Abstract
Type 17 helper T (Th17) cells are implicated in the pathogenesis many of human autoimmune diseases. Development of Th17 can be enhanced by the activation of aryl hydrocarbon receptor (AHR) whose ligands include the environmental pollutant dioxin, potentially linking environmental factors to the increased prevalence of autoimmune disease. We report here that nitric oxide (NO) can suppress the proliferation and function of polarized murine and human Th17 cells. NO also inhibits AHR expression in Th17 cells and the downstream events of AHR activation, including IL-22, IL-23 receptor, and Cyp1a1. Conversely, NO did not affect the polarization of Th17 cells from mice deficient in AHR. Furthermore, mice lacking inducible nitric oxide synthase (Nos2−/−) developed more severe experimental autoimmune encephalomyelitis than WT mice, with elevated AHR expression, increased IL-17A, and IL-22 synthesis. NO may therefore represent an important endogenous regulator to prevent overexpansion of Th17 cells and control of autoimmune diseases caused by environmental pollutants.
Type 17 helper T (Th17) cells have recently been defined as a new lineage of CD4+ T cells that produce IL-17. Th17 cells are associated with pathogenesis of human autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and psoriasis (1, 2). Thus, there likely exist rigorous endogenous control mechanisms to limit Th17 development. We now show that nitric oxide (NO) can potently inhibit Th17 proliferation and function.
NO is a key mediator of a variety of biological functions, including vascular relaxation, platelet aggregation, neurotransmission, tumoricidal and microbicidal activities, and immunosuppression (3–5). It is also associated with some of the most important immunopathologies, including rheumatoid arthritis, diabetes, systemic lupus erythematosus, and septic shock. NO is derived from the guanidino nitrogen atom(s) and molecular oxygen in a reaction catalyzed by the enzyme nitric oxide synthase (NOS). There are three forms of NOS. The endothelial form (eNOS or NOS3) and neuronal form (nNOS or NOS1) produce the amount of NO required for physiological functions. The cytokine-inducible form (iNOS or NOS2) is activated by a number of immunological stimuli, such as IFN-γ, TNF-α, and LPS, and catalyses the high output of NO, which can be cytotoxic and kill intracellular pathogens.
NO plays a key role in the immune response (3, 5). NO down-regulates the expression of selectins (P and E), vascular cell adhesion molecule-1, and intracellular adhesion molecule-1 induced by cytokine activation on the vessel wall, resulting in inhibition of rolling of leukocytes along the endothelium and migration of cells from vessels to the tissues (6). We have previously reported that low doses of NO selectively enhanced Th1-cell differentiation and expansion under Th1 polarizing conditions. This was mediated by enhanced IL-12 receptor (IL-12R) β2 expression through a cGMP-dependent pathway (7). We have also shown that NO can induce a subset of CD4+CD25+ Foxp3− regulatory T (NO-Treg) cells from CD4+CD25− T cells via p53, IL-2, and OX40 in a cGMP-independent manner (8). NO-Tregs suppressed the proliferation of freshly purified CD4+CD25− effector cells in vitro and ameliorated the effector T cell-mediated colitis and collagen-induced arthritis in the mouse in an IL-10–dependent manner. Both Th1 and Treg cells are pivotal to autoimmune diseases, indicating that NO may be a key player in modulating inflammatory disease.
Th17 cell differentiation is dependent on IL-6, IL-1, and TGF-β (with potential species-specific differences) and is enhanced by activation of the aryl hydrocarbon receptor (AHR) (9–11). AHR ligands include the environmental pollutant dioxin and the ultraviolet-B light-induced tryptophan metabolite 6-formylindolo [3, 2-b] carbazole (FICZ), potentially linking environmental factors to the increased prevalence of autoimmune diseases.
We now show that NO potently inhibits the proliferation and function of mouse and human Th17 cells. Furthermore, we show that NO suppresses the expression of AHR in Th17 cells independent of IL-2, IL-10, retinoid-related orphan receptor α (RORα), and RORγt. NO had no effect on the polarization of Th17 cells from CD4+ T cells of Ahr−/− mice. In vivo, Nos2-deficient mice developed exacerbated experimental autoimmune encephalomyelitis (EAE) accompanied by elevated numbers of Th17 cells and AHR expression in the spinal cord. Thus, NO is an endogenous negative regulator of Th17-cell development and may prevent autoimmune diseases, including those induced by environmental toxins.
Results
NO Suppresses Th17-Cell Polarization.
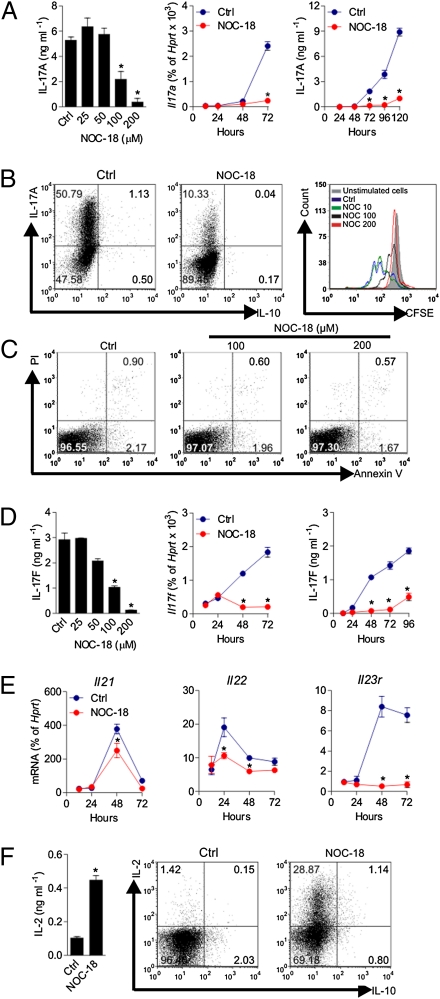
CD4+ T cells were purified (>98% pure) from the lymph nodes and spleens of BALB/c mice or B6 mice by immunomagnetic beads and polarized under Th17 differentiation conditions (TGF-β + IL-6 + IL-1β or these cytokines + anti–IL-2 + anti–IL-4 and anti–IFN-γ) in the presence of graded concentrations of an NO-donor (NOC-18) for up to 5 d. NO inhibited the production of IL-17A protein and expression of mRNA in a dose- and time-dependent manner. The maximum inhibition was achieved at 200 μM NOC-18, and the inhibition was evident from 72 h after Th17 polarization (Fig. 1A). NO inhibited the percentage of IL-17–producing T cells but had no apparent effect on the low number of IL-10–producing cells as determined by intracellular flow cytometry (FACS). NO also blocked the Th17 polarization-driven cell cycle as estimated by FACS on [5-(-6) carboxyfluorescein diacetate succinimidyl ester] (CFSE) dilution (Fig. 1B). The NO-mediated inhibition of Th17 was not attributable to increased apoptosis or necrosis of the treated cells as evident by the similar percentage of viability (propidium iodide-negative and Annexin V-negative cells) of the NO-treated and control untreated cells (Fig. 1C). Despite the inhibitory effect of NO on cell cycle during the first 3 d of culture, NO did not reduce but did increase the total number of T cells at the end of the culture (before culture: 1.5 × 105 cells/mL; at the end of the 5-d culture: without NO, 3.5 ± 0.3 × 105/mL; with 100 μM NOC-18, 6.3 ± 1.5 × 105/mL; with 200 μM NOC-18, 10.7 ± 1.1 × 105/mL). NO also inhibits the protein synthesis and mRNA expression of IL-17F in a dose- and time-dependent manner (Fig. 1D). The inhibition of IL-17F by NO was evident at 48 h and earlier than that for IL-17A. NO also blocked the expression of IL-21, IL-22, and IL-23R (Fig. 1E). In contrast, NO (at 200 μM NOC-18) markedly increased the synthesis of IL-2 during the Th17 polarization as determined by flow cytometry using a cytokine secretion assay and in the culture supernatant harvested at day 5 of the culture (polarizing condition without anti–IL-2) (Fig. 1F). IFN-γ and IL-4 production was low and barely detectable by ELISA or by intracellular staining in culture with or without anti–IFN-γ and anti–IL-4 antibodies. NOC-18 has a half-life of 20 h in solution. To demonstrate that the effect on Th17 is mediated by NO produced by NOC-18 and not by other stable residual products of NOC-18, we incubated the medium containing NOC-18 (200 μM) at 37° C and 5% (vol/vol) CO2 for 5 d. We then compared the conditioned medium (CM) with freshly prepared NOC-18 solution for its effect on Th17 development. Whereas the freshly prepared NOC-18 medium suppressed the polarization of Th17 from CD4+ T cells, the spent CM did not (Fig. S1A). We have also further titrated the concentration of NOC-18 used. At concentrations below 60 μM, NOC-18 modestly but not significantly affected the polarization of Th17; NOC-18 is cytotoxic beyond 300 μM (Fig. S2). We then determined whether the cells could respond to IL-23 during the suppression. In the primary Th17 polarization culture in the presence of NO, the cells were not responsive to exogenously added IL-23 (Fig. S3A). We also investigated whether the continuous presence of NO is required to maintain the suppression of Th17-cell development. Th17 cells were harvested after 5 d of polarization in the presence of 200 μM NOC-18 and restimulated with IL-23 and anti-CD3 with or without freshly added NOC-18. The NO-treated Th17 cells recovered from suppression in the absence of additional NO but remained suppressed in the presence of NO (Fig. S3 B and C). We next compared the effect of NO on Th1-, Th2-, and Th17-cell polarization in parallel cultures. In the condition in which NO markedly suppressed Th17 polarization, NO had little effect on Th1 differentiation but enhanced the low levels of Th2 development (Fig. S4).
Fig. 1.
NO suppresses the polarization of Th17 cells. Purified CD4+ T cells from BALB/c mice were cultured in round-bottom 96-well plates with mitomycin C-treated antigen-presenting cells as well as anti-CD3, anti-CD28, IL-6, TGF-β, and IL-1 with graded concentration of an NO donor (NOC-18) for up to 120 h. In some cultures, anti–IL-2, anti–IL-4, and anti–IFN-γ antibodies were also added with similar results, except with a higher percentage of IL-17+ cells. (A) IL-17A in the culture supernatant was measured by ELISA, and Il17a expression in the cells was determined by qPCR. Ctrl, control. (B) IL-17– and IL-10–producing cells were determined by intracellular staining (day 4), and cell cycle was estimated by CFSE dilution (day 3) using flow cytometry (FACS). (C) Viability of the cells cultured above was determined by propidium iodide and Annexin V staining and measured by FACS on day 4 of culture. (D) Production of IL-17F was determined by ELISA, and the expression of Il17f mRNA was measured by qPCR. (E) mRNA levels of Il21, Il22, and Il23r in cells were determined by qPCR. (F) Concentrations of IL-2 in the culture supernatant (day 4, 200 μM NOC-18) were determined by ELISA, and the percentage of IL-2+ and IL-10+ cells was assayed by FACS using a cytokine secretion assay. Data are expressed as mean ± SEM (n = 5), representative of three independent experiments. *P < 0.05 compared with control without NOC-18.
Together, these results demonstrate that at the concentrations used, NO selectively suppressed Th17-cell proliferation and the cytokines and cytokine receptor associated with IL-17. Furthermore, the NO-mediated suppression was not attributable to cytotoxicity or NO-induced metabolites.
NO Also Suppresses the Proliferation of Established Th17 Cells.
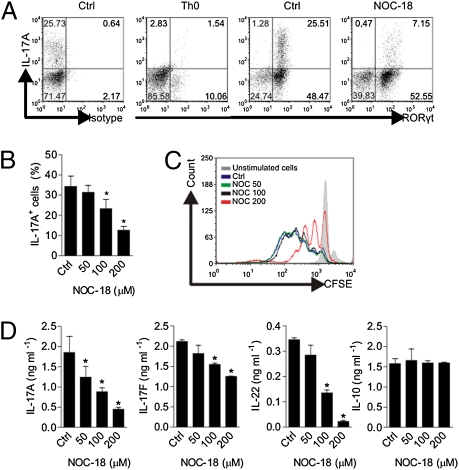
We next investigated whether NO can also affect the functions of established Th17 cells. CD4+ T cells from BALB/c mice were polarized as above for 5 d. The cells were washed and then recultured for 3 d with anti-CD3, IL-23, and IL-1β as indicated in the presence of graded concentrations of NOC-18. The cells were then harvested for intracellular IL-17A and RORγt (a major transcription factor of Th17) staining and the concentrations of cytokines in the culture supernatant determined by ELISA. Around 75% of Th17 cells were RORγt+. NO inhibited the percentage of IL-17+ RORγt+ cells in a dose-dependent manner and had only a modest effect on the IL-17− RORγt+ cells (Fig. 2 A and B). NO also inhibited the proliferation of the polarized Th17 cells as shown by CFSE staining analysis in a dose-dependent manner (Fig. 2C). The concentrations of IL-17A, IL-17F, and IL-22 in the culture supernatant were also significantly reduced by NO in a dose-dependent manner (Fig. 2D). In contrast, the concentration of IL-10 in the culture supernatant was not affected by NO (Fig. 2D). These results demonstrate that NO selectively inhibits the proliferation and functions of established Th17 cells.
Fig. 2.
NO suppresses the function of established Th17. CD4+ T cells were polarized for 5 d as in Fig. 1. The cells were then recultured for 3 d with IL-23 (20 ng/mL), anti-CD3 (4 μg/mL), and anti-CD28 (1.5 μg/mL) antibodies in the presence of the NO donor NOC-18 (200 μM) (A) or as indicated. (A) Percentages of IL-17A+ cells and RORγt+ CD4+ T cells in the control and NO-treated cells, together with the isotype control and unactivated cells (Th0), were determined by intracellular staining using FACS, and representative data are shown. Ctrl, control. (B) Quantitation of the FACS data in A. (C) Cell cycle of the established Th17 cells treated with graded concentrations of NOC-18 was determined by CFSE staining. (D) IL-17A, IL-17F, IL-22, and IL-10 concentrations in the culture supernatant were measured by ELISA. Data are mean ± SEM (n = 5), representative of four independent experiments. *P < 0.05 (compared with control without NOC-18).
NO Suppresses Th17 Differentiation Independent of IL-2, IL-10, cGMP, or ETS1.
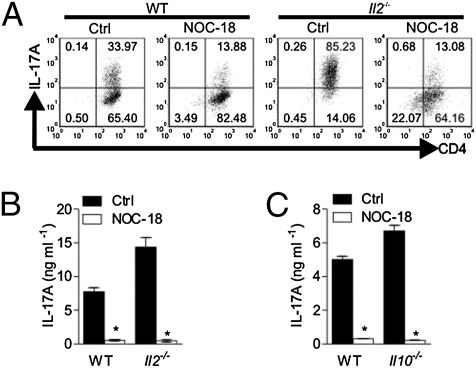
Various cytokines regulate the polarization of Th17 cells: TGF-β, IL-6, IL-1β, and IL-21 are positive inducers, whereas IL-2, IL-4, IL-27, IL-35, and IFN-γ are negative regulators (12–20). Because NO treatment led to a marked increase in IL-2 synthesis (Fig. 1F), we investigated the potential role of IL-2 in the NO-mediated inhibition of Th17 development. As expected (19), the number of Th17 cells polarized was dramatically enhanced in CD4+ T cells from Il2−/− mice compared with WT cells (Fig. 3A). However, NO inhibited the polarization of Th17 cells to a similar extent whether the cells were from the WT mice or the Il2−/− mice (Fig. 3 A and B). NO also inhibited the polarization of Th17 cells from CD4+ cells of IL-10–deficient mice to the same degree as that of the WT mice (Fig. 3C). These results demonstrate that IL-2 and IL-10 are unlikely the mediators of NO-induced inhibition of Th17 development. IFN-γ, IL-4, and IL-27 were not detected in the culture supernatants. Furthermore, NO was equally effective in suppressing Th17 polarization in the presence of anti–IFN-γ and anti–IL-4 antibodies (Fig. 1A), thus excluding the potential role of these cytokines in NO-mediated suppression of Th17. We then examined the possibility that NO may exert its influence via the activation of soluble guanylyl cyclase (sGC), thus elevating cGMP, a well-established NO-effector pathway (21). CD4+ T cells from BALB/c mice were cultured for 3 d under the same Th17 polarizing condition as above with 0.5–1 mM 8-Bromo–cGMP (8-Br–cGMP, an analog of cGMP). 8-Br–cGMP had no effect on IL-17 synthesis (Fig. S5). Conversely, we polarized Th17 cells in the presence of NO and 10 μM [1H-oxodiazolo-(1,2,4)-(4,3-a) guinoxaline-1-one] (ODQ), a competitive inhibitor of the activation of sGC. ODQ was not able to reverse the inhibitory effect of NO on IL-17 production (Fig. S5). Similar negative results were obtained with established Th17 cells. Therefore, NO is unlikely to affect Th17 polarization and function via cGMP. We also examined the effect of NO on Ets1, a recently discovered transcriptional activator that is a negative regulator of Th17 development (22). CD4+ T cells from BALB/c mice were polarized to Th17 with or without NOC-18. Cells were harvested 12 or 24 h later, and Ets1 mRNA expression was determined by quantitative PCR (qPCR). NO did not affect the expression of Ets1 (Fig. S6). Together, these results show that NO suppresses Th17 differentiation independent of IL-2, IL-10, cGMP, or ETS-1.
Fig. 3.
NO suppresses Th17 independent of IL-2 or IL-10. CD4+ T cells from WT B6 mice or B6 Il2−/− or Il10−/− mice were purified and polarized to Th17 in the presence of 200 μM NOC-18 for 4 d as in Fig. 1. IL-17A+ and CD4+ T cells were determined by FACS (A). IL-17A concentrations in the culture supernatants of Il2−/− (B) or Il10−/− cells (C) were determined by ELISA. Data are mean ± SEM (n = 5), representative of two independent experiments. *P < 0.05 (compared with control without NOC-18). Ctrl, control.
NO Suppresses the Expression of AHR on Th17 Cells.
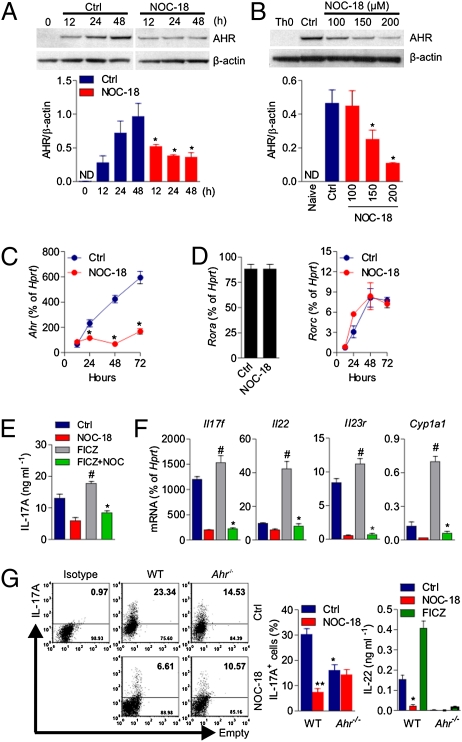
Recently, it has been reported that AHR expression and activation enhanced the development of Th17 cells (9–11). We therefore investigated whether NO would have an effect on AHR expression during Th17 differentiation. CD4+ T cells were polarized under the Th17 condition as described above in the presence of 200 μM NOC-18. The cells were harvested at regular intervals for up to 48 h, and the synthesis of AHR was determined by Western blot analysis. As expected, naive cells did not produce a detectable level of AHR, whereas differentiating Th17 cells expressed AHR in a time-dependent manner (Fig. 4A). NO markedly inhibited the production of AHR in a time-dependent (Fig. 4A) and dose-dependent (Fig. 4B) fashion. Protein synthesis was significantly suppressed by 100 μM NOC-18 and reduced by at least fivefold by 200 μM NOC-18. NO also potently inhibited the Ahr mRNA in a time-dependent manner (Fig. 4C). The suppression of AHR by NO was evident 24 h after Th17 polarization, and this was sustained for at least 72 h.
Fig. 4.
NO suppresses the expression of AHR. (A and B) CD4+ T cells from BALB/c mice were cultured (for 3 d, unless indicated otherwise) as in Fig. 1 in the presence of NOC-18 (200 μM, unless indicated otherwise). AHR protein synthesis was analyzed by Western blot in a time- and dose-dependent manner and was also expressed as the ratio to the housekeeping protein β-actin. Ctrl, control; ND, not detectable. Data are mean ± SEM (n = 3). *P < 0.05. The expression of Ahr (C) and Rora and Rorc (D) mRNA was also analyzed by qPCR. Data are mean ± SEM (n = 3), representative of three independent experiments. *P < 0.05 (compared with control without NOC-18). (E and F) CD4+ T cells from BALB/c mice were polarized to Th17 for 3 d as above in the presence of NOC-18 (200 μM) ± FICZ (200 nM). The concentration of IL-17A in the culture supernatant was determined by ELISA (E), and the expression of mRNA of Il17f, Il22, Il23r, and Cyp1a1 was determined by qPCR (F). Data are mean ± SEM (n = 3), representative of three independent experiments. #P < 0.05 compared with control; *P < 0.05 compared with the group with FICZ but without NOC-18. (G) CD4+ T cells from WT B6 mice or B6 Ahr−/− mice were polarized to Th17 for 4 d ± NOC-18 (100 μM) in flat-bottom 96-well plates. IL-17A+ cells were analyzed by intracellular staining, and IL-22 concentration was determined by ELISA. Data are mean ± SEM (n = 3). *P < 0.05; **P < 0.01 compared with WT mice not treated with NOC-18. The difference between NOC-18–treated WT cells and NOC-18–treated Ahr−/− cells was not significant.
The orphan nuclear receptors, RORα and RORγt (encoded by Rora and Rorc, respectively, in mice) have been identified as the key transcription factors that determine the differentiation of Th17 (23, 24). We therefore investigated the effect of NO on the expression of Rora and Rorc. NO had no effect on the expression of Rora or Rorc at any of the time points tested during the differentiation of Th17 cells (Fig. 4D).
AHR, also known as dioxin receptor, is a transcription factor member of the basic helix-loop-helix periodicity/AHR nuclear translocator/single–minded (Per/ARNT/Sim) family (25). AHR binds to its high-affinity ligands, such as FICZ; translocates to the nucleus and dimerizes with ARNT; and could cause a variety of toxicological effects (26). The best-studied downstream target of AHR is the Cyp1a1 gene family that encodes cytochrome p450 family drug-metabolizing enzymes. We therefore investigated whether NO would also affect the expression of the downstream events of AHR activation. As expected, FICZ enhanced the synthesis of IL-17A and increased the expression of Il17f, Il22, Il23r, and cyp1a1 mRNA during Th17-cell polarization (Fig. 4 E and F). NO markedly suppressed the enhanced IL-17A synthesis induced by FICZ (Fig. 4E). NO also significantly suppressed the FICZ-induced enhanced mRNA expression of Il17f, Il22, Il23r, and Cyp1a1 (Fig. 4F). We also found that NO has no effect on Rorc expression whether CD4+ T cells were exposed to FICZ or not during Th17 polarization (Fig. S7). These results are consistent with an earlier report that the FICZ-AHR activation pathway did not affect the expression of Rorc during Th17 polarization (10). Together, these results indicate that NO suppresses Th17 development, at least in part, via the inhibition of AHR expression. To test this possibility directly, CD4+ T cells from WT or Ahr−/− mice were polarized to Th17 in the presence or absence of NOC-18. As expected, Ahr−/− cells produced 50% less Th17 than the WT cells (10). NO markedly suppressed the Th17 polarization of WT cells but failed to suppress the Th17 polarization of Ahr−/− cells (Fig. 4G). IL-22 was not produced by Ahr−/− cells (Fig. 4G).
We further examined the effect of NO on the expression of IL-1R, IL-6R, and TGF-βRII. NO reduced the expression of IL-1R1, had no effect on IL-6R, and modestly enhanced the expression of TGF-βRII (Fig. S8A). These results indicate that NO may also inhibit Th17-cell development via the down-regulation of IL-1R1. We then investigated the effect of NO on Th17 polarization in T cells from Il1r1−/− mice (27). IL-1R–deficient cells produced about 50% less Th17 than the WT cells. NO completely and similarly suppressed Th17 polarization in WT and Il1r1−/− cells (Fig. S8 B and C), indicating that IL-1R1 is unlikely to be the major target in the NO-mediated suppression of Th17 polarization. We then investigated the effect of NO on the expression of Foxp3, IRF4, and Stat3 phosphorylation, all of which have been implicated to play a significant role in Th17 differentiation (17, 24, 28, 29). Furthermore, it has been suggested that iNOS inhibits IL-6–mediated STAT3 activation (30). Under the Th17 polarizing condition, NO had no effect on Foxp3 (Fig. S9A) or IRF4 (Fig. S9C) expression but significantly inhibited Stat3 phosphorylation (Fig. S9B).
Together, these results suggest that NO is unlikely to affect Th17 differentiation via modulating Foxp3 or IRF4 expression; rather, NO suppresses Th17 differentiation and function, at least in part, via the inhibition of AHR expression and Stat3 phosphorylation. The molecular mechanism by which NO inhibits AHR expression is unknown at present. It is also unclear whether NO suppresses the functions of other AHR-bearing cells. These questions are now amenable to investigation.
NO Suppresses Human Th17-Cell Differentiation.
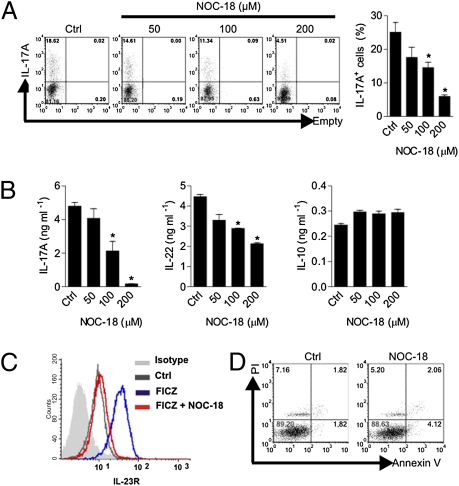
Next, we investigated whether NO also has an effect on the polarization of human Th17 cells. CD4+ T cells were purified from the peripheral blood of healthy donors by immunomagnetic beads and cultured for 4 d with anti-human (h) CD3, anti-hCD28, hIL-6, hTGF-β, hIL-1β, hIL-21, hIL-23, anti–hIFN-γ, and anti–hIL-4 in the presence of graded concentrations of NOC-18. NO suppressed the polarization of IL-17–producing cells in a dose-dependent manner (Fig. 5A). When the culture supernatants were analyzed by ELISA, NO again potently suppressed IL-17 and IL-22 synthesis but had no effect on IL-10 production (Fig. 5B). The AHR agonist FICZ strongly enhanced the expression of IL-23R. This increase was abrogated by NO (Fig. 5C). There was also no difference in the percentage of viable cells between the NO-treated culture and the control untreated cells, with a low percentage of propidium iodide-positive and Annexin V-positive cells in both cultures (Fig. 5D). These results therefore recapitulate the observation seen with the murine cells, again indicating the selective suppressive effect of NO on human Th17-cell differentiation and that this effect was not attributable to potential cytotoxic effects of NO.
Fig. 5.
NO suppresses human Th17 cell differentiation. CD4+ T cells were purified from peripheral blood of healthy donors and cultured for 4 d with bead-coated anti-CD3 + anti-CD28, IL-6, TGF-β, IL-1β, IL-21, IL-23, anti–IFN-γ, and anti–IL-4 antibodies in the presence of graded concentrations of NOC-18. (A) Intracellular FACS analysis of IL-17A. Ctrl, control. (B) Concentrations of IL-17A, IL-22, and IL-10 were determined by ELISA. (C) Expression of IL-23R was also determined by FACS. The histogram shows isotype control (gray), Th17 polarized without NOC-18 or FICZ (green), Th17 polarized with 2 μM FICZ (blue), and Th17 polarized with 100 μM FICZ + NOC-18 (red). (D) Percentage of viable cells at the end of culture was analyzed by propidium iodide and Annexin V staining. Data are mean ± SEM (n = 5), representative of three independent experiments. *P < 0.05 (compared with control without NOC-18).
Nos2−/− Mice Developed Exacerbated EAE, Elevated Numbers of Th17 Cells, and Enhanced AHR Expression.
Th17 plays a prominent role in the pathogenesis of autoimmune diseases, such as EAE, and NO has been reported to suppress EAE (31–35). We therefore investigated the effect of NO on Th17 development and functions in the murine model of EAE in vivo. WT and Nos2−/− mice of the C57BL/6 background were immunized with the myelin oligodendrocyte glycoprotein (MOG35–55) peptide in complete Freund's adjuvant. Nos2−/− mice developed earlier and more severe EAE compared with WT mice (Fig. S10A). Whereas WT mice developed EAE with a mean day of onset of 14 d, Nos2−/− mice developed EAE with earlier kinetics (onset at 11.5 d). Serum collected on day 17 from the Nos2−/− mice contained a higher concentration of IL-17A than that of the WT mice (Fig. S10B). The draining lymph node cells of the Nos2−/− mice also produced larger amounts of IL-17A than produced by the cells from the WT mice in the recall response to MOG35–55 antigen in vitro (Fig. S10C). Cells from both groups of mice produced similarly high levels of IL-17A in response to the polyclonal T-cell mitogen, Con A, demonstrating antigen specificity. There was, however, no difference in the amount of IFN-γ produced by both strains of mice, showing that the effect of NO is cell type-specific and in agreement with an earlier finding that AHR is principally expressed on Th17 cells (10). Our results are in agreement with a recent report (which used a reverse protocol) that mice given an NO donor (GSNO) developed less severe EAE accompanied by reduced IL-17 but not IFN-γ production (36).
In addition, we found that the spinal cord tissue from Nos2−/− mice expressed a significantly higher level of Ahr, Il17a, and Il22 compared with that of the WT mice (Fig. S10D). There was no difference in the Il21 mRNA between the two groups. Histological examination shows that the spinal cord of the Nos2−/− mice contained markedly more CD3+ IL-17A+ T cells compared with the WT mice (Fig. S10E). A higher magnification version of the pictures is provided in Fig. S11. Sections were also stained with H&E and examined under light microscopy. Nos2−/− mice exhibited significantly more perivascular and subpial infiltrates of different size compared with the WT mice.
To obtain direct evidence that the exacerbated disease in EAE Nos2−/− mice was mediated by enhanced Th17 response, we treated EAE Nos2−/− mice with a neutralizing anti–IL-17 antibody. As expected, Nos2−/− mice developed significantly more severe EAE compared with the WT mice. The disease in the Nos2−/− mice was completely abolished by the treatment with the anti–IL-17 antibody (Fig. S10F). Together, these results indicate that NO produced during an inflammatory disease may attenuate the disease development by inhibiting Th17 differentiation associated with the suppression of AHR in vivo.
Discussion
Data reported here demonstrate that NO can suppress the proliferation and functions of Th17 cells, at least in part, via inhibition of the expression of AHR. This finding provides a hitherto unrecognized function of NO, a molecule of pervasive and pivotal pathophysiological roles. Our results also demonstrate a mechanism for preventing excessive activation of Th17 cells, and hence inflammatory diseases. This observation also suggests that endogenously produced NO could attenuate environmental-linked autoimmunity.
NOS2, which catalyses the production of high levels of NO, is induced by cytokines like IFN-γ and TNF-α following an adaptive immune response and is expressed in a variety of cells. Although NOS2 is not readily detected in human peripheral blood monocytes, its overall in vivo role in humans is not in doubt. NOC-18 at a rate of 100–200 μM constantly releases 200–400 nM NO with a half-life of 20 h (37). This dose of NO occurs in vivo in sites of acute infection and inflammation (38) and has been used routinely in experiments in vitro (8, 39). The expression of iNOS at the site of inflammation during EAE has been extensively reported (33, 40–42). We show here by qPCR and immunofluorescent staining that Th17 is expressed at the site of inflammation. Whether Th17 cells differentiated at the site of inflammation is unknown, and investigation is limited by currently available technology. The continuous presence of NOS2 in the lymph nodes may be sufficient to suppress the differentiation of Th17 in the lymph nodes. Furthermore, NO may also suppress fully differentiated Th17 cells at the site of inflammation. We have not used Nos1- and Nos3-deficient mice in our study because the low levels of NO generated by the endogenous NOS1 and NOS3 are unlikely to affect a highly inflammatory disease model like EAE.
It is important to note that NO is not cytotoxic at the dose used, because there was no evidence of any increase in apoptosis or necrosis under the culture conditions. Furthermore, the NO-treated cells produced elevated levels of IL-2 synthesis and no reduction of IL-10 production. These findings also indicate a high degree of selectivity of the action of NO in the polarization of Th17 cells. The effect of NO on Th17 development appears to be independent of the classic sGC-cGMP pathway, because at the concentrations known to be physiologically effective, 8-Br–cGMP had no effect on Th17 differentiation and ODQ could not reverse the inhibitory effect of NO. It is also unlikely that NO mediates the suppression via IL-2, IL-10, or IFN-γ, because NO could effectively suppress Th17 differentiation in the absence of these cytokines.
It should be noted that NO down-regulates Th17 proliferation but does not reprogram Th17 cells. Following a resting period in the absence of NO, Th17 cells can resume proliferation in the presence anti-CD3 and IL-23. This finding suggests that NO, generated during inflammation, could limit collateral damage by shutting down Th17 cells for as long as it takes without permanently eliminating the cells, which may be required for defense against future infections.
Our results provide direct evidence that the suppressive effect of NO on Th17 cell proliferation and function is associated with the inhibition of Ahr mRNA transcription and AHR protein synthesis. NO also inhibits the expression of the known downstream events of AHR-ligand binding, including IL-22 and CYP1a1, reinforcing the notion that NO suppresses the expression of AHR. Furthermore, NO has no apparent effect on RORα or RORγt expression, the canonical pathway of Th17 differentiation. This, however, is in agreement with a previous report that AHR enhances Th17 polarization independent of Rorc (10) and further supports the notion that the NO-mediated suppression of Th17 cells is closely associated with the inhibition of AHR expression. Nevertheless, given the pleiotropic nature of NO, it is likely that NO may affect other molecules in Th17 polarization. NO also suppressed the expression of IL-1R1 and IL-23R (but not IL-6R or TGF-βRII). IL-1 is a key driver of Th17 polarization (14, 43, 44). However, because NO similarly suppresses Th17 polarization of WT as well as Il1r1−/− cells, IL-1R1 is unlikely a major target of NO. In contrast, NO markedly suppressed Th17 proliferation in WT cells but not in Ahr−/− cells. It is therefore likely that AHR plays a major role in the NO-mediated inhibition of Th17 differentiation.
In our hands, the expression of IL-23R is, at least in part, linked to the activation of AHR. FICZ markedly promoted IL-23R expression in murine and human Th17 cell differentiation, and this enhancement was abrogated by NO. NO also significantly suppressed Stat3 phosphorylation. It is not clear whether Stat3 modulates RORγt gene transcription, but activated Stat3 binds directly to the Stat-binding sites in the IL-17 gene promoter and increases IL-17 gene transcription (28). Thus, the inhibition of Stat3 phosphorylation may represent an additional mechanism by which NO suppresses Th17 differentiation independent of RORγt.
Our results demonstrate that NO could effectively inhibit Th17 proliferation and function, and hence attenuate inflammation. This finding opens the intriguing possibility that NO could be a constitutive negative regulator of a range of inflammatory diseases initiated by, among others, environmental factors. The demonstration that human Th17 cell function is similarly affected by NO further suggests the therapeutical potential of NO in inflammation.
Materials and Methods
Mice.
Full details of mouse strains and induction of EAE are presented in SI Materials and Methods
Cell Culture.
Cell culture was carried out in vitro with purified CD4+ T cells according to generally used protocols as cited in the text. NOC-18 was added at the beginning of the cultures (SI Materials and Methods).
Induction and Measurement of EAE.
EAE was induced with MOG35–55 peptide in complete Freund's adjuvant containing Myobacterium tuberculosis H37Ra (Difco Laboratories). Clinical signs of EAE were assessed daily according to scores based on a 10-point scale or a 5-point scale (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Dr. Brigitta Stockinger for providing the Il2−/− and Ahr−/−cells and Dr. Jean Langhorne for providing the Il10−/− cells (both from the National Institute of Medical Research); and Dr. Bernard Ryffel (Centre National de la Recherche Scientifique) for providing the il1r1−/− cells. This work was supported by The Wellcome Trust, the Medical Research Council of the United Kingdom, the European Union (F.Y.L.), and by the State of São Paulo Research Foundation, Brazil (F.Q.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.I. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100667108/-/DCSupplemental.
References
- 1.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 5.Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–iii40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Caterina R, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedbala W, et al. Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP. Proc Natl Acad Sci USA. 2002;99:16186–16191. doi: 10.1073/pnas.252464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedbala W, et al. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci USA. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 10.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 11.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 14.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 16.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 18.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Niedbala W, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 21.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtake F, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 27.Labow M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 28.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brüstle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 30.Villavicencio RT, et al. Induced nitric oxide inhibits IL-6-induced stat3 activation and type II acute phase mRNA expression. Shock. 2000;13:441–445. doi: 10.1097/00024382-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kahn DA, Archer DC, Gold DP, Kelly CJ. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J Exp Med. 2001;193:1261–1268. doi: 10.1084/jem.193.11.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zielasek J, et al. Administration of nitric oxide synthase inhibitors in experimental autoimmune neuritis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995;58:81–88. doi: 10.1016/0165-5728(94)00192-q. [DOI] [PubMed] [Google Scholar]
- 33.Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Nitric oxide via an inducible isoform of nitric oxide synthase is a possible factor to eliminate inflammatory cells from the central nervous system of mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:107–116. doi: 10.1016/s0165-5728(96)00194-4. [DOI] [PubMed] [Google Scholar]
- 34.Dalton DK, Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: Evidence from EAE in iNOS KO mice. J Neuroimmunol. 2005;160:110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Fenyk-Melody JE, et al. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 36.Nath N, Morinaga O, Singh I. S-nitrosoglutathione a physiologic nitric oxide carrier attenuates experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:240–251. doi: 10.1007/s11481-009-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954–10961. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- 39.Macphail SE, et al. Nitric oxide regulation of human peripheral blood mononuclear cells: Critical time dependence and selectivity for cytokine versus chemokine expression. J Immunol. 2003;171:4809–4815. doi: 10.4049/jimmunol.171.9.4809. [DOI] [PubMed] [Google Scholar]
- 40.Rajan AJ, Klein JD, Brosnan CF. The effect of gammadelta T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. J Immunol. 1998;160:5955–5962. [PubMed] [Google Scholar]
- 41.Staykova MA, Paridaen JT, Cowden WB, Willenborg DO. Nitric oxide contributes to resistance of the Brown Norway rat to experimental autoimmune encephalomyelitis. Am J Pathol. 2005;166:147–157. doi: 10.1016/S0002-9440(10)62240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol. 2007;72:934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 43.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulen MF, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.