Abstract
Recently we have established that the kidney tubular epithelium is repaired by surviving epithelial cells. It is not known, however, whether a population of intratubular adult progenitor cells are responsible for this epithelial repair after acute kidney injury. In this study, we used an unbiased DNA analog-based approach that does not rely on candidate markers to track multiple rounds of cell division in vivo. In the proximal tubule, robust thymidine analog incorporation was observed postinjury. Cell division was stochastic and enriched among cells that were injured and dedifferentiated. There was no evidence for the presence of a population of specialized progenitors that repeatedly divide in response to injury. Instead, these results indicate that after injury, new epithelial cells arise from self-duplication of surviving cells, most of which are injured. Because the renal papilla contains DNA label-retaining cells and has been proposed as a stem cell niche, we examined the proliferative behavior of these putative progenitors after ischemia-reperfusion injury. Although label-retaining cells in the renal papilla diminished with time after ischemia-reperfusion injury, they neither proliferated nor migrated to the outer medulla or cortex. Thus, nonlethally injured cells repopulate the kidney epithelium after injury in the absence of any specialized progenitor cell population.
During adult homeostasis, kidney cell proliferation is very low (1). After acute injury, kidney epithelial cells rapidly reenter the cell cycle and this high proliferative capacity has been interpreted to reflect an intrinsic ability of surviving epithelial cells to adapt to the loss of neighboring cells by proliferating, ultimately replacing the cells that died as a result of the insult (2, 3). This process has been proposed to occur by a process of dedifferentiation of the surviving proximal tubule epithelial cells. This model has been challenged by more recent data (reviewed in ref. 4), including the description of adult renal stem and progenitor cells in lower vertebrates (5, 6), which suggest a role for resident stem or progenitor cells in kidney repair. Here we examine the replicative history of renal epithelial cells to address this important question.
To clarify the origin of reparative epithelia after acute kidney injury, we previously used genetic lineage analysis and determined that extratubular cells do not appreciably contribute to epithelial repair after acute kidney injury (7), ruling out the possibility of a bone marrow-derived or renal interstitial cell-derived epithelial progenitor cell population. These findings do not exclude the existence of an intratubular progenitor cell, however, because such a cell would have been labeled by our genetic strategy. Recent evidence indicates that such an intratubular epithelial progenitor could exist. In renal papilla, epithelial slow-cycling label-retaining cells (LRCs) have been characterized, and proposed to migrate toward the cortex during renal repair (8, 9). In the murine renal cortex, candidate stem-cell markers including label retention (10, 11), Oct4 expression (12), podocalyxin promoter activity (13), and NFATc1 expression (14), have been suggested to identify epithelial progenitors, and the identification of Kruppel-positive intratubular progenitors in Drosophila malpighian tubules provides indirect support for this concept (15).
The purpose of this study was to distinguish between proximal tubule repair by a specialized intratubular progenitor population or by self-duplication of surviving epithelia. To evaluate these two possibilities, we adapted and validated a DNA analog-based lineage analysis technique to track sequential rounds of renal epithelial cell division after ischemia-reperfusion injury (IRI). This unbiased approach was used to trace the fate of cortical tubule cells and papillary LRCs and to ask whether a rapidly proliferating progenitor population among surviving epithelial cells could be detected. The results indicate that cell division in the cortex and outer medulla occurs predominantly among injured and dedifferentiated epithelial cells, and this proliferation is stochastic and reflects self-duplication rather than selective activation of an intratubular epithelial progenitor population. Furthermore, papillary LRCs do not contribute to proximal tubule epithelial cells after acute injury.
Results
Validation of Thymidine Analog Assay Specificity.
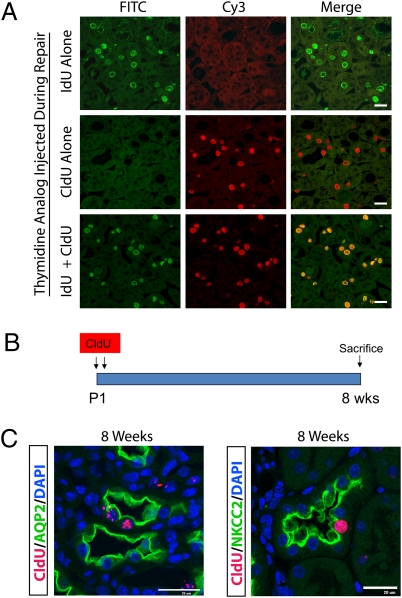
To accurately quantify two sequential rounds of DNA synthesis in the same cell, we used the thymidine analogs, 5-chloro-2-deoxyuridine (CldU) and 5-iodo-2-deoxyuridine (IdU), in a strategy validated in solid organs, including pancreas (16), heart (17), and adult, uninjured kidney (18). To assess the specificity of CldU and IdU in our renal IRI model, we performed unilateral IRI and killed mice 48 h after injury. Three hours before being killed, the mice were injected with either IdU alone, CldU alone, or the combination of IdU and CldU. A protocol was optimized that allowed for specific detection of each thymidine analog during repair. Using this optimized protocol (see Materials and Methods), kidney sections from all three conditions were costained with both anti-IdU and anti-CldU antibodies. In mice that received IdU alone, strong IdU-positive nuclei were detected in the outer medulla with no CldU positivity (Fig. 1A). Similarly, mice that received CldU alone showed strong CldU nuclear staining but no IdU signal. In mice that received both IdU and CldU, there was excellent concordance between nuclear IdU and CldU signal. This experiment shows that our thymidine analog protocol exhibits excellent specificity for distinguishing IdU and CldU nuclear staining.
Fig. 1.
Thymidine analog detection after either IRI or pulse chase. (A) To validate the specificity of thymidine analog labeling, IdU and CldU was injected alone or in combination 3 h before killing during repair. Kidney sections from outer medulla were probed with both anti-IdU (green) and anti-CldU (red) antibodies. When either IdU or CldU was injected alone there was no cross-reactivity, and when both IdU and CldU were injected together, there was concordant nuclear staining. (B) Newborn mice at P1 and P2 were injected with CldU followed by an 8-wk chase, to identify slow cycling cells in kidney. (Scale bar, 25 μm.) (C) LRCs were located in papilla, primarily in epithelial cells. ∼80% of LRC were AQP2-positive collecting duct cells, with the rest from other tubule segments such as NKCC2-positive thick ascending limb. (Scale bar, 25 μm.)
Slow Cycling Cells Identified by CldU Retention.
Stem cells retain DNA labels either through slow cycling or asymmetric segregation of chromosomes. The latter theory, also called the “immortal-strand hypothesis,” proposes that stem cells avoid the accumulation of mutations that arise during DNA replication by retaining parental DNA strands during asymmetric cell division (19). In kidney, the renal papilla has been identified as a site of DNA LRCs and therefore proposed as a stem cell niche (8). We next asked whether CldU could be used to identify such LRCs, and to do so we briefly pulsed newborn mice with CldU and chased for 8 wk in a protocol similar to that used by others to identify LRCs in adult mouse and rat kidney (Fig. 1B) (8, 9, 20). As expected, LRCs were located primarily in the tip and midportion of the renal papilla, where they represented 8.5 ± 1.0% (average ± SE) of tubular cells, with ∼80% of LRC in an aquaporin-2–positive collecting duct, and the rest in noncollecting duct epithelia, such as NKCC2-positive cells of the thick ascending limb of the loop of henle (Fig. 1C).
Cells That Proliferate After Ischemia Reperfusion in the Cortex and Outer Medulla Are Not LRCs.
In the outer medulla and cortex, there was a very low number of tubular LRCs, and this number did not significantly change after IRI, indicating that there are very few LRCs in the cortex or outer medulla and papillary LRCs do not migrate from the papilla toward the cortex (Fig. 2 A–C). In contrast, substantial IdU incorporation could be detected beginning 24 h after injury and increasing thereafter, from 0.3 ± 0.1% in uninjured cortex to 26.9 ± 4.1% of tubular cells by 48 h after IRI (Fig. 2D). Similar results were obtained when mice were subject to bilateral IRI and analyzed 48 h after IRI (Fig. S1).
Fig. 2.
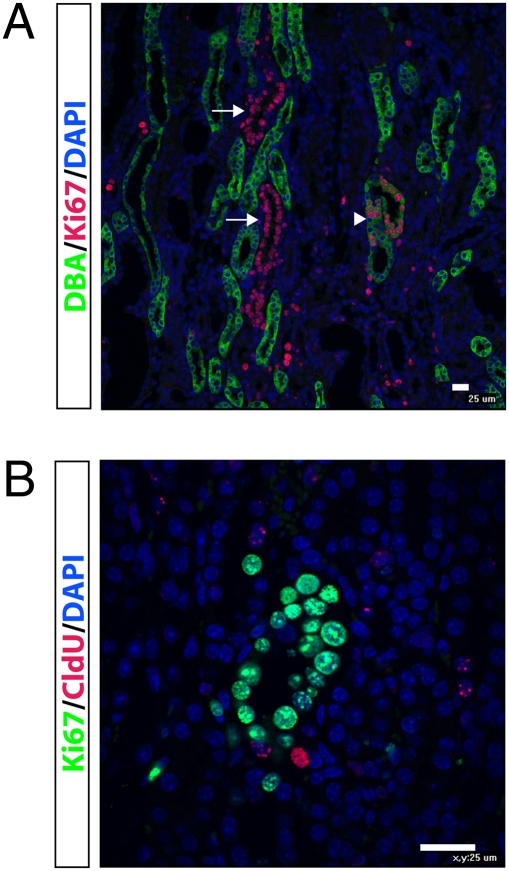
Papillary LRCs do not proliferate or migrate after IRI. (A) Groups of newborn mice (n = 3 per group) were injected with CldU at P1 and P2 and chased for 8 wk. Then, unilateral IRI was performed, and mice were killed at either 12, 24, 36, or 48 h after injury. IdU was injected 3 h before killing in each condition. (B) In the outer medulla, IdU (green) incorporation began 24 h after injury and increased thereafter. There were very rare CldU (red) positive cells in the outer medulla, and they neither incorporated IdU nor increased in number over time. In papilla, CldU-positive LRCs did not incorporate IdU during repair, although several IdU-positive cells could be found in papilla beginning 24 h after injury (arrows). (Scale bar, 25 μm.) (C) The percentage of LRCs in the cortex did not change during repair, indicating that there was no migration of LRCs from papilla to cortex. In contrast, the percentage of LRCs in the papilla decreased over the 48-h time course. *P = 0.003, 0 h compared with 48 h. (D) There was a dramatic increase in IdU-positive epithelial cells in the cortex, but only few cells in papilla divided after IRI. *P < 0.05, **P < 0.01, cortex compared with papilla. (E) Using the cell-cycle marker Ki67, there was a dramatic increase in cycling cells in cortex during repair, but very few Ki67-positive cells in renal papilla. *P < 0.05, **P < 0.01, cortex compared with papilla.
LRCs in Papilla Tip and Midportion Do Not Proliferate or Migrate to Cortex After Injury.
To determine whether papillary LRCs selectively proliferate after injury, as would be expected for a progenitor population, mice that had received pulsed CldU at P1 and P2 were subject to unilateral IRI at 8 wk of age. IdU was administered 3 h before being killed, which was performed at 12, 24, 36, and 48 h after IRI (Fig. 2A). Papillary LRCs were readily detected in uninjured kidneys. After IRI, the number of papillary LRCs gradually decreased from 8.5 ± 1.0% before injury to 4.6 ± 5.6% of tubular cells 48 h after injury (Fig. 2 B and C). No IdU-positive cells were observed in uninjured papilla, and very rare IdU-positive cells were found in the papilla by 48 h after IRI at 0.2 + 1.4% of tubular nuclei, but no papillary cells were positive for both CldU and IdU (Fig. 2 B and D). These results suggest that papillary LRCs in the tip and midportion of the papilla do not proliferate in the first 48 h following IRI.
Although recent data refute the immortal-strand hypothesis in the best-characterized adult stem-cell population—hematopoietic stem cells (21)—the foregoing experiment leaves open the possibility that papillary LRCs did not incorporate IdU because they retain the older, CldU-labeled, DNA strands. To address this possibility, we costained papilla and cortex for the mitogenic marker Ki67, which shows nuclear staining in cells that have entered the cell cycle. If papillary LRCs selectively proliferate during repair even as they retain CldU-labeled chromosomes, they will still appear as Ki67-positive cells within the papilla. Although Ki67-positive cells in the cortex increased from 0.6 ± 0.2% to 40.0 ± 7.0% by 48 h after IRI (P < 0.0001), there was a very modest increase in papilla, from undetectable in uninjured papilla to 0.5 ± 2.8% at 48 h after injury (Fig. 2E) (P = NS). No papillary cells were observed to be positive for both CldU and Ki67 at any time point, indicating that papillary LRCs do not selectively proliferate after IRI.
By 48 h after IRI, there were occasional collecting ducts and noncollecting tubules located in the papilla base and inner medulla, where the majority of epithelial nuclei were Ki67-positive (Fig. 3A). To determine whether these proliferating cells derived from CldU-positive LRCs, we costained for CldU and Ki67. Although occasional CldU-positive LRCs could be found in this region, some even adjacent to these “chains” of proliferating cells (Fig. 3B), we did not detect any epithelia positive for both CldU and Ki67. Because we did not observe CldU-positive cells that were also positive for either IdU or Ki67 in the papilla tip or midportion, these results together suggest that the chains of Ki67-positive cells in the papilla base do not derive from LRC.
Fig. 3.
Chains of proliferating cells in the upper papilla do not derive from LRCs. (A) Forty-eight hours after IRI, tubules can be seen in which nearly all epithelial cell nuclei are Ki67-positive (red). These tubules are located in the base of the papilla, adjacent to the inner medulla. Some of these proliferating tubules are DBA-positive, indicating a collecting duct (arrowhead), and others are DBA-negative (arrows). (B) Costaining of CldU (red) and Ki67 (green) shows that LRCs are not contributing to the Ki67-positive cells, even though CldU-positive LRCs can be found adjacent to proliferating tubules. (Scale bar, 25 μm.)
Proximal Tubule Cell Division After Injury Is Stochastic.
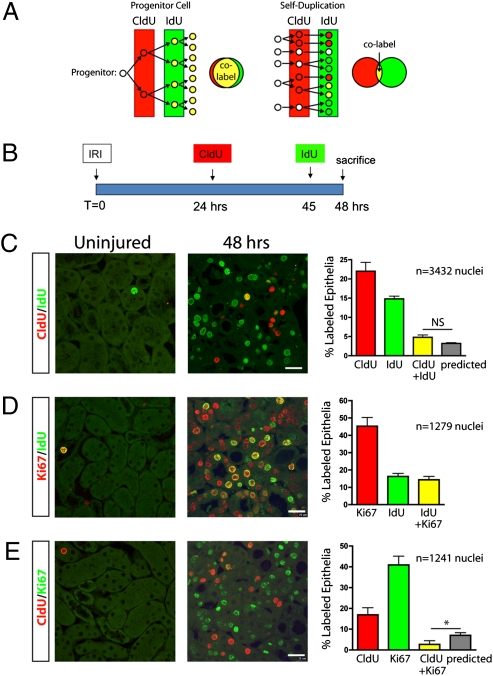
To test whether an intratubular progenitor cell is responsible for generation of new proximal tubule epithelia after IRI, we treated mice with a single injection of CldU 24 h after unilateral IRI followed by a single injection of IdU 45 h after IRI with killing at 48 h (Fig. 4A). If an intratubular progenitor selectively proliferates during repair, its progeny will divide rapidly and thereby incorporate both CldU and IdU. Surviving epithelial cells that are not progenitors will not divide at all, leaving a large population of double-labeled cells. In contrast, if proximal tubules repair by self-duplication of all surviving epithelial cells, proliferation will be random, and the fraction of double-labeled cells will be low and can be predicted by multiplying the percentage of CldU-positive cells times the percentage of IdU-positive cells (Fig. 4A). CldU is cleared within hours under normal conditions, and by separating the CldU and IdU injections by 21 h we attempted to minimize the possibility that double labeling with this experimental protocol could simply reflect slow clearance of CldU (21).
Fig. 4.
Proximal tubule repair does not involve an intratubular progenitor population. (A) If an intratubular progenitor cell generates new epithelial cells during repair, such progenitors would selectively proliferate after injury resulting in a high percentage of colabeled nuclei after sequential CldU and IdU pulses. If all surviving epithelial cells are capable of proliferating through self-duplication, then cell division is stochastic and a certain fraction of cells will incorporate CldU, and a different fraction epithelial cells will incorporate IdU, with few cells incorporating both labels. (B) Adult mice were subject to unilateral IRI, and injected with CldU 24 h after injury and IdU 3 h before being killed at 48 h after injury. (C) Sham-operated kidneys showed little CldU (red) or IdU (green) uptake, but injured kidneys had substantial CldU and IdU uptake. A smaller fraction of cells was positive for both CldU and IdU, indicating sequential cell division in the prior 24 h, and this fraction was not different from that predicted if cell division were stochastic (%predicted = %CldU-positive × %IdU-positive). (D) Nearly all cells that incorporated IdU 3 h before being killed were also Ki67-positive, as expected. (E) The number of Ki67-positive cells was more than double the number of CldU-positive cells; however, very few cells were positive for both CldU and Ki67, and this number was significantly less than that predicted if cell division were stochastic. *P < 0.05. n = 3 mice for each condition. (Scale bar, 25 μm.)
CldU was administered 24 h after IRI and IdU was administered 45 h after IRI, just before killing at 48 h (Fig. 4B). Few CldU- or IdU-labeled nuclei were seen in uninjured kidney. At 48 h after injury, there were 22.0 ± 2.3% CldU-positive and 14.8 ± 0.7% IdU-positive nuclei (Fig. 4C). There were 4.8 ± 0.6% copositive nuclei, a fraction that was not different from the 3.2 ± 0.2% copositive nuclei predicted if cell division is stochastic (predicted = CldU% × IdU%).
Because Ki67 staining identifies cells that have reentered the cell cycle, including those in G1 that have not progressed through the S-phase, we also measured Ki67 staining in this experiment. All IdU-positive cells were expected to be Ki67-positive, and this is what we observed (Fig. 4D). Cells that were Ki67-positive but IdU-negative represent cells in G1 that have not yet progressed through the S-phase. When we examined CldU and Ki67, few copositive cells were observed: although 16.9 ± 3.5% of proximal tubule nuclei were CldU-positive and 40.9 ± 4.2% were Ki67-positive, only 2.7 ± 1.7% stained for both CldU and Ki67, a number that was significantly less than the 7.0 ± 1.32% predicted. These results indicate that within the first 48 h after injury, at least 55% (CldU% + Ki67% − copositive%) of outer medullary epithelia have either reentered the cell cycle (Ki67 expression) or completed the S-phase (CldU uptake).
Cell Division Occurs Primarily in Injured and Dedifferentiated Epithelia.
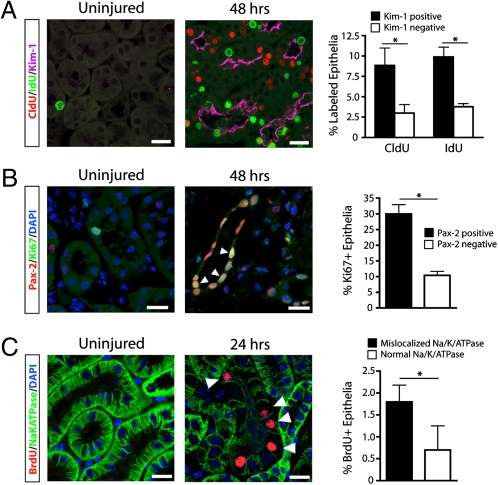
Our results indicate that most if not all proximal tubule epithelial cells are capable of proliferating after injury but do not answer whether injured but surviving epithelia or uninjured bystanders are more likely to proliferate after IRI. To address this question, we costained for CldU, IdU, and Kidney Injury Molecule-1 (Kim-1), an apical phosphatidylserine receptor that is strongly induced in injured proximal tubule epithelia and undetectable in uninjured kidney (22, 23). We found that 75.0 ± 18.1% of CldU-positive cells and 72.3 ± 8.9% of IdU-positive cells coexpressed Kim-1 (Fig. 5A) (P < 0.05 for both, compared with Kim-1–negative cells). Because Kim-1 is not expressed in thick ascending limbs or collecting ducts, this fraction may be an underestimate because we did not exclude these segments from the analysis and some portion of cells in those tubule segments may proliferate after injury.
Fig. 5.
Cell division occurs primarily in injured, dedifferentiated proximal tubule epithelial cells. (A) Mice treated with sequential CldU and IdU pulses and killed 48 h after injury show little thymidine analog uptake and no Kim-1 staining in sham-operated kidneys. In injured kidneys, there was strong apical Kim-1 staining. Tubules that were positive for Kim-1 were more likely to have divided over the previous 24 h (CldU) or 3 h (IdU), indicating that injured tubules are more likely to proliferate during repair. *P < 0.01. n = 3 mice for each condition. (B) Injury induces nuclear Pax2 expression in outer medullary tubular epithelia. Ki67-positive cycling epithelia were more likely to express the dedifferentiation marker Pax2. *P < 0.001. n = 4 mice for each condition. (C) Mice treated with a single pulse of BrdU before being killed 24 h after injury show reduced or absent basolateral Na/K/ATPase staining in the majority of BrdU-positive cells, indicating that proliferating cells are dedifferentiated at this time point. *P < 0.05. n = 3 mice for each condition. (Scale bars, 25 μm.)
To corroborate this finding, we next investigated whether dividing epithelia expressed Pax-2, a transcription factor transiently expressed during renal development that is reexpressed in dedifferentiated proximal tubule after acute injury (24, 25). Of the Ki67-positive epithelial cells, 74.3 ± 7.2% coexpressed Pax-2 (Fig. 5B) (P < 0.001 compared with Pax-2–negative cells), consistent with a previous observation that BrdU/Pax-2 copositive cells can be found in rat tubules after acute injury (26). Reduced or mislocalized expression of basolateral Na/K/ATPase, a marker of terminal epithelial differentiation, is an additional marker of epithelial dedifferentiation (27, 28). We analyzed the expression pattern of the Na/K/ATPase 24 h after ischemia-reperfusion in mice that received BrdU 3 h before being killed. We observed substantially reduced or absent basolateral Na/K/ATPase expression in 71.9 + 15.4% of BrdU-positive cells (Fig. 5C) (P < 0.05 compared with cells with normal Na/K/ATPase). Taken together, these results indicate that the majority of proliferating epithelial cells are injured and dedifferentiated in the repairing kidney.
Discussion
There are three main findings from the present work. First, epithelial proliferation after ischemic injury occurs by self-duplication of differentiated cells, not by an intratubular progenitor cell mechanism. Second, the cells most likely to proliferate within the proximal tubule are those that are injured and dedifferentiated rather than uninjured bystanders. Third, our data do not suggest that slow-cycling cells in the papilla proliferate or that they or their progeny migrate to the proximal tubule after IRI.
The CldU/IdU-labeling technique does not rely on candidate stem-cell markers to identify putative progenitors and this allowed an assessment of the replicative history of slow-cycling cells in the papilla, which lack any other distinguishing marker aside from thymidine analog retention itself. Indeed, papillary LRCs express markers of terminal differentiation, such as aquaporin-2, which is not consistent with a progenitor cell identity that should be undifferentiated. The nondependence on stem-cell markers is an important attribute of this technique because it is well-known that the injured cell can dedifferentiate and express a host of proteins in vivo that are typical of earlier stages of development. Furthermore, once the cells are placed in culture they also assume markers of early development, which likely do not reflect pluripotency and stem-cell character in vivo.
If an intratubular epithelial progenitor located within a proximal tubule contributed substantially to the generation of new epithelial cells during repair, most proliferating epithelial cells should have been positive for both CldU and IdU. We observed infrequent copositive cells at a frequency that was not different from what would occur if cell division were stochastic. The fraction of CldU- and Ki67-copositive cells was even lower, and may be the more accurate reflection of epithelial proliferative history in our experiment because we cannot rule out that unilateral IRI might have slowed clearance of CldU leaving a low concentration present as IdU was injected, which would tend to increase the number of CldU- and IdU-copositive cells. Such slow clearance would bias our results toward a small increase in double-labeled cells, as has been previously reported (21). The observation that proximal tubule cells undergo cell cycle arrest after division in growing rats is consistent with our low measured frequency of CldU- and Ki67-copositive cells after IRI (18). Taken together, our observations do not support even a minor contribution of specialized intratubular progenitors to epithelial repair.
We determined that a majority of proliferating cells after IRI were injured and dedifferentiated because dividing cells coexpressed Kim-1 and Pax-2, and had reduced and mislocalized expression of the Na/K/ATPase. The induction of injury marker expression among repairing epithelial cells has implications for the interpretation of experiments analyzing putative adult epithelial stem-cell markers after injury. If a candidate stem-cell marker is induced in terminally differentiated cells after injury, then it is an injury marker and not a stem-cell marker. For example, NFATc1 was described recently as an intratubular progenitor cell marker in well-performed experiments that showed an important role for NFATc1 in recovery from acute injury (14). Because lineage-marked NFATc1-positive cells expanded after injury, these cells were interpreted as progenitors. However, our results argue against the existence of an intratubular progenitor and therefore suggest that expansion of NFATc1-Cre–labeled cells may reflect induction of nFatc1 promoter activity in injured cells that subsequently proliferate—similar to Kim-1—rather than selective clonal expansion of an intratubular progenitor.
Although thymidine analog-based pulse-chase has been used to identify stem cells in the hematopoietic system (29), skin (30), mammary gland epithelium (31), and heart (32), this technique also has limitations. Label retention is neither sensitive nor specific for identifying stem cells: less than 6% of hematopoietic stem cells retain label, and fewer than 0.5% of LRCs in the hematopoietic system are in fact stem cells (21). These findings raise the possibility that papillary LRCs are a similarly heterogeneous population and that we might have missed the small number of LRCs that are truly renal progenitors. Although we cannot rule this possibility out, it seems unlikely because we did not observe cycling papillary LRCs, either in the tip and midportion or in the papilla base. Moreover, even if the papillary LRCs that are progenitors are rare, we should have been able to detect their progeny (even if they migrated out of the papilla tip) using our dual-labeling approach. We did not find evidence for such a “transit-amplifying population” anywhere in the kidney.
It has been proposed that papillary LRCs migrate from the tip to its base, where they undergo proliferative expansion. Although we did observe tubules in this region where almost all nuclei were Ki67-positive, they did not derive from papillary LRCs because we could not detect cells copositive for CldU and either IdU or Ki67 in the papilla tip, midportion, or base. We also did not observe a corresponding uptake of IdU in the region where we observed chains of Ki67-positive epithelial cells, suggesting that these cells may be progressing through G1 very slowly or are potentially arrested in G1 as a consequence of DNA damage or reactive oxygen species (33). In any case, the results do not support these Ki67-positive cells as direct participants in outer medullary proximal tubule repair in the first 48 h after injury.
Papillary LRCs decreased after IRI. The reasons for this finding, consistent with other reports (8), are unclear. We found very little cell division in papilla so dilution of label through DNA synthesis cannot explain the loss of LRCs. There is very little apoptotic cell death in papilla as well (8). We do not find evidence for a migration of papillary LRCs to the cortex, but these LRCs might be migrating out of the kidney entirely. Although the mechanism for this finding requires further investigation, we did not find any evidence that the loss of papillary LRCs after IRI reflects mobilization of a progenitor pool through proliferation or migration to outer medulla. This conclusion is consistent with a recent study that proposed that papillary label retention reflects the early quiescence of papilla during development rather than a stem-cell pool (34).
In summary, we have shown that proximal tubule epithelial cells expand by self-duplication of surviving cells, not by replication of a specialized intratubular progenitor pool. Most of these cells are injured. In the future it will be important to understand the molecular mechanisms regulating epithelial dedifferentiation and proliferation after acute injury.
Materials and Methods
Animal Experiments.
All mouse studies were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Harvard University. Male C57Bl6 mice, aged 8 to 10 wk were anesthetized with pentobarbital sodium (60 mg/kg body weight) before surgery. At least three mice for each time point were used. Body temperatures were controlled at 36.5 to 37.5 °C throughout the procedure. Kidneys were exposed through flank incisions, and mice were subjected to ischemia by clamping the renal pedicle with nontraumatic microaneurysm clamps (Roboz), which were removed after 27 min. One milliliter of 0.9% NaCl was administered intraperitoneally 2 h after surgery.
CldU (Sigma-Aldrich) and IdU (MP Biomedicals) were made fresh as 20-mg/mL stocks in sterile 0.9% saline warmed to 45 °C, as described (35). Thymidine analogs were injected at 50 mg/kg intraperitoneally. Mice were anesthetized, killed, and immediately perfused via the left ventricle with ice-cold PBS for 2 min. Kidneys were hemisectioned and fixed in 10% neutral buffered formalin at 4 °C for 16 h and mounted in paraffin sections, taking care to orient the papilla for optimal sectioning.
Immunofluorescence Microscopy.
Detection of CldU and IdU was accomplished using modifications of published protocols (16, 18, 36). Paraffin sections (4 μM) were rehydrated, nuclear membranes permeabilized with 0.2% Triton X-100 in PBS for 5 min, and antigens retrieved by treatment with heated citrate (Antigen Unmasking Solution; VectorLabs). After blocking in 5% normal goat serum in PBS, sections were incubated overnight at 4 °C with both mouse anti-BrdU (which binds IdU strongly but CldU weakly, clone B44, 1:200; Becton-Dickenson) and rat anti-BrdU (which binds to CldU but not IdU, clone BU1/75, 1:1,500; Abcam). Next, slides were incubated in warmed high-salt TBS-T buffer (0.5 M NaCl, 36 mM Tris-HCl, 0.5% Tween-20, pH 8) and incubated with shaking at 225 rpm, 37° for 20 min. Secondary antibodies with minimal cross reactivity to other species were used. FITC-conjugated goat anti-mouse IgG (Jackson Laboratories) was used to reveal IdU, and Cy3-conjugated goat anti-rat IgG (Jackson Laboratories) was used to reveal CldU, both at 1:500.
The following additional antibodies were used: aquaporin-2 (Rabbit, 1:1,000, Cat. No. 15116; Abcam), DBA-lectin (FITC coupled, 1:500, Cat. No. FL-1031; VectorLabs), Ki67 (Rabbit, 1:200, Cat. No. VP-RM04; VectorLabs), Ki67 (FITC-coupled, rabbit, 1:75, Cat. No. 27619; Abcam), NKCC2 (Rabbit, 1:100, Cat. No. NKCC21-A; Alpha Diagnostic International), Kim-1 (Goat, 1:500, Cat. No. AF1817; R&D Systems), Na/K/ATPase (Rabbit, 1:500, Cat. No. 76020; Abcam), and Pax2 (Rabbit, 1:500, Cat. No. 79389; Abcam). Sections were mounted in Vectashield containing 40, 6-diamino-2-phenylindole (VectorLabs). Images were taken on a Nikon C1 D-Eclipse confocal microscope using standard procedures.
Quantification of Labeled Tubular Cells.
Two areas of kidney were quantitated for thymidine analog uptake. The combined outer medulla and inner cortex were assessed because this is the region with most proliferative activity after IRI. In papilla, the mid- and tip-regions were examined and counted together, because these are the regions where LRC depletion with aging occurs, suggestive of the true papillary progenitor niche (9). Ten high-power fields from each mouse were examined from each kidney region, and epithelial cell nuclei counted based on DAPI staining. Interstitial cell nuclei were not counted. CldU, IdU, and copositive cells were and expressed as a percentage of the total number of tubular epithelial cells as assessed by DAPI-positive intratubular nuclei.
Statistical Analyses.
The results are expressed as the means ± SEM. Nuclei were counted from 10 high-power fields for each kidney, and each experiment consisted of at least three mice per group. Statistical analyses were performed using two-way ANOVA followed by a Bonferroni test. P values less than 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
We thank Jake Kushner for advice on the CldU/IdU assay. This work was supported by a seed grant from the Harvard Stem Cell Institute and National Institutes of Health Grants DK73628, DK88923, and DK84316 (to B.D.H.), and DK864406, DK39773, and DK72381 (to J.V.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100629108/-/DCSupplemental.
References
- 1.Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int. 1978;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 3.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little MH, Bertram JF. Is there such a thing as a renal stem cell? J Am Soc Nephrol. 2009;20:2112–2117. doi: 10.1681/ASN.2009010066. [DOI] [PubMed] [Google Scholar]
- 5.Elger M, et al. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver JA, et al. Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol. 2009;20:2315–2327. doi: 10.1681/ASN.2008111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006;17(1):188–198. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura S, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 13.Appel D, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langworthy M, Zhou B, de Caestecker M, Moeckel G, Baldwin HS. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:C807–C813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 19.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 20.Vogetseder A, Karadeniz A, Kaissling B, Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005;124(2):97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 21.Kiel MJ, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimura T, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imgrund M, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 1999;56:1423–1431. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva S, Céspedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 26.Maeshima A, Maeshima K, Nojima Y, Kojima I. Involvement of Pax-2 in the action of activin A on tubular cell regeneration. J Am Soc Nephrol. 2002;13:2850–2859. doi: 10.1097/01.asn.0000035086.93977.e9. [DOI] [PubMed] [Google Scholar]
- 27.Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol. 1998;275:C711–C731. doi: 10.1152/ajpcell.1998.275.3.C711. [DOI] [PubMed] [Google Scholar]
- 28.Kuehn EW, Park KM, Somlo S, Bonventre JV. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol. 2002;283:F1326–F1336. doi: 10.1152/ajprenal.00166.2002. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 30.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 31.Welm BE, et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245(1):42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 32.Urbanek K, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol. 1998;18:378–387. doi: 10.1128/mcb.18.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams DC, Oxburgh L. The long-term label retaining population of the renal papilla arises through divergent regional growth of the kidney. Am J Physiol Renal Physiol. 2009;297:F809–F815. doi: 10.1152/ajprenal.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 36.Aten JA, Bakker PJ, Stap J, Boschman GA, Veenhof CH. DNA double labelling with IdUrd and CldUrd for spatial and temporal analysis of cell proliferation and DNA replication. Histochem J. 1992;24:251–259. doi: 10.1007/BF01046839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.