Abstract
Growing hypoxic and anoxic areas in coastal environments reduce fish habitat, but the interactions and impact on fish in these areas are poorly understood. Using “natural tag” properties of otoliths, we found significant correlations between the extent of Baltic Sea hypoxia and Mn/Ca ratios in regions of cod (Gadus morhua) otoliths corresponding to year 1 of life; this is associated with elevated bottom water dissolved manganese that increases with hypoxia. Elevated Mn/Ca ratios were also found in other years of life but with less frequency. We propose that cod exhibiting enhanced Mn/Ca ratios were exposed to dissolved manganese from hypoxia-induced redox dynamics in nursery areas. Neolithic (4500 B.P.) cod otoliths (n = 12) had low levels of Mn/Ca ratios, consistent with low hypoxia, but a single otolith dated to the younger Iron Age had a distinct growth band with an elevated Mn/Ca ratio. Sr/Ca patterns reflecting changes in environmental salinity and temperature were similar in both modern and Stone Age otoliths, indicating consistent migration habits across time, and Ba/Sr ratios in modern cod otoliths indicate increasing use of a more saline habitat with age. Using elemental ratios, numerous existing archival collections of otoliths could provide the means to reconstruct hypoxia exposure histories and major patterns of fish movement near “dead zones” globally.
Keywords: otolith chemistry, life-history tracers
The semi-enclosed Baltic Sea has experienced hypoxia periodically since the transition from the freshwater Ancylus Lake to the Littorina Sea ∼8,000 y ago (1). Hypoxia, defined as oxygen depleted to <2 mL·l−1, is deleterious to fish and invertebrate life (2); the Baltic Sea has the largest anthropogenically caused “dead zone” in the world (2, 3), with areal extents ranging from 25 to 67,000 km2 in 1969–2008 and a mean of 49,000 km2 (4). Although hypoxia arises naturally in the Baltic because of particular salinity and temperature conditions, its intensity has been exacerbated over the past half century by nutrient loading from the watershed (3). Among the negative effects has been a severe reduction in the available volume of water suitable for spawning of cod, a demersal species. Baltic cod require salinities of 11 practical salinity units (PSU; found only in deeper Baltic waters) and oxygen >2 mL·l−1 for successful fertilization and egg development (5). Together with overfishing and resulting shifts in trophic dominance (6), hypoxic reduction of spawning volume has greatly reduced Baltic cod stocks.
Recent efforts to rebuild Baltic cod stocks have included detailed studies of cod movement using models and data storage tags (DSTs) (7, 8) to identify critical habitat needs better, with an eye toward developing marine protected areas (MPAs). In fact, DSTs placed on large (>45 cm) cod revealed that some cod visited hypoxic areas, presumably to feed on zoobenthos (9).
Although DSTs have greatly enhanced our understanding of cod movements, they have the disadvantage of high cost and are not deployable on small fish, such that detailed data on early life stages cannot be obtained. Teleost fishes carry with them natural tags in the form of otoliths (ear-stones), however, which form part of their hearing and balance system. Otoliths, composed of aragonite (CaCO3) precipitated on a protein matrix, grow incrementally and provide an unsurpassed record of age and growth of fishes (10) as well as a suite of trace elemental and isotopic chemistries associated with specific growth bands. If assumptions are met (11), these chemistries can provide lifetime environmental histories of individual fishes.
We report here on studies of trace elements in Baltic cod otoliths (Materials and Methods and SI Appendix) that show striking patterns of variation, particularly when displayed as elemental maps (Fig. 1). Such maps are produced via scanning X-ray fluorescence microscopy (SXFM) using high-energy X-rays as the excitation source (12). The trace elements strontium, manganese, and barium are well known to vary in otoliths in relation to calcium (10), but the mechanisms of uptake and incorporation have been debated. Most trace elements are thought to be taken up from water via the gills, introduced into the endolymph fluid surrounding the otolith from the blood, and finally incorporated via enzymatically controlled precipitation into the otolith (13). Otolith uptake of Sr and Ba is well documented as being affected by temperature and salinity (13–15), but such is not the case with Mn (14). Dissolved manganese, which can be Mn2+ or Mn3+ (16), is often associated with hypoxic or anoxic waters and sediments, including in the Baltic Sea (17). Unlike iron, which oxidizes rapidly when in contact with oxygen and precipitates, manganese may remain dissolved for periods of days (18). Thus, dissolved manganese that fluxes out of hypoxic or anoxic sediments may become available to be taken up by benthic fish, such as juvenile cod.
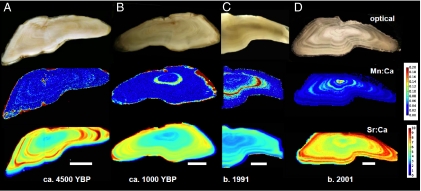
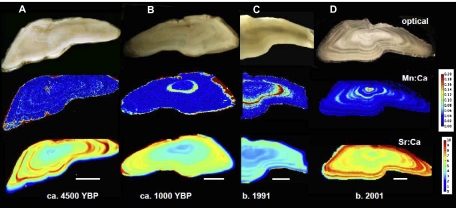
Fig. 1.
Transverse sections of Baltic Sea cod otoliths from four different time periods: the Neolithic (A), late Iron Age/Middle Age (B), early 1990s when the areal extent of hypoxia was low (C), and 2001 when it was high (D). Regions in the core correspond to the juvenile stage. Note that Mn is high in the Neolithic and Iron Age otoliths in small cracks as well as along the edge, where it was in contact with soil pore water. The 1991 otolith is portrayed in a partial map. (Scale bar: 1 mm.)
Results
We selected cod otoliths from the archival collection at the Institute for Marine Research Karlskrona Laboratory, Swedish Board of Fisheries, to represent fish born in three periods with different hypoxia intensity: the 1980s, early 1990s, and late 1990s through early 2000s. Of these time periods, the early 1990s had the least hypoxia and the last period had the most severe (3). The cod originated in Eastern Baltic Sea Fishing Subdivision 25 (SI Appendix, Fig. S1), the most important fishing area and one that contains all life stages of cod. Additionally, we drew from an unusual collection of Baltic cod otoliths from the Neolithic period circa 4500 B.P. (19). These last were from a settlement on the southwestern side of the island of Gotland in Sweden, and although diagenesis had affected some elements, the calcium and strontium appeared intact, as did much of the manganese (Fig. 1A). We also recently obtained and analyzed two cod otoliths from a site dated to the younger Iron Age (circa 700–1000 B.P.), located in southern Gotland. We hypothesized that the Neolithic otoliths would provide a baseline, because the data presented by Zillén et al. (1) indicated no hypoxia in the area where the fish lived during that part of the Neolithic. Conversely, we hypothesized that evidence of hypoxia might be observed in the Iron Age otoliths, because this period coincides with a time of intermittent hypoxia (1). For one otolith, this was the case (Fig. 1B).
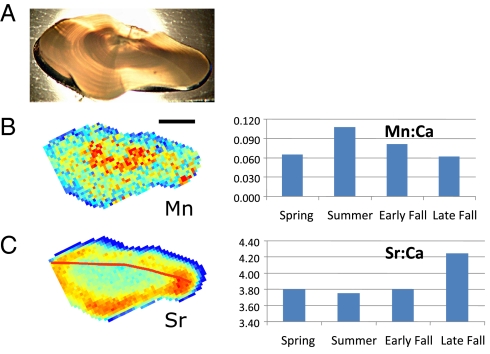
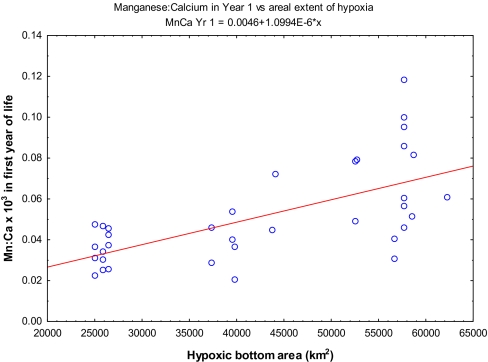
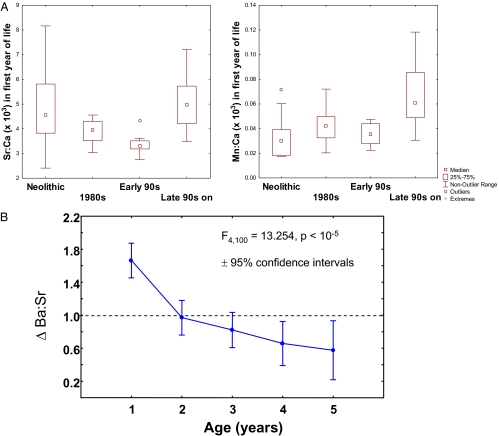
In modern cod otoliths, we found that Mn (as a ratio of Ca) often was highest in the first year of the otolith record of individual cod (Fig. 1D) but, in some cases, was very high in the second or even third year (Fig. 1C and SI Appendix, Fig. S2). The patterns appear to be seasonal, and careful examination of daily increments in a subyearling fish otolith showed that elevated Mn developed during summer and declined in the fall, whereas strontium was low in spring and summer but increased in late fall (Fig. 2). Otolith Mn/Ca ratios in the zone corresponding to the first year of life increased with increasing hypoxic area in the Baltic (R = 0.65, P < 0.001; Fig. 3), although the relationship is noisy. Neolithic cod had comparable first-year Mn/Ca ratios to the fish born before 1998, but those born after 1998 had significantly higher Mn/Ca ratios than the other groups [Tukey's honestly significant difference (HSD) test, P < 0.013; Fig. 4A].
Fig. 2.
Otolith of a young-of-the-year Baltic cod, with a length of 100 mm, captured November 17, 2008, in a special trawl survey. (Scale bar = 1 mm.) (A) Photograph of transverse section through the otolith. (B) Manganese elemental map (Left) and Mn/Ca ratios (×103, Right) parsed out by season. (C) Strontium elemental map (Left) and Sr/Ca ratios (×103, Right) parsed out by season. The red line represents transects of data that were extracted by ArcGIS for preparing the graphs. Examination of daily increments (not visible) indicated that the fish was born in May.
Fig. 3.
Otolith Mn/Ca ratio (×103) vs. hypoxic area. Data on hypoxic areal extent were obtained from O. P. Savchuk (Baltic Nest Institute, Stockholm Resilience Centre, Stockholm, Sweden).
Fig. 4.
(A) Box plots of Mn/Ca and Sr/Ca for each time period studied. (B) General trend in predominance of Ba vs. Sr (Δ Ba/Sr), defined as the mean annual Ba/Sr ratio of an individual observation divided by the average Ba/Sr ratio of all individuals combined.
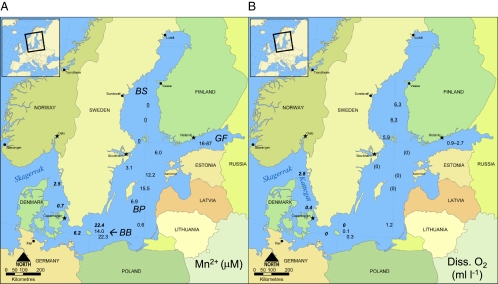
That dissolved manganese follows hypoxia can be seen in Fig. 5. A survey of bottom waters during a period when large areas of the Baltic proper were anoxic (2007–2009) shows persistent Mn, albeit with variability. In contrast, the well-oxygenated Bothnian Sea in the northern part of the basin shows negligible to no dissolved Mn. Temporal variability is well documented (3, 17, 20) because of alternating ventilation and stagnation periods. The actual concentration of bottom water Mn during periods of hypoxia will depend on the duration of hypoxia, the previous buildup of Mn-oxides in the sediment during more oxic periods, the possible precipitation as Mn-carbonates in the sediment, and lateral water exchange at a given site. The result is heterogeneous dissolved Mn in the bottom water from site to site, and this may contribute to the variability observed in Fig. 3. We also note that in recent years, virtually all cod spawning areas have been exposed to hypoxia; thus, complete avoidance of these waters by young cod is unlikely.
Fig. 5.
Dissolved Mn (A, μM) and oxygen (B, mL·l−1) in bottom waters at 17 sites in the Baltic Sea as determined in August/September 2007 (bold, italic), September/October 2008 (underlined), or May/June 2009. Numbers between brackets indicate sulfidic bottom waters. For 2 sites, one in the Kattegat and the other in the Baltic Proper, no oxygen data are available for the time of sampling. The results highlight that low oxygen concentrations and elevated Mn in the bottom waters generally go hand in hand in the Baltic Sea. In areas where bottom waters are well-oxygenated, such as the Bothnian Sea, no dissolved Mn was observed. Insets depict Europe and the Baltic region. BB, Bornholm Basin, the primary area of cod spawning; BP, Baltic Proper; BS, Bothnian Sea; Diss., dissolved; GF, Gulf of Finland.
In contrast, cod otolith Sr/Ca ratios showed similar patterns across all time periods, typically with lowest levels occurring in the first year of life, increasing for several years, and declining slightly thereafter (SI Appendix, Fig. S3). As in Mn/Ca ratios, seasonal variations are apparent (Fig. 2), and in early years, elevated Sr/Ca ratios corresponded to low periods of Mn/Ca ratios. First-year Sr/Ca ratios were lowest in the fish born in the early 1990s (Tukey's HSD test, P < 0.005) and were most elevated in the Neolithic period and in the period from the late 1990s through the early 2000s (Fig. 4A). Sr/Ca ratios in cod are known to increase with declining temperature (21), but there also appears to be a correlation with salinity in our data, because the “low hypoxia early 1990s” was somewhat less saline than in adjacent time periods (3). Because aqueous Sr/Ca ratios vary with salinity in the Baltic (22), we suggest that cod otolith Sr/Ca ratios vary as a function of both salinity and water temperature, but further research is needed to distinguish their relative effects. Given that deeper, saltier water in the Baltic is also colder, however, we infer that elevated Sr/Ca ratios indicate exposure to colder, saltier water.
We observed two common patterns of Ba/Ca ratios in the modern cod otoliths. In early life, Ba/Ca ratios were elevated relative to Sr/Ca ratios; however, later on in life, Ba/Ca ratios often tracked Sr/Ca ratios (SI Appendix, Fig. S4). To capture this statistically, we computed the overall Ba/Sr ratio of all the otolith transects and then examined the mean annual Ba/Sr ratio as a ratio of the overall ratio (Δ Ba/Sr) for years 1–5 across all modern otoliths. Values of Δ Ba/Sr were highest in the first year and declined asymptotically thereafter but also became more variable (Fig. 4B).
Discussion
Cod are born in relatively deep, saline water in the Baltic Sea and are subsequently advected to nursery areas. Little is reported about use of specific juvenile habitats. Nevertheless, cod are known from other geographical regions to use inshore nursery areas as juveniles to feed, grow, and avoid predation (23, 24). In the Baltic drainage, Ba/Sr ratios are elevated in soils (∼3.24) (25) but are comparatively very low in sea water [∼0.01, calculated from published data (22, 26)]. We interpret the elevated Δ Ba/Sr in the first-year portion of Baltic cod otoliths as evidence of use of nursery grounds with exposure to lower salinity, consistent with the influence of riverine inputs. This is also consistent with the Sr/Ca ratios. At times, these grounds may be subject to low-oxygen conditions, particularly in summer, allowing Mn2+ (or possibly Mn3+) to diffuse out of the sediments into the water column (18, 27). As cod mature, they undertake directed migrations offshore to deeper, colder waters, but we have also observed evidence of fish spending later years in low-salinity environments, possibly northward in the Bothnian Gulf. We do not know why the Mn/Ca ratio often declines with increasing age (Fig. 1D) but hypothesize that it could be a growth effect (a proposed model is presented in SI Appendix) or an averaging effect of the fixed-size analytical beam traveling over increasingly tighter growth increments.
In this limited study, Baltic cod otoliths appear to track hypoxia intensity through their Mn/Ca ratios, watershed influence through Δ Ba/Sr, and correlated salinity and temperature through Sr/Ca ratios. We did not emphasize here within-year dynamics, but each individual otolith bears a detailed history of its environment that can be interpreted through trace elemental ratios. These could be combined with other proven tracers, such as strontium isotopes, for more precise identification of source waters (28) or with DSTs in larger fish for direct confirmation of location. Furthermore, we have observed periods of elevated Mn/Ca ratios in cod otoliths from other areas (North Sea and Norwegian poll fjord) as well as in those of other species (Salmonidae, Clupeidae) in the Baltic and in Oneida Lake, a system known to have elevated Mn and periods of hypoxia. Elevated Mn/Ca ratios in sciaenid fishes in the Pamlico Sound and the Chesapeake Bay were also attributed to redox dynamics (29, 30). Given the numerous and large archival collections of cod otoliths, there is potential to gain much better understanding of cod's interaction with hypoxia, particularly in early life, and to aid in designing MPAs that protect all life stages, including juveniles. Finally, this approach offers the potential to examine more closely the relationship of many other fish species (and fisheries) to hypoxia (31), which is much needed in light of the growing instances of dead zones worldwide (32).
Materials and Methods
Otoliths.
Modern otoliths were obtained from a national archival collection maintained by the Institute for Marine Research Karlskrona Laboratory, Swedish Board of Fisheries. These were originally collected within International Council for the Exploration of the Seas (ICES) Eastern Baltic Fisheries Subdivision 25, a region where all life stages of cod occur and which is the most important fishing area in the Baltic Proper (SI Appendix, Fig. S1).
Otoliths from the middle Neolithic period, circa 3400 to 2300 B.C. (in Sweden) were obtained from the late Stone Age site Ajvide (57° 16’ North, 18° 7’ East) situated on the southwest coast of Gotland (SI Appendix, Fig. S1), a large island in the center of the Baltic Sea (19). At the time of occupancy, the settlement was located on the shore of a wide shallow bay directly connected to the open sea. The site is currently 1 km from the shore and 12 m above sea level (asl). The site area of Ajvide comprises 200,000 m2, of which partial areas have been continuously excavated since 1983 (33). The site has been subdivided into three areas: area C, area D-upper, and area D-lower, where C represents the oldest phase of the settlement and D-lower represents the youngest phase of the settlement. Hitherto, 2,200 kg of faunal remains have been unearthed from these areas. The rich cultural layers at Ajvide contain a variety of mammal, bird, and fish species, but bones of harp seal, herring, and cod dominate the faunal assemblage (34, 35). Large amounts of skeletal elements of cod, including a considerable number of otoliths, have been recovered from area D-upper (2900 to 2700 B.C.). Well-preserved otoliths from the D-upper area were selected for this study.
Finally, two well-preserved otoliths were obtained from a site (Sundre in southern Gotland, 56° 54.5’ North, 18° 7.7’ East) dated to the late Iron Age/Middle Age (circa 700–1000 B.P.). The site is an old fishing village with numerous cultural relicts, mammal bones, and a few fish remains (36).
All otoliths were embedded and sectioned transversely, and they were subsequently ground down to expose the core; polished to 1–3 μm; cleaned by ultrasonication; and mounted on clean, fused quartz disks.
Elemental Analysis.
Otoliths were analyzed using two different methods. The first was SXFM at the Cornell High Energy Synchrotron Source, which was conducted on the F3 bending magnet beam line. This work permitted us to develop high-resolution elemental maps (Fig. 1). A double-bounce multilayer monochromator with 0.6% bandpass was used to produce a 16.1-keV X-ray beam, which was focused with a single-bounce glass capillary optic (37, 38) to produce a photon flux of circa 1011 counts per second in a spot ca. 20–30 μm in diameter at the sample. Scans were made initially at 10 and 17.5 keV to augment lower- and higher-energy fluorescence, respectively; however, we later found that 16.1 keV produced a good compromise. The beam was focused to a spot on the specimen, and the fluorescence spectrum was integrated for a fixed amount of time (typically 1–10 s); the beam was then moved to an adjacent spot, and the process was repeated until the entire surface of the sample had been mapped. The fluorescent X-rays were collected using an energy-dispersive Vortex silicon drift detector (Sii NanoTechnology USA Inc.). An aluminum foil attenuator was placed over the detector to reduce the overwhelming intensity attributable to calcium fluorescence so as to increase sensitivity to elements present in trace amounts. Calibrations were carried out using a variety of certified reference materials, including National Institute of Standards and Technology Standard Reference Materials 8704 (Buffalo River sediment) and 1572 (citrus leaves) as well as the MACS-3 carbonate standard developed at the US Geological Survey Reference Materials Project (39). We also developed our own otolith standard by pulverizing 54 large otoliths from freshwater drum (Aplodinotus grunniens) and pressing a pellet; concentrations of major, minor, and trace elements in this pulverized material were analyzed three separate times in solution with a Perkin–Elmer Elan DRC-e inductively coupled plasma mass spectrometer (ICPMS) at the State University of New York College of Environmental Science and Forestry. Spectra were analyzed to produce maps of elemental mass fractions using routines provided by the PyMCA library (40). Detection limits depend on element, integration time, and how images are binned but were generally ca. 0.1–1 ppm.
The element maps enabled us to determine places in the otolith sections that would produce uninformative results. For example, we discovered that manganese is poorly acquired in material directly below the sulcus but otherwise showed uniform patterns of uptake within annular rings. In the case of the Neolithic otoliths, we could readily determine whether diagenetic effects were present and whether or not data could be extracted to avoid them (e.g., in small cracks where elements had entered from the soil).
Given the spatial nature of the data, we imported the data files into a geographical information system (41), drew transects from the otolith core to the outer edge, and extracted the elemental data. Because otoliths grow incrementally throughout a fish's lifetime, these transects are analogous to time series.
Elemental map collection was augmented by collection of transect data using the ICPMS connected to a NewWave UP-193–nm solid-state laser (Electro Scientific Industries, Inc.). Operating parameters for the laser were 50% power (circa 1 GW/cm−2, pulse duration <3 ns), 10 Hz, 100-μm spot size, and 3 μm·s−1 travel time. NIST-612, MACS-3, and the otolith pellet were used as standards. Precision was 10% or better, and limits of detection were sub-parts per million for Ba, Ca, and Sr and ∼1 ppm for Mn. One-minute washouts were performed between samples to remove residues. Drift corrections were performed as necessary. Cross-validations and calibrations were performed among standards to correct for matrix effects. Cross-validations and calibrations were also performed among samples analyzed with SXFM to ensure comparability between the two methods.
Baltic Bottom Water Chemistry.
Seventeen sites in the Baltic Sea, covering a range of water depths, were visited during cruises in August/September 2007 and September/October 2008 with Research Vessel (RV) Skagerak and during a cruise with RV Aranda in May/June 2009. Sediment cores (30–45 cm of sediment and at least 10 cm of overlying water) were recovered using a multicorer. Bottom water samples were taken from the overlying water in a multicore at each site using a 20-mL plastic syringe. The syringes were immediately closed with a small three-way tap and were transferred to a glove box under nitrogen. Here, the bottom water was filtered (0.45-μm pore size) and collected in a 15-mL plastic tube (Greiner) or Nalgene bottle. Subsamples were taken for analysis of dissolved manganese (acidified with 10 μL of 37% HCl per milliliter of subsample) in 15-mL plastic tubes and stored at 5 °C. Dissolved manganese (assumed to be present as Mn2+ and possibly as Mn3+) (16, 42) in the bottom water was measured on a Perkin–Elmer Optima 3000 Inductively Coupled Plasma-Optical Emission Spectrometer. In 2007 and 2008, oxygen concentrations in the bottom water of the multicores were measured using Unisense microelectrodes combined with Winkler titrations (Unisense Science). Details on the sediment biogeochemistry of the sites sampled in 2007 are given by Mort et al. (43). In 2009, bottom water samples were obtained with a plastic Nansen water sampler (Hydrobios) at 1 m above the sediment-water interface and analyzed for oxygen using Winkler titrations and for sulfide using spectrophotometry (44). At the sites of this 2009 cruise, the bottom water was always low in oxygen or even sulfidic. Given that Baltic Sea bottom waters typically become more reducing with water depth (3, 19, 42), concentrations of oxygen at the actual sediment-water interface at the 2009 sites are expected to have been similar or lower.
Supplementary Material
Acknowledgments
We thank S. Baden, D. Bilderback, T. Hayden, C. Humborg, M. Pace, D. Swaney, and B. Walther for discussions and comments on earlier drafts, O. Savchuk for hypoxia data, and D. Driscoll for help with ICPMS analysis. This work is based on research conducted at the Cornell High Energy Synchrotron Source, which is supported by the National Science Foundation and the National Institutes of Health/National Institute of General Medical Sciences under National Science Foundation Award DMR-0225180. Partial support was from National Science Foundation Grant DEB-0238121, the Netherlands Organization for Scientific Research (NWO-Vidi-grant), the European Union-Bonus project HYPoxia mitigation for Baltic Sea Ecosystem Restoration (HYPER), and the Baltic Sea 2020.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 8931.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100684108/-/DCSupplemental.
References
- 1.Zillén L, Conley DJ, Andrén T, Andrén E, Björck S. Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth Sci Rev. 2008;91:77–92. [Google Scholar]
- 2.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 3.Conley DJ, et al. Hypoxia-related processes in the Baltic Sea. Environ Sci Technol. 2009;43:3412–3420. doi: 10.1021/es802762a. [DOI] [PubMed] [Google Scholar]
- 4.Savchuk OP. In: Chemical Structure of Pelagic Redox Interfaces: Observation and Modeling (Hdb Env Chem) Yakushev EV, editor. Berlin: Springer; 2010. [Google Scholar]
- 5.Vallin L, Nissling A, Westin L. Potential factors influencing reproductive success of Baltic cod, Gadus morhua: A review. Ambio. 1999;28:92–99. [Google Scholar]
- 6.Casini M, et al. Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc Natl Acad Sci USA. 2009;106:197–202. doi: 10.1073/pnas.0806649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuenfeldt S, Hinrichsen H-H, Nielsen A, Andersen KH. Reconstructing migrations of individual cod (Gadus morhua L.) in the Baltic Sea by using electronic data storage tags. Fish Oceanogr. 2007;16:526–535. [Google Scholar]
- 8.Hinrichsen H-H, Kraus G, Böttscher U, Köster F. Identifying eastern Baltic cod nursery grounds using hydrodynamic modelling: Knowledge for the design of marine protected areas. ICES J Mar Sci. 2009;66:101–108. [Google Scholar]
- 9.Neuenfeldt S, Andersen KH, Hinrichsen H-H. Some Atlantic cod Gadus morhua in the Baltic Sea visit hypoxic water briefly but often. J Fish Biol. 2009;75:290–294. doi: 10.1111/j.1095-8649.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- 10.Campana SE, Thorrold SR. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci. 2001;58:30–38. [Google Scholar]
- 11.Elsdon TS, et al. Otolith chemistry to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations, and inferences. Oceanogr Mar Biol Ann Rev. 2008;46:297–330. [Google Scholar]
- 12.Limburg KE, Huang R, Bilderback DH. Fish otolith trace element maps: New approaches with synchrotron microbeam X-ray fluorescence. Xray Spectrom. 2007;36:336–342. [Google Scholar]
- 13.Campana SE. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar Ecol Prog Ser. 1999;188:263–297. [Google Scholar]
- 14.Martin GB, Wuenschel MJ. Effect of temperature and salinity on otolith element incorporation in juvenile gray snapper Lutjanus griseus. Mar Ecol Prog Ser. 2006;324:229–239. [Google Scholar]
- 15.McCulloch M, Cappo M, Aumend J, Müller W. Tracing the life history of individual barramundi using laser ablation MC-ICP-MS Sr-isotopic and Sr/Ba ratios in otoliths. Mar Freshw Res. 2005;56:637–644. [Google Scholar]
- 16.Trouwborst RE, Clement BG, Tebo BM, Glazer BT, Luther GW., 3rd Soluble Mn(III) in suboxic zones. Science. 2006;313:1955–1957. doi: 10.1126/science.1132876. [DOI] [PubMed] [Google Scholar]
- 17.Neretin LN, Pohl C, Jost G, Leipe T, Pollehne F. Manganese cycling in the Gotland Deep, Baltic Sea. Mar Chem. 2003;82:125–143. [Google Scholar]
- 18.Pakhomova SV, et al. Fluxes of iron and manganese across the sediment-water interface under various redox conditions. Mar Chem. 2007;107:319–331. [Google Scholar]
- 19.Olson C. Neolithic Fisheries: Osteoarchaeology of Fish Remains in the Baltic Sea Region. Theses and Papers in Osteoarchaeology 5. Stockholm: Stockholm University; 2008. [Google Scholar]
- 20.Turnewitsch R, Pohl C. An estimate of the efficiency of the iron- and manganese-driven dissolved inorganic phosphorus trap at an oxic/euxinic water-column redoxcline. Global Biogeochem Cycles. 2010;24:GB4025. [Google Scholar]
- 21.Townsend DW, Radtke RL, Malone DP, Wallinga JP. Use of strontium:calcium ratios for hind-casting larval cod Gadus morhua distributions relative to water masses on Georges Bank. Mar Ecol Prog Ser. 1995;119:37–44. [Google Scholar]
- 22.Andersson PS, Wasserburg GJ, Ingri J. The sources and transport of Sr and Nd isotopes in the Baltic Sea. Earth Sci Planet Lett. 1992;113:459–472. [Google Scholar]
- 23.Borg Å, Pihl L, Wennhage H. Habitat choice by juvenile cod (Gadus morhua) on sandy soft bottoms with different vegetation types. Helgol Meersunters. 1997;51:197–212. [Google Scholar]
- 24.Fahay MP, Berrien PL, Johnson DL, Morse WW. Woods Hole: Northeast Fisheries Science Center; 1999. Atlantic Cod, Gadus morhua, Life History and Habitat Characteristics. National Oceanic and Atmospheric Administration Technical Memorandum NMFS-NE-124. [Google Scholar]
- 25.Reimann C, et al. Baltic soil survey: Total concentrations of major and selected trace elements in arable soils from 10 countries around the Baltic Sea. Sci Total Environ. 2000;257:155–170. doi: 10.1016/s0048-9697(00)00515-5. [DOI] [PubMed] [Google Scholar]
- 26.Brügmann L, Bernard PC, van Grieken R. Geochemistry of suspended matter from the Baltic Sea. 2. Results of bulk trace metal analysis by AAS. Mar Chem. 1992;38:303–323. [Google Scholar]
- 27.Slomp CP, Malschaert JFP, Lohse L, van Raaphorst W. Iron and manganese cycling in different sedimentary environments on the North Sea continental margin. Cont Shelf Res. 1997;9:1083–1117. [Google Scholar]
- 28.Kennedy BP, Folt CL, Blum JD, Chamberlain CP. Natural isotope markers in salmon. Nature. 1997;387:766–767. [Google Scholar]
- 29.Thorrold SR, Shuttleworth S. In situ analysis of trace elements and isotope ratios in fish otoliths using laser ablation sector field inductively coupled plasma mass spectrometry. Can J Fish Aquat Sci. 2000;57:1232–1242. [Google Scholar]
- 30.Dorval E, Jones CM, Hannigan R, van Montfrans J. Relating otolith chemistry to surface water chemistry in a coastal plain estuary. Can J Fish Aquat Sci. 2007;64:411–424. [Google Scholar]
- 31.Utne-Palm AC, et al. Trophic structure and community stability in an overfished ecosystem. Science. 2010;329:333–336. doi: 10.1126/science.1190708. [DOI] [PubMed] [Google Scholar]
- 32.Breitburg DL, Hondorp DW, Davias LA, Diaz RJ. Hypoxia, nitrogen, and fisheries: Integrating effects across local and global landscapes. Ann Rev Mar Sci. 2009;1:329–349. doi: 10.1146/annurev.marine.010908.163754. [DOI] [PubMed] [Google Scholar]
- 33.Burenhult G. Remote Sensing II. Thesis and Papers in North European Archaeology 13:b. Stockholm: Stockholm University; 2001. Applied techniques for the study of cultural resources and the localization, identification and documentation of sub-surface prehistoric remains in Swedish archaeology; pp. 7–16. [Google Scholar]
- 34.Storå J, Ericson PGP. Faunal history and human exploitation of the Harp Seal (Phoca Groenlandica) in the Baltic Sea during the Subboreal Stone Age. Mar Mamm Sci. 2004;20:115–133. [Google Scholar]
- 35.Olson C, Walther Y. Neolithic cod (Gadus morhua) and herring (Clupea harengus) fisheries in the Baltic Sea—In the light of fine-mesh sieving: A comparative study of subfossil fishbone from the late Stone Age sites at Ajvide, Gotland, Sweden and Jettböle, Åland, Finland. Env Archaeol. 2007;12:175–185. [Google Scholar]
- 36.Carlsson D. Hamnar och fiskelägen i Sundre under vikingatid—medeltid (Harbors and fishing camps in Sundre during the Viking and Medieval periods) ArkeoDok Rapport. 2008;2008:1–36. (in Swedish) [Google Scholar]
- 37.Bilderback DH, et al. Monocapillary optics developments and applications. Adv X-ray Anal. 2003;46:320–325. [Google Scholar]
- 38.Cornaby SW. Ithaca, NY: Cornell University; 2008. The Handbook of X-Ray Single-Bounce Monocapillary Optics, Including Optical Design and Synchrotron Applications. PhD thesis. [Google Scholar]
- 39.Wolf RE, Wilson SA. US Geological Survey Reference Materials Program: US Geological Survey Fact Sheet 2007-3056. Denver, CO: US Geological Survey; 2007. [Google Scholar]
- 40.Solé VA, Papillon E, Cotte M, Walter PH, Susini J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta. B At Spectrosc. 2007;6:263–268. [Google Scholar]
- 41.ESRI . ArcGIS Version 9.3. Redlands, CA: ESRI; 2009. [Google Scholar]
- 42.Yakushev E, Pakhomova S, Sorenson K, Skei J. Importance of the different manganese species in the formation of water column redox zones: Observations and modeling. Mar Chem. 2009;117:59–70. [Google Scholar]
- 43.Mort HP, Slomp CP, Gustafsson BG, Andersen TJ. Phosphorus recycling and burial in Baltic Sea sediments with contrasting redox conditions. Geochim Cosmochim Acta. 2010;74:1350–1362. [Google Scholar]
- 44.Fonselius SH. In: Determination of Hydrogen Sulphide. Methods of Seawater Analysis. Grasshoff K, Ehrhardt M, Kremling K, editors. Weinheim, Germany: Verlag Chemie; 1983. pp. 73–80. [Google Scholar]