic parasitic models has not been extensively addressed (24, 25). Although PD-1 expression during toxoplasmosis has been recently reported (26), its role in mediating CD8+ T-cell dysfunction or pathogenesis as shown in chronic viral models like lymphocytic choriomeningitis virus (LCMV), hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV (27), is currently unknown. Analysis of CD8+ T cells revealed a progressive increase of PD-1 expression and frequency during the chronic stage of parasite infection (Fig. 2). Of the two PD-1 ligands, PDL-1 and PDL-2, the mean fluorescence intensity (MFI) of the former but not the latter increased during the course of chronic infection (Fig. S3).
Fig. 2.
PD-1 is expressed at high levels on CD8+ T cells in both lymphoid and nonlymphoid tissue during late-chronic Toxoplasmosis. PD-1 expression on CD8+ T cells from tissues (spleen, brain, and liver) was measured at weeks 3, 5, 7, and 10 postinfection. Filled histogram represents staining with isotype-control antibody. The data represent three experiments with at least four mice per group.
Because increased PD-1 MFI correlated with decreased frequency of splenic IFN-γ+CD8+ T cells, we hypothesized that PD-1hiCD8+ T cells would have reduced capacity to release IFN-γ. To address this theory, we measured the proportion of IFN-γ–producing cells within PD-1hiCD8+ and PD-1intCD8+ T-cell population. No significant difference in cytokine-positive cells between the two populations was noted (Fig. S4). Although this finding suggests that PD-1 expression does not directly regulate IFN-γ production in CD8+ T cells, the role of other factors, such as the extent and duration of PD-1 cross-linking and frequency of PD-1–PDL-1 engagement in regulating CD8+ T-cell–mediated IFN-γ production, cannot be ruled out. Irrespective of the time point, very few PD-1lo cells produced IFN-γ, indicating that an overwhelming majority of CD8+ T cells in this subset are naive. Although we did not observe a significant difference in frequency of IFN-γ+CD8+ T cells between the PD-1hi and PD-1int CD8+ T-cell subsets, we hypothesized that the reduced frequency of splenic IFN-γ+CD8+ T cells during late-chronic infection could be because of apoptosis of PD-1hi cells. As recent studies have suggested that apoptosis can be coupled to cell-cycle progression (28, 29), we incubated splenocytes from week 7-infected mice for 5 h at 37 °C and stained for CD8, PD-1, Ki-67, and active caspase-3, the active form of the apoptosis-related cysteine peptidase. Although cycling PD-1hiCD8+ T cells exhibited a higher expression of active caspase-3 than noncycling PD-1hiCD8+ T cells, PD-1int/lo cells had a much lower MFI than cycling or noncycling PD-1hi cells (Fig. S4), suggesting that cycling PD-1hiCD8+ T cells exhibit poor survival.
Because PD-1 has been reported to be transiently expressed in acute viral infections (27), increased PD-1 MFI during later stages of chronic Toxoplasmosis could be because of a generation of new PD-1–expressing effectors in response to parasite recrudescence. To address this theory, we used a BrdU pulse-chase approach and then measured PD-1 expression on BrdU+ and Brdu− CD8+ T cells during the early-chronic and late-chronic phases (Fig. S5). Dramatic increase in PD-1 MFI and frequency of PD-1hi cells was noted only in BrdU-labeled fraction. This finding suggests that PD-1 up-regulation is a graded process and the majority of PD-1hiCD8+ T cells at week 7 postinfection are not new effector cells generated in response to parasite recrudescence.
Considering that a majority of PD-1hiCD8+ T cells during the late-chronic phase are not newly generated effectors, we wanted to address if the control of infection during the early or later phases of infection would result in reduced CD8 exhaustion. To evaluate this, we treated mice with sulfamethoxazole and trimethoprim at various time points postinfection, as show in Fig. S6 (19). Because reduced antigen burden in drug-treated mice can result in increased naive T-cell numbers, which can potentially confound interpretation of data, we analyzed brain, a tissue known to recruit CD8+ T cells in a strictly antigen-specific manner (30). Only early initiation of drug treatment resulted in decreased PD-1 MFI on CD8+ T cells during the late-chronic phase (Fig. S6). Moreover, CD8+ T cells from these mice exhibited a higher proportion of IFN-γ+GranzymeB− and IFN-γ+GranzymeB+ CD8+ T cells (Fig. S6). However, IFN-γ−GranzymeB+ CD8+ T cells underwent a moderate decrease in these animals (Fig. S6). This finding may be attributed to attenuated inflammation or antigen load in drug-treated mice, which can result in suboptimal development or maintenance of Granzyme B-expressing short-lived effector subset (31, 32). Combined, these data suggest that control of infection during the early phases of infection may be critical for minimizing induction of CD8 dysfunction during the chronic phase.
Blockade of PD-1–PDL-1 Pathway Reinvigorates CD8+ T-Cell Response.
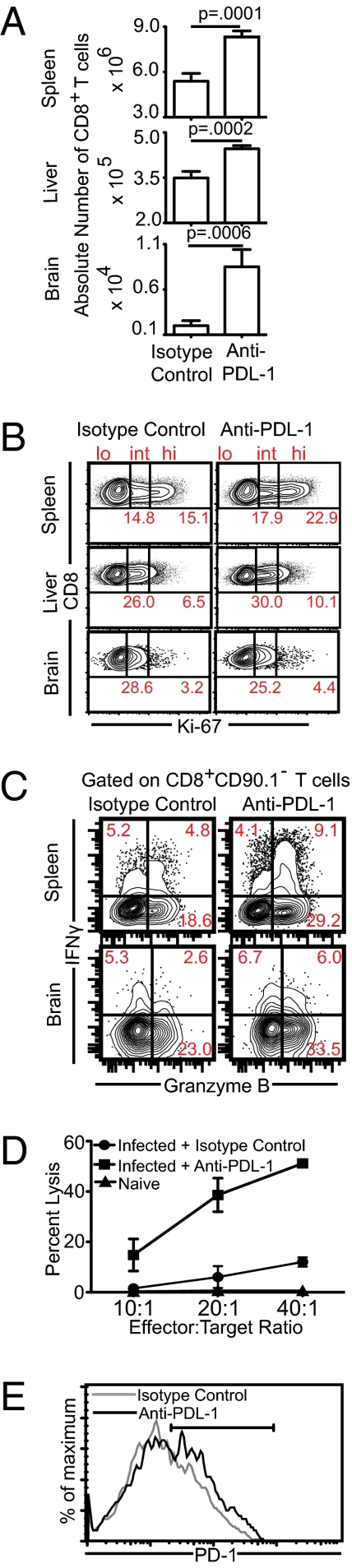
Next, we addressed if a dysfunctional CD8+ T-cell response could be revived by blocking the PD-1–PDL-1 pathway. To address this, animals were administered a nondepleting anti–PDL-1 antibody starting at week 5 postinfection for a period of 2 wk and, thereafter, the CD8+ T-cell response in the spleen, liver, and brain were quantified (33). Although significant increase in absolute number of CD8+ T cells was noted in all of the tissues examined, the most dramatic increase occurred in brain (Fig. 3A). To determine if this increase was because of enhanced proliferation, we measured Ki-67 expression in the CD8+ T cells. Unlike the spleen and liver, we were unable to detect increased frequency of cycling CD8+ T cells in anti–PDL-1 treated groups in the brain (Fig. 3B). Taken together, these data suggest that one of the mechanisms for increased brain CD8+ T cells in anti–PDL-1 treated mice is not in situ proliferation but possibly increased recruitment of these cells to the tissue.
Fig. 3.
Blockade of PD-1–PDL-1 pathway reinvigorates CD8+ T-cell response in both lymphoid and nonlymphoid tissue in chronically infected mice. (A) Chronically infected mice were treated with anti–PDL-1 for 2 wk and then killed at week 7 postinfection. The absolute number of CD8+ T cells was evaluated in the spleen, liver, and brain. (B) The frequency of proliferating CD8+ T cells was assessed by intracellular staining for Ki-67. (C) IFN-γ and Granzyme B production by CD8+ T cells was evaluated in splenocytes or brain cells from infected mice in the presence of TLA. (D) Antigen-specific cytotoxicity of splenic CD8+ T-cells was measured by 51Cr-release assay. (E) PD-1 expression on splenic CD8+ T cells from anti–PDL-1 or isotype control antibody-treated mice was evaluated by flow cytometry. The data represent three experiments with at least four mice per group.
As IFN-γ production (34) and cytotoxicity (35) are known to be critical for control of toxoplasmosis, we examined if blockade with anti–PDL-1 augmented these functions within the CD8+ T-cell population. Anti–PDL-1 treatment increased the frequency of IFN-γ+Granzyme B+ and IFN-γ−Granzyme B+ CD8+ T cells in the spleen and brain, as well as ex vivo cytotoxicity of splenic CD8+ T cells (Fig. 3 C and D). We then measured, if anti–PDL-1 treatment decreased the frequency of PD-1hi cells. Surprisingly, PD-1–PDL-1 blockade caused a further increase in the frequency of PD-1–expressing CD8+ T cells (Fig. 3E). Because we demonstrated that high PD-1 expression was associated with apoptosis, we determined if rescue of CD8+ T-cell response by PD-1–PDL-1 blockade was because of reduced apoptosis. To address this under steady-state conditions without confounding effects of proliferation and graded PD-1 increase, we evaluated apoptotic CD8+ T cells in splenocytes from chronically infected mice incubated for 5 h with anti–PDL-1 or control antibody (Fig. S7). Anti–PDL-1 treatment mediated reduction in apoptosis occurred primarily in the PD-1int subset and only moderate decrease in apoptosis was noted in the PD-1hi subset. To further address if anti–PDL-1 treatment was capable of rescuing preexisting PD-1 expressing CD8+ T cells in vivo, we adoptively transferred sorted PD-1+CD8+ T cells from chronically infected CD45.1 mice (week 5–6) to similarly infected CD45.2 mice and then treated recipients with anti–PDL-1 antibody. Anti–PDL-1 treatment increased the absolute number of donor cells in both spleen and brain (Fig. S8). However, although the recipient CD8+ T-cell population (comprising of both PD-1+ and PD-1−) expanded sixfold at the effector site (vis-à-vis isotype-treated), the donor population underwent over 20-fold expansion. This finding suggests that anti–PDL-1 treatment preferentially rescues preexisting PD-1–expressing cells.
Blockade of the PD-1–PDL-1 Pathway Controls Toxoplasma Recrudescence.
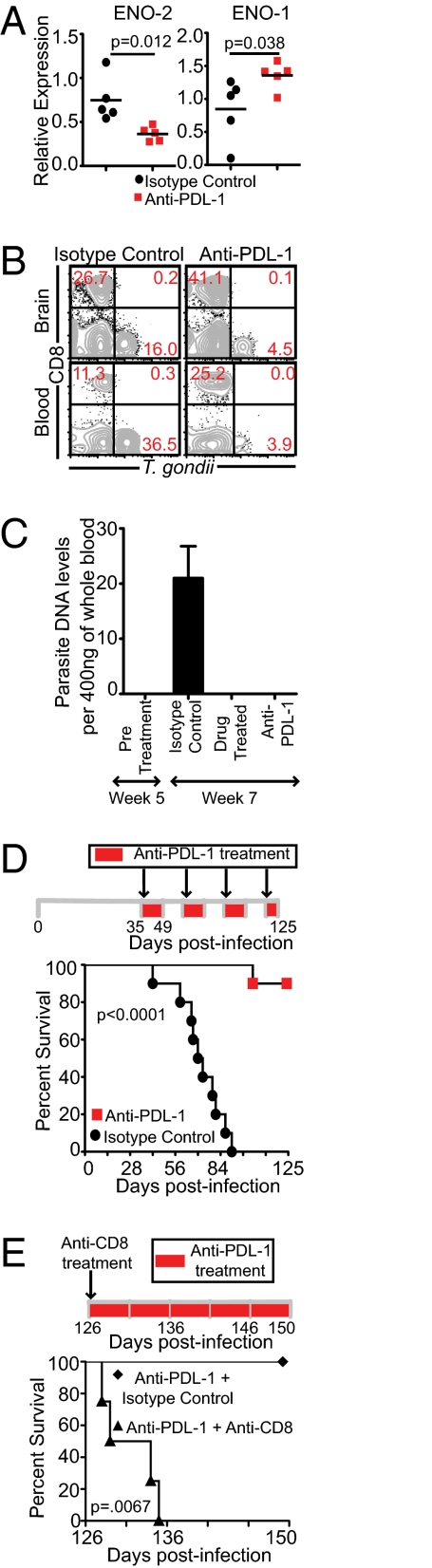
Finally, we wanted to address if this augmented CD8+ T-cell response was able to control recrudescence by examining the gene expression of Toxoplasma parasites in brains of anti–PDL-1 or control antibody treated mice. Anti–PDL-1 treated mice revealed more of a bradyzoite-specific than tachyzoite-specific gene expression (Fig. 4A). Moreover, anti–PDL-1 treated mice exhibited a much lower frequency of Toxoplasma-infected leukocytes in brain and blood than control group (Fig. 4B). As previously stated, because encysted parasites can persist for life in immunocompetent host brains without causing pathology, we examined parasite genomic DNA in blood (6). Detection of parasite DNA in blood by PCR has been used to diagnose cerebral toxoplasmosis in patents with AIDS (36). As shown in Fig. 4C, parasite genomic DNA was only detectable in control antibody-treated and not in drug or anti–PDL-1 treated blood. Next, we monitored long-term survival of anti–PDL-1 treated mice. Surprisingly, although 100% of control mice died by day 91 postinfection, 90% of anti–PDL-1 treated mice survived until the termination (day 125 postinfection) of the experiment (Fig. 4D). Furthermore, to demonstrate that increased survival of anti–PDL-1 treated mice was CD8-dependent, the surviving mice were treated with anti-CD8 antibody. As shown in Fig. 4E, CD8 depletion abrogated the survival of anti–PDL-1 treated animals. Taken together, the above data suggest that anti–PDL-1 treatment augments suboptimal CD8+ T-cell response in chronically infected mice, which prevents reactivation of infection leading to host survival.
Fig. 4.
Anti–PDL-1 treatment controls parasite reactivation and prevents mortality in chronically infected mice. (A) Relative expression of ENO-1 and ENO-2 was computed for each sample. Transcript levels at day 10 postinfection was taken as 1. (B) T. gondii-infected cells in the brain and blood leukocyte-gated samples (n = 5) were assayed by flow cytometry. (C) Parasite genomic DNA level was computed in whole blood before initiation of treatment (week 5 postinfection) or 2 wk after initiation of treatment (week 7 postinfection) (n = 4–5). (D) Anti–PDL-1 antibody was injected every third day, starting at week 5 postinfection (n = 10). To minimize the development of an allogeneic response, the antibody was administered with 2-wk interruptions until termination of the experiment. (E) Surviving anti–PDL-1 treated mice from the previous experiment were administered anti–PDL-1 along with anti CD8 (n = 4) or anti–PDL-1 and control antibody (n = 4) every third day unitl the conclusion of the experiment. Survival was monitored on a daily basis. Data represent two experiments.
Anti–PDL-1 Treatment Up-Regulates Eomes in CD8+ T Cells from Chronically Infected Mice.
T-box factors, T-bet and Eomesodermin, play a critical role in development, survival, and function of CD8+ T cells (37, 38). Studies from our laboratory suggest that anti–PDL-1 treatment dramatically augments expression of Eomes but not T-bet (unaffected vis-à-vis acutely infected mice) in cycling CD8+ T cells (Fig. S9). Incidentally, the critical role of Eomes in mediating CD8+ T-cell responsiveness to IL-15 (38), a cytokine crucial for survival of memory as well as effector CD8+ T cells, and Granzyme B (39–41) expression in T cells has been recently elucidated. Significantly, our data show that CD8+ T-cell dysfunction affects Granzyme B expression more than IFN-γ production. Significance of Eomes expression in our current model will have to await further investigation.
Discussion
CD8+ T-cell exhaustion has been reported in several chronic viral infections, like LCMV, HBV, HCV, HIV, and Simian Immunodeficiency Virus, which are characterized by high levels of persisting viremia (42). This exhaustion is manifested by the gradual loss of CD8+ T-cell effector functions (cytokine production, cytotoxicity, proliferation, and recall responses) and, in extreme situations, CD8+ T cells can be physically deleted (43). In contrast, in infection models like murine cytomegalovirus, characterized by low levels of persistent viremia, antigen-specific T cells remain functional and respond vigorously to viral challenge (42, 44). In models of reactivation like HSV that induce latent infection punctuated by periods of reactivation, functional long-lived CD8+ T-cell response is generated (45). Based on these findings, it has been suggested that CD8+ T-cell exhaustion occurs only in the presence of uncontrolled persistent viremia (42, 46–48). Our current report documents that CD8+ T-cell dysfunction is not restricted to viral infections alone but can be extended to intracellular protozoan parasite, like T. gondii. Although CD8+ T-cell dysfunction observed in the present study is similar in certain facets to certain viral pathogens, in its dependence on PD-1–PDL-1 interaction, it discounts the conventional paradigm that CD8+ T-cell exhaustion occurs only in infectious disease models with uncontrolled persistent pathogen load. Moreover, notwithstanding the fact that the role of the PD-1–PDL-1 pathway has been addressed in chronic viral models of CD8+ T-cell exhaustion, its significance in bacterial or parasitic models of CD8+ T-cell dysfunction has not been extensively investigated.
Our studies demonstrate that despite a potent development of CD8+ T-cell immunity during the early stages of chronic infection, endogenous CD8+ T-cell response is unable to control parasite reactivation at the later time points. This finding is in agreement with a previous study by Gazzinelli et al. (49), which demonstrated that parasite reactivation in B6 mice during late-chronic phases is associated with increased expression of tachyzoite-specific genes in brain. Additionally, there is an increase in parasite levels in the periphery. A recent study has demonstrated the CD11b+ leukocytes can act as shuttles for parasite migration during the acute infection phase (22). Hence, it would be interesting to investigate in future studies whether during parasite reactivation, CD11b+ leukocytes, which are preferentially parasitized during the late-chronic phase, potentially serve a similar function and its role in mediating disease pathogenesis. Cumulatively, our results show that long-term exposure to a persistent pathogen despite initial control of parasitemia can lead to CD8+ T-cell dysfunction and parasite reactivation. This CD8 exhaustion with concomitant PD-1 up-regulation is characterized by reduced effector-cell numbers, decreased polyfunctionality, increased apoptosis, and poor recall response and expansion. Preliminary studies using drug treatment suggest that control of infection during the early but not later phases is critical for minimizing PD-1 up-regulation and CD8 dysfunction. This finding suggests that high antigen burden or inflammation (including anti-inflammatory cytokines) or both that occurs during acute phase of infection may be primarily responsible for defective development of a long-lived robust CD8 response during the chronic phase (42, 50). Based on a recent study by Chang et al. (32) demonstrating that T-cell fate imprinting can occur as early as the first cell division, it is hardly surprising that T-cell microenvironment (cytokine, antigen burden, and so forth) during the early phase of Toxoplasma infection can have such a profound effect during the later phases. Our findings are further supported by a study in an LCMV model where antigen burden has been shown to be the key determinant for development of CD8 dysfunction (42). However, any control of infection or antigen burden can potentially affect inflammation. Hence, in future studies it will be critical to discriminate the differential role of inflammatory milieu and antigen burden in mediating CD8 dysfunction.
Recent reports have identified role of IL-10 in mediating dysfunctional CD8+ T-cell response during LCMV infection (51). However, anti-IL10R treatment of mice carrying chronic Toxoplasma infection led to their increased mortality because of a strong inflammatory response (52). This finding suggests that any strategy for preventing Toxoplasma reactivation must involve an appropriate balance between enhanced proinflammatory mediators without excessive suppression of anti-inflammatory factors. Interestingly, anti–PDL-1 treatment not only augmented the CD8+ T-cell response but also prevented mortality in chronically infected mice by controlling parasite reactivation.
How blockade of the PD-1–PDL-1 pathway modulates CD8+ T-cell response at the molecular level is poorly understood in infectious disease models. Recent studies have shown that differential TCR signal strength can affect not only memory CD8 programming but also development of a potent polyfunctional CD8 response (53, 54). Considering that PD-1 ligation has been shown to inhibit TCR signaling via down-regulation of ZAP-70 and PKCθ in vitro, it is possible that anti–PDL-1 treatment restores robust CD8 response during chronic Toxoplasma infection via increased TCR sensitivity (55). Although the mechanistic basis of CD8 dysfunction and PD-1 up-regulation need to be investigated more thoroughly in future studies, the data presented in this article provide significant insights into the role of PD-1–PDL-1 pathway in mediating CD8+ T-cell dysfunction and concomitant parasite reactivation. These results are especially significant in view of a minimal role played by CTLA-4, a related inhibitory molecule in regulating T-cell response during TE (56). Our findings have important implications in the use of PD-1–PDL-1 blockade in combination with drug regimen for controlling TE in immunocompromised patients, as well as use of similar therapy in other dysfunction-induced reactivation-type diseases.
Materials and Methods
Mice, Parasites, and in Vivo Antibody Treatment.
Female C57BL/6, CD45.1, and CD90.1 mice were purchased from the National Cancer Institute or the Jackson Laboratory. Animal studies were carried out in agreement with Institutional Animal Care and Use Committee approved guidelines. Unless otherwise mentioned, T. gondii cysts of ME49 strain were used for i.g. infection throughout. Some mice were injected in vivo with the following antibodies i.p.: anti-PDL-1 (MIH5) or anti-CD8 (2.43) or isotype-matched control antibodies.
Lymphocyte Isolation, Cell Surface Staining, and Intracellular Staining.
Single cell suspension was prepared from spleen, liver, blood, and brain using standard protocol. For cytokine detection, 5 × 105 naïve congenic splenocytes was mixed with equal number of brain or splenic cells from infected mice, and restimulation was similarly carried out in the presence of TLA.
Real-Time RT-PCR and PCR.
From total infected mouse brain RNA, real-time RT-PCR was performed with primers for ENO-1, ENO-2, SAG-1, and BAG-1 on Toxoplasma specific actin normalized samples. Transcript levels relative to day 10 post infection (day 10 post infection relative transcript level = 1.0) were calculated according to the Pfaffl method of quantitation (57). Parasite DNA quantitation (fg of DNA) was calculated after real-time PCR by comparing Ct values for the B1 gene in experimental samples to a serial dilution of genomic parasite DNA equivalents as previously described (26).
Statistical Analysis.
Statistical analysis was performed used Student's t test or Log-rank test with P < 0.05 taken as statistically significant.
Additional details and techniques are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Azuma for providing MIH5 hybridoma. This work was supported by National Institutes of Health Grant AI-33325 (to I.A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015298108/-/DCSupplemental.
References
- 1.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Grant IH, Gold JW, Rosenblum M, Niedzwiecki D, Armstrong D. Toxoplasma gondii serology in HIV-infected patients: The development of central nervous system toxoplasmosis in AIDS. AIDS. 1990;4:519–521. [PubMed] [Google Scholar]
- 3.Zangerle R, Allerberger F, Pohl P, Fritsch P, Dierich MP. High risk of developing toxoplasmic encephalitis in AIDS patients seropositive to Toxoplasma gondii. Med Microbiol Immunol (Berl) 1991;180:59–66. doi: 10.1007/BF00193846. [DOI] [PubMed] [Google Scholar]
- 4.Jones JL, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill Summ. 1999;48:1–22. [PubMed] [Google Scholar]
- 5.Vidal JE, et al. Cerebral toxoplasmosis in HIV-positive patients in Brazil: Clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS. 2005;19:626–634. doi: 10.1089/apc.2005.19.626. [DOI] [PubMed] [Google Scholar]
- 6.Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18:198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 7.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deckert-Schlüter M, et al. Toxoplasma encephalitis in congenic B10 and BALB mice: Impact of genetic factors on the immune response. Infect Immun. 1994;62:221–228. doi: 10.1128/iai.62.1.221-228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 10.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su C, et al. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- 12.Hughes HP, Kasper LH, Little J, Dubey JP. Absence of a role for natural killer cells in the control of acute infection by Toxoplasma gondii oocysts. Clin Exp Immunol. 1988;72:394–399. [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson DJ, Huskinson-Mark J, Araujo FG, Remington JS. A morphological study of chronic cerebral toxoplasmosis in mice: Comparison of four different strains of Toxoplasma gondii. Parasitol Res. 1994;80:493–501. doi: 10.1007/BF00932696. [DOI] [PubMed] [Google Scholar]
- 14.Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci. 2009;364:2749–2761. doi: 10.1098/rstb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: Localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell. 2007;6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzierszinski F, Mortuaire M, Dendouga N, Popescu O, Tomavo S. Differential expression of two plant-like enolases with distinct enzymatic and antigenic properties during stage conversion of the protozoan parasite Toxoplasma gondii. J Mol Biol. 2001;309:1017–1027. doi: 10.1006/jmbi.2001.4730. [DOI] [PubMed] [Google Scholar]
- 17.Fux B, et al. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect Immun. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tait ED, et al. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torre D, et al. Italian Collaborative Study Group. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Antimicrob Agents Chemother. 1998;42:1346–1349. doi: 10.1128/aac.42.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss LM, et al. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson DJ, Hutchison WM. The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Arch A Pathol Anat Histopathol. 1987;411:39–43. doi: 10.1007/BF00734512. [DOI] [PubMed] [Google Scholar]
- 22.Courret N, et al. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammit DJ, Cauley LS, Pham QM, Lefrançois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 25.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson EH, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 28.Janumyan YM, et al. Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003;22:5459–5470. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen JL, et al. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2006;2:e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galea I, et al. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 33.Dias P, et al. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: Role of PD-1-PD-L1 interactions. J Virol. 2010;84:8241–8249. doi: 10.1128/JVI.00784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 35.Hakim FT, et al. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 36.Lamoril J, et al. Detection by PCR of Toxoplasma gondii in blood in the diagnosis of cerebral toxoplasmosis in patients with AIDS. J Clin Pathol. 1996;49:89–92. doi: 10.1136/jcp.49.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 38.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 39.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 48.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 50.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 51.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jankovic D, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teixeiro E, et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 54.Almeida JR, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 56.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J Immunol. 1999;163:3354–3362. [PubMed] [Google Scholar]
- 57.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.