Abstract
Immunodominant T-cell responses are important for virus clearance. However, the identification of immunodominant T-cell peptide + HLA glycoprotein epitopes has been hindered by the extent of HLA polymorphism and the limitations of predictive algorithms. A simple, systematic approach has been used here to screen for immunodominant CD8+ T-cell specificities. The analysis targeted healthy HLA-A2+ donors to allow comparison with responses to the well-studied influenza matrix protein 1 epitope. Although influenza matrix protein 1 was consistently detected in all individual samples in our study, the response to this epitope was only immunodominant in three of eight, whereas for the other five, prominent CD8+ T-cell responses tended to focus on various peptides from the influenza nucleoprotein that were not presented by HLA-A2. Importantly, with the four immunodominant T-cell epitopes identified here, only one would have been detected by the current prediction programs. The other three peptides would have been either considered too long or classified as not containing typical HLA binding motifs. Our data stress the importance of systematic analysis for discovering HLA-dependent, immunodominant CD8+ T-cell epitopes derived from viruses and tumors. Focusing on HLA-A2 and predictive algorithms may be too limiting as we seek to develop targeted immunotherapy and vaccine strategies that depend on T cell-mediated immunity.
According to the World Health Organization (WHO), influenza viruses cause 0.5 million deaths each year (http://www.who.int/mediacentre/factsheets/fs211/en/index.html), despite the fact that vaccines are readily available, at least in the wealthier countries. Although these products are modified annually, the protective antibody response can be ineffective because of the surface HA and neuraminidase (NA) proteins being mismatched with the currently circulating viruses. Furthermore, the widely used inactivated vaccines do not prime memory CD8+ T cells, reflecting that immunogenic, internal (to the virus) proteins have been excluded or that the adjuvants used tend to stimulate antibody responses (1). Experiments with animal models have shown that the recall of memory CD8+ cytotoxic T lymphocytes (CTLs) leads to early influenza A virus (IAV) clearance and reduced clinical impairment (2, 3), whereas emerging evidence further suggests that CTLs provide a measure of protection against human influenza (4).
Although IAV has been studied for many decades, relatively few immunogenic peptide + MHC (pMHC) glycoprotein complexes (epitopes) have been characterized for the vast array (>2,000) of human class I (MHCI: HLA-A, -B, and -C) and (>1,000) class II (MHCII: HLA-DR, -DP, and -DQ) alleles (http://www.ebi.ac.uk/imgt/hla/allele.html). Those that are published are readily accessed through the web-based Immune Epitope Database (IEDB) (5). To date, most epitopes have been identified using HLA binding motifs and peptide prediction algorithms. For example, working from >4,000 computer-defined peptides with HLA binding motifs (for six HLA-A and -B and one HLA-DR), Assarsson et al. (6) identified 54 novel epitopes, including 38 that were CD8+ CTL-specific. However, it is not known whether these are immunodominant or subdominant within any given CD8+ T-cell response.
Immunodominance describes the situation where, although many pMHC complexes are potentially available, T-cell responses are reproducibly focused on one or a few key epitopes. A case in point is the massively overdominant H-2Db/nucleoprotein (NP)366–374-specific secondary CTL response in IAV-infected H-2b mice (all published and defined minimal epitopes are shown as subscripted amino acid positions, such as NP44–52; other peptide sequences are shown as in-line text, such as NP37–54) (7, 8). In humans, the immunodominance of the HLA-A2–restricted response to the conserved IAV matrix 158–66 (M158A2) has been well-established over the years (9, 10). Understanding immunodominance is important, because the available evidence suggests that, after virus challenge, these numerically prominent CTL populations provide more effective protection than the smaller subdominant sets (2). Immunodominant, HIV-specific CTLs limit virus replication in many asymptomatic or slow-progressing individuals. Furthermore, the preferential selection of virus mutants within both targeted HLA binding and T-cell receptors (TCR) interaction motifs (11, 12) points to the significance of such epitopes in virus control and suggests a focus for CTL vaccination and monitoring strategies.
Practically, however, such a simple proposal is greatly confounded by the fact that MHCI and MHCII glycoproteins are, excluding the immunoglobulins and the TCRs, the most polymorphic molecules known in both humans and mice. Some believe that this extraordinary diversity reflects a process of pMHC-determined, odorant-based mate selection (13). Others have argued that the greater the range of MHC types, then the more likely it is that a population will be able to deal with a novel, invading pathogen (9, 10, 14). Even so, whatever the selective pressure, the reality from the aspect of developing better immunotherapy or vaccination protocols is that it is essential to identify optimally immunogenic pMHC complexes. The present analysis, thus, uses a systematic approach for detecting CTL epitopes that are immunodominant, or prominent, in healthy HLA-A2+ individuals that have been infected at some time with one or other strains of IAV. In general, when evaluated in the context of predictive models, the findings do not support the idea that in silico analysis alone is sufficient to determine the major epitopes targeted in CTL-mediated immunity.
Results
Identifying Immunodominant Epitopes in Healthy HLA-A2+ Individuals.
Previous attempts at dealing with the extraordinary diversity of potential CTL targets have focused on screening thousands of algorithm-predicted, motif-containing peptides for chosen HLA alleles to find novel epitopes. However, accuracy is known to be low, and the approach does not reproducibly identify immunodominant pMHCI epitopes, despite most prediction programs having integrated scoring systems. In addition, many of the known immunodominant determinants from both viruses and tumors are either unusually long or contain no typical MHC binding motifs (15, 16). The broad alternative is to do extensive peptide screens, although this requires a massive effort for outbred, HLA-diverse human populations.
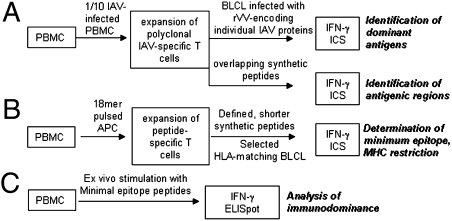
The systematic protocol that we have developed for the identification of immunodominant human T-cell responses to IAV is shown in Fig. 1. The first step was to generate B lymphoma cell lines (BLCLs) using EBV transformation and then, to type their HLA-A, -B, and -C alleles by PCR. The A/Puerto Rico/8/34 (PR8) strain of IAV infects most peripheral blood mononuclear cells (PBMCs), and therefore, polyclonal CTL lines were generated by coincubating IAV-infected PBMC as antigen-presenting cells (APCs) with autologous PBMC (responders) at a ratio of 1:9 in the presence of IL-2. Then, 12–15 d later, each expanded CTL line was assessed for the capacity to recognize autologous BLCLs infected with a panel of 11 recombinant vaccinia viruses (rVVs) encoding individual IAV proteins (17). The steps taken to ensure that these rVVs infected BLCLs to an equivalent extent are shown in Fig. S1.
Fig. 1.
The T-cell epitope screening protocol.
After the protein had been identified for a particular line, specificity was further screened (Fig. 1A) with synthetic 18mer overlapping peptides. This approach relies on the superior MHC binding property of minimal peptides (18) generated by serum proteases (19). T-cell lines were then raised to the particular 18mer peptide to define both the minimal peptide and the presenting HLA molecule (Fig. 1B). Finally, ex vivo memory CTL precursor (CTLp) frequencies were compared side by side with those for the immunodominant M158A2 CTLps from the same PBMC sample using both intracellular cytokine staining (ICS) and ELISpot assays (Fig. 1C). The approach, thus, identifies pMHCI epitopes and then places them in context by defining the magnitude of CTL memory populations relative to the M158A2 responders that have tended to dominate thinking about IAV and HLA-A2. Clearly, the protocol (Fig. 1) can be used for any antigenic system and all HLA types.
Most Immunodominant CTLs in HLA-A2+ Donors Are IAV-Nucleoprotein–Specific.
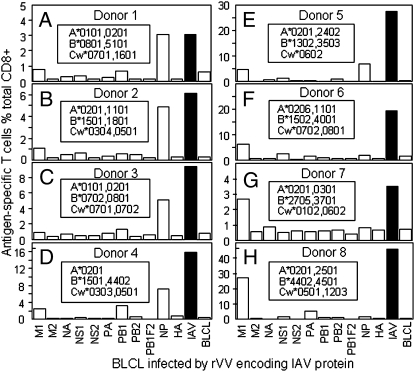
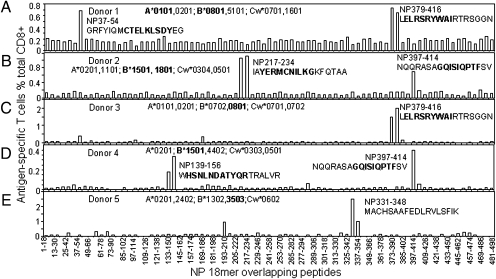
The first screen of an HLA-A2+ donor gave the unexpected result that more T cells in the polyclonal CTL line (donor 1) (Fig. 2A) responded to IAV-NP than IAV-M. This pattern repeated for four of seven more individuals (Fig. 2 B–H), with the fact that this did not reflect any problem with M158A2 presentation being validated by the finding that three of eight lines reacted most strongly to M (Fig. 2 F–H). The polyclonal CTL lines from the five donors where NP-specific responses were either larger than or comparable with those for M1 (Fig. 2 A–E) were then used to further screen overlapping NP 18mer peptides (Fig. 3). It was clear that most of these responses were focused on either one or two of the NP 18mers (Fig. 3), specifically NP regions 37–54 (Fig. 3A), 133–156 (Fig. 3D), 211–228 (Fig. 3B), 331–354 (Fig. 3E), 373–396 (Fig. 3 A and C), and 397–414 (Fig. 3 B and D).
Fig. 2.
Most immunodominant T cells are NP-specific. Polyclonal T-cell lines were raised using IAV-infected PBMCs. After 12–15 d, these lines were tested for their reactivity to autologous BLCLs infected (MOI = 10) with single rVVs encoding individual IAV proteins. The total IAV responses stimulated by IAV-infected, autologous BLCLs are shown in black bars for easier comparison. A–H correspond to donors 1–8, and the donor HLA alleles are shown in Insets.
Fig. 3.
Mapping immunodominant NP epitopes. The same IAV-specific T-cell lines used in Fig. 2 derived from five donors with either higher or equivalent CD8+ T-cell responses to NP than to M1 were also screened for their specific response to the 81 overlapping 18mer NP peptides at a final concentration around 1 μM in an ICS assay. The identified 18mer sequences are shown, and the subsequently identified minimal epitopes are bold (Figs. 1 and 2). A–E correspond to donors 1–5.
Various HLA Molecules Present Immunodominant T-Cell Epitopes Derived from NP.
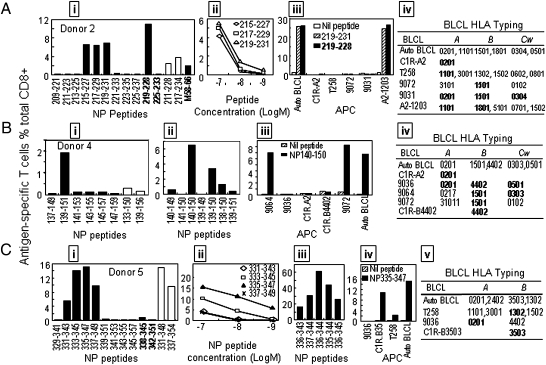
Because 18mer peptide screen identified the regions that contained the immunodominant T-cell epitopes, appropriate 13mer overlapping peptides (within the active 18mer sequence) were then used to probe the minimal epitopes recognized by the NP-specific CTLs. These responses were then compared with those that recognize the control immunodominant M158A2 epitope. Additionally, the screen was extended to include all of the known IAV peptides that match the PR8 NP sequence (IEDB listing) (Table S1). These published NP sequences are identified in bold in Figs. 3 and 4 and Fig. S2. As shown in Fig. S2A, when the 13mers within the NP37–54 and NP379–396 18mers (Fig. 3) were tested in parallel with the previously reported HLA-A1/NP44–52 (CTELKLSDY), HLA-B8/NP380–388 (ELRSRYWAI), and HLA-A2/M158–66 (GILGFVFTL) minimum epitopes using the donor 1 polyclonal T-cell line, it was clear that the minimum peptides NP44–52 and NP380–388 stimulated more antigen-specific T cells than the corresponding 18mers and that the responses were much bigger than those to M158A2 (Fig. S2A, white bars in i). The HLA-A1/NP44–52 and HLA-B8/NP380–388 responses were further validated (Fig. S2A, ii and iii) using C1R (20) APC lines that express HLA-A1 or HLA-B8. Both the NP44–52 and NP380–388 peptides are known to possess binding motifs for HLA-A1 [position 2 requires a Threonine (p2T) and p9Y] and HLA-B8 (p3K/R and p9L/I), respectively, and they had been identified earlier by algorithm prediction (21, 22).
Fig. 4.
Various HLA molecules present immunodominant NP peptides. (A) The 13mer peptides within the NP209–237 18mers (donor 2) were screened, with the 18mer results being shown as open bars (i). Three 13mer overlapping peptides were titrated to compare their activity (ii), and HLA-matched BLCLs were used to identify the HLA presenting the NP219–231 13mer (iii and iv). (B) The 13mer peptides within NP133–150 (donor 4) were screened as in A (B, i). The minimal NP140–150 peptide and its amino acid-truncated and -extended variants were tested under serum-free conditions (ii), and the HLA restriction profile for NP140–150 was determined using HLA matched BLCLs (iii, iv). (C) The 13mer peptides within the NP331–348 18mer (donor 5) were screened as in A (C, i). Four 13mers were titrated to determine their activity (ii); single amino acid-truncated and -extended peptides of NP336–344 were tested under serum-free conditions (iii), and the HLA restrictions of 13mer containing NP336–344 were analyzed as in A (iii and iv).
The polyclonal T-cell line from donor 2 was then used to further characterize the two immunodominant responses detected by the 18mer screen (Fig. 3B). Three 13mers spanning segments of NP217–234 (NP215–227, NP217–229, and NP219–231) stimulated comparable levels of T-cell activation (Fig. 4A, i and ii). Of these, NP219–228 (YERMCNILKG), which had been previously published and was included in our screen (Table S1), was found to be as potent as the 13mer NP219–231 (Fig. 4A, iii). According to the in silico prediction, NP219–228 should bind to HLA-B*4402 (6). However, donor 2 does not express B*4402, and NP219–228 was, in fact, presented by HLA-B*1801 (Fig. 4A, iii and iv). A typical binding motif for HLA-B*1801 is p1D, p5L, and p9Y/F, and therefore, NP219–228 would not have been identified as an HLA-B*1801 candidate by the currently available computer programs.
The immunogenic determinant within NP397–414 (Fig. 3B) was shown to be shared by NP401–413 and NP403–415 (AGQISIQPTFS) but not NP405–417 (QISIQPTFSVQR), indicating that GQISIQPTFS likely contains the minimum sequence subsequently shown to be presented by HLA-B*1501 (Fig. S2B, ii and iii). The B*1501 molecule selects binding peptides according to a p2Q/L, p5I/V, or p9F/Y motif. Thus, NP404–412 (GQISIQPTF) displays a perfect binding motif for HLA-B*1501 and is most likely the minimum epitope. Indeed, when the full NP sequence was subjected to B*1501 epitope prediction by the http://www.syfpeithi.de/ site and the IEDB site tool (http://www.iedb.org/), GQISIQPTF was predicted as the second- and fourth-best B*1501 binder, respectively.
The single immunodominant response to NP373–390 found for the HLA-2+/B*0801+ donor 3 (Fig. 3C) was confirmed as being the same immunodominant HLA-B8/NP380–388 shown for donor 1 (Fig. S2 A and C). Furthermore, the response to HLA-B8/NP380–388 was sixfold larger than that to M158A2 (3.0% vs. 0.5%) (Fig. S2C, i).
With donor 4 (Fig. 3D), the NP139–156 18mer-specific T cells were stimulated by NP139–151 but not NP137–149 or NP141–153. Each differed from NP139–151 by 2 aa at either end (Fig. 4B, i). Comparison of the overlapping sequences, thus, indicated that the likely immunogenic sequence is NP140–150 (HSNLNDATYQR). This was subsequently confirmed (Fig. 4B, ii) by responses to peptides with truncation and/or extension at either end of this 11mer, with the analysis being done in the absence of serum proteases to avoid amino acid trimming (19). Subsequent investigation established that NP140–150 is presented by HLA-B*1501 (Fig. 4B, iii, iv). As mentioned earlier, B*1501 selects peptides according to p2Q/L, p5I/V, and p9F/Y, and therefore, this 11mer would not have been predicted by any computer program because of the unusual length and the lack of a binding motif for B*1501.
With donor 5, the immunodominant T cells responded to the NP331–348 and NP337–354 18mers (Fig. 3E), recognizing four 13mers: NP331–343, NP333–345, NP335–347, and NP337–349 (Fig. 4C, i). Peptide titration (Fig. 4C, ii) indicated that NP333–345 and NP335–347 contain the minimal immunogenic sequence, with the other two perhaps missing a single terminal amino acid (Fig. S2D shows the sequence alignment of the four peptides). Thus, we tested the candidate NP336–344 (AAFEDLRVL) peptide and those with a single amino acid truncation or extension at either end in the absence of FCS (Fig. 4C, iii) to establish that NP336–344 was indeed the minimal epitope as the extensions/truncations reduced T-cell stimulating capacity. Furthermore, using the peptide-pulsed CR1.B3503 cell line, we showed that this peptide is presented by HLA-B*3503 (Fig. 4C, iv and v).
Ex Vivo Assessment of Immunodominant CTLp Frequencies.
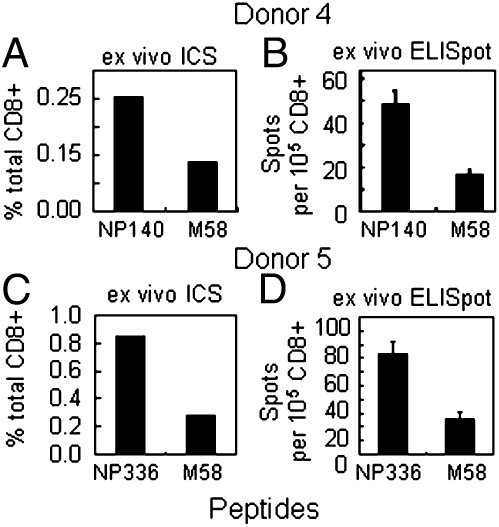
The fact that the preceding dissection used in vitro-expanded CTLs raises the possibility that the different T-cell sets within these polyclonal lines could have changed in relative size during the culture period. The PBMCs from donors 4 and 5 showed relatively small difference in response magnitude after restimulation of the IAV-expanded CTLs with rVV.M1 and rVV.NP (Fig. 2 D and E). Going back to frozen aliquots of the original PBMC samples, CD8+ T cells were purified directly ex vivo and then stimulated in vitro with the appropriate peptides for IFN-γ ICS and ELISpot analysis. For the same donor, the numbers of CD8+ T cells responding to B*1501/NP140–150 (Fig. 5A) and B*3503/NP336–344 were about two to three times more abundant than those specific for A*0201/M158–66 (Fig. 5).
Fig. 5.
Ex vivo CTLp analysis for two immunodominant T-cell responses. The M58, NP140–150 (A and B) and NP336–344 (C and D) peptides were used to stimulate purified CD8+ T cells from donors 4 and 5, respectively, for 5 h in the presence of Brefeldin A in an ICS (A and C) or an overnight ELISpot assay (B and D).
Discussion
The first unexpected finding from this analysis was that the well-studied, highly conserved HLA-A2/M158–66 CD8+ T-cell response, although consistently detected in all eight donors, was not numerically immunodominant in >50% of the samples tested. For several reasons, this cannot be considered a function of biased sampling. First, the PR8 IAV used here infected most PBMCs to give APCs that were effective stimulators for the expansion of polyclonal, IAV-specific T-cell lines. Second, the 11 rVVs that allowed us to identify the immunogenic IAV proteins infected the APCs to comparable extents, which was shown by the equivalent production of an mAb-detected, early rVV gene product and the rVV-derived antigenic peptides recognized by specific murine T-cell lines (Fig. S1), indicating that any bias that would confound antigen identification was unlikely. Third, and perhaps most importantly, immunodominance hierarchies inferred from the in vitro analysis of two PBMC samples that showed similar rankings for CTLs recognizing M158A2 and the NP epitopes were additionally validated when T-cell populations were tested directly ex vivo for specific memory CTLp frequencies. This relegation of M158A2 in the human CTL response league is not unprecedented, because using a much more limited approach, Boon et al. (23) also found that the M158A2-specific set was smaller (or comparable in size) compared with a B*3501-restricted response in individuals with almost totally matched HLA-A and -B alleles.
Virus-specific T-cell responses tend to focus on more conserved determinants drawn predominantly from internal proteins, such as NP, basic polymerase 1 (PB1), and M1 in the IAVs. This profile has been established from both in vitro functional studies (6, 24) and biochemical work with peptides eluted from IAV-infected cells on HPLC (25). However, the relative importance of different IAV epitopes is still far from clear when it comes to immunogenicity and protection. Although a great deal of attention has been given to NP (24, 26, 27), Assarsson et al. (6) found, in humans, that both CD4+ and CD8+ T-cell responses are substantially targeted to epitopes derived from the PB1 and M1 proteins. The present analysis does not, however, provide unqualified support for this conclusion and emphasizes the importance of the IAV NP in CTL immunogenicity. Although NP, HA, and M1 are the most abundant IAV proteins (28), we do not understand why NP (then M) is particularly prominent, whereas HA hardly figures in the influenza CTL immunity equation. Such a lack of immunodominant CD8+ T-cell response to HA in the studied donors is unlikely a result from any lack of IAV exposure, because most adult Australians had been exposed to H1 IAV strains during the past 10 y as shown in Table S2.
Unlike HIV, the IAVs do not establish as persistent infections. In HIV, HLA-dependent (14) CD8+ T-cell immunodominance hierarchies are correlated with both transient protection and immune escape, with concurrent fitness loss in the overall CTL response (11, 12, 14). The need with influenza is also to correlate epitope specificity with protection (5), which translates, in turn, into identifying the viral peptides that give immunodominant responses in the context of defined, common HLA glycoproteins. At least for Caucasians, a vaccination (or immunotherapy) strategy targeted to HLA-A2 would cover about one-half of the population. As a consequence, research activity relating to epitope discovery in humans has been significantly biased to peptides that are predicted to bind to HLA-A2 and its super alleles. In fact, HLA-A2–restricted epitopes account for 39% of total IAV-derived components in the IEDB database (http://www.iedb.org/), with the current count for A2 being 48 (including 3 from NP) from all of the IAV strains studied thus far. Most are highly conserved between the IAVs that provided these epitopes and the PR8 strain used here (Table S3).
Interestingly, only one HLA-A2 epitope (M158A2) was found to be immunodominant in the present analysis, although in less than one-half of the HLA-A2+ donors. Similarly, there are 38 epitopes derived from NP, and the majority of the peptides shared sequences between the original IAV strains and the PR8 strain used in this study (Table S4). Again, in our assays, only three of the previously published NP peptides were shown to be immunodominant within a given donor. Beyond that, the systematic approach used here allowed for the identification of four unreported immunodominant epitopes: B*1501/NP140–150 (donor 4), B*1801/NP219–228 (donor 2), B*3503/NP336–344 (donor 5), and B*1501/NP404–412 (donor 2). These epitopes are summarized in Table S5. The results suggest that M158A2 immunodominance is dependent on which other HLA alleles are expressed within any given individual, a phenomenon that is well-recognized for HIV immunity (14). Therefore, even for Caucasians, the analysis of CTL response profiles needs to extend beyond HLA-A2.
A recent report claimed high accuracy of epitope prediction for CTL responses to rVV-derived peptides presented in the context of mouse H-2Kb/Db, because combined, peptide-stimulated T-cell activation accounted for nearly 100% of that triggered by the rVV-infected APCs (29). However, Yuen et al. (30) showed, using the same H-2b (C57BL/6J) mice, that at least one predicted epitope was incorrect and that the response to rVV-infected APCs may represent as much as a 50% underestimate. Even taking that possible 50% discrepancy into consideration, the clear divergence in prediction accuracy between human and murine systems is not easily explained by current knowledge. Although tentative, perhaps, it seems possible that binding motifs containing middle anchors (true for both H-2Kb and H-2Db) may confer tighter peptide selection and thus, better predictive outcomes.
Because of the relative small number of samples and the extreme nature of HLA diversity, the present study was not designed primarily to probe the general rules for HLA-dependent immunodominance. However, because M158–66 is highly conserved in most IAV strains (6, 9) and T cells specific for M158A2 can be detected in both memory populations and recall responses (31), it seems reasonable to assume that CTLs with this specificity would have been primed during an initial IAV exposure and then restimulated with each subsequent experience of natural IAV infection. Thus, if an alternative NP-specific response is more prominent than that to M158A2, it is likely that this reflects more optimal NP-specific stimulation rather than lack of M158A2 antigenicity.
Interestingly, there is a complete lack of immunogenic IAV peptide presentation, both from published studies and the present analysis, for any HLA-Cw allele. As in the host response to HIV (14), HLA-Cw is unlikely to play much part at the population level when it comes to influenza immunity. However, for those three individuals in which M158A2-specific T cells were immunodominant, the data suggest that other HLA alleles expressed by the same donors (including A*1101, B*1502, B*4001, A*0301, B*2705, B*3701, A*2501, B*4402, and B*4501) do not drive substantial IAV-specific CTL expansion. Conversely, where the M158A2-specific T-cell response was less prominent, other HLA-peptide complexes (all involving NP in this analysis) may simply be more immunogenic.
When it comes to identifying immunogenic peptides and defining pMHCI immunodominance hierarchies, the present systematic approach is clearly powerful. Even so, only one PR8 virus was analyzed, and it is obviously important to screen for NP variants that emerge in pandemic and seasonal IAVs (32). Comparative sequence analysis of the antigenic peptides identified in the current study showed a high level of NP219–228 and NP336–344 conservation for circulating IAV strains, both locally (in Australia, n = 23 H1N1 and n = 40 H3N2) and worldwide (Table S2). However, more variation was observed in the immunogenic NP140–150 and NP404–412 sequences (Table S2), suggesting either that these epitopes may be subject to greater selection pressure or that mutants have simply emerged by chance. Whichever is the case, any NP-related escape could potentially result in the response overall defaulting to M158A2.
Taken together, using a systematic screening approach to probe immunodominant CD8+ T-cell responses to IAV in HLA-A2+ donors led to the confirmation of three previously published pMHCI complexes and beyond that, allowed for the discovery of four unpublished immunodominant IAV epitopes. Interestingly, none of these epitopes involved HLA-A2, and with the exception of B*1501/NP404–412 displaying a perfect binding motif, three of four would not have been predicted by the currently available programs. This reinforces the perception that many other HLA-A and -B glycoproteins have the capacity to present immunogenic and even immunodominant peptides derived from NP and/or other IAV proteins. Focusing exclusively on HLA-A2 and M158–66 is too limiting. Particularly when it comes to identifying novel immunodominant response, peptide binding motifs cannot provide all of the answers (15).
Materials and Methods
PBMC Samples.
Eight HLA-A2–positive buffy coats were obtained from the Australian Red Cross. PBMC samples were isolated by Ficoll–Hypaque gradient and stored in liquid nitrogen until use. HLA typing was performed by the Victorian Transplantation and Immunogenetics Service (VTIS).
Viruses.
The Mount Sinai strain of PR8 IAV was propagated in 10-d-old embryonated chicken eggs. Allantoic fluids were harvested 2 d after infection, and aliquots were stored at −80 °C until use. rVV-containing individual HA, NA, NP, M1, M2, PB1, PB1F2, PB2, acidic polymerase (PA), nonstructural protein 1 (NS1), and NS2 IAV genes were gifts from Jonathan Yewdell and Jack Bennink (National Institutes of Health, Bethesda, MD). The viruses were propagated using a TK- cell line and were stored at −80 °C until use. These proteins are also derived from the PR8 IAV.
Synthetic Peptides and Antibodies.
All peptides were synthesized by Chiron Mimotopes; IAV-NP overlapping 18mers with 6-aa shifts and 13mers with 2-aa shifts were synthesized as cleaved pin peptides. Other PR8 NP-derived peptides were synthesized according to the previously published sequences as crude, desalted peptide (purity > 75%; sequences are shown in Table S1). All peptides were dissolved in DMSO. Anti-CD3 (PE Cy5.5), anti-CD8 (APC), and anti–IFN-γ (PE) mAbs were purchased from BD. Other antibodies are described in the appropriate sections.
Cell Culture.
All cells were cultured in RF-10 consisting of RPMI-1640 supplemented with 10% FCS, 2-ME (5 × 10−5 M), and antibiotics. Donor BLCLs were established using standard EBV transformation. The other human BLCL lines were made available from the International HLA Workshop and the VTIS. The C1R cells transfected with various HLA molecules were provided by James McCluskey (A1, A2, and B8; Department of Microbiology and Immunology, Melbourne University, Melbourne, Australia) and Scott Burrows (B4402 and B3503; Queensland Institute of Medical Research, Brisbane, Australia).
Bulk T-Cell Cultures.
Aliquots of 0.5 × 106 PBMCs were infected with IAV at a multiplicity of infection (MOI) of 10 for 1 h at 37 °C in acidic FCS-free RPMI-1640 followed by RF-10 for 3 h. After being washed two times, the infected PBMCs (as APCs) were cocultured with 4.5 × 106 PBMCs (as responders) in RF-10 with 20 U/mL rIL-2 (Roche). Peptide-specific CTL lines were generated by pulsing PBMCs (5 × 106) with 1 μM NP peptide at 37 °C for 30 min, and then, washed cells were cultured in RF-10 containing IL-2 for 12–15 d unless otherwise stated.
ICS and ex Vivo IFN-γ ELISpot Assay.
The IFN-γ ICS assay used a standard protocol (16), whereas for ELISpot analysis, PBMC CD8+ T cells purified (>95%) with MACS beads (Miltenyi Biotec) were added with the respective minimal peptides to 96-well nitrocellulose plates (Millipore) coated with an IFN-γ capture Ab (Mabtech); 18 h later, the wells were washed with PBS containing 0.05% Tween 20. Biotinylated detection Ab (Mabtech), streptavidin-conjugated alkaline phosphatase, and its substrate (Sigma-Aldrich) were used to develop the IFN-γ spots.
HLA Restriction Assays.
Determining the identity of the presenting HLA alleles depended on the use of multiple, partial HLA class I-matched BLCLs or C1R HLA transfectants. These cells were pulsed with the peptide of interest, and then, they were washed extensively and used as APCs in an ICS assay.
Bioinformatics Analysis.
Protein sequences were aligned and amino acid differences were scored to determine the sequence conservation between local circulating and vaccine strains for the newly identified NP-derived peptides. The National Center for Biotechnology Information (NCBI) influenza virus database (http://www.ncbi.nlm.nih.gov/genomes/FLU/Database/nph-select.cgi?go=1) was used (December 1, 2010) with the search criteria Australia, NP, H3N2/H1N1, non-identical sequences excluded, full length only, from 2000 to 2010, which identified H3N2 (n = 40) and H1N1 (n = 24) sequences. Selected NP sequences representing influenza strains previously used in the trivalent vaccine were also accessed from the NCBI influenza virus resource database (Table S2 footnote has strain names). Protein sequences were aligned using the NCBI database, peptide regions were mapped, and frequency of mutation was determined across the various sequence groups.
Supplementary Material
Acknowledgments
S.V. is a recipient of the Australian Postgraduate Award, and K.K. is an National Health and Medical Research Council R. D. Wright Fellow. W.C. is an NHMRC Senior Research Fellow (603104). This project was partly supported by NHMRC Program Grant 567122 (to P.C.D. and W.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105624108/-/DCSupplemental.
References
- 1.Rimmelzwaan GF, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine. 2000;19:1180–1187. doi: 10.1016/s0264-410x(00)00310-8. [DOI] [PubMed] [Google Scholar]
- 2.Oukka M, et al. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J Immunol. 1996;157:3039–3045. [PubMed] [Google Scholar]
- 3.Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhaney JE, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 5.Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci USA. 2007;104:246–251. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assarsson E, et al. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol. 2008;82:12241–12251. doi: 10.1128/JVI.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn KJ, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 8.Belz GT, Altman JD, Doherty PC. Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia. Proc Natl Acad Sci USA. 1998;95:13812–13817. doi: 10.1073/pnas.95.23.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMichael AJ, Gotch FM, Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986;164:1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon AC, et al. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J Virol. 2002;76:582–590. doi: 10.1128/JVI.76.2.582-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie AJ, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 12.Ammaranond P, et al. A new variant cytotoxic T lymphocyte escape mutation in HLA-B27-positive individuals infected with HIV type 1. AIDS Res Hum Retroviruses. 2005;21:395–397. doi: 10.1089/aid.2005.21.395. [DOI] [PubMed] [Google Scholar]
- 13.Eklund AC, Belchak MM, Lapidos K, Raha-Chowdhury R, Ober C. Polymorphisms in the HLA-linked olfactory receptor genes in the Hutterites. Hum Immunol. 2000;61:711–717. doi: 10.1016/s0198-8859(00)00132-4. [DOI] [PubMed] [Google Scholar]
- 14.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrows SR, Rossjohn J, McCluskey J. Have we cut ourselves too short in mapping CTL epitopes? Trends Immunol. 2006;27:11–16. doi: 10.1016/j.it.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Ebert LM, et al. A long, naturally presented immunodominant epitope from NY-ESO-1 tumor antigen: Implications for cancer vaccine design. Cancer Res. 2009;69:1046–1054. doi: 10.1158/0008-5472.CAN-08-2926. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 18.Schumacher TN, et al. Peptide selection by MHC class I molecules. Nature. 1991;350:703–706. doi: 10.1038/350703a0. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski S, et al. Multiple pathways are involved in the extracellular processing of MHC class I-restricted peptides. J Immunol. 1993;151:4033–4044. [PubMed] [Google Scholar]
- 20.Zemmour J, Little AM, Schendel DJ, Parham P. The HLA-A,B “negative” mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992;148:1941–1948. [PubMed] [Google Scholar]
- 21.DiBrino M, et al. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J Immunol. 1993;151:5930–5935. [PubMed] [Google Scholar]
- 22.Suhrbier A, Schmidt C, Fernan A. Prediction of an HLA B8-restricted influenza epitope by motif. Immunology. 1993;79:171–173. [PMC free article] [PubMed] [Google Scholar]
- 23.Boon AC, et al. Preferential HLA usage in the influenza virus-specific CTL response. J Immunol. 2004;172:4435–4443. doi: 10.4049/jimmunol.172.7.4435. [DOI] [PubMed] [Google Scholar]
- 24.Lee LY, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl A, et al. HLA class I molecules consistently present internal influenza epitopes. Proc Natl Acad Sci USA. 2009;106:540–545. doi: 10.1073/pnas.0811271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 27.Kreijtz JH, et al. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82:5161–5166. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw ML, Stone KL, Colangelo CM, Gulcicek EE, Palese P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008;4:e1000085. doi: 10.1371/journal.ppat.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moutaftsi M, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 30.Yuen TJ, et al. Analysis of A47, an immunoprevalent protein of vaccinia virus, leads to a reevaluation of the total antiviral CD8+ T cell response. J Virol. 2010;84:10220–10229. doi: 10.1128/JVI.01281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touvrey C, et al. Dominant human CD8 T cell clonotypes persist simultaneously as memory and effector cells in memory phase. J Immunol. 2009;182:6718–6726. doi: 10.4049/jimmunol.0803095. [DOI] [PubMed] [Google Scholar]
- 32.Rimmelzwaan GF, Kreijtz JH, Bodewes R, Fouchier RA, Osterhaus AD. Influenza virus CTL epitopes, remarkably conserved and remarkably variable. Vaccine. 2009;27:6363–6365. doi: 10.1016/j.vaccine.2009.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.