Abstract
Most processing of sensation involves the cortical hemisphere opposite (contralateral) to the stimulated limb. Stroke patients can exhibit changes in the interhemispheric balance of sensory signal processing. It is unclear whether these changes are the result of poststroke rewiring and experience, or whether they could result from the immediate effect of circuit loss. We evaluated the effect of mini-strokes over short timescales (<2 h) where cortical rewiring is unlikely by monitoring sensory-evoked activity throughout much of both cortical hemispheres using voltage-sensitive dye imaging. Blockade of a single pial arteriole within the C57BL6J mouse forelimb somatosensory cortex reduced the response evoked by stimulation of the limb contralateral to the stroke. However, after stroke, the ipsilateral (uncrossed) forelimb response within the unaffected hemisphere was spared and became independent of the contralateral forelimb cortex. Within the unaffected hemisphere, mini-strokes in the opposite hemisphere significantly enhanced sensory responses produced by stimulation of either contralateral or ipsilateral pathways within 30–50 min of stroke onset. Stroke-induced enhancement of responses within the spared hemisphere was not reproduced by inhibition of either cortex or thalamus using pharmacological agents in nonischemic animals. I/LnJ acallosal mice showed similar rapid interhemispheric redistribution of sensory processing after stroke, suggesting that subcortical connections and not transcallosal projections were mediating the novel activation patterns. Thalamic inactivation before stroke prevented the bilateral rearrangement of sensory responses. These findings suggest that acute stroke, and not merely loss of activity, activates unique pathways that can rapidly redistribute function within the spared cortical hemisphere.
Keywords: ischemia, plasticity, in vivo imaging, recovery of cortical function, thalamocortical circuit
During stroke, neurons that are deprived of their normal substrates can show signs of structural damage after as little as 2 min of ischemia (1, 2). Over time, surviving brain tissue is thought to compensate for regions lost to stroke (1, 3–5). It is generally assumed that recovery is a process that occurs over weeks and involves both the formation of new structural circuits and the alternative use of spared circuits (6). Recovery after a small stroke may involve spared peri-infarct tissue with function similar to the infarct (1, 7). In contrast, after a large stroke, tissue with similar function may only be found at more distant sites, such as the premotor cortex (for motor cortex stroke) (8, 9) or regions within the unaffected contralateral hemisphere (10) where structural remodeling can be observed (11). Although the canonical view of sensory and motor processing is that body parts are controlled by neurons in the cerebral hemisphere on the opposite side of the body, ipsilateral pathways in which, for example, the right hemisphere processes information from the right side of the body are also present (12, 13) and may provide a means to recovery. Evidence suggests that stroke recovering animals and patients can exhibit widespread changes in activity patterns that can even extend to the unaffected hemisphere (14–16). These altered circuits work within the intact contralesional (opposite to stroke) hemisphere (10), leading to less lateralized (less crossed) activation.
It is currently unclear to what extent these widespread changes in activity reflect new circuits that develop through structural plasticity over weeks (7, 17, 18) or whether they might reflect latent circuits that are uncovered through disinhibition (19) or alterations brought about by propagating ischemic depolarizations that may occur within the first hours after stroke (20). Recently, we have used voltage-sensitive dye imaging to assess how patterns of sensory-evoked synaptic activity change after targeted infarcts to individual arterioles (21). Voltage-sensitive dye (VSD) imaging reveals both subthreshold and suprathreshold activity with high spatiotemporal resolution (22). A limitation of VSD imaging methods is that the signals are from the superficial layers of the cortex. However, one should take into account the fact that the activity in superficial layers could arise from neurons in deep cortical layers because of their dendritic arborization (23). Indeed, pyramidal neurons in layer V have apical dendrites that reach superficial layers and therefore, contribute to the signal (24). In addition, recent studies have shown that, although sensory-evoked activity originates mainly at the granular layer of cortex, the activity distributes rapidly among all cortical lamina (25, 26).
Our previous findings show that rapid changes in sensory processing within the peri-infarct zone can occur within minutes of stroke induction (21). However, whether these changes can extend to the contralateral hemisphere is not known. Here, we extend these results by examining how targeted ischemia affects the regional spread of sensory activation within both hemispheres. Using a large bilateral craniotomy preparation, we show that targeted ischemia to even a single arteriole causes alterations in the patterns of sensory-evoked activity that extend beyond peri-infarct areas into somatotopic regions of the unaffected hemisphere as early as 30 min after stroke onset. These findings strongly suggest that existing sensory pathways are capable of redistributing activity to even the contralateral hemisphere. Because these changes in activity are observed at very early time points and even within the unaffected hemisphere, they may be an unavoidable consequence of acute stroke leading to an unmasking of existing pathways and not the result of poststroke maladaptive behavioral experience.
Results
Bilateral Changes in the Pattern of Sensory-Evoked Activity in Response to Stimulation of the Stroke-Affected Limb.
Fore- and hindlimb stimulation-evoked VSD signals were recorded in both cortical hemispheres before and after targeted ischemia to individual pial arterioles that supplied the primary forelimb sensory representation (Fig. 1 A–D). Successful occlusion was confirmed using laser speckle imaging (Fig. 1 C–F) as described (21). The ischemic area, measured at the cortical surface, averaged 0.46 ± 0.06 mm2 (n = 25 mice) (Fig. S1). A single 5-ms tap stimulus was delivered to either left or right limbs (hind- and forelimbs) with a piezoelectric device. Before focal ischemia, stimulation of the limbs produced stereotypical depolarizing signals within the somatosensory representation of the corresponding limbs (Fig. 2A). VSD signals appeared within 11–15 ms of stimulation in the hemisphere contralateral to stimulation; then, these signals propagated to neighboring regions within the somatosensory (hindlimb and barrel) and later, curved to the midline area (Figs. 2A and 3A and Movie S1). Both fore- and hindlimb stimulation-evoked VSD signals appeared to follow a preferred path involving motor, cingulate, and retrosplenial cortices (Figs. 2A and 3A, Movies S1 and S2, forelimb activation pattern, and Fig. S2A, hindlimb activation pattern). The propagation of sensory-evoked depolarization along these routes was similar to the propagation of spontaneous ongoing activity (27) and may suggest that evoked and ongoing activity can produce spatially similar patterns.
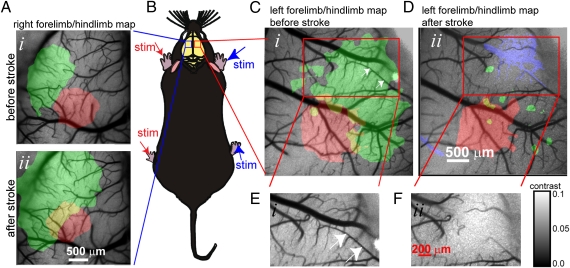
Fig. 1.
Targeted ischemia and bilateral assessment of blood flow and sensory function. Experimental setup showing sensory stimuli delivered to all four limbs of a urethane-anesthetized mouse during voltage-sensitive dye imaging. (A) Pseudocolor maps are shown of forelimb (FL; green) and hindlimb (HL; red) sensory responses in the left (unaffected) somatosensory cortex in response to stimulation of right (unaffected) limbs before (Upper) and 45 min after (Lower) targeted ischemia within opposite hemisphere. Yellow color in A is the overlap of forelimb and hindlimb maps. Note that neither ischemia nor sensory deficits extends to the unaffected hemisphere. (B) Schematic showing C57BL6 mouse after bilateral craniotomy. (C and D) Maps of VSD fluorescence changes within the sensorimotor cortex in response to stimulation of left forelimb (FL; green) and hindlimb (HL; red) before (C) and 30 min after (D) targeted ischemia to select pial arteriole segments (white arrows in C) that supply the forelimb area within the right hemisphere. Loss of blood flow (determined from the difference of speckle signals as shown in D) is overlaid in blue. (E and F) Laser speckle images of the area in C and D (darker tone indicates more flow) report regional blood flow and successful occlusion of the targeted pial arteriole (calibration bar indicates speckle contrast; SD/mean). Note that targeted ischemia was selective to the forelimb area and produced sensory deficits that were larger than the area of ischemia.
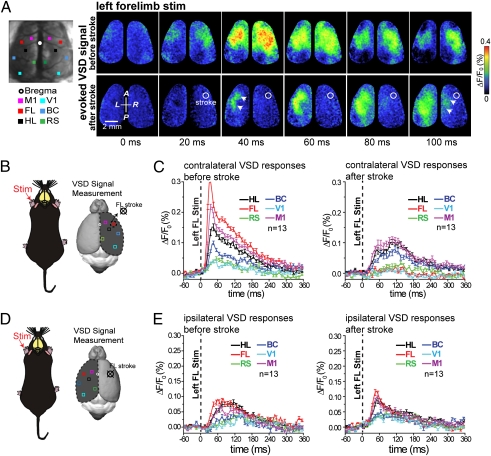
Fig. 2.
Bilateral assessment of sensory processing in response to stimulation of the affected limb in mice before and immediately after acute focal stroke. (A Left) Targeted ischemia was induced as in Fig. 1, and responses were monitored in both cortical hemispheres at the indicated 5 × 5-pixel (0.11 mm2) regions of interest. (Upper Right) Cortical VSD fluorescence signal in response to tactile stimulation of the left forelimb 40 min before stroke induction. (Lower Right) VSD fluorescence signal in response to tactile stimulation of the left forelimb 30 min after targeted photothrombotic focal stroke (Fig. 1) in the cortical area within the right hemisphere that represents the anterior forelimb map. The stroke focus, determined by speckle imaging, is outlined by the white circle. Note that the ipsilateral response is still present within the left hemisphere despite the loss of early activity within the cortical representation of contralateral forelimb area. (B and C) Regions of interest where plots of average VSD response made from the stroke-affected hemisphere in response to stimulation of the left forelimb (contralateral response) from 13 mice were made are indicated with small 5 × 5-pixel boxes placed over a reconstruction of a C57B16 brain. (D and E) Regions of interest where plots of VSD response were made from the unaffected hemisphere (ipsilateral response). Notice that the averaged ipsilaterally evoked VSD responses were not significantly different in amplitude or time course from prestroke responses (statistical quantitative changes in cortical responsiveness shown in Fig. S3).
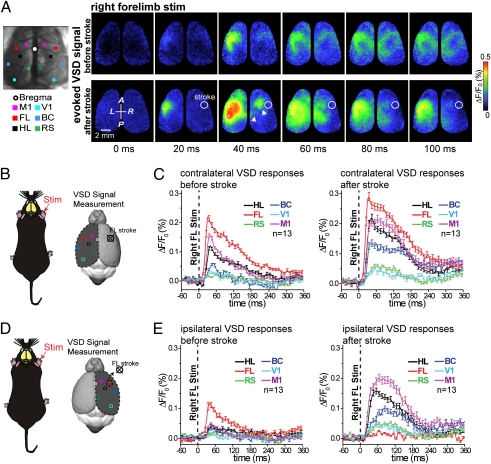
Fig. 3.
Assessment of sensory processing in response to stimulation of the unaffected limb after acute ischemia. (A Left) Targeted ischemia was made as in Fig. 1. (Right) Pseudocolor images of the bilateral sensory response (VSD images) to stimulation of the right forelimb (unaffected limb) before (Upper) and 45 min after (Lower) a targeted stroke was induced within the right hemisphere forelimb area (outlined with white circle). (B) Schematic showing the location of regions of interest used for assessing responses within the unaffected hemisphere resulting from stimulation of the unaffected forelimb. (B and C) Quantification of responses from indicated regions of interest mediated by the unaffected limb within the unaffected hemisphere. Plots are the average of 13 mice. (D) Schematic showing the location of regions of interest used for assessing responses within the affected hemisphere resulting from stimulation of the unaffected forelimb. (E) Quantification of sensory responses to forelimb stimulation within the stroke-affected hemisphere while stimulating the unaffected paw (right forelimb stimulation measurements in right hemisphere). Statistical quantification of unaffected forelimb-evoked VSD cortical response measured in both hemispheres is shown in Fig. S5.
Approximately 10–15 ms after the earliest VSD response in contralateral forelimb cortex, the sensory-evoked response was observed within somatotopic regions of the hemisphere ipsilateral to stimulation (Figs. 2A and 3A). At later times, ∼50 ms after the forepaw stimulation, the sensory-evoked VSD depolarization spread, although weakly, to distal cortical regions, including contralateral and ipsilateral visual and retrosplenial cortices (Figs. 2 A–E and 3 A–E and Movies S1 and S2) (27, 28). Spreading activity in somatosensory cortex in response to a single-tap forepaw stimulation is not a property of anesthesia and seems to be a feature of brain function. Work by Ferezou et al. (28) used VSD imaging in both anesthetized and head-fixed quiet awake mice to directly image the membrane potential dynamics of sensorimotor cortex. Their results show that whisker deflections in quiet awake mice evoked a similar pattern of activity as observed in anesthetized mice. Before stroke, consistent with previous data (28, 29), the ipsilateral signal was attributed to the spread of activity from the opposite hemisphere through the corpus callosum. After stroke, VSD responses were greatly reduced within the targeted forelimb representation in response to stimulation of either the left (Fig. 2 A and C) or right (Fig. 3 A and C) limb (Figs. S3 A and B and S5 A and B). The ischemic area is indicated by a white circle within the response images (Fig. 2A).
Consistent with our previous results (21), the contralateral limb-stimulated VSD signal was reduced in the stroke core but now, is concentrated within adjacent peri-infarct sensory and motor cortex (Fig. 2 A and C and Fig. S3 A and B), possibly through preserved connections from afferent sensory pathways (30). These changes were associated with stimulation of the forelimb and were not reproduced when the hindlimb was stimulated (Fig. S3 A–C). Although the left forelimb-stimulated VSD response in the stroke-affected right hemisphere (contralateral response) was greatly reduced (P < 0.001; n = 13 mice) (Fig. 2C and Fig. S3B), left forelimb-stimulated ipsilateral responses were still present (within the left hemisphere) despite the loss of activity within the right hemisphere (Fig. 2 A, D, and E). These ipsilaterally evoked VSD responses were not significantly different in amplitude or time course from prestroke responses (Fig. S3 C and D) and in some cases, were even enhanced compared with the prestroke case.
These changes were associated with the stimulation of the forelimb and were not induced when the hindlimb was stimulated (Fig. S4 A–C). The presence of ipsilateral responses (measured in the left hemisphere to left forepaw stimulation) in the animal with a stroke on the right side was surprising, because the contralateral (right) forelimb area was ischemic and could not relay this signal across the corpus callosum as expected in naïve animals (28, 29, 31).
Changes in Sensory-Evoked Activity Extend to the Unaffected Limb.
When the unaffected forelimb ipsilateral to stroke was stimulated (for example, when a stroke was made within the right hemisphere and the right forelimb was stimulated), we also observed significant changes in VSD responses (Fig. 3A and Movie S2). Most notably within the unaffected hemisphere, the contralateral forelimb response was significantly enhanced in peak amplitude (n = 13 mice; P < 0.01) and integrated area (n = 13 mice; P < 0.001) (Fig. 3 B and C and Fig. S5 A and B). The effect was also observed in areas neighboring the forelimb area such as the motor, hindlimb, barrel, and retrosplenial cortex. These effects indicate that reduced activity in the stroke-affected hemisphere alters the excitability of the unaffected hemisphere, perhaps through a loss of feed-forward interhemispheric inhibition (32–36). Of note, these changes in forelimb-evoked cortical activation patterns within the unaffected hemisphere were not present when the relatively unaffected hindlimb was stimulated (Fig. S4 A–D).
Routing of Sensory Signals from the Unaffected Paw to the Stroke-Affected Hemisphere.
In animals with a stroke on the right side, we also assessed the ability of the unaffected limb (right limb) to evoke activity ipsilaterally within the stroke-affected hemisphere (Fig. 3A). We found that forelimb-derived ipsilateral signals were enhanced in related peri-infarct areas such as hindlimb, motor, and barrel cortex but were absent in the stroke-affected forelimb area (Fig. 3 D and E and Fig. S5 C and D). These results suggest that diffuse off-target ipsilateral signals are enhanced in the stroke-affected hemisphere. The exception was the forelimb area itself; it cannot respond, because it is ischemic. Notably, right hindlimb stimulation-derived ipsilateral signals were unchanged within stroke-affected hemispheres, except for the spread to the ischemic forelimb area (Fig. S2 A, F, and G).
Pharmacological Inhibition of Activity Within One Hemisphere only Partially Mimics the Effect of Stroke.
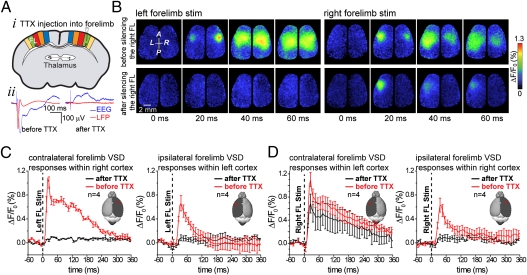
It is possible that stroke simply produces a deficit in function that leads to less transcallosal activity and interhemispheric inhibition (32, 35, 36) within the unaffected hemisphere. Alternatively, stroke leads to events (perhaps ischemia itself or inflammation) (37, 38) that may actively promote a reassignment of circuits in a manner not strictly predicted by a simple reduction in activity. To address these possibilities, we sought to mimic an aspect of stroke's effect on the unaffected hemisphere by pharmacologically blocking activity within either cortex or thalamus in the absence of an ischemic lesion. Injection of 30 μM tetrodotoxin (TTX) into the granular layer of forelimb representation of cortex (n = 4) (Fig. 4A, i) completely blocked the contralateral sensory response within the injected hemisphere (P < 0.005; n = 4) (Fig. 4 A, ii and B). Consistent with previous findings (35) and contrary to our results after stroke, this treatment blocked the ipsilateral response within the uninjected hemisphere (P < 0.01; n = 4) (Fig. 4C) and reduced [VSD peak amplitude (ΔF/F0) before and after injection of TTX into the cortex; P < 0.05 (n = 4) in forelimb area] the sensory response within the unaffected hemisphere when the unaffected forepaw was stimulated (Fig. 4 B and D).
Fig. 4.
Cortical silencing with tetrodotoxin does not reproduce the effects of targeted ischemia. (A, i) Cartoon showing experimental setup where tetrodotoxin is injected into the right forelimb map. (ii) Local field potential and cortical EEG data showing a loss of sensory-evoked response after tetrodotoxin (30 μM) injection. (B) Example VSD images before and 35 min after tetrodotoxin injection in response to stimulation of the forelimb contralateral (Left) or ipsilateral (Right) to the injection site. Note the absence of ipsilateral VSD signal within unsilenced hemisphere in response to stimulation of left forelimb (in contrast to stroke observation) (Fig. 2). (C and D) Quantification of TTX effects on VSD responses to affected (C) and unaffected (D) forelimb stimulation after injection of tetrodotoxin into the right forelimb region.
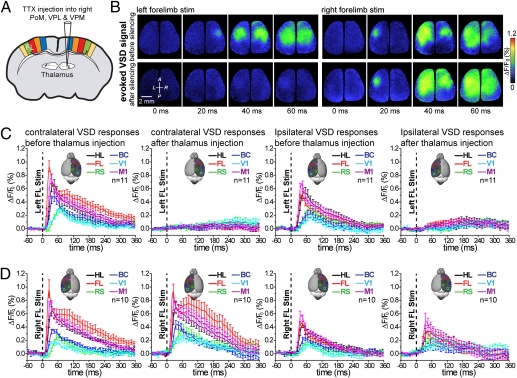
It is also possible that acute stroke leads to a rearrangement of sensory processing through thalamic nuclei, resulting in a different balance of interhemispheric signaling (39–42). To address this possibility, TTX (30 μM) was injected into the right thalamic nuclei [ventral posteriolateral (VPL), ventral posteriomedial (VPM), and posterior medial nucleus (PoM); n = 11] (Fig. 5A). After this treatment, ipsilateral responses to left forelimb stimulation (measured within the left hemisphere) were blocked (P < 0.001; n = 11 mice) (Fig. 5 B and C). This result is consistent with the ipsilateral response in nonstroke animals being derived through transcallosal projections (28, 29, 43). The lack of an ipsilateral response contrasted sharply with the sustained or even enhanced (within spared medial areas) ipsilateral response observed within the stroke experienced animals (Fig. 2 A and D). Together, our results with both cortical and thalamic silencing imply that, within 30–50 min of a stroke, ipsilateral responses are generated by a mechanism that may use an alternative pathway within the stroke-affected hemisphere.
Fig. 5.
Thalamic inactivation does not reproduce circuit level changes in sensory processing after ischemia. (A) Experimental setup showing the site of TTX injection into the right thalamic nuclei expected to mediate forelimb-stimulated cortical responses. (B Left) Cortical VSD fluorescence signal in response to tactile stimulation of the left forelimb before and 25 min after silencing contralateral thalamic nuclei using TTX (30 μM) injection. (Right) Example of VSD images showing that TTX (30 μM) injection into the thalamus does not lead to disinhibition of the unaffected paw. Notably, within this animal, transcallosal ipsilateral responses are still apparent. (C and D) Quantification of sensory responses for forelimb-derived signals either contralateral (C) or ipsilateral (D) to the TTX-injected thalamus. As expected, TTX injection blocks the forelimb-mediated response contralateral to injection site but fails to lead to disinhibition of the nonstroke (unaffected) hemisphere.
We also assessed whether silencing the thalamus in one hemisphere would lead to disinhibition within the other hemisphere by assessing the effect of stimulating the unaffected limb (TTX in right thalamus and stimulating the right forelimb). Under these conditions, we did not observe changes in the peak amplitude (n = 10 mice) or integrated areas of VSD responses within the uninjected hemisphere (Fig. 5 B and D). Moreover, within these animals, transcallosal ipsilateral responses (Fig. 5 B and C) were not enhanced, contrary to our results obtained with stroke. These results reinforce the idea that acute stroke leads to selective changes within the circuits used, an effect that is not necessarily mimicked by a loss of input (40).
Stroke-Induced Interhemispheric Rearrangements of Sensory-Evoked Activity Patterns Can Occur in the Absence of Transcallosal Connections.
To further address the source of interhemispheric rearrangements of sensory-evoked activity patterns after stroke, we used a line of mice that lacks a corpus callosum (n = 7) (27, 44). One potential caveat of using acallosal mice is that features of this mutant animal may be abnormal, and therefore, the effects observed after stroke lesions may be unique to this strain. In these mice, the callosal fibers fail to cross the midline, making instead a different structure (the longitudinal bundle of Probst) (45). This structure provides redundant ipsilateral cortico-cortical connections (46). Surprisingly, these acallosal mice showed similar rearrangements in interhemispheric activity as WT mice after forelimb sensory cortex stroke, suggesting that subcortical pathways may be important for redistribution of sensory processing within 1 h after stroke (Fig. S6 A–C). In particular, these animals also showed enhanced responses to stimulation of the unaffected forepaw in the hemisphere opposite to stroke [VSD peak amplitude (ΔF/F0) and integrated amplitude (ΔF/F0.S) before and after stroke; P < 0.01 in forelimb (FL), hindlimb (HL), barrel cortex (BC), and primary motor cortex (M1) areas] (Fig. S6 D–F).
Stroke-Induced Rapid Interhemispheric Redistribution of Sensory Processing Is Dependent on Thalamic Activity.
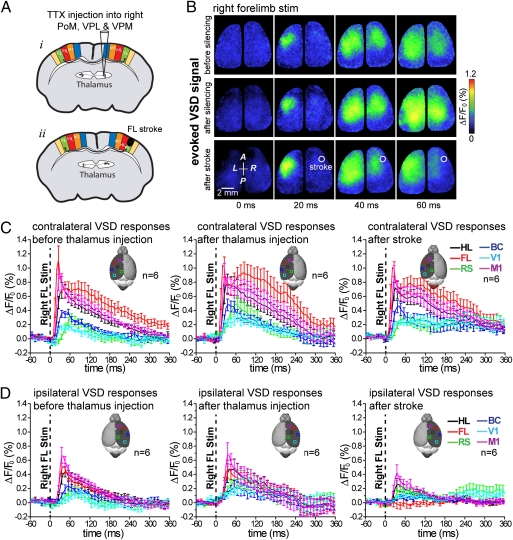
Interhemispheric subcortical (47–51) or direct noncrossed ipsilateral fibers (52) have been reported in animals without stroke. Unmasking of these ipsilateral pathways could be a mechanism of the rapid functional rearrangement of sensory-evoked responses after stroke that we observed. To address these possibilities, TTX (30 μM) was first injected into the right thalamic nuclei (as described before) to inhibit thalamic activity. A photothrombotic stroke was then induced within forelimb representation of somatosensory cortex within the same hemisphere as thalamic inactivation (Fig. 6A). As expected, thalamic silencing blocked (n = 6; P < 0.001) all residual sensory response to stimulation within the injected hemisphere produced by the stimulation of the contralateral (left) paw (Fig. 5C and Fig. S7B). Ipsilateral responses to left forelimb stimulation that were normally present after stroke (Fig. 2A) (measured within the left hemisphere) were blocked in these animals [VSD peak amplitude (ΔF/F0) before thalamus injection and after stroke in FL, HL, BC, M1, RS, and V1 areas; n = 6; P < 0.001] (Fig. S7 B and C) where the right thalamus was inactivated.
Fig. 6.
Thalamic silencing before focal stroke prevents the enhanced cortical response to the unaffected forelimb. (A, i) Experimental setup showing the site of tetrodotoxin injection into the right thalamic nuclei. (ii) A photothrombotic ischemia was then created within forelimb representation of somatosensory cortex within the same hemisphere as thalamic inactivation. (B) Cortical VSD fluorescence signal in response to tactile stimulation of the right forelimb in control (Top), after tetrodotoxin (30 μM) injection into thalamic nuclei (Middle), and finally, 27 min after photothrombotic stroke within cortical representation of right forelimb (Bottom). Note that, in this animal, the ipsilateral VSD response (dependent on transcallosal activity) resulting from stimulation of the right (unaffected) forelimb is still apparent after thalamic inactivation within most of the right hemisphere, but it was not detected within the stroke-affected forelimb area after ischemia. (C and D) Quantification of either contralateral or ipsilateral sensory responses to stimulation of unaffected forelimb in control after TTX-injected thalamus and finally, after forelimb stroke. Averaged data from six mice is shown.
In addition, we assessed the role of the thalamus in mediating enhanced cortical responses to the unaffected paw (Fig. 6 B–D). Forelimb area stroke made after thalamic silencing (n = 6 mice) did not lead to an enhancement of the peak amplitude or integrated area of VSD responses within the unaffected hemisphere (left) when the right (unaffected) forelimb was stimulated (Fig. 6 B and C). Moreover, within these animals, ipsilateral responses produced by right forelimb stimulation were not enhanced (Fig. 6 B and D), contrary to our results obtained when animals were given a stroke (Fig. 3 A–F).
Discussion
Diffuse Widespread Cortical Connectivity May Be Called on After Stroke.
In addition to consensus circuits, such as primary sensory areas, sensation-induced signals are routed to and within the brain along multiple diffuse pathways (28, 30, 43). Diffuse connectivity may be called on to reroute signals after injury such as stroke. For example, somatosensory stimuli from a specific sensory system are preferentially routed through the thalamus to primary and secondary sensory areas dedicated to that body part, but they can also exhibit widely divergent activation patterns (28, 30, 34, 43, 53). VSD imaging in mice revealed surprisingly widespread intracortical connectivity between related regions of the cortex, such as sensory and motor areas (7, 21, 27, 28, 30). Thus, diffuse connectivity together with redundancy in neuronal processing may facilitate recovery from stroke damage and suggest a need to look for the circuits that mediate recovery over wide regions of cortex or even elsewhere, such as the thalamus or spinal cord (42, 54–57).
When Does Circuit Level Compensation Occur After Stroke?
Evidence from our laboratory (7) and others (9, 14, 17, 18, 55, 57) supports the idea that changes over weeks in the structural circuitry of the peri-infarct region, as well as more distant areas, could contribute to recovery from stroke. For the most part, this evidence stems from the use of anterograde and retrograde tracers (that define axonal projections) and visualization of local dendritic spine turnover within the peri-infarct area or contralateral hemisphere (9, 11, 17, 18, 58). In these studies, the tracers indicate connections to the peri-infarct zone from regions within the affected hemisphere as well as projections from the peri-infarct zone to other sites (7, 14, 17). However, this evidence for structural plasticity must reconcile the fact that rearrangements in the patterns of sensory-evoked activity can even occur at very short time points that are too early for the sprouting of connections to have an effect (21, 33, 59, 60). Furthermore, although structural connections are apparent at later time points, whether they or existing connections are used during the processing of sensation has been difficult to unequivocally establish (10, 61, 62).
Rapid Compensatory Changes in Cortical Circuit Use Because of Loss of Function.
The appearance of new structural connections that form over weeks during recovery from stroke is supported by data from a number of different studies (7, 14, 17, 63, 64). However, it is also possible to view the stroke recovery process not as a response to damaging events such as apoptosis and necrosis but as a form of compensation (homeostatic plasticity) because of loss of function (1, 65). Stroke would produce a local loss of function that could lead to the removal of surrounding inhibition and patterns of activation using existing disinhibited spared circuits. Previous work in animal models (66, 67) and patients (68) has suggested that, after stroke, the cortex is relatively disinhibited, whereas other recent work implies increased tonic GABAergic inhibition (69). Disinhibition is supported by electrophysiological data as well as neurochemical changes in both excitatory and inhibitory transmitter systems (66, 67, 70). Disinhibition of this type leading to network changes has been observed in the absence of stroke with local inactivation of cortex (32, 35, 36). This mechanism would fit our data, because it would be expected to be observed immediately after stroke induction and would not be dependent on the time required for the formation of new structural connections. A question that arises is whether loss of activity after stroke is different from loss of activity after a noninjurious event, such as cooling (32, 36) or pharmacological blockade (35). During stroke in addition to loss of function within the ischemic core, there will be injurious events, such as inflammation, and waves of spreading depolarization (14, 20, 70) that may have very different effects than merely a loss of function. Interestingly, in our work, we cannot recreate all aspects of the alterations in sensory processing induced by stroke using the injection of TTX into either the cortex or thalamus. Notably, silencing the thalamus did not lead to enhancement of responses mediated by stimulation of the unaffected limb (the limb on the same side as the injection).
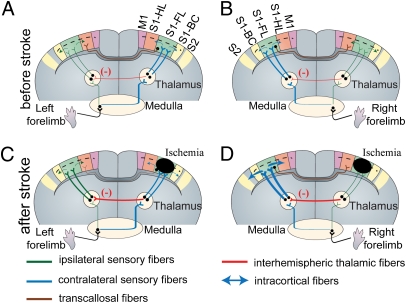
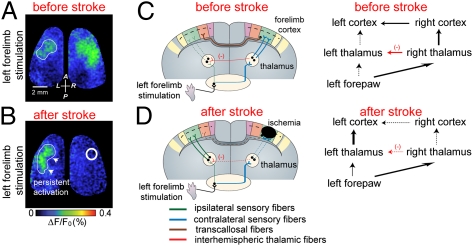
One open question is how stroke can elicit changes in widespread areas of the brain and particularly, within the contralesional hemisphere. It is possible that stroke elicits unique propagating electrophysiological events that lead to changes in sensory processing over wide regions of brain. Regarding mechanism, waves of depolarization can originate in areas adjacent to a stroke core and propagate into homotopic brain regions soon after injury onset (20, 71). These recurrent depolarizing waves can propagate between hemispheres without the use of corpus callosal fibers (72). It is possible that propagating depolarizing waves reduce the tonic cortico-reticular inhibitory influence and thus, increase the spontaneous frequency of ipsilesional thalamic reticular neurons. This is in accord with the results of the work by Bures et al. (73), which showed that the functional elimination of the cortex during cortical spreading depression produces a clear cutout increase of the firing frequency of ipsilesional thalamic reticular neurons. The thalamic reticular nucleus is a net-like structure comprised of inhibitory neurons that envelops the thalamus and can gate thalamic relays (74). Neuroanatomical tracing studies in rodents (47–49), cat (50), and primates (51), together with electrophysiological evidence (75), provide support for reticuloreticularis connections between thalami in both hemispheres. Increased firing frequency of ipsilesional thalamic reticular neurons could lead to inhibition of contralesional thalamic reticular neurons and thus, disinhibition of thalamic relay neurons within contralesional VPL and VPM nuclei (Fig. 7 and Fig. S8). Based on our hypothesis, disinhibition of thalamic relay neurons within the unaffected hemisphere could render them more sensitive to incoming sensory stimuli within thalamocortical circuits, resulting in the increased responsiveness of the contralesional hemisphere that we observe (Fig. 7 and Fig. S8).
Fig. 7.
Cartoon example showing possible interhemispheric rerouting of sensory processing within the first hours of targeted ischemia to the forelimb sensory map. (A and B) Control (before stroke): sensory axons coming from the left (A) or right (B) forepaw cross either to the contralateral side (blue) or continue through an uncrossed ipsilateral pathway (green). Note that ipsilateral sensory pathways are weak compared with contralateral pathways. The contralateral pathways are routed to the primary forelimb somatosensory cortex through excitatory thalamocortical pathways (blue) from contralateral thalamus and then, spread throughout nearby cortical regions such as the HL, BC, and M1 area (blue arrow). Sensory signals then spread within <15 ms to the homotopic cortical region within the opposite hemisphere through transcallosal pathways. Uncrossed ipsilateral pathways (green) make synapses on ipsilateral thalamus and cortex, respectively. Interhemispheric reticuloreticularis connections (47–51, 75) between thalami in either hemisphere are shown in red (detailed diagram in Fig. S8). (C) After stroke in the right FL area, contralateral thalamocortical inputs are disrupted, which leads to blockade of activity spread through the corpus callosum. Ischemia may also increase the influence of interhemispheric thalamic reticuloreticularis inhibition (red) and thus, unmask the ipsilateral pathways (green; from left forepaw) through disinhibition of thalamic relay neurons (inhibition of inhibitory neurons) within contralesional hemisphere. (D) The excitability of thalamocortical pathways contralateral to stroke, which carry the sensory information of unaffected (right) forepaw, may be enhanced (blue) because of down-regulation of interhemispheric thalamic inhibition (red).
Possible Roles of Changes in Signal Lateralization in Recovery.
We observe that changes in signal processing that occur immediately after stroke can extend to both hemispheres. Notably, we observe that the ipsilateral sensory response is normally dependent on the activity within the contralateral hemisphere, whereas after stroke, ipsilateral responses are preserved and apparently become independent of the contrateral hemisphere. This finding suggests that normally poorly functioning noncrossed responses (52) are augmented after stroke to make up for the lack of transcalossal input from the damaged hemisphere (noncrossed projections).
These results bring up the question of whether the less lateralized (crossed) responses that we observe are detrimental to the recovery process. An emerging consensus from human imaging studies is that the most successful recovery occurs in individuals that exhibit relatively normal lateralized patterns of sensory activation within the lesioned hemisphere, whereas patients with larger stroke, who often show bilateral cortical activation, typically have less complete recovery (4, 76). Ipsilateral activation may, thus, indicate an inability of compensatory mechanisms to restore normal, predominantly lateralized sensory activation. An important point to remember is that these studies are done on patients at time points often weeks to months after stroke, making it difficult to extrapolate this work to the findings that we have in the setting of acute stroke after 1 h. Nonetheless, we believe that our results, although admittedly difficult to translate to longer time points, do suggest that wide-scale bihemispheric circuit level rearrangements could potentially occur in the absence of new structural connectivity. We should also note that we cannot rule out that long-range changes in structural projections contribute to altered sensory processing and function observed during stroke recovery (5, 7). Such long-range changes are well-described after spinal cord injury and stroke, and agents that promote axonal outgrowth have clear permissive effects on the recovery process (55, 58, 62, 69). A challenge in future work will be link areas with altered function to new structural connections. Perhaps such investigations will be facilitated by using optogenetic excitation and inhibition combined with promoters that are activated during stroke recovery (17).
Methods
Methodological details are in SI Methods. Adult male C57BL6J mice were used (n = 32) for most experiments, whereas I/LnJ acallosal mice (n = 7; Jackson Laboratory) were used for a subset of these experiments. Animal protocols were approved by the University of British Columbia Animal Care Committee. Anesthesia was induced with urethane (0.12% wt/wt). For in vivo imaging, a large (7 × 8 mm; bregma 2.5 to −4.5 mm and lateral 0–4 mm) bilateral cranial window was performed over the cortex (SI Methods). For in vivo VSD imaging, the dye, RH1692 (Optical Imaging) (22), was dissolved in Hepes-buffered saline: optical density of 5–7 (measured at 550 nm) applied to the exposed cortex for 60–90 min (SI Methods). VSD signal responses to stimulation were calculated as the normalized difference to the average baseline recorded before stimulation (ΔF/F0) (SI Methods). To periodically assess blood flow, we imaged laser speckle contrast (67) as described in SI Methods. Focal stroke was induced in surface vessels as we described (66, 68) by the photothrombosis with Rose Bengal. We injected the photosensitizing dye into the tail vein. Within 10 min after injection, we targeted individual surface arterioles to induce photothrombotic blockage of blood flow using a 0.7- to 1.4-mW, 532-nm beam from a diode pumped laser (MGM-20; Beta Electronics).
Supplementary Material
Acknowledgments
We thank Pumin Wang and Cindy Jiang for surgical assistance, Alexander Goroshkov and Jeff LeDue for assistance with optics, Jamie Boyd for assistance with computer programming, Matthew Fingas for help with early experiments, and Allen Chan, David McVea, Diana Lim, and Shangbin Chen for critical reading of the manuscript. This work was supported by a Michael Smith Foundation for Health Research postdoctoral fellowship, a Heart and Stroke Foundation of Canada and Canadian Institutes of Health Research (CIHR) Focus on Stroke postdoctoral fellowship (to M.H.M.), and Heart and Stroke Foundation of British Columbia and Yukon Grant in Aid and CIHR Operating Grant MOP-48695 (to T.H.M). T.H.M. is funded by a Human Frontier Science Program Grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 8932.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101914108/-/DCSupplemental.
References
- 1.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 5.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Merzenich MM, et al. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 7.Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–1734. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: A potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 9.Dancause N, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 11.Takatsuru Y, et al. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci. 2009;29:10081–10086. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez CL, et al. Evidence for bilateral control of skilled movements: Ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20:3442–3452. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelles G, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke. 1999;30:1510–1516. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- 16.Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 2008;18:638–647. doi: 10.1093/cercor/bhm096. [DOI] [PubMed] [Google Scholar]
- 17.Li S, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostany R, et al. Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci. 2010;30:14116–14126. doi: 10.1523/JNEUROSCI.3908-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall PD. The presence of ineffective synapses and the circumstances which unmask them. Philos Trans R Soc Lond B Biol Sci. 1977;278:361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]
- 20.Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci. 2010;30:9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigler A, Mohajerani MH, Murphy TH. Imaging rapid redistribution of sensory-evoked depolarization through existing cortical pathways after targeted stroke in mice. Proc Natl Acad Sci USA. 2009;106:11759–11764. doi: 10.1073/pnas.0812695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoham D, et al. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron. 1999;24:791–802. doi: 10.1016/s0896-6273(00)81027-2. [DOI] [PubMed] [Google Scholar]
- 23.Chemla S, Chavane F. Voltage-sensitive dye imaging: Technique review and models. J Physiol Paris. 2010;104:40–50. doi: 10.1016/j.jphysparis.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Grinvald A, Hildesheim R. VSDI: A new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- 25.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohajerani MH, McVea DA, Fingas M, Murphy TH. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci. 2010;30:3745–3751. doi: 10.1523/JNEUROSCI.6437-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferezou I, et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Frostig RD, Xiong Y, Chen-Bee CH, Kvasnák E, Stehberg J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci. 2008;28:13274–13284. doi: 10.1523/JNEUROSCI.4074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 32.Clarey JC, Tweedale R, Calford MB. Interhemispheric modulation of somatosensory receptive fields: Evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- 33.Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci USA. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones EG. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci. 2000;23:1–37. doi: 10.1146/annurev.neuro.23.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Ebner FF. Balancing bilateral sensory activity: Callosal processing modulates sensory transmission through the contralateral thalamus by altering the response threshold. Exp Brain Res. 2006;172:397–415. doi: 10.1007/s00221-005-0337-y. [DOI] [PubMed] [Google Scholar]
- 36.Blankenburg F, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Block F, Dihné M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75:342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- 39.Parker JL, Dostrovsky JO. Cortical involvement in the induction, but not expression, of thalamic plasticity. J Neurosci. 1999;19:8623–8629. doi: 10.1523/JNEUROSCI.19-19-08623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox K, Wallace H, Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Philos Trans R Soc Lond B Biol Sci. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paz JT, Christian CA, Parada I, Prince DA, Huguenard JR. Focal cortical infarcts alter intrinsic excitability and synaptic excitation in the reticular thalamic nucleus. J Neurosci. 2010;30:5465–5479. doi: 10.1523/JNEUROSCI.5083-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Livy DJ, Wahlsten D. Tests of genetic allelism between four inbred mouse strains with absent corpus callosum. J Hered. 1991;82:459–464. doi: 10.1093/oxfordjournals.jhered.a111128. [DOI] [PubMed] [Google Scholar]
- 45.Ozaki HS, Shimada M. The fibers which course within the Probst's longitudinal bundle seen in the brain of a congenitally acallosal mouse: A study with the horseradish peroxidase technique. Brain Res. 1988;441:5–14. doi: 10.1016/0006-8993(88)91377-7. [DOI] [PubMed] [Google Scholar]
- 46.Ozaki HS, Wahlsten D. Cortical axon trajectories and growth cone morphologies in fetuses of acallosal mouse strains. J Comp Neurol. 1993;336:595–604. doi: 10.1002/cne.903360411. [DOI] [PubMed] [Google Scholar]
- 47.Battaglia G, Lizier C, Colacitti C, Princivalle A, Spreafico R. A reticuloreticular commissural pathway in the rat thalamus. J Comp Neurol. 1994;347:127–138. doi: 10.1002/cne.903470110. [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Raos V, Bentivoglio M. Connections of the thalamic reticular nucleus with the contralateral thalamus in the rat. Neurosci Lett. 1992;147:85–88. doi: 10.1016/0304-3940(92)90780-b. [DOI] [PubMed] [Google Scholar]
- 49.Raos V, Bentivoglio M. Crosstalk between the two sides of the thalamus through the reticular nucleus: A retrograde and anterograde tracing study in the rat. J Comp Neurol. 1993;332:145–154. doi: 10.1002/cne.903320202. [DOI] [PubMed] [Google Scholar]
- 50.Rinvik E. Thalamic commissural connections in the cat. Neurosci Lett. 1984;44:311–316. doi: 10.1016/0304-3940(84)90041-7. [DOI] [PubMed] [Google Scholar]
- 51.Paré D, Steriade M. The reticular thalamic nucleus projects to the contralateral dorsal thalamus in macaque monkey. Neurosci Lett. 1993;154:96–100. doi: 10.1016/0304-3940(93)90180-s. [DOI] [PubMed] [Google Scholar]
- 52.Armand J, Kuypers HG. Cells of origin of crossed and uncrossed corticospinal fibers in the cat: A quantitative horseradish peroxidase study. Exp Brain Res. 1980;40:23–34. doi: 10.1007/BF00236659. [DOI] [PubMed] [Google Scholar]
- 53.Kaas JH. Is most of neural plasticity in the thalamus cortical? Proc Natl Acad Sci USA. 1999;96:7622–7623. doi: 10.1073/pnas.96.14.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 55.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaas JH, Florence SL, Jain N. Subcortical contributions to massive cortical reorganizations. Neuron. 1999;22:657–660. doi: 10.1016/s0896-6273(00)80725-4. [DOI] [PubMed] [Google Scholar]
- 58.Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- 59.Sober SJ, Stark JM, Yamasaki DS, Lytton WW. Receptive field changes after strokelike cortical ablation: A role for activation dynamics. J Neurophysiol. 1997;78:3438–3443. doi: 10.1152/jn.1997.78.6.3438. [DOI] [PubMed] [Google Scholar]
- 60.Aguilar J, et al. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh A, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 63.Baron JC. Perfusion thresholds in human cerebral ischemia: Historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(Suppl 1):2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 64.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 65.Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 66.Schiene K, et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 67.Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: Mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22:1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- 68.Blicher JU, Jakobsen J, Andersen G, Nielsen JF. Cortical excitability in chronic stroke and modulation by training: A TMS study. Neurorehabil Neural Repair. 2009;23:486–493. doi: 10.1177/1545968308328730. [DOI] [PubMed] [Google Scholar]
- 69.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centonze D, et al. Synaptic plasticity during recovery from permanent occlusion of the middle cerebral artery. Neurobiol Dis. 2007;27:44–53. doi: 10.1016/j.nbd.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Mun-Bryce S, Roberts L, Bartolo A, Okada Y. Transhemispheric depolarizations persist in the intracerebral hemorrhage swine brain following corpus callosal transection. Brain Res. 2006;1073–1074:481–490. doi: 10.1016/j.brainres.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 72.Fifková E. Thalamic spreading depression in the rat. Electroencephalogr Clin Neurophysiol. 1966;20:68–76. doi: 10.1016/0013-4694(66)90142-8. [DOI] [PubMed] [Google Scholar]
- 73.Bures J, Buresova O, Weiss T, Fifkova E. Excitability changes in non-specific thalamic nuclei during cortical spreading depression in the rat. Electroencephalogr Clin Neurophysiol. 1963;15:73–83. doi: 10.1016/0013-4694(63)90040-3. [DOI] [PubMed] [Google Scholar]
- 74.Fuentealba P, Steriade M. The reticular nucleus revisited: Intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 76.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]