Abstract
Cellular imbalances of cholesterol and fatty acid metabolism result in pathological processes, including atherosclerosis and metabolic syndrome. Recent work from our group and others has shown that the intronic microRNAs hsa-miR-33a and hsa-miR-33b are located within the sterol regulatory element-binding protein-2 and -1 genes, respectively, and regulate cholesterol homeostasis in concert with their host genes. Here, we show that miR-33a and -b also regulate genes involved in fatty acid metabolism and insulin signaling. miR-33a and -b target key enzymes involved in the regulation of fatty acid oxidation, including carnitine O-octaniltransferase, carnitine palmitoyltransferase 1A, hydroxyacyl-CoA-dehydrogenase, Sirtuin 6 (SIRT6), and AMP kinase subunit-α. Moreover, miR-33a and -b also target the insulin receptor substrate 2, an essential component of the insulin-signaling pathway in the liver. Overexpression of miR-33a and -b reduces both fatty acid oxidation and insulin signaling in hepatic cell lines, whereas inhibition of endogenous miR-33a and -b increases these two metabolic pathways. Together, these data establish that miR-33a and -b regulate pathways controlling three of the risk factors of metabolic syndrome, namely levels of HDL, triglycerides, and insulin signaling, and suggest that inhibitors of miR-33a and -b may be useful in the treatment of this growing health concern.
Keywords: lipid homeostasis, posttranscriptional regulation, cardiovascular disease
Many diseases result from perturbations in lipid homeostasis, including atherosclerosis, type II diabetes, and metabolic syndrome (1–4). The intracellular and membrane levels of fatty acids and cholesterol are under constant surveillance and are coordinated with de novo lipid biosynthesis by endoplasmic reticulum (ER)-bound sterol regulatory element-binding proteins (SREBPs) (5–7). The SREBP family of basic helix–loop–helix–leucine zipper (bHLH-LZ) transcription factors consists of SREBP-1a, SREBP-1c, and SREBP-2 proteins that are encoded by two unique genes, Srebp-1 and Srebp-2 (5–7). The SREBPs differ in their tissue-specific expression, their target gene selectivity, and the relative potencies of their trans-activation domains. SREBP-1c regulates the transcription of genes involved in fatty acid metabolism, such as fatty acid synthase (FASN) (5–7). SREBP-2 regulates the transcription of cholesterol-related genes, such as 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR), which catalyzes a rate-limiting step in cholesterol biosynthesis, and the low-density lipoprotein receptor (LDLr), which imports cholesterol from the blood (5–7). Increased SREBP activity causes cholesterol and fatty acid accumulation and down-regulates the SCAP/SREBP pathway by feedback inhibition. In this way, lipid metabolism within cells is tightly regulated.
In addition to classical transcriptional regulators, a class of noncoding RNAs, termed microRNAs (miRNAs), has emerged as critical regulators of gene expression acting predominantly at the posttranscriptional level (8–10). These short (22 nt) double-stranded regulatory noncoding RNAs are encoded in the genome, and most are processed from primary transcripts by the sequential actions of Drosha and Dicer enzymes (8–10). In the cytoplasm, mature miRNAs are incorporated into the cytoplasmic RNA-induced silencing complex (RISC) and bind to partially complementary target sites in the 3′ UTRs of mRNA. miRNA targeting of mRNAs inhibits their expression through mRNA destabilization, repression of translation, or a combination of both processes (8–10).
We and others provided identification of a highly conserved miRNA family, miR-33, within the intronic sequences of the Srebp genes in organisms ranging from Drosophila to humans (11–14). Two miR-33 genes are present in humans: miR-33b, which is present in intron 17 of the Srebp-1 gene on chromosome 17, and miR-33a, which is located in intron 16 of the Srebp-2 gene on chromosome 22. In mice, however, there is only one miR-33 gene, which is conserved with human miR-33a and located within intron 15 of the mouse Srebp-2 gene.
We recently showed that miR-33a is cotranscribed with its host gene Srebp-2 like many intronic miRNAs, and it targets genes involved in cholesterol export, including the adenosine triphosphate binding cassette (ABC) transporters ABCA1 and ABCG1 and the endolysosomal transport protein Niemann-Pick C1 (NPC1) (14). This regulatory function of miR-33a ensures that the cell is protected under low sterol conditions from additional sterol loss. In addition to this role in maintaining cholesterol homeostasis, we now show that miR-33a and -b also regulate fatty acid metabolism and insulin signaling. We identify putative binding sites for miR-33 in the 3′ UTR of carnitine O-octaniltransferase (CROT), carnitine palmitoyltransferase 1A (CPT1a), hydroxyacyl-CoA-dehydrogenase (HADHB), AMP kinase subunit-α (AMPKα), and insulin receptor substrate 2 (IRS2) and show that miR-33a and -b specifically inhibit the expression of these genes. The physiological relevance of this targeting is revealed by miR-33 overexpression in hepatic cells, which reduces both fatty acid oxidation and insulin signaling. Furthermore, inhibition of endogenous miR-33 increases the expression of CROT, CPT1a, HADHB, AMPKα, and IRS2 and up-regulates fatty acid oxidation and insulin signaling. Together, these data suggest that feedback loops involving SREBPs and miR-33a and -b balance cholesterol metabolism, fatty acid oxidation, and insulin signaling, three of the major risk factors of metabolic syndrome (1, 3, 15).
Results
miR-33 Targets Genes Regulating β-Oxidation of Fatty Acid and Insulin Signaling.
We have previously described the presence of miR-33a in the Srebp-2 gene. miR-33a is found within the same intron of Srebp-2 from many animal species, including large and small mammals, chickens, and frogs. Interestingly, the fruit fly D. melanogaster also has a highly conserved mature form of miR-33a, but these organisms do not synthesize sterols. SREBP in flies regulates fatty acid metabolism (16), which is reminiscent of the function of the Srebp-1 gene in mammals (6). As shown in Fig. S1 A and B, miR-33b is synchronously expressed with SREBP-1c in human hepatic Huh7 cells treated with an agonist of the liver X receptor (LXR), a transcriptional regulator of Srebp-1c expression. Kinetic analysis of miR-33b induction revealed a concomitant increase in miR-33b and SREBP-1c expression, consistent with their coregulation. Thus, we postulated that miR-33a and -b, which differ only in 2 nt (Fig. S1C), might, therefore, regulate genes involved in lipid metabolism. To search for potential targets, we performed gene ontology and biological association analyses using Pathway Studio 7 software (Ariadne Genomics) and looked for enrichment of specific target genes associated with lipid metabolism. As shown in Fig. S2 (red box), several genes involved in fatty acid β-oxidation have predicted targets for miR-33, including CROT, CPT1a, HADHB and AMPKα (17–20). CROT, a carnitine acyltransferase that catalyzes the reversible transfer of fatty acyl between CoA and carnitine, provides a crucial step in the transport of medium and long-chain acyl-CoA out of the mammalian peroxisome to the cytosol and mitochondria (17–20). CPT1a is a mitochondrial enzyme that mediates the transport of long fatty acids across the membrane by binding them to carnitine, and it is the rate-limiting enzyme that regulates fatty acid oxidation (17–20). HADHB is the β-subunit of the mitochondrial trifunctional protein, which catalyzes the last three steps of mitochondrial β-oxidation of long-chain fatty acids, whereas AMPKα stimulates hepatic fatty acid oxidation and ketogenesis (17–20). Interestingly, we also identified IRS2, a component of the insulin-signaling pathway, as a potential target of miR-33.
To determine whether miR-33b targets these predicted target genes, we generated reporter constructs with the luciferase coding sequence fused to the 3′ UTRs of these genes. miR-33b markedly repressed the activity of the Crot, Cpt1a, Hadhb, Ampkα, and Irs2 3′ UTR luciferase constructs (Fig. S3). Furthermore, mutation of the miR-33 target sites in these constructs relieved miR-33b repression of the 3′ UTR of Crot, Cpt1a, Hadhb, Ampkα, and Irs2, consistent with a direct interaction of miR-33b with these sites (Fig. S3). Mutation of both miR-33 sites in the 3′ UTR of Crot was necessary to completely reverse the inhibitory effects of miR-33 (Fig. S3).
We next determined the effect of miR-33 on mRNA and protein expression of CROT, CPT1a, HADHB, AMPKα, IRS2, and lipid-related genes that lack predicted miR-33 binding sites. Transfection of Huh7 cells with miR-33b (32-fold increase expression) significantly inhibited the mRNA levels of CROT, CPT1a, HADHB, AMPKα, and IRS2 (Fig. 1A). Notably, inhibition of endogenous miR-33b using anti–miR-33b oligonucleotides (threefold decrease expression) increased the mRNA expression of CROT, CPT1a, AMPKα, and IRS2 in Huh7 cells (Fig. 1B), consistent with a physiological role for miR-33b in regulating the expression of these genes. Similar regulation of these genes by miR-33b was also seen at the protein level (Fig. 1 C and D). We next investigated the effect of manipulating miR-33 levels in vivo in mice using lentiviruses encoding premiR-33, anti–miR-33, or a control. Efficient lentiviral delivery was previously confirmed (14). Consistent with our in vitro results, miR-33 reduced hepatic CROT, HADHB, CPT1a, IRS2, and ABCA1 mRNA expression (Fig. S4). Conversely, mice expressing anti–miR-33 showed a modest increase of CROT, CPT1a, IRS2, and ABCA1 mRNA expression, although the effect was not statistically significant (Fig. S4).
Fig. 1.
Posttranscriptional regulation of IRS2, AMPKα, CROT, CPT1a, and HADHB by miR-33b. Quantitative RT-PCR expression profile of selected miR-33 predicted target and other related genes in human hepatic Huh7 cell line (A) after overexpressing miR-33b and (B) after endogenous inhibition of miR-33b by anti–miR-33b. Western blot analysis of HepG2 cells (C) overexpressing or (D) inhibiting endogenous miR-33b. Heat shock protein (HSP)90 bands are the loading control. (E) Specificity of miR-33b on fatty acid metabolism-related genes. qRT-PCR array analysis of fatty acid metabolism-related genes from HepG2 cells transfected with Con miR or miR-33. Data are the mean ± SEM and are representative of more than or equal to three experiments. *P ≤ 0.05.
To show the specificity of miR-33b targeting of CROT, CPT1a, HADHB, AMPKα, and IRS2, we assessed the effect of miR-33b overexpression in HepG2 cells on other fatty acid metabolism-related genes using an array that included 84 genes involved in fatty acid transport and biosynthesis, ketogenesis, and ketone body metabolism. Whereas CROT, CPT1a, and AMPKα were predictably down-regulated by miR-33, we did not observe changes in the expression of non–miR-33 targets (Fig. 1E). Furthermore, other genes containing putative miR-33 binding sites such as citrate synthase (CS) and HMGCR were not affected either at the mRNA level (Fig. S5A) or by 3′ UTR activity (Fig. S5B).
miR-33a and miR-33b Have Similar Targeting Effects on Genes Regulating Cholesterol Metabolism, β-Oxidation of Fatty Acids, and Insulin Signaling.
miR-33a and -b have identical seed sequences but differ in 2 of 19 nt of the mature RNA (Fig. S1C). To determine whether miR-33a and -b have similar effects on CROT, CPT1a, HADHB, AMPKα, Sirtuin 6 (SIRT6), and IRS2 protein expression, we transfected Huh7 cells with a control miR, miR-33a, or -b. As seen in Fig. S6, miR-33a and -b inhibited CROT, CPT1a, HADHB, AMPKα, SIRT6, and IRS2 protein expression to a similar extent. In addition, both miR-33a and -b significantly inhibited the 3′ UTR activity of Crot, Cpt1a, Hadhb, Ampkα, and Irs2 with only modest differences, indicating that the 2-nt variation in the mature forms of miR-33a and -b does not appreciably affect the gene targeting by these miRNAs.
miR-33 Inhibits Cellular Fatty Acid Oxidation.
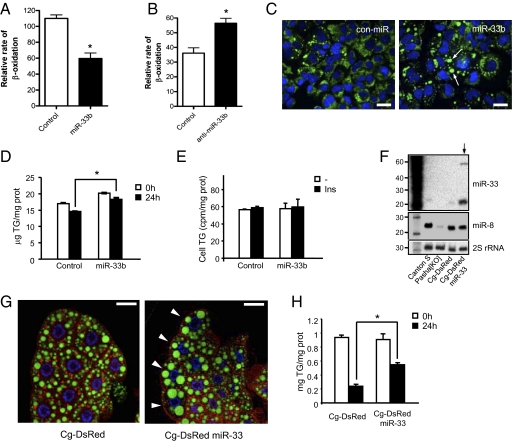
To evaluate the effects of miR-33a and -b on fatty acid β-oxidation, we measured the release of [14C]-carbon dioxide from the oxidation of [14C]-oleate. miR-33b overexpression (27-fold increase) markedly reduced the fatty acid β-oxidation in Huh7 (Fig. 2A) cells. Conversely, inhibition of endogenous miR-33b expression using anti–miR-33 (2.6-fold increase) increased the rate of fatty acid β-oxidation (Fig. 2B). We next evaluated the accumulation of neutral lipids and lipid droplet formation in Huh7 cells incubated with oleate for 24 h and then starved for the next 24 h. In agreement with the reduced fatty acid β-oxidation rates observed in hepatic cells overexpressing miR-33b, Huh7 cells transfected with miR-33b accumulated more triglycerides (TAG) in larger lipid droplets (Fig. 2 C and D). The increase in triglyceride content was independent of changes in triglyceride synthesis rates. As seen in Fig. 2E, miR-33 expression did not alter basal and insulin-induced triglyceride synthesis.
Fig. 2.
miR-33b regulates human hepatic β-oxidation and lipid homeostasis in Drosophila. (A and B) Relative rate of β-oxidation from Huh7 cells transfected with miR-33 (A) and anti–miR-33b (B). (C) Analysis of neutral lipid accumulation of Huh7 cells transfected with Con-miR or miR-33b and stained with Bodipy (green) and DAPI (blue). (D) Analysis of triglyceride content of Huh7 cells transfected with miR-33b at 0 and 24 h of starvation. (E) Analysis of triglyceride synthesis of Huh7 cells transfected with Con-miR or miR-33 and stimulated or not stimulated with insulin. (F) Northern blot analysis of miR-33, miR-8, and 2S rRNA of transgenic Drosophila overexpressing miR-33 or control transgene in the fat body [genotype: Cg-gal4, upstream activating sequence (UAS)-myrRFP, and UAS-transgene; abbreviated as Cg > transgene]. miR-8 is used as the control. (G) Neutral lipid accumulation in the fat body of Cg > DsRed and Cg > DsRed-miR-33 stained with Bodipy (green), Hoechst 33352 (blue), and the transgene (red). (Scale bar: 30 μm.) (H) Analysis of triglyceride content of transgenic Drosophila overexpressing miR-33 or control transgene in the fat body before and after starvation.
Because CPT1a is also a target of miR-33 in Drosophila, we set out to determine if miR-33 plays a role in maintaining lipid homeostasis in Drosophila. To do this, we generated a transgenic fly that overexpressed miR-33 in the fat body (Fig. 2F). We hypothesized that miR-33–overexpressing flies would retain TAG and fatty acids upon starvation as a consequence of reduced fatty acid oxidation, which should manifest as an increase in lipid storage. Accordingly, fat bodies from starved miR-33 transgenic flies, dissected and stained with Bodipy, showed large lipid droplets in many cells compared with control flies (Fig. 2G). Similarly, flies overexpressing miR-33 accumulated more TAG upon starvation (Fig. 2H), indicating that the function of miR-33 in regulating fatty acid oxidation is conserved from Drosophila to humans.
miR-33 Regulates Insulin Signaling.
To further explore our observation that miR-33 inhibits IRS2 expression, we next assessed the effect of miR-33 on insulin signaling. IRS2 is a cytoplasmic signaling molecule that mediates the effects of insulin, insulin-like growth factor 1, and other cytokines by acting as a molecular adaptor between receptor tyrosine kinases and downstream effectors (21–23). To test the role of miR-33 in regulating insulin signaling, we analyzed the effect of miR-33 overexpression on two of the main downstream effectors of IRS2: the PI3K/AKT and rat sarcoma (RAS)/RAF/ERK pathways (21–23). As seen in Fig. 3 A and B, Huh7 cells transfected with miR-33b showed reduced AKT and ERK phosphorylation after insulin stimulation, indicative of reduced IRS2 function. Similar results were observed when we analyzed the AKT activation using an in vitro kinase assay (Fig. 3C). To determine whether or not IRS2 overexpression rescues the miR-33 overexpression effect on AKT phosphorylation, we transfected Huh7 cells with IRS2 cDNA that lacked the 3′ UTR sequence. As seen in Fig. 3 D and E, IRS2 expression rescued the AKT activation on insulin stimulation in miR-33b–overexpressing cells.
Fig. 3.
miR-33b regulates insulin signaling. (A and B) miR-33b impairs insulin signaling by reducing AKT phosphorylation in hepatic Huh7 cells. (C) In vitro AKT kinase assay of postinsulin-stimulated and immunoprecipitated total AKT from Huh7 cells. GSK-3 fusion protein was used as a substrate and assayed for phosphor-GSK-3α/β (Ser-219). (D and E) IRS2 overexpression rescues Akt phosphorylation in miR-33b–transfected cells after insulin stimulation. (F) miR-33b overexpression reduces 2-deoxyglucose (2-DOG) uptake in Huh7 cells treated with insulin. (G) Quantitative RT-PCR array analysis of insulin signaling related genes from Huh7 cells transfected with Con miR or miR-33. Data are the mean ± SEM and are representative of more than or equal to three experiments. *P ≤ 0.05.
To gain insights into the role of miR-33 in regulating insulin signaling, we assessed the effect of miR-33b on 2-deoxyglucose uptake after insulin stimulation. As seen in Fig. 3F, miR-33b overexpression reduced insulin-induced 2-deoxyglucose (2-DOG) uptake in Huh7 cells. We also assessed FoxO1 cellular localization in insulin-stimulated cells. FoxO1 is a well-defined target downstream of the conserved insulin/target of rapamycin (TOR) signaling network that has important roles in the regulation of processes as diverse as cellular growth, stress resistance, and energy homeostasis (24). To date, several proteins are known to interact with FoxO transcription factors, regulating their intracellular localization and/or activity. One of the best documented is the AKT/protein kinase B (PKB) kinase, which phosphorylates FoxO in three conserved sites, leading to FoxO cytoplasmatic retention and transcriptional inactivation. To test the effect of miR-33 on FoxO1 localization, we transfected Huh7 with a con-miR or miR-33b and transduced the cells with a FoxO1-GFP adenovirus. FoxO1-GFP localized primarily to the nucleus in starved cells transfected with Con-miR (Fig. S7A Upper) or miR-33b (Fig. S7B Upper). Treatment with insulin induced FoxO1-GFP translocation from the nucleus to the cytoplasm in Con-miR–transfected cells (Fig. S7A Lower), whereas cells transfected with miR-33b had the cellular distribution reversed (Fig. S7B Lower). Interestingly, IRS2 overexpression rescued the effect of miR-33 overexpression on FoxO1 intracellular localization (Fig. S7 C and D). Together, these experiments identify miR-33 as an important regulator of the insulin-signaling pathways.
In addition, we also assessed the effect of miR-33b overexpression in Huh7 cells on other insulin-related genes using an array that included 84 genes involved in carbohydrate and lipid metabolism and target genes for insulin signaling. As expected, IRS2 was down-regulated in Huh7 cells transfected with miR-33b (Fig. 3D). Moreover, glucokinase (GCK), fibroblast growth factor receptor substrate 2 (FRS2), acetyl-CoA carboxylase-α (ACACA), and peroxisome proliferator-activated–γ (PPARG) were also inhibited (Fig. 3D). Interestingly, FRS2 is also a predicted target of miR-33a and -b. FRS2 has been suggested to participate in insulin signaling by recruiting Src-homology-phosphatase 2 (SHP2) and hence, could function as a docking molecule similar to insulin receptor substrate proteins.
In addition to IRS2 and FRS2, our bioinformatic analysis identified a third miR-33 predicted target involved in glucose homeostasis: the histone deacetylase SIRT6 (25, 26). SIRT6-deficient mice develop normally but succumb to lethal hypoglycemia early in life, suggesting an important role of SIRT6 in regulating glucose metabolism (25, 26). Interestingly, it has also been recently reported that hepatic-specific disruption of SIRT6 in mice results in fatty liver formation because of enhanced glycolysis and triglyceride synthesis (27). To confirm that miR-33b targets SIRT6, we cloned the Sirt6 3′ UTR into a luciferase reporter construct. miR-33b markedly repressed the 3′ UTR activity of Sirt6 (Fig. S8A), and mutation of the miR-33 target sites in the 3′ UTR relieved miR-33b repression of Sirt6, consistent with a direct interaction of miR-33b with these sites (Fig. S8A). Furthermore, miR-33b overexpression significantly inhibited the SIRT6 mRNA and protein levels in Huh7 cells (Fig. S8 B and C), whereas inhibition of endogenous miR-33b by anti–miR-33 increased the expression of SIRT6 (Fig. S8 B and C). Although these data are consistent with a physiological role for miR-33b in regulating SIRT6 expression, this is unlikely to contribute to the regulation of fatty acid metabolism by miR-33b, because inhibition of SIRT6 expression by siRNA only modestly decreased fatty acid β-oxidation (Fig. S8D).
Additional experiments, including RIPseq and proteomics, are warranted to understand the miR-33 regulatory network and its implication in lipid and carbohydrate metabolism.
Discussion
We and others have recently established that, during sterol-limited states, miR-33a is coincidentally generated with Srebp-2 transcription and works to increase cellular cholesterol levels by limiting cholesterol export through the down-regulation of ABC transporters, ABCA1 and ABCG1 (12–14). Importantly, these pathways regulate circulating HDL levels through their roles in HDL biogenesis and cellular cholesterol efflux (12–14). We now show that a second member of the miR-33 family, miR-33b, is coregulated with the human Srebp1 gene and targets genes involved in fatty acid oxidation and insulin signaling. Together, these results suggest a paradigm in which miR-33a and miR-33b act in concert with their host genes, Srebp-2 and Srebp-1, to boost intracellular cholesterol and fatty acid levels by balancing transcriptional induction and posttranscriptional repression of lipid metabolism genes. Notably, we show that miR-33a and miR-33b have overlapping gene targets, suggesting that cotranscription of Srebp-2 and miR-33a or Srebp-1 and miR-33b would be predicted to regulate both cholesterol metabolism and fatty acid oxidation; this establishes a model of reciprocal regulation of cholesterol and fatty acid metabolism by SREBPs (Fig. 4).
Fig. 4.
Potential role of SREBPs and miR-33a and -b in metabolic syndrome. In hepatocytes, conditions of low intracellular cholesterol (or statins) induce SREBP-2, leading to increased lipoprotein uptake and endogenous cholesterol biosynthesis. Hyperinsulinemia or insulin resistance induces SREBP-1, leading to increased fatty acid and triglycerides synthesis. The activation of Srebps induces miR-33a and -b expression, leading to decreased HDL cholesterol levels by targeting ABCA1, reduced insulin signaling by targeting IRS2, and reduced cellular β-oxidation by targeting different fatty acid oxidation enzymes. Therapeutic inhibition of miR-33 might result in increased plasma HDL cholesterol levels, reduced VLDL secretion, and increased insulin signaling, thus improving the prognosis of patients with metabolic syndrome.
The presence of miR-33a in the intron of Srebp-2 is remarkably conserved in many species, including the fruit fly D. melanogaster. This was notable, because Drosophila neither synthesizes sterols nor expresses ABCA1. Interestingly, the Srebp gene of Drosophila controls fatty acid production, and our bioinformatic analysis of miR-33a and -b target genes revealed an enrichment of genes involved in fatty acid metabolism. We identify herein six miR-33 target genes that regulate fatty acid metabolism and insulin signaling, including CPT1a, CROT, HADHB, AMPKα, SIRT6, and IRS2. Importantly, we show that miR-33 overexpression reduces fatty acid oxidation in hepatic cell lines. While this manuscript was in preparation, Gerin et al. (11) reported similar effects of miR-33 on CROT, CPT1a, and HADHB expression. Finally, we show that miR-33 transgenic flies show increased lipid accumulation in tissues, suggesting that miR-33 regulation of fatty acid metabolism pathways is evolutionarily conserved.
Our work also identifies miR-33a and -b as regulators of insulin signaling. By inhibiting expression of IRS2 in hepatic cells, miR-33a and -b reduce the activation of downstream insulin signaling pathways, including AKT and ERK. Although our previous work established that miR-33a and Srebp-2 are cotranscribed during states of cholesterol depletion (14), the changes in Srebp-2 and miR-33a transcription were quite small in this setting. By contrast, Srebp-1c is transcribed at extremely high levels in response to insulin (6, 28), which may be particularly relevant in the setting of metabolic syndrome, where insulin resistance is accompanied by increased triglycerides and plasma levels of very low density lipoprotein (VLDL) as well as reduced HDL levels (1, 3). Our data indicate that miR-33a and -b impact pathways influencing three of the primary risk factors in this disease, namely insulin resistance, low HDL, and high triglycerides/VLDL. Importantly, we show that antagonism of endogenous miR-33a and -b up-regulates fatty acid oxidation and response to insulin in hepatocytes, suggesting that miR-33a and -b may be an attractive therapeutic target for metabolic syndrome. Although our previous work in mice supports this contention by showing that inhibition of miR-33a and -b effectively increases HDL (14), the functional relevance of the miR-33b/Srepb-1 association cannot easily be determined using traditional models of insulin resistance, such as rats or mice, because the Srebp1 genes of these animal models lack miR-33b.
Recently, siRNAs and miRNAs have gained considerable attention as therapeutic targets (29–32). Different strategies have been developed to modulate miRNA effects for therapeutic purposes. Inhibition of miR expression can be achieved by using antisense oligonucleotides antagomirs or their chemically modified versions, 2’-O-methyl-group (OMe)–modified oligonucleotides and locked nucleic acids (LNA) anti-miRs, as well as by inhibiting the production of the mature forms by disrupting their processing (29–32). There is tremendous therapeutic potential for the treatment of cardiovascular diseases by either overexpression or inhibition of miRNAs. Our results suggest that antagonism of endogenous miR-33 may be useful as a therapeutic strategy for treating metabolic syndrome and nonalcoholic fatty liver disease (NAFLD), which is, by far, the most common liver ailment. Although the biology of miRNAs regulating lipid metabolism and insulin signaling is still an exciting frontier in cardiovascular medicine, therapeutic miRNA manipulations are emerging as promising players in the treatment of disease. Additional research and specialty clinical trials, however, are needed to translate these therapies into clinical practice.
Materials and Methods
A detailed description of procedures is provided in SI Materials and Methods.
Bodipy Staining and Triglyceride Analysis of Fat Body Larvae and Northern Blot Analysis for miRNAs.
Larval starvation was performed as described previously (33). Fat bodies were dissected in PBS, incubated in 1 μg/mL Bodipy 493/503 and 10 μg/mL Hoechst 33352 in 1× PBS for 20 min, mounted on a glass slide with spacers in 50% glycerol in PBS, and imaged by confocal microscopy within 2 h. Single confocal sections are shown. Triglyceride levels were calculated as previously described (32). Small RNA Northern blots were performed as described previously (34).
Immunohistochemistry.
Huh7 cells were transfected with miR-33 or Con-miR as described above and incubated with 1 mM oleate for 12 h. Then, cells were washed two times with cold PBS and starved for the next 24 h. Cells were fixed for 1 h in 4% paraformaldehyde/PBS and stained for 1 h with Bodipy 493/503 in PBS. The coverslips were then mounted on glass slides with Gelvatol/DAPI and analyzed with an epifluorescence microscope (Axiovert; Carl Zeiss MicroImaging) with a 40× objective. Analysis of different images was performed using openlab software (Improvision). In another set of experiments, we transfected Huh7 cells with miR-33 or Con-miR for 24 h. Then, cells were infected with FoxO1-GFP as previously described (35) for 24 h and starved for the next 24 h before insulin stimulation. We captured microscope images on a fluorescence microscope using 488-nm laser excitation for GFP. Images were captured with a 40× objective and analyzed with the Image J software (National Institutes of Health). To calculate relative nuclear fluorescence, we divided nuclear fluorescence by the total amount of cellular fluorescence. All quantitative values represent averages from at least 30 cells per well. Data are the mean ± SEM and are representative of three independent experiments.
Supplementary Material
Acknowledgments
We thank Domenico Accili for providing the FoxO1 adenovirus construct. This work was supported by the Deutsche Forschungsgemeinschaft (Exc 257); Neurocure (D.C.-S. and E.E.); American Heart Association Grants SDG-0835481N (to Y.S.) and SDG-0835585D (to C.F.-H.); National Institutes of Health Grants 1P30HL101270-01, R01HL58541 (to E.A.F.), RO1AG20255 (to K.J.M.), R01GM083300 (to E.C.L.), R01HL16063 and RO1HL107953 (to C.F.-H.); and the Alfred Bressler Scholar Fund (to E.C.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.L.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102281108/-/DCSupplemental.
References
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 6.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne TF. Sterol regulatory element-binding proteins (SREBPs): Key regulators of nutritional homeostasis and insulin action. J Biol Chem. 2000;275:32379–32382. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 11.Gerin I, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terán-García M, Bouchard C. Genetics of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:89–114. doi: 10.1139/h06-102. [DOI] [PubMed] [Google Scholar]
- 16.Rawson RB. The SREBP pathway—insights from Insigs and insects. Nat Rev Mol Cell Biol. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- 17.Brady PS, Ramsay RR, Brady LJ. Regulation of the long-chain carnitine acyltransferases. FASEB J. 1993;7:1039–1044. doi: 10.1096/fasebj.7.11.8370473. [DOI] [PubMed] [Google Scholar]
- 18.Eaton S, et al. The mitochondrial trifunctional protein: Centre of a beta-oxidation metabolon? Biochem Soc Trans. 2000;28:177–182. doi: 10.1042/bst0280177. [DOI] [PubMed] [Google Scholar]
- 19.Mannaerts GP, Van Veldhoven PP, Casteels M. Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals. Cell Biochem Biophys. 2000;32:73–87. doi: 10.1385/cbb:32:1-3:73. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay RR, Gandour RD. Selective modulation of carnitine long-chain acyltransferase activities. Kinetics, inhibitors, and active sites of COT and CPT-II. Adv Exp Med Biol. 1999;466:103–109. [PubMed] [Google Scholar]
- 21.Haeusler RA, Accili D. The double life of Irs. Cell Metab. 2008;8:7–9. doi: 10.1016/j.cmet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72–78. doi: 10.1016/j.tem.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 23.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 24.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 25.Xiao C, et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285:36776–36784. doi: 10.1074/jbc.M110.168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong L, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown MS, Ye J, Goldstein JL. Medicine. HDL miR-ed down by SREBP introns. Science. 2010;328:1495–1496. doi: 10.1126/science.1192409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmén J, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 30.Elmén J, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esau C, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 33.Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.