Abstract
The ribosomal incorporation of nonnative amino acids into polypeptides in living cells provides the opportunity to endow therapeutic proteins with unique pharmacological properties. We report here the first clinical study of a biosynthetic protein produced using an expanded genetic code. Incorporation of p-acetylphenylalanine (pAcF) at distinct locations in human growth hormone (hGH) allowed site-specific conjugation with polyethylene glycol (PEG) to produce homogeneous hGH variants. A mono-PEGylated mutant hGH modified at residue 35 demonstrated favorable pharmacodynamic properties in GH-deficient rats. Clinical studies in GH-deficient adults demonstrated efficacy and safety comparable to native human growth hormone therapy but with increased potency and reduced injection frequency. This example illustrates the utility of nonnative amino acids to optimize protein therapeutics in an analogous fashion to the use of medicinal chemistry to optimize conventional natural products, low molecular weight drugs, and peptides.
Keywords: protein engineering, endocrinology, bio-better
Throughout history, natural products have served as a vital source of medicinal agents. However, chemical synthesis is often required to introduce structural changes that lead to improved therapeutic index, pharmacokinetics, potency, stability, delivery, and bioavailability. The more nascent field of protein therapeutics has followed a similar evolutionary path in a more compressed time frame. Animal-sourced protein therapeutics such as insulin and growth hormone have given way to recombinant DNA (rDNA)-based production. More recently, site-directed mutagenesis has allowed the optimization of natural protein sequence and structure for therapeutic and industrial applications. However, compared to chemical synthesis, the structural and functional diversity of native biosynthesis is severely limited. The 20 canonical amino acids derived from strict evolutionary pressure have evolved protein sequences for physiological, but not therapeutic, purposes. Moreover, although solid phase protein synthesis and semisynthesis offers the promise of increased structural diversity, reports of clinical results have yet to emerge, and there are likely to be significant costs associated with manufacturing at scale.
Technology that marries the efficiency and fidelity of rDNA-directed protein biosynthesis with the chemical diversity accessible through modern synthetic chemistry has recently been developed (1). Here we show that this methodology can be applied to the development of an improved human therapeutic. Human growth hormone (hGH) is a proven treatment for pathological short stature (2–4) and other growth-associated abnormalities. However, while hGH therapy is highly efficacious, it requires daily subcutaneous injection and frequent dose adjustment to minimize adverse effects. Efforts thus far to modify the protein and its formulation have failed to produce a sustained-action hGH with the efficacy, safety, and tolerability of daily subcutaneous injection (5, 6). Herein we report the pharmacological properties of a previously unreported hGH molecule biosynthesized with a genetically encoded nonnative amino acid.
Results and Discussion
Synthesis of hGH Variants and in Vitro Activity.
Key to the design of an hGH with sustained action is the identification of a location where a polymer such as PEG can be attached to suppress nonproductive renal clearance with minimal perturbation of inherent receptor interactions. Building on previous structural work characterizing the hGH receptor-binding interface (7–9), 19 surface-exposed amino acids distal from the site 1 and 2 receptor-binding domains were selected for substitution with p-acetylphenylalanine (pAcF) and subsequent PEG attachment. pAcF is a nonnative phenylalanine derivative with a p-acetyl group that has chemical reactivity orthogonal to the common 20 amino acids and efficiently forms a covalent oxime bond with an amino-oxy terminated PEG polymer (10, 11). Its structural similarity to native amino acids Phe and Tyr reduces the likelihood of conformational perturbations that could lead to loss of function, instability, or immunogenicity. Moreover, pAcF has previously been biosynthetically incorporated into proteins in response to the amber nonsense codon TAG with an orthogonal Methanococcus jannaschii derived aminoacyl-tRNA synthetase/cognate amber suppressor tRNA pair (12).
Table 1 lists the 20 hGH amino acid positions selected for pAcF substitution. One variant, G120pAcF, served as negative control, as changes at this amino acid are known to disrupt receptor signaling (8, 13). All 20 pAcF-substituted hGH variants were produced in Escherichia coli and purified with an N-terminal His6 tag. Expression titers of pAcF variants ranged from 20–70% WT-hGH. After purification, pAcF-His6hGH variants were conjugated with amino-oxy PEG to produce PEGylated pAcF-His6hGH analogs in yields of 80–97%. The proteins were further purified by anion-exchange chromatography to remove unreacted PEG, non-PEGylated hGH, and residual host cell proteins. Site-specific incorporation of pAcF and site-selective PEGylation were confirmed by tryptic peptide mapping (Fig. S1 and SI Materials and Methods). The stability of the resulting oxime bond was assessed at physiological pH over a 1 yr time period. Less than 1% total release was observed rendering the conjugate suitable for once weekly or less frequent administration (Fig. S2).
Table 1.
Affinity and bioactivity measurements
| Kon | Koff | Kd | pSTAT5 EC50 | |
| Variant | × 10-5 1/M ∗ s | × 104 1/s | nM | nM |
| WHO hGH | 6.4 | 3.8 | 0.6 | 0.4 ± 0.1 (n = 8) |
| His6hGH | 9.0 | 5.6 | 0.6 | 0.6 ± 0.3 (n = 3) |
| Rat GH | 0.33 | 83 | 250 | > 200,000 |
| Y35pAcF | 7.8 | 5.3 | 0.7 | 0.7 ± 0.2 (n = 4) |
| PEG-Y35pAcF | 0.9 | 0.7 | 0.7 | |
| E88pAcF | 6.8 | 5.4 | 0.8 | 0.9 |
| Q91pAcF | 6.6 | 4.9 | 0.7 | 2.0 ± 0.6 (n = 2) |
| F92pAcF | 8.6 | 5.0 | 0.6 | 0.8 ± 0.4 (n = 9) |
| PEG-F92pAcF | 1.3 | 0.9 | 0.7 | |
| R94pAcF | 5.6 | 6.0 | 1.1 | 0.7 |
| S95pAcF | 0.7 | 3.1 | 4.3 | 16.7 ± 1 (n = 2) |
| N99pAcF | 2.2 | 3.8 | 1.7 | 8.5 |
| Y103pAcF | ∼0.06 | ∼6 | > 100 | 130,000 |
| Y111pAcF | 8.4 | 4.8 | 0.6 | 1.0 |
| G120pAcF | 1.1 | 23 | 20 | > 200,000 |
| G131pAcF | 6.3 | 5.3 | 0.9 | 0.8 ± 0.5 (n = 3) |
| PEG-G131pAcF | 1.0 | 2.9 | 2.9 | |
| P133pAcF | 6.4 | 4.9 | 0.8 | 1.0 |
| R134pAcF | 8.4 | 5.8 | 0.7 | 0.9 ± 0.3 (n = 4) |
| PEG-R134pAcF | 0.7 | 1.7 | 2.4 | |
| T135pAcF | 7.2 | 4.5 | 0.6 | 0.9 |
| G136pAcF | 6.2 | 4.3 | 0.7 | 1.4 |
| F139pAcF | 6.8 | 4.4 | 0.7 | 3.3 |
| K140pAcF | 7.2 | 3.7 | 0.5 | 2.7 + 0.9 (n = 2) |
| Y143pAcF | 7.8 | 6.7 | 0.9 | 0.8 ± 0.3 (n = 3) |
| PEG-Y143pAcF | 1.3 | 3.3 | 2.5 | |
| K145pAcF | 6.4 | 5.0 | 0.8 | 0.6 ± 0.2 (n = 3) |
| PEG-K145pAcF | 2.1 | 1.5 | 0.7 | |
| A155pAcF | 5.8 | 4.4 | 0.8 | 1.3 |
KD values were measured with Biacore using a mutant rat GH receptor.
STAT5 phosphorylation was measured in human IM-9 cells post treatment.
The effects of site-specific pAcF substitution and PEGylation on hGH receptor binding were measured with surface plasmon resonance (Table 1). Additionally, the potency of the pAcF-substituted hGH variants, relative to native hGH and an N-terminal histine-tagged analog (His6hGH), was evaluated in a human IM-9 cell based assay of JAK2-mediated STAT5 phosphorylation in response to hGH receptor binding. (Table 1; see SI Materials and Methods for details). As previously reported, rat GH did not function in the human IM-9 cell based assays and the G120pAcF substitution yielded inactive protein (8, 13). Sixteen of the 20 pAcF-substituted His6hGH variants demonstrated appreciable receptor-binding affinity and signaling through STAT5 phosphorylation. The six pAcF variants substituted at Y35, F92, Q131, R134, Y143, and K145 were virtually indistinguishable from the native-sequence control and were chosen for further study.
A linear 30 kDa PEG was chosen as the conjugation partner with the goal of achieving a molecular size suitable for minimizing renal clearance yet maintaining acceptable viscosity. Receptor binding and STAT5 phosphorylation assays confirmed that 30 kDa PEGylation was not disruptive to the function of the six PEGylated pAcF-His6hGH proteins (Table 1 and Table 2). Slower kon rates were observed for PEGylated analogs, and seven- to 10-fold increases in EC50 were also observed. Measurement of in vitro biological activity by cellular proliferation in mouse BAF3 cells containing a modified rat GH-receptor (Table 2) illustrated improved preservation of potency relative to STAT5 phosphorylation (EC50 increases of twofold to sixfold).
Table 2.
Bioactivity of hGH variants and PEGylated analogs
| pSTAT5 | Proliferation | |
| Variant | EC50, nM | EC50, nM |
| WHO hGH* | 1.0 | 1.0 |
| Y35pAcF | 1.6 ± 0.4 (n = 2) | 1.6 ± 0.8 (n = 3) |
| PEG-Y35pAcF | 13.5 ± 4.9 (n = 2) | 5.4 ± 2.8 (n = 4) |
| F92pAcF | 1.3 ± 0.5 (n = 2) | 1.4 ± 0.7 (n = 4) |
| PEG-F92pAcF | 8.8 ± 3.0 (n = 6) | 4.1 ± 0.9 (n = 3) |
| Q131pAcF | 2.3 ± 1.8 (n = 2) | 2.1 ± 1.1 (n = 3) |
| PEG-Q131pAcF | 23.8 ± 1.7 (n = 2) | 4.6 ± 2.4 (n = 3) |
| R134pAcF | 1.1 ± 0.2 (n = 2) | 1.7 ± 0.3 (n = 3) |
| PEG-R134pAcF | 11.3 ± 1.1 (n = 2) | 2.5 ± 0.7 (n = 4) |
| Y143pAcF | 1.6 ± 0.1 (n = 2) | 1.8 ± 0.6 (n = 2) |
| PEG-Y143pAcF | 12.3 ± 0.9 (n = 2) | 6.6 ± 2.7 (n = 3) |
| K145pAcF | 2.3 ± 0.5 (n = 2) | 3.0 ± 1.4 (n = 2) |
| PEG-K145pAcF | 20.6 ± 9.8 (n = 2) | 5.3 ± 3.5 (n = 3) |
*Expressed as unity for between-assay comparison.
Pharmacokinetic Analysis of hGH Variants.
In a pharmacokinetic analysis, the six PEGylated pAcF-His6hGH analogs were injected subcutaneously at 1 mg/kg (20 mM NaCitrate, 270 mM Glycine, 0.5% Mannitol, pH 6.0) into normal rats (male, Sprague–Dawley). All six displayed longer terminal t1/2 values (4–6 h) relative to native WHO hGH standard, His6-hGH or a pAcF-variant (Fig. S3). Notably, the PEGylated Y35pAcF-His6hGH analog exhibited a 3.8-fold increase in Cmax and a 5.7-fold increase in AUC0-> inf when compared to the PEGylated G131pAcF analog. This difference represents the dynamic range observed between the best and worst performing single site PEGylated analogs, despite near equivalent in vitro performance. Additional distinctions were noted in the terminal t1/2 of the PEGylated Y143pAcF and K145pAcF His6hGH analogs, where prolongation of clearance up to 38% was observed compared to the PEGylated R134pAcF His6hGH analog.
Pharmacodynamic Evaluation of hGH Variants.
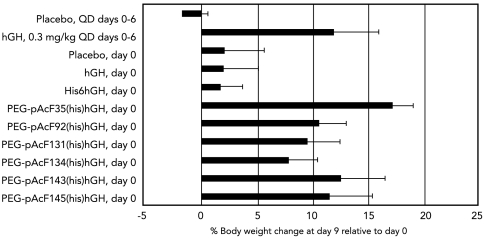
The pharmacodynamic performance of the six PEGylated His6hGH analogs was assessed in a standard hypophysectomized rat assay. Cohorts of hypophysectomized rats, matched by initial weights and pretreatment growth rates (see Materials and Methods), were treated with one of several therapies: daily subcutaneous injection of native hGH for 7 d (0.3 mg/kg); or a single, weekly administration (2.1 mg/kg) of native hGH, His6hGH, or one of six PEGylated His6hGH analogs. When evaluated at day 9, the single weekly dose of either hGH or His6hGH was indistinguishable from daily injection of placebo. In contrast, PEGylated Y35pAcF-His6hGH given once weekly displayed sizable and sustained growth-promoting activity, reflected in relative body weight gain. Notably, this analog exceeded the performance of the other PEGylated molecules (Fig. 1). Weekly administration of PEGylated Y35pAcF-His6hGH produced a response that was statistically equivalent to daily hGH therapy when all mean pairs were compared using a Tukey–Kramer honestly significant difference test. Similar analysis revealed that PEGylated Y35pAcF-His6hGH produced a statistically larger body weight gain than PEGylated analogs F92pAcF (p < 0.05), G131pAcF (p < 0.01), and R134pAcF -His6hGH (p < 0.01). The rank order of total % body weight increase correlated with AUC0-> inf demonstrating that the PEGylated hGH analogs with greater exposure produced larger increases in body weight (Table 3).
Fig. 1.
PEGylated His6hGH analog-induced growth in hypophysectomized rats. Six site-selective PEGylated His6hGH analogs were compared for their ability to induce weight gain in hypophysectomized rats (N = 5–11 per group). Error bars represent standard deviation. Unless otherwise indicated, hGH, His6hGH, and the PEGylated His6hGH analogs were dosed once on day 0 at 2.1 mg/kg. PEGylated Y35pAcF-His6hGH produced the largest body weight increase but was not significantly better than daily hGH administration at 0.3 mg/kg when all mean pairs were compared using a Tukey–Kramer honestly significant difference test.
Table 3.
Pharmacokinetic parameter values for single-dose (1 mg/kg s.c.) administration in normal male Spargue–Dawley rats
| Parameter |
|||||||
| Compound | n | Cmax (ng/ml) | Terminal t1/2 (h) | AUC0-> inf (ng × hr/ml) | MRT (h) | Cl/f (ml/hr/kg) | Vz/f (ml/kg) |
| WHO hGH | 3 | 529 (±127) | 0.53 (±0.07) | 759 (±178) | 1.29 (±0.05) | 1,368 (±327) | 1051 (±279) |
| His6hGH | 4 | 680 (±167) | 0.61 (±0.05) | 1,033 (±92) | 1.30 (±0.17) | 974 (±84) | 853 (±91) |
| PEG-Y35pAcF-His6hGH | 4 | 1,885 (±1,011) | 4.85 (±0.80) | 39,918 (±22,683) | 19.16 (±4.00) | 35 (±27) | 268 (±236) |
| PEG-F92pAcF-His6hGH | 6 | 663 (±277) | 4.51 (±0.90) | 10,539 (±6,639) | 15.05 (±2.07) | 135 (±90) | 959 (±833) |
| PEG-Q131pAcF-His6hGH | 5 | 497 (±187) | 4.41 (±0.27) | 6,978 (±2,573) | 14.28 (±0.92) | 161 (±61) | 1,039 (±449) |
| PEG-R134pAcF-His6hGH | 3 | 566 (±204) | 4.36 (±0.33) | 7,304 (±2,494) | 12.15 (±1.03) | 151 (±63) | 931 (±310) |
| PEG-Y143pAcF-His6hGH | 5 | 803 (±149) | 6.02 (±1.43) | 17,494 (±3,654) | 18.83 (±1.59) | 59 (±11) | 526 (±213) |
| PEG-K145pAcF-His6hGH | 5 | 634 (±256) | 5.87 (±0.09) | 13,162 (±6,726) | 17.82 (±0.56) | 88 (±29) | 743 (±252) |
Concentration vs. time curves were evaluated by noncompartmental analysis (Pharsight, version 4.1). Values shown are averages (+ standard deviation).
Cmax: maximum concentration; terminal t1/2: terminal half-life; AUC0-> inf: area under the concentration-time curve extrapolated to infinity; MRT: mean residence time; Cl/f: apparent total plasma clearance; Vz/f: apparent volume of distribution during terminal phase.
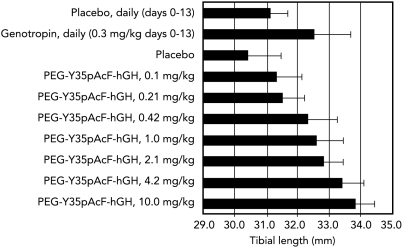
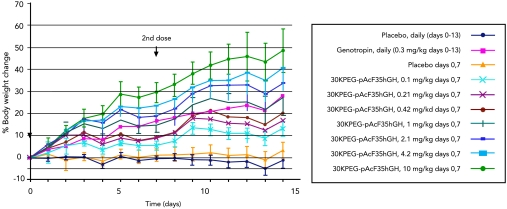
The in vivo pharmacology of PEGylated Y35pAcF-hGH, expressed in E.coli without a His6 tag and purified by a commercially suitable process, was assessed in hypophysectomized rats (see Materials and Methods). Seven weekly doses (0.1, 0.21, 0.42, 1.0, 2.1, 4.2, and 10.0 mg/kg) of PEGylated Y35pAcF-hGH were compared to daily administration of Genotropin (0.3 mg/kg) and placebo (n = 10 per group). The study lasted two weeks, and three parameters of pharmacodynamic activity were measured: tibia bone growth, body weight gain, and insulin-like growth factor 1 (IGF-1) levels. Dose-dependent increases in tibia bone length (Fig. 2) and body weight (Fig. 3) were observed. The PEGylated Y35pAcF-hGH analog produced increases in tibia length of 0.9, 1.1, 1.9, 2.2, 2.4, 3.0, and 3.4 mm at the respective study doses. All animals dosed at greater than 0.42 mg/kg-wk demonstrated significant bone growth compared to placebo (p < 0.0001 for 0.42–2.1 mg/kg-wk and p < 0.000001 for doses 4.2 and 10 mg/kg-wk). Daily administration of Genotropin at 0.3 mg/kg produced an approximately 25% increase in body weight and a 1.4-mm increase in tibia bone length. In contrast, a similar 25% increase in body weight was achieved with a weekly dose of 1.0 mg/kg of PEGylated Y35pAcF-hGH. Equivalent increases in tibia length were achieved at an even lower weekly dose of just 0.42 mg/kg. Therefore, weekly therapy with PEGylated Y35pAcF-hGH in the hypophysectomized rat model was shown to be protein sparing, approxmiately 2 to 5 times more potent than daily Genotropin. The in vitro and in vivo pharmacology demonstrate that control of conjugation site provides homogeneous analogs with differential performance that clearly identifies one optimal candidate for clinical study.
Fig. 2.
Dose-dependent increase in tibia length with PEGylated Y35pAcF-hGH. Rats were treated with placebo daily or on days 0 and 7, with 0.3 mg/kg genotropin, or with increasing doses of PEGylated Y35pAcF-hGH on days 0 and 7. Solid bars indicate the mean of measured tibia bone length. Error bars represent standard deviation. N = 10 rats per treatment group, one tibia measurement per rat.
Fig. 3.
Dose-dependent weight gain in rats treated with PEGylated Y35pAcF-hGH. Rats were treated with placebo daily or on days 0 and 7, with 0.3 mg/kg genotropin, or with increasing dose of PEGylated Y35pAcF-hGH on days 0 and 7. Solid lines indicate the mean percentage change in body weight. Error bars represent standard deviation. N = 10 rats per treatment group, one daily weight measurement per rat.
Clinical Evaluation.
Given the promising rodent pharmacology of PEGylated Y35pAcF-hGH, a clinical study of this candidate was initiated. The duration of action and level of IGF-1 stimulation derived from PEGylated Y35pAcF-hGH was studied in adult GH-deficient (AGHD) patients. Protein manufactured for use in human clinical trials was designated ARX201.
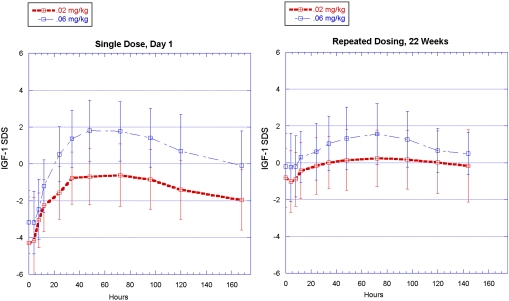
Twenty-two AGHD subjects who had not received GH replacement therapy in the previous 6 mo were given weekly injections of ARX201. Drug doses were titrated based on IGF-1 responses and/or safety findings (see Materials and Methods). Administration of ARX201induced rapid normalization of IGF-1 levels, with standard deviation scores largely remaining between -1 and +1.5 throughout the study (14, 15) (Fig. 4). This demonstrates that IGF-1, the primary effector of growth hormone activity, can be brought back to normal levels, and that these levels are maintained without significant excursions during the course of administration, thus confirming appropriate growth hormone replacement. The half-life of the administered drug was 89–102 h. Mean truncal fat mass decreased by 0.7 kg (5.6%) and mean total body fat declined by 0.4 kg (1.3%), as assessed by dual energy X-ray absorptiometry (DEXA). Mean lean body mass increased by 1.5 kg (3.6%). Mean HbA1c levels were maintained within the normal range: 6.0% at screening, 5.4% at week 26. One subject was removed from the study at investigator discretion due to elevated insulin levels, and there was one case of unrelated accidental death. In addition, ARX201 was well-tolerated, with only transient minor injection site reactions reported. No neutralizing antibodies to either PEGylated GH or to native GH were detected throughout the study. These early phase clinical efficacy and safety observations support advanced clinical investigation with weekly ARX201.
Fig. 4.
ARX201-induced increase in IGF-1 standard deviation scores (SDS) in GH-deficient human clinical trial subjects. ARX201 (starting dose 0.02 and 0.06 mg/kg) was administered weekly by subcutaneous injection for up to 26 weeks, and induced an IGF-1 SDS increase into the normal range within one day.
Conclusions
We report here the biosynthesis and characterization of a unique, sustained-action analog of hGH, created by substituting a nonnative amino acid, pAcF, for the native tyrosine 35 by which a single 30 kDa linear PEG molecule was covalently conjugated. The production of this molecule was straightforward and has been currently scaled to > 1,000 L fermentation to support completion of advanced Phase IIB studies and an eventual commercial supply. Preclinical rodent and initial human clinical studies with ARX201 indicated efficacy and safety that matched or exceeded daily therapy with native hormone, taking into account the expected influence of on-target GH-mediated modulation of intermediary metabolism. Site-selective PEGylation differentially extended hGH pharmacokinetics in rodents with the best analog demonstrating once weekly dosing potential in humans.
Importantly, this technology allows one to rapidly optimize the physical, biological, and pharmacological properties of a protein conjugate through recombinant-based protein chemistry that is unrestricted in site of conjugation. This constitutes a significant advance relative to conventional conjugation methods that are restricted by the native 20 amino acids (16–18). For example, the modification of lysines by electrophiles that is widely used in research settings results in mixtures of positional isomers even when the stoichiometry is controlled to provide one PEG per protein. Furthermore, each positional isomer is likely to have different efficacy, half-life, immunogenicity, and the like. Because chromatographic purification is not commercially viable (19), one is left with the challenge of optimizing the pharmacology of a heterogeneous mixture of drug substance (in contrast to the careful SAR studies historically used to optimize chemically defined small molecule drugs). An alternative is to exploit the low pKa of the amino terminus to direct conjugation, which is the basis of a mono-PEGylated GCSF drug (20). This approach is not broadly applicable because nonspecific reactions with other amines can occur; moreover, it is N-terminally restricted, which is rarely the preferred site of conjugation for reasons pertaining to receptor potency, bioavailability, and biophysical stability. Finally, while site-specific cysteine conjugation has been reported (17), it is well recognized to be inherently much more difficult because an unpaired additional thiol is prone to oxidation and catalysis of disulfide bond shuffling. Consequently, site selection is compromised and commercial production problematic.
Indeed, in the case of hGH, significant differences in potency, Cmax, T1/2, and AUC were observed for analogs that differed only in the site of PEG attachment. Separately, we have observed similar results for granulocyte colony-stimulating factor (G-CSF) and interferon (IFN) alpha and beta. The precise molecular mechanism by which PEGylation at different sites gives rise to such dramatic changes in pharmacology is unknown. PEGylation may differentially shield charged surfaces, provide protection against protease cleavage, degradation, and absorption, and clearance mechanisms. PEGylation may also change a protein’s isoelectric point, which has been shown to affect pharmacokinetics (21, 22). Similar challenges associated with the site-specific conjugation of proteins are also likely to apply to other protein conjugates as well, including antibody-drug conjugates, where the site of conjugation and the ability to both quantify and optimize the therapeutic index of homogeneous molecules are desirable.
Of broader significance is the precedent this work establishes in demonstrating the power of an expanded genetic code to enable novel, site-specific, posttranslational modification of recombinant proteins. Using hGH as an example, we have provided a general strategy that simplifies and facilitates the rapid recombinant-based introduction of chemical functionality at defined positions within a protein in order to optimize its therapeutic performance. The technology described here represents a significant step forward in the emerging field of protein medicinal chemistry and may be extended to allow site-selective protein conjugation of a variety of effector molecules such as toxins, peptides, oligonucleotides, and other complementary proteins. Importantly, like the case with small molecules, structural analogs are being generated that are homogeneous with pure chemical activities, rather than mixtures, with distinct efficacy and safety profiles. Application of this technology in mammalian and other eukaryotic recombinant expression systems has been reported (23, 24) and holds promise to further expand its utility for protein therapeutics.
Materials and Methods
Production of pAcF-Containing His6hGH Variants.
hGH variants with a TAG codon at each selected position were generated by site-directed PCR mutagenesis and inserted into a plasmid (pBR322) containing the lac promoter, a His6 tag and ampR. Each plasmid was transfected into DH10B(fis3) cells (Invitrogen Corporation) containing the expanded genetic code system components for pAcF incorporation, an amber codon-suppressing tRNATyr under the control of the lpp promoter and pAcF-specific aminoacyl tRNA synthetase under the control of the E. coli GlnRS promoter as previously described (12). Transformed DH10B(fis3) cells were grown in defined medium (glucose minimal medium supplemented with leucine, isoleucine, trace metals, and vitamins) with 100 μg/mL ampicillin at 37 °C. When the OD600 reached approximately 1, pAcF was added to a final concentration of 3.3 mM, and the temperature was lowered to 28 °C. After 0.75 h, protein expression was induced with IPTG at a final concentration of 0.25 mM. Cells were grown an additional 8 h at 28 °C, pelleted, and frozen at -80 °C until further processing. Expression titers of pAcF variants ranged from 20–70% of WT-hGH. A similar system was optimized for high density fermentations that have been carried out at > 1,000 liter scale with titers ranging from 500–800 mg/L.
Protein Purification and PEGylation.
For production of PEGylated His6hGH analogs, frozen cell paste was resuspended in lysis buffer (20 mM Tris, pH 8.5) and passed through a Microfluidizer (Microfluidics) two times at 15,000 psi with cooling. After centrifugation, the His-tagged hGH protein in the supernatant was purified using the ProBond Nickel-Chelating Resin (Invitrogen) as recommended, followed by anion-exchange chromatography(Q Sepharose Fast Flow column, 10 mM Bis-Tris, pH 6.5, 0–100 mM NaCl gradient). The purified pAcF-containing His6hGH was concentrated to 8 mg/mL and buffer exchanged to the PEGylation reaction buffer (20 mM sodium acetate, 150 mM NaCl, 1 mM EDTA, pH 4.0). PEG-Oxyamine powder was added to the His6hGH solution in 5X molar excess and the reaction was carried out at 28 °C with gentle shaking for 36–48 hrs. The yield of protein PEGylation was site-dependent and ranged from 80–97%. PEGylated His6hGH was purified from unreacted PEG and His6hGH by anion-exchange chromatography (Source 30Q column, 10 mM Tris, pH 7.0, 0–100 mM NaCl gradient). Purity was examined by Bis-Tris 4–12% NuPAGE with MES SDS running buffer under nonreducing conditions (Invitrogen), Coomassie blue staining and densitometry. The PEGylated His6hGH protein was greater than 95% pure, with endotoxin levels in each variant less than 5 EU/animal dose by KTA2 kit analysis (Charles River Laboratories).
For PEGylated Y35pAcF-hGH, frozen cell paste harvested from fermentations performed at 5–1,000 L was resuspended in 50 mM Tris, 10 mM EDTA, and 0.07% Triton X-100 at pH 8. Following lysis and centrifugation, anion-exchange capture (Q Sepharose Fast Flow column, 10 mM Bis-Tris, pH 6.5, 0–100 mM NaCl gradient) and a hydrophobic interaction column (Phenyl Sepharose High Performance column, binding buffer -20 mM Tris, 400 mM NaCitrate, pH 7.0, elution buffer -10 mM Tris, pH 7.0) were used to purify Y35pAcF-hGH from the supernatant. The PEGylation and post-PEGylation purification methods were as described above.
Genotropin is manufactured by Pfizer and was purchased from a CRO.
Animals.
Animal experimentation was conducted in an AAALAC accredited facility using protocols approved by the Institutional Animal Care and Use Committee of St. Louis University. Male (270–450 g) CD IGS Sprague–Dawley rats and hypophysectomized male Sprague–Dawley rats (pituitaries surgically removed at age 3–4 weeks) were obtained from Charles River Laboratories. Rats were caged individually in rooms with a 12-h light/dark cycle and provided certified Purina rodent chow 5001 and water ad libitum. Animals were allowed to acclimate for three weeks, during which time body weight was monitored. Animals that lost less than 2 g of body weight or gained less than 8 g body weight before the start of the study were included and randomized to treatment groups.
Animal Dosing and Plasma Collection.
In some studies, indwelling carotid catheters were surgically installed prior to randomization. For the hypophysectomized rat models, a single dose of compound was administered subcutaneously in a dose volume of 0.5 mL/kg 20 mM NaCitrate, 270 mM Glycine, 0.5% Mannitol, pH 6.0. Dosed compound concentrations (mg/kg) reflect the weight of the hGH protein without the attached PEG. Blood samples were collected into EDTA-coated tubes at various time points, and plasma collected and stored at -80 °C after microcentrifugation.
Pharmacokinetics and Pharmacodynamic Analysis.
Compound concentrations were measured using an antibody sandwich GH ELISA kit from Biosource or Diagnostic Systems Laboratories (for PEGylated Y35pAcF-hGH only). Because each PEGylated hGH analog produced a different concentration-dependent signal in these assays, concentrations were calculated using a standard curve generated from the corresponding dosed compound. Pharmacokinetic parameters were estimated using the modeling program WinNonlin version 4.1 (Pharsight). Noncompartmental analysis with linear-up/log-down trapezoidal integration was used, and concentration data were uniformly weighted. Hypophysectomized rats were dosed subcutaneously either daily or weekly. Throughout the study rats were sequentially weighed, anesthetized, bled, and dosed (hGH only) daily. Blood was collected from the orbital sinus using a heparinized capillary tube and placed into an EDTA-coated microcentrifuge tube. Plasma was isolated by centrifugation and stored at -80 °C until analysis. Rat IGF-1 concentrations were measured using a competitive binding enzyme immunoassay kit (Diagnostic Systems Laboratories). For tibia bone length measurements, animals were sacrificed on day 14 using CO2, and both tibias from all animals were collected and immersion-fixed in 10% neutral-buffered formalin. One tibia from each animal was radiographed 8 d later and length was measured from the radiograph.
Tolerability, Safety, Half-Life, and IGF-1 Response in Adult GH-Deficient (AGHD) Patients Treated with PEGylated Y35pAcF-hGH.
The protocol and consent form for this phase I-II study was approved by the Bioethics Committee of the Medical Centre of Postgraduate Education (Warsaw, Poland) and the study conducted according to the Declaration of Helsinki. Signed, informed consent was obtained from all patients. PEGylated Y35pAcF-hGH prepared for use in humans was designated ARX201. Twenty-two subjects (13 men and 9 women; median age 39.5 yrs) who had not received GH replacement therapy in the previous 6 mo were given weekly subcutaneous injections of ARX201 for up to 26 weeks. The starting dose was either 0.02 or 0.06 mg/kg/week (11 patient-years exposure), and doses were titrated based on IGF-1 response and/or safety findings. Safety was assessed throughout the trial by monitoring adverse events (AEs), electrocardiograms (ECGs), safety laboratory results (hematology, serum chemistry profile, liver enzymes, and urinalysis), glucose metabolism (blood glucose, insulin and hemoglobin A1c [HbA1c]), cortisol, prolactin, thyroid function, lipids, IGF-I, immunogenicity (antibody development), local tolerability to injections, physical examinations, vital signs, fundoscopy, and concomitant medication. Routine clinical assessments and laboratory measurements were made during the course of the study, including measurements of hemoglobin A1C, a glycosylated hemoglobin whose levels are modulated by ambient glucose levels, and which is used to determine glycemic status over the previous 2–3 mo. Mean truncal fat mass and mean lean body mass were measured by dual energy X-ray absorptiometry (DEXA).
Supplementary Material
Acknowledgments.
We thank Russell Driver, Andrew Paulsel, and Ian Warner for expert technical contributions; Ashton Cropp for advice about improving protein production efficiency; John Wallen and Cris Calsada for helpful discussions about experimental design and project direction; Dannete Powell and Vijay Hingorani for invaluable contributions to the design, conduct, and analysis of the clinical aspects of this work; Jeanne McAdara-Berkowitz, Melanie Nelson, and Paula Dunagin for expert assistance in manuscript preparation; and Jason Pinkstaff for help with the manuscript and figures. Troy Wilson made it possible to complete this work.
Footnotes
Conflict of interest statement: The authors declare a conflict of interest (such as defined by PNAS policy). This work was funded by Ambrx, Inc. (La Jolla, CA) and in part by Merck Serono (Geneva). As indicated in the author list, some of the authors are current or former employees of Ambrx, Celgene, Amylin Pharmaceuticals, or Applied Molecular Evolution. P.G.S. and R.D.D. are members of Ambrx’s board of directors and cofounders of the company and own company stock. A.R.H. is an advisor and consultant to Ambrx and Merck Serono and owns options for Ambrx. T.D., D.C.L., and B.E.K. are former employees of Ambrx and own stock in the company.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100387108/-/DCSupplemental.
References
- 1.Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science. 2001;292:498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 2.Haffner D, et al. Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. New Engl J Med. 2000;343:923–930. doi: 10.1056/NEJM200009283431304. [DOI] [PubMed] [Google Scholar]
- 3.Leschek EW, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140–3148. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld RG, et al. Growth hormone therapy of Turner’s syndrome: Beneficial effect on adult height. J Pediatr. 1998;132:319–324. doi: 10.1016/s0022-3476(98)70452-4. [DOI] [PubMed] [Google Scholar]
- 5.Clark R, et al. Long-acting growth hormones produced by conjugation with polyethylene glycol. J Biol Chem. 1996;271:21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman AR, et al. Efficacy of a long-acting growth hormone (GH) preparation in patients with adult GH deficiency. J Clin Endocrinol Metab. 2005;90:6431–6440. doi: 10.1210/jc.2005-0928. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham BC, Wells JA. Comparison of a structural and a functional epitope. J Mol Biol. 1993;234:554–563. doi: 10.1006/jmbi.1993.1611. [DOI] [PubMed] [Google Scholar]
- 9.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 10.Brustad EM, et al. A general and efficient method for the site-specific labeling of proteins for single molecule fluorescence resonance energy transfer. J Am Chem Soc. 2008;130:17664–17665. doi: 10.1021/ja807430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleissner MR, et al. Site-directed spin labeling of a genetically encoded unnatural amino acid. Proc Natl Acad Sci USA. 2009;106:21637–21642. doi: 10.1073/pnas.0912009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhang Z, Brock A, Schultz PG. Addition of the keto functional group to the genetic code of Escherichia coli. Proc Natl Acad Sci USA. 2003;100:56–61. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuh G, et al. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992;256:1677–1680. doi: 10.1126/science.256.5064.1677. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman AR, et al. Efficacy and tolerability of an individualized dosing regimen for adult growth hormone replacement therapy in comparison with fixed body weight-based dosing. J Clin Endocrinol Metab. 2004;89:3224–3233. doi: 10.1210/jc.2003-032082. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee A, Monson JP, Jonsson PJ, Trainer PJ, Shalet SM. Seeking the optimal target range for insulin-like growth factor I during the treatment of adult growth hormone disorders. J Clin Endocrinol Metab. 2003;88:5865–5870. doi: 10.1210/jc.2002-021741. [DOI] [PubMed] [Google Scholar]
- 16.DeFrees S, et al. GlycoPEGylation of recombinant therapeutic proteins produced in Escherichia coli. Glycobiology. 2006;16:833–843. doi: 10.1093/glycob/cwl004. [DOI] [PubMed] [Google Scholar]
- 17.Cox GN, et al. A long-acting, mono-PEGylated human growth hormone analog is a potent stimulator of weight gain and bone growth in hypophysectomized rats. Endocrinology. 2007;148:1590–1597. doi: 10.1210/en.2006-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen MH, et al. Pegylated long-acting human growth hormone is well-tolerated in healthy subjects and possesses a potential once-weekly pharmacokinetic and pharmacodynamic treatment profile. J Clin Endocrinol Metab. 2010;95:3411–7. doi: 10.1210/jc.2009-2813. [DOI] [PubMed] [Google Scholar]
- 19.Foser SJ, et al. Isolation, structural characterization, and antiviral activity of positional isomers of monpegylated interferon α-2a (PEGASYS) Prot Expr Purif. 2003;30:78–87. doi: 10.1016/s1046-5928(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 20.Kinstler O, et al. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2002;54:477–485. doi: 10.1016/s0169-409x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 21.Holash J, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi H, et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 1999;59:422–430. [PubMed] [Google Scholar]
- 23.Chin JW, et al. An expanded eukaryotic genetic code. Science. 2003;301:964–7. doi: 10.1126/science.1084772. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto K, et al. Site-specific incorporation of an unnatural amino acid into proteins in mammalian cells. Nucleic Acids Res. 2002;30:4692–9. doi: 10.1093/nar/gkf589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.