Abstract
T cell egress from the thymus is essential for adaptive immunity, yet the requirements for and sites of egress are incompletely understood. We have shown that transgenic expression of sphingosine-1-phosphate receptor-1 (S1P1) in immature thymocytes leads to their perivascular accumulation and premature release into circulation. Using an intravascular procedure to label emigrating cells, we found that mature thymocytes exit via blood vessels at the corticomedullary junction. By deleting sphingosine kinases in neural crest-derived pericytes, we provide evidence that these specialized vessel-ensheathing cells contribute to the S1P that promotes thymic egress. Lymphatic endothelial cell-derived S1P was not required. These studies identify the major thymic egress route and suggest a role for pericytes in promoting reverse transmigration of cells across blood vessel endothelium.
The thymus is an essential site of T cell development and tolerance induction. CD4 and CD8 double-negative (DN) precursors develop into double-positive (DP) thymocytes in the thymic cortex, and cells positively selected for weak recognition of self major histocompatibility (MHC)-peptide complexes give rise to semi-mature single-positive (SP) thymocytes that localize to the medulla. Strongly self-reactive semi-mature SP thymocytes are negatively selected whereas cells that pass this tolerance checkpoint undergo further maturation in the medulla. Over a period of a few days, SP thymocytes upregulate the transcription factor Kruppel-like factor (KLF) 2 and KLF2 target genes, including the G protein-coupled receptor sphingosine-1-phosphate receptor-1 (S1P1) and the adhesion molecule CD62L, and mature SP cells exit the thymus in an S1P1-and sphingosine-1-phosphate (S1P)-dependent manner (1–3). The route by which egress occurs has not yet been identified. Electron microscopy studies have occasionally (non-quantitatively) identified cells crossing blood vessels at the corticomedullary junction (4–7). Thymic progenitor cells are thought to enter the thymus at this location (8, 9), however, and it was not possible in these studies to determine whether the transmigrating cells were entering or exiting the thymus. Thymic lymphatics have also been implicated as a route of egress (7, 10–13); whether egress occurs predominantly via blood vessels or lymphatics has not been resolved (2, 3).
S1P1 is sufficient to mediate immature thymocyte egress
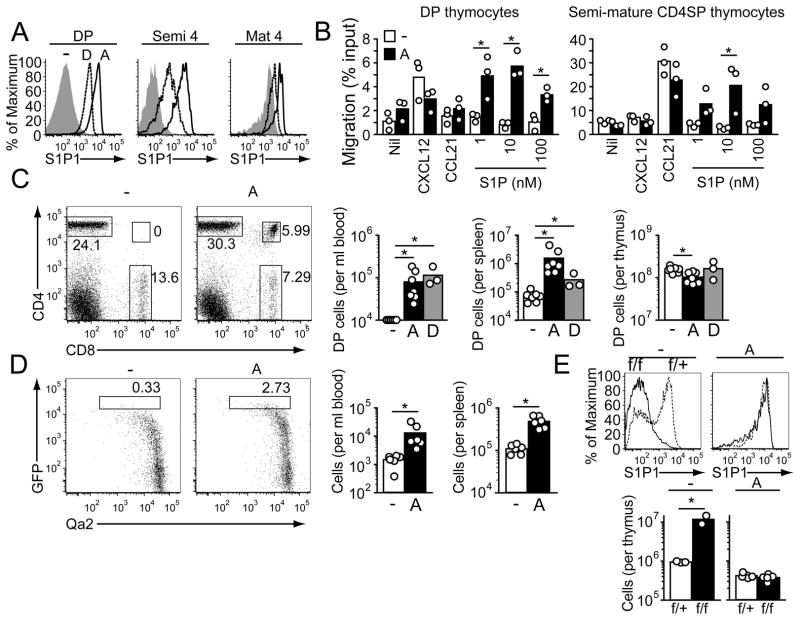
To examine whether S1P1 upregulation is the key maturation event occurring in SP thymocytes that is necessary for their egress, we asked whether premature expression of S1P1 on immature thymocytes was sufficient to promote egress. Transgenic mice carrying an S1P1 transgene under control of the Lck proximal promoter and immunoglobulin heavy chain (Eμ) enhancer (14), which together direct transgene expression in developing thymocytes and B cells, were generated and screened for S1P1 expression in thymocytes. One line (line A) showed abundant expression in DP thymocytes and semi-mature SP thymocytes (Fig. 1A), and a second line (line D) had lower but detectable expression in these cells (Fig. 1A). In chemotaxis assays S1P1A transgenic DP and semi-mature SP thymocytes showed migratory responses to S1P whereas control cells did not (Fig. 1B). Analysis of blood and spleen samples from S1P1 transgenic mice revealed the presence of substantial numbers of DP thymocytes (Fig. 1C), and there was a reduction of these cells within the thymus (Fig. 1C). The reduction was greatest for the more mature CD3hiCCR7hi DP cells, and these cells were also enriched among DP cells that reached the spleen and were more responsive to S1P in chemotaxis assays (Fig. S1). Post-selection DP thymocytes also migrate more rapidly in vivo (15), which suggests that factors affecting overall cell motility may influence egress efficiency. To determine whether there was premature egress of SP thymocytes, we intercrossed S1P1A transgenic mice with RAG-GFP reporter mice (16); in these mice GFP is under the control of recombination-activating gene-1 (RAG-1) regulatory elements and GFP is expressed in RAG-1+ DP thymocytes and then gradually decays over a period of days as the thymocytes mature (17, 18). Enumeration of CD4 T cells in the periphery that had the RAG-GFPhi Qa2int phenotype of recent thymic emigrants (17) revealed they were present in elevated frequencies and numbers in the transgenic mice (Fig. 1D). To directly test whether S1P1 was the sole KLF2-target gene needed for egress of mature SP thymocytes, we bred S1P1A transgenic and KLF2f/f -CD4Cre mice (19), which selectively delete KLF2 in thymocytes at the DP stage, and enumerated mature S1P1+ cells in the thymus. The S1P1 transgene was not expected to be KLF2-regulated because it is under the control of the Lck promoter and Eμ enhancer. Indeed, no difference in S1P1 staining was detected between KLF2f/f -CD4Cre S1P1A transgenic and wild-type S1P1A transgenic mature SP thymocytes (Fig. 1E). In contrast to findings in KLF2-deficient non-transgenic mice, where mature SP thymocytes accumulate [Fig. 1E and (20)], there was no accumulation of mature SP thymocytes in KLF2-deficient S1P1 transgenic mice (Fig. 1E) indicating that egress of the transgenic SP cells was not KLF2-dependent. These observations establish that S1P1 is sufficient to promote egress of KLF2-negative DP cells and KLF2-deficient mature SP thymocytes, demonstrating that S1P1 is the essential KLF2-target gene needed for emigration.
Fig. 1.
Transgenic S1P1 expression promotes egress of DP and KLF2-deficient SP thymocytes. (A) Flow cytometric analysis of S1P1 expression by DP, semi-mature and mature SP thymocytes in S1P1A and S1P1D transgenic and control (-) mice. Semi-mature CD4 SP cells (semi 4) were gated as CD62L− HSAhi, and mature CD4 SP cells (mat 4) as CD62LhiHSAint/lo. (B) Transwell migration of S1P1A transgenic and control DP and semi-mature SP thymocytes to S1P, CXCL12 (0.3 μg/ml) and CCL21 (1.0 μg/ml). Bars indicate means and circles represent values from individual mice. (C) Flow cytometric detection of DP thymocytes in blood of S1P1A transgenic mice. Bar graph shows numbers of cells in the blood, spleen and thymus of S1P1A and S1P1D transgenic and control mice with bars showing means and circles values for individual mice. Data points on axis were below threshold of detection. (D) Flow cytometric detection of semi-mature RAG-GFPhi Qa2 int CD4 T cells in blood of S1P1A and control RAG-GFP mice. Graphs on right show enumeration of these cells in blood and spleen. (E) Number of (lower graphs) and S1P1 expression by (upper plots) mature CD62Lhi Qa2int CD4 SP thymocytes in KLF2f/+ or KLF2 f/f CD4Cre+ control (−) and S1P1A transgenic (A). Data in A–E are representative of at least 3 experiments of each type.
Perivascular accumulation of S1P1 transgenic thymocytes
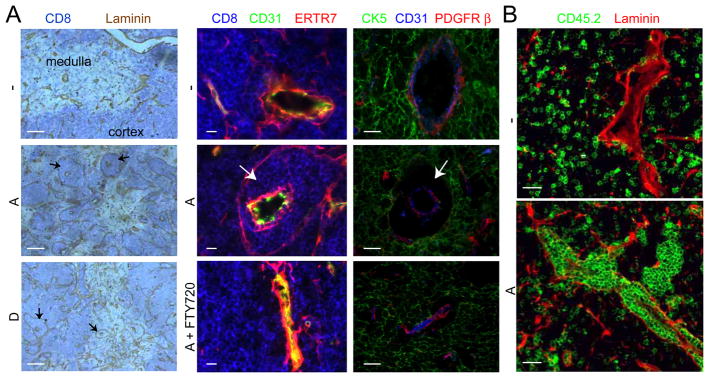
In sections of S1P1 transgenic thymi, unusual perivascular accumulations of thymocytes were observed, most strikingly in the cortex but also detectable in the medulla (Fig. 2A). Transgenic cells accumulated between the epithelial basement membrane associated with CK5+ epithelial cells and ERTR7+ cells and the basement membrane of the blood vessels and associated ERTR7+ and PDGFRβ+ pericytes, the specialized support cells that ensheathe blood vessels (Fig. 2A). In mixed bone marrow chimeras, transgenic cells were localized in these perivascular channels, bounded by laminin-containing epithelial and endothelial basement membranes, whereas wild-type cells were excluded (Fig. 2B). Treatment with FTY720 to disrupt thymocyte S1P1 function (3) dramatically reduced the perivascular accumulations, causing cells to disperse from the perivascular region and return to the thymic parenchyma (Fig. 2A). These observations indicate that the perivascular accumulations were maintained by ongoing S1P responses and suggest that there is local availability of S1P in the immediate vicinity of thymic blood vessels. The much greater accumulation of cells in perivascular channels of transgenic mice than occurs in wild-type mice (8, 11, 21) may reflect both the higher number of S1P1-expressing cells and the higher S1P1 surface abundance and greater S1P sensitivity of the transgenic cells (Fig. 1, A and B). The greater accumulation around cortical than medullary vessels may indicate that despite abundant S1P1-transgene expression, DP thymocytes are less efficient at egressing than SP thymocytes, or cortical vessels are less efficient at supporting egress.
Fig. 2.
Perivascular accumulations of S1P1-transgenic thymocytes. (A) Immunohistochemical analysis of thymus sections from S1P1-transgenic and control mice with or without 24 h FTY720 treatment. Sections were stained with antibodies to detect the indicated markers. CD8 highlights the DP-rich cortical regions, ERTR7, cells associated with endothelial and epithelial basement membranes, CD31, the endothelium, CK5, medullary epithelium and PDGFRβ, pericytes. Arrows point to perivascular thymocyte accumulations. Scale bars represent 200 μm (left column) or 10 μm (middle and right columns). (B) Immunofluorescence of thymic sections from bone marrow chimeras reconstituted with a 50:50 mix of wild-type CD45.1 cells and wild-type (−) or transgenic (A) CD45.2 cells. Thymocytes of wild-type or transgenic CD45.2 origin are identified by CD45.2 staining (green). Laminin staining (red) highlights the endothelial and epithelial basement membranes. Scale bars indicate 25 μm. Data in A and B are representative of at least 3 mice analyzed in 3 experiments.
Autoimmunity in S1P1 transgenic mice
To explore the possibility that premature egress might be associated with diminished negative selection and autoimmunity, various organs of the S1P1 transgenic mice were examined for cellular infiltrates (Fig. S1). Large lymphoid infiltrates were observed in salivary gland, lacrimal gland and lung at a substantially higher frequency in the S1P1A transgenic mice than in littermate control mice (Fig. S1). Recent findings have shown elevated S1P1 expression diminishes T-regulatory (Treg) cell development (22) and we also observed reduced Treg numbers in the thymi of S1P1A transgenic mice (Fig. S1). Peripheral Treg frequencies, however, were not affected (Fig. S1) and previous studies have shown that even small numbers of Treg can protect from disease (23). Thus, although we cannot exclude an effect of the S1P1A transgene on peripheral Treg function, our results are consistent with the possibility that premature thymic egress of developing T cells is associated with insufficient negative selection and autoimmunity.
Intravascular labeling identifies emigrating thymocytes
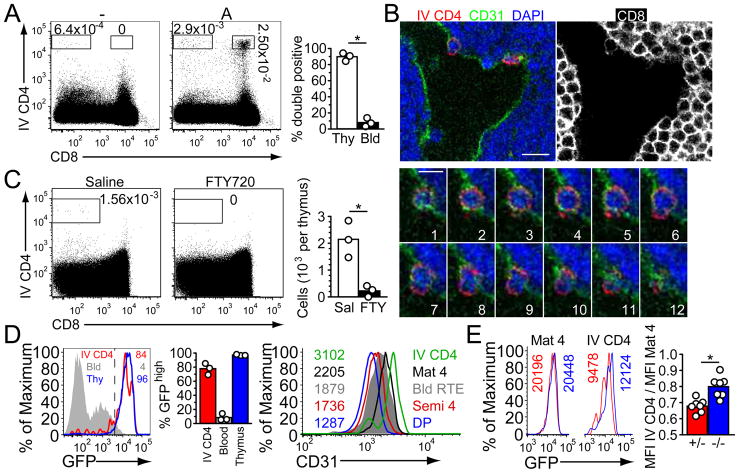
The heightened frequency of emigrating thymocytes in S1P1A transgenic mice led us to ask if we could detect thymocytes in the act of egress. A previous study has shown that very short treatment with labeled antibodies, particularly if conjugated to the large fluorophore phycoerythrin (PE), achieves selective labeling of cells exposed to the vascular compartment in bone marrow (24). Mice were injected intravenously with PE-conjugated CD4 antibody (CD4PE) and a few minutes later the thymus was isolated and prepared for flow cytometric analysis and immunofluorescence microscopy. In S1P1A transgenic mice we observed a low but reproducible frequency of DP thymocytes that became CD4PE-labeled (Fig. 3A). No DP cells became labeled in nontransgenic littermate controls (Fig. 3A). The labeled DP cells in transgenic thymi did not simply reflect blood contamination because the frequency of DP cells amongst the CD4PE-labeled cells was highly enriched compared to their frequency amongst CD4PE-labeled cells in venous blood (Fig. 3A). When DP cells were transferred directly into the blood they disappeared with a half-life of about 3 hours (Fig. S2), suggesting they are lost rapidly once they leave the thymus and enter general circulation. In sections, the S1P1A transgenic cells labeled following intravenous CD4PE antibody treatment were located in blood vessels distributed in both the cortex and medulla (Fig. 3B and Fig. S2). In some cases the CD4PE-labeled cells were found to be spanning the CD31+ endothelium, apparently caught in the act of crossing (Fig. 3B).
Fig. 3.
In vivo labeling identifies emigrating CD4 SP thymocytes. (A) Flow cytometric detection of intravascularly CD4PE-labeled DP cells in the thymus of S1P1A transgenic (A) versus control (−) mice. Thymocytes were isolated 5 minutes after CD4PE antibody injection. Bar graph shows the average percent of CD4PE-positive cells in transgenic mice that were also CD8-positive, from n= 3 mice. Circles represent individual mice. (B) Immunofluorescence of thymic section from an S1P1A transgenic mouse pretreated with CD4PE. Panels 1–12 show a confocal z-series through an emigrating DP cell (0.3μm z step). Single color panel shows CD8 staining, indicating cortical location of transmigration. Scale bars represent 10 (top) or 5 (bottom) μm. Data are representative of at least 3 mice analyzed in 3 experiments. (C) Flow cytometric detection of intravascularly CD4PE-labeled SP cells in the thymus of non-transgenic mice and their absence after 12 h FTY720 treatment. Bars in right graph show mean of 3 experiments (n=3 mice). (D) RAG-GFP intensity and CD31 staining on the indicated CD4PE-labeled cells. Left histogram plot shows GFP fluorescence in intravascularly CD4PE-labeled cells in thymus, mature CD62Lhi Qa2int CD4 SP thymocytes, and CD4 T cells in blood, and numbers indicate % GFPhi for each cell type. Middle graph shows summarized data for 3 mice aged 5 to 6 weeks. Right histogram plot shows CD31 staining with inset fluorescent intensities for the indicated thymic populations and RAG-GFPhi blood recent thymic emigrants (RTE). Data are representative of 5 mice analyzed in 5 experiments. (E) RAG-GFP intensity of CD69−/− (blue) and CD69+/− (red) mature CD4 SP and IV CD4PE+ thymocytes. Bottom bar graph shows ratios of GFP intensity comparing mature CD4 SP and IV CD4PE+ thymocytes for individual mice. These data represent 7 mice analyzed in 7 experiments.
By collecting large (~5 million event) flow cytometric files it was possible to observe intravascularly CD4PE-labeled SP thymocytes in normal mice (Fig. 3, A and C). Using RAG-GFP reporter mice to track thymocyte maturity (17), the majority (~80%) of intravascularly CD4PE-labeled cells in the young adult thymus had higher amounts of GFP than cells in the blood, indicating that these were cells that had just emigrated but not yet left the thymus, rather than corresponding to cells already present in blood circulation (Fig. 3D). These thymic cells also expressed higher amounts of CD31 than cells in the blood or the most mature cells in the thymus (Fig. 3D) suggesting they had transiently acquired or upregulated CD31 during egress. We used this labeling technique to examine mice lacking CD69, a protein that regulates S1P1 function in peripheral T cells (25) and that is expressed on semi-mature SP thymocytes but is of unknown function in the thymus (26, 27). Intravascularly CD4PE-labeled cells in the thymus of CD69−/− RAG-GFP+ mice had higher amounts of GFP than cells in control RAG-GFP+ mice, indicating they were less mature and based on estimates of GFP half-life (18) had emigrated several hours prematurely (Figs. 3E and S3). CD69−/− mice also had fewer mature SP cells in the thymus and more recent thymic emigrants in the periphery (Fig. S3). These observations indicate CD69 regulates the timing of thymocyte egress.
Thymocytes emigrate by blood vessels at the corticomedullary junction
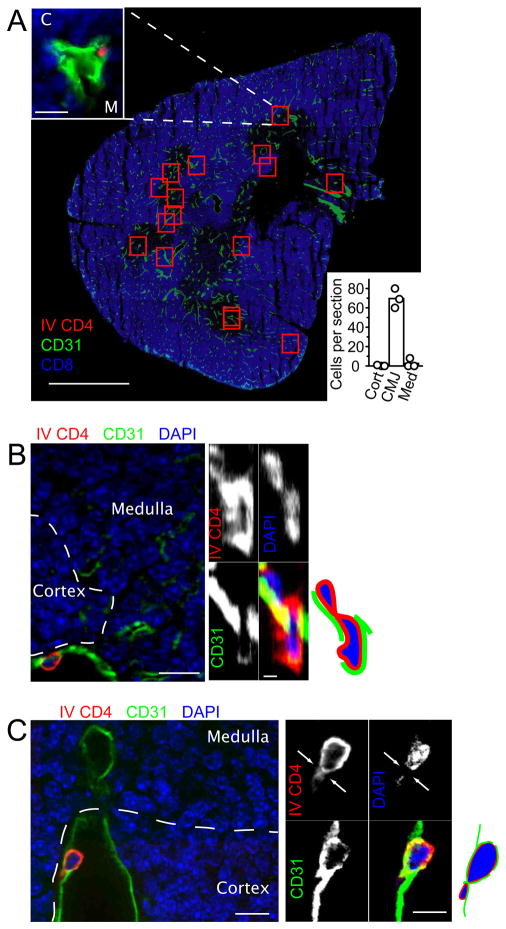
Immunofluorescence analysis of sections from in vivo labeled wild-type thymus revealed rare PE-labeled cells in vessels near the corticomedullary junction (Fig. 4). Enumeration of CD4PE-labeled cells across 30 μm thick thymic cross-sections revealed a mean of 70 cells per section (Figs. 4A and S4A) with the great majority being located in vessels within 50 μm of the corticomedullary junction (Fig. 4B). Using the number of cells present in the volume of one section to extrapolate the number present in the volume of the whole organ (~0.025 cubic centimeters), we calculated that roughly 2500 labeled cells are present per thymus. This number agrees closely with that obtained by flow cytometry (Fig. 3C). If we assume that thymocyte reverse transmigration has a similar speed to other T cell transmigration events and takes approximately 3 minutes (28, 29), then we estimate 0.8 million CD4 SP cells (or ~1 million total SP cells) exit the thymus per day. This number is in close agreement with the estimate from intra-thymic FITC labeling experiments that ~1% of total thymocytes (~1 million cells) egress per day (30). Close examination of in vivo labeled thymic sections revealed examples of cells that appeared to be in the act of reverse transmigrating across the vascular endothelium into the blood (Figs. 4, B and C and S4, B and C). Analysis of serial sections confirmed that the cells were projecting through the CD31+ endothelium (Movies S1 and S2). In summary, by selectively labeling egressing cells we demonstrate that the majority of thymocytes emigrate via blood vessels at the corticomedullary junction.
Fig. 4.
In vivo labeling identifies CD4 SP thymocytes emigrating at the cortico-medullary junction. (A–C) Immunofluorescence of thymic sections from non-transgenic mice injected with anti-CD4PE, also stained to detect vascular endothelium with anti-CD31 (green) and with anti-CD8 to highlight cortical regions (A) or with DAPI (B–C) to detect nuclei (blue). (A) Tiled view of an entire thymic lobe section with CD4PE+ cells highlighted by red squares and an example shown in the top left inset. Bottom right inset displays enumeration of CD4PE-labeled cells in thymic cross sections, assigned as being located in vessels within 50 μm of the corticomedullary junction (CMJ), in the cortex (Cort or “C”) or in the medulla (Med or “M”). Scale bars indicate 25 μm (inset image) and 800 μm (main image). (B, C) High power views of transmigrating thymocytes. Large left panel displays a single 0.24 μm-thick xy optical section. Single color panels: upper left, IV CD4; upper right, DAPI; lower left, CD31; lower right, 3 color overlay. Single panels are 0.1 μm-thick yz optical sections in (B) and 0.24 μm-thick xy optical sections in (C). Diagrams on right of each set of panels included for clarity. Scale bars indicate 10 μm for large left panel and 1 (B) or 5 (C) μm for small right panels. Data are representative of thymic sections from at least 3 mice analyzed in 3 experiments.
Neural crest-derived pericytes promote thymocyte egress
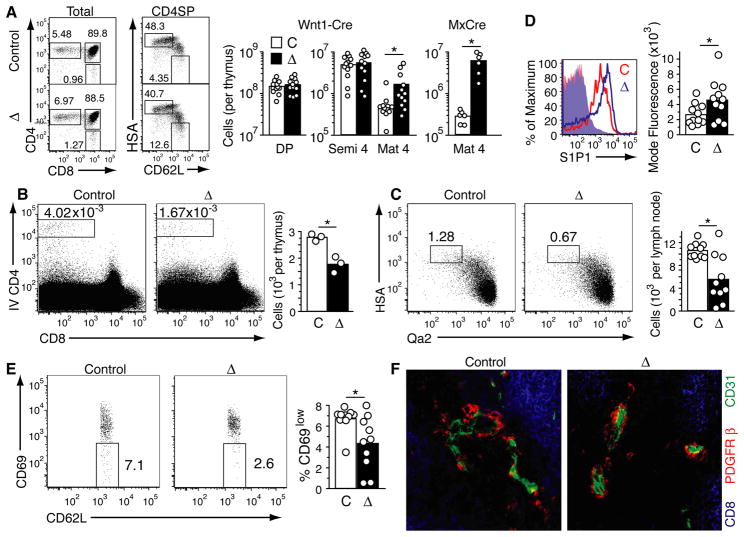
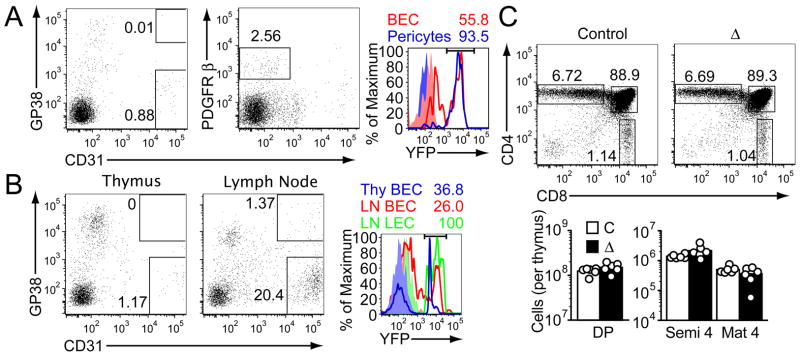
S1P is present at high concentration in blood and low concentration in the thymus (3). Previous work established that the major source of S1P necessary for thymic egress is radiation-resistant (31). This suggested, surprisingly, that blood S1P is not sufficient to promote normal egress and S1P production by a local stromal cell type might be required. Pericytes are a diverse population of cells that closely ensheathe blood vessels, but not lymphatics, and can lack or express α-smooth muscle actin (α-SMA) (32, 33). Recently it was discovered that thymic perivascular cells are unusual in having a neural crest origin (34, 35). For simplicity and in accord with a prior study (35) we refer to the neural crest-derived perivascular cells as pericytes. Thymocytes undergoing reverse (basolateral-to-apical) transmigration are likely to encounter pericytes prior to engaging the endothelium. This prediction, and our finding that S1P1 transgenic thymocytes accumulate adjacent to pericytes (Fig. 2A) led us to hypothesize that S1P produced by neural crest-derived pericytes might be required for thymic egress. Wnt1-Cre, an established neural crest-specific Cre (36), causes efficient recombination and expression of the Rosa26eYFP locus in the majority of thymic pericytes (34, 35). To test for a possible role of pericytes as a radiation-resistant S1P source necessary for thymocyte egress, Wnt1-Cre mice were intercrossed with Sphk1f/− Sphk2−/− mice (31), to generate mice with neural crest-derived cells lacking the two kinases required for production of S1P. Wnt1-Cre+ Sphk1f/− or f/f Sphk−/−2 mice were viable and showed no gross abnormalities, suggesting intrinsic Sphk-activity may not be essential for neural crest function during development. However, analysis of Wnt1-Cre+ Sphk1f/− orf/f Sphk2−/− mice revealed a selective accumulation of CD62LhiHSAint/lo mature SP thymocytes compared to littermate controls that retained at least one intact copy of Sphk1 or Sphk2 (Fig. 5A). This accumulation was partial compared with that observed in polyI:C-treated MxCre Sphk-deficient mice (Fig. 5A) that lack sphingosine kinases in a wide range of IFNα/β-responsive cell types and have undetectable plasma S1P (31). Plasma S1P amounts were unaffected in Wnt1-Cre Sphk-deficient mice (Fig. S5). Intravenous CD4PE antibody treatment showed a reduced number of vascularly exposed CD4 SP thymocytes in the mice lacking Sphk activity in neural crest-derived cells (Fig. 5B), and there were reduced numbers of HSAint Qa2int recent thymic emigrants (17) in peripheral lymph nodes (Fig. 5C). Mature T cell numbers in lymph nodes were in the normal range (Fig. S5), perhaps due to incomplete activity of Wnt1-Cre in neural crest-derived pericytes (34) or the sufficiency of plasma S1P to promote a significant amount of thymic egress (31). S1P1 surface abundance was slightly elevated on mature SP thymocytes in Wnt1-Cre Sphk-deficient mice, consistent with reduced exposure to S1P (Fig. 5D). Despite the accumulation of mature SP thymocytes in Wnt1Cre Sphk-deficient mice (Fig. 5A) there was a lower frequency of CD69-negative cells amongst this population compared to littermate controls (Fig. 5E). This may reflect a requirement for S1P exposure for full CD69 downregulation during thymocyte maturation. Immunohistochemical analysis established that the pericyte distribution in the thymus of Wnt1-Cre Sphk-deficient mice was normal (Fig. 5F). To determine whether the thymic egress block in polyI:C-treated MxCre Sphk-deficient mice could also reflect gene deletion in pericytes, we examined Rosa26eYFP reporter expression in isolated cells by flow cytometry (Fig. 6A). Pericytes, identified as PDGFRβ+ CD31− CD45− cells (34) showed near complete reporter gene activation whereas blood endothelial cells showed partial reporter gene activation (Fig. 6A). The findings from the Wnt1-Cre and MxCre Sphk-deficient mice suggest that pericytes contribute to the S1P required for thymocyte egress. These results do not exclude the possibility that Sphk ablation affects pericyte function in additional ways.
Fig. 5.
Sphingosine kinase acivity is required in neural crest-derived pericytes for thymic egress. (A) Flow cytometric analysis of total thymocytes (left panels) and enumeration of thymocyte subsets (right panels) in the thymus of Wnt1-Cre Sphk-deficient (◇), control (C) and polyI:C-treated MxCre Sphk-deficient (MxCre) mice. Left dot plots show total thymocytes, and right dot plots show CD4 SP thymocytes. Semi-mature CD4 SP cells (semi 4) were gated as CD62L− HSAhi, and mature CD4 SP cells (mat 4) as CD62LhiHSAint/lo. Numbers indicate the percentage of cells in indicated gate. Cell numbers are summarized in bar graphs on the right and are from 12 Wnt1-Cre Sphk-deficient and control mice aged 5 to 14 weeks and 6 MxCre Sphk-deficient and control mice aged 10 to 21 weeks analyzed in 11 and 4 experiments, respectively. (B) Flow cytometric analysis (left panel) and enumeration of intravascularly CD4PE-labeled cells in Wnt1-Cre Sphk-deficient and control thymi. Data are from 3 mice aged 5–6 weeks, analyzed in 3 experiments. (C) Flow cytometric analysis of HSAint Qa2int recent thymic emigrants in the peripheral lymph nodes of Wnt1-Cre Sphk-deficient mice. Ten 5–20 week old mice were analyzed in 10 experiments. (D) S1P1 surface abundance on mature CD4 SP thymocytes in the indicated Wnt1-Cre Sphk-deficient or control mice. (E) Flow cytometric analysis of frequency of CD69lo cells amongst mature HSAint CD62Lhi CD4 SP thymocytes in Wnt1-Cre Sphk-deficient mice. (F) Normal appearance of pericytes in Wnt1-Cre Sphk-deficient mice. Sections were stained to detect the indicated markers. Scale bar indicates 25 μm. Data in D–F are from at least three mice each.
Fig. 6.
Ablation of sphingosine kinase acivity in lymphatic endothelium does not inhibit thymic egress. (A) Flow cytometric analysis of digested thymus tissue from polyI:C-treated MxCre x Rosa26eYFP reporter mice showing MxCre activity in PDGFRβ+ CD31− pericytes and CD31+ GP38− blood vessel endothelium (BEC). CD31 GP38 lymphatic endothelial cells were undetectable. (B) Flow cytometric analysis of digested tissues from LYVE1-Cre x Rosa26eYFP reporter mice showing CD31 and GP38 staining (left panels) and extent of Cre activity as revealed by YFP expression (right histogram) in blood endothelial cells (BEC) and lymphatic endothelial cells (LEC). In (A) and (B), plots were pregated on CD45-negative cells, numbers indicate frequency of cells in gates, and shaded histograms show Cre− control cells. In (B), digested tissue was negatively selected using anti-CD8 antibody to enrich for stromal cells. (C) Flow cytometric analysis and quantitation showing lack of thymocyte accumulation in LYVE1-Cre Sphk-deficient mice. Data are from 6 mice analyzed in 6 experiments.
Lymphatic S1P is not required for thymic egress
Some studies have suggested a major role for lymphatic vessels in thymocyte egress (2, 7, 10–13, 21). In our studies, LYVE1+ lymphatic vessels were rarely detected in cross sections of adult C57Bl/6 mouse thymi and gp38+CD31+ lymphatic endothelial cells were very rare by flow cytometric analysis of enzyme digested thymi, being outnumbered by gp38− CD31+ blood vessel endothelium by at least 50 to 1 (Fig. 6A and B). Lymphatic endothelial cells are a necessary S1P source for lymphocyte egress into lymph node lymphatics (37). To further test whether lymphatic endothelium played a role in thymic egress, we analyzed LYVE-1Cre Sphk1f/f Sphk2−/− mice. LYVE-1Cre causes efficient floxed gene ablation in lymphatic endothelial cells and partial ablation in blood vasculature (37). Analysis of LYVE-1Cre x Rosa26eYFP mice revealed yellow fluorescent protein (YFP) expression in ~40% of thymic blood vessel endothelial cells (Fig. 6B); there were too few lymphatic endothelial cells in the thymus to permit reporter analysis. In lymph nodes, however, all the lymphatic endothelial cells were YFP reporter+ (Fig. 6B) as expected (37). In LYVE-1Cre+ Sphk-deficient mice there was no increase in mature SP thymocyte numbers in the thymus (Fig. 6C). These observations and the concordance between the number of cells egressing into blood vessels and exiting from the thymus as a whole lead us to the conclusion that lymphatic vessels do not play a major role in thymic egress.
Discussion
In summary, we provide evidence that S1P1 is the only target that KLF2 must upregulate in mature SP thymocytes to promote their emigration. Using an intravascular labeling approach, we establish that T cells emigrate from the thymus via blood vessels at the corticomedullary junction. This non-invasive technique for enumerating and phenotyping emigrating thymocytes also allowed us to estimate that CD69 delays thymic egress by several hours, likely helping ensure cells complete selection and maturation events before reaching circulation. Unlike most vascular beds, thymic blood vessels are ensheathed by neural crest-derived pericytes (34, 35). We provide evidence of an active role for these support cells in promoting T cell reverse transmigration. These data help explain the seemingly discrepant findings that thymocyte egress occurs into the blood, yet plasma S1P alone appears insufficient to promote normal egress (31). Endothelial cells can produce S1P in vitro (38) and we do not exclude the possibility that endothelial cell S1P works together with pericyte and plasma S1P in promoting thymocyte egress. Postcapillary venules at the corticomedullary junction have large (10–50 μm) diameters (4) and are thin walled, with only a single layer of ensheathing α-SMA+ pericytes (35), perhaps indicating specialization to support thymocyte egress. Our findings exclude a major role for lymphatic vessels in thymocyte egress in the young adult mouse, and the lack of LYVE1+ lymphatics in the human thymic medulla suggests a similar conclusion for human thymocyte egress (39). However, it remains possible that lymphatics contribute to egress from thymi of aged or diseased animals, where they are sometimes more prevalent (11, 40). Perivascular channels containing thymocytes have been seen in thymi of a number of species including humans (5, 7, 11, 41). Our findings indicate that thymocyte accumulation in these channels can be S1P-mediated. We suggest that with their rich content of extracellular matrix, perivascular channels provide a space where pericyte-and vessel-derived S1P is protected from rapid degradation by cell-associated enzymes (1, 3), facilitating formation of an egress-promoting S1P gradient. An important question is whether S1P secretion is a unique property of the neural crest-derived thymic pericytes, or whether it also occurs in other vascular beds. We propose that the differential propensity of pericytes to secrete S1P may be a key factor determining whether S1P receptor-expressing cells undergo reverse transmigration across blood vessels.
Supplementary Material
Acknowledgments
We thank J. Lingrel for making KLF2f/f mice available and K. Hogquist for sending these mice, S. Coughlin for Sphk1f/− 2−/− mice, B. Black for providing Wnt1-Cre mice, T. Pham, Ying Xu and J. An for technical help, F. Schaufele of the Diabetes Endocrinology Research Center Imaging Core for help with the confocal microscope core, N. Killeen for help generating the S1P1 transgenic mice and O. Bannard, S. Coughlin, M. Anderson, and A. Weiss for comments on the manuscript. M.A.Z. was supported by the UCSF Medical Scientist Training Program. J.G.C. is an investigator of the Howard Hughes Medical Institute. This work was supported in part by NIH grant AI74847.
References and Notes
- 1.Rivera J, Proia RL, Olivera A. Nat Rev Immunol. 2008;8:753. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich MA, Hogquist KA. J Immunol. 2008;181:2265. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachariah MA, Cyster JG. F1000 Biology Reports. 2009;1:60. doi: 10.3410/B1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raviola E, Karnovsky MJ. J Exp Med. 1972;136:466. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sainte-Marie G, Leblond CP. Blood. 1964;23:275. [PubMed] [Google Scholar]
- 6.Toro I, Olah I. J Ultrastruct Res. 1967;17:439. doi: 10.1016/s0022-5320(67)80134-5. [DOI] [PubMed] [Google Scholar]
- 7.Ushiki T. Cell Tissue Res. 1986;244:285. doi: 10.1007/BF00219204. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T. Int Immunol. 2007;19:745. doi: 10.1093/intimm/dxm041. [DOI] [PubMed] [Google Scholar]
- 9.Petrie HT, Zuniga-Pflucker JC. Annu Rev Immunol. 2007;25:649. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 10.Kato S. Cell Tissue Res. 1988;253:181. doi: 10.1007/BF00221753. [DOI] [PubMed] [Google Scholar]
- 11.Kato S. Microsc Res Tech. 1997;38:287. doi: 10.1002/(SICI)1097-0029(19970801)38:3<287::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Kotani M, Kawakita M, Fukanogi M, Yamashita A, Seiki K. Okajimas Folia Anat Jpn. 1967;43:61. doi: 10.2535/ofaj1936.43.2_61. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka M, Pabst R, Dudler L, Cooper M, Yamaguchi K. Thymus. 1990;16:29. [PubMed] [Google Scholar]
- 14.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Embo J. 1997;16:7019. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Borgne M, et al. Nat Immunol. 2009;10:823. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. J Immunol. 1999;163:6355. [PubMed] [Google Scholar]
- 17.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Nat Immunol. 2004;5:418. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 18.McCaughtry TM, Wilken MS, Hogquist KA. J Exp Med. 2007;204:2513. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinreich MA, et al. Immunity. 2009;31:122. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson CM, et al. Nature. 2006;442:299. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 21.Odaka C, Morisada T, Oike Y, Suda T. Cell Tissue Res. 2006;325:13. doi: 10.1007/s00441-005-0139-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, et al. Nat Immunol. 2009;10:769. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu N, Hori S. Proc Natl Acad Sci U S A. 2007;104:8959. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Nat Immunol. 2009;10:403. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiow LR, et al. Nature. 2006;440:540. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 26.Lauzurica P, et al. Blood. 2000;95:2312. [PubMed] [Google Scholar]
- 27.Murata K, et al. Int Immunol. 2003;15:987. doi: 10.1093/intimm/dxg102. [DOI] [PubMed] [Google Scholar]
- 28.Smith ME, Ford WL. Immunology. 1983;49:83. [PMC free article] [PubMed] [Google Scholar]
- 29.McGettrick HM, et al. J Leukoc Biol. 2009;85:98. doi: 10.1189/jlb.0508301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scollay RG, Butcher EC, Weissman IL. Eur J Immunol. 1980;10:210. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 31.Pappu R, et al. Science. 2007;316:295. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 32.Bergers G, Song S. Neuro Oncol. 2005;7:452. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krueger M, Bechmann I. Glia. Vol. 58. p. 1. [DOI] [PubMed] [Google Scholar]
- 34.Foster K, et al. J Immunol. 2008;180:3183. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- 35.Muller SM, et al. J Immunol. 2008;180:5344. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 36.Chai Y, et al. Development. 2000;127:1671. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 37.Pham TH, et al. J Exp Med. 2009 [Google Scholar]
- 38.Venkataraman K, et al. Circ Res. 2008;102:669. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drumea-Mirancea M, et al. J Cell Sci. 2006;119:1396. doi: 10.1242/jcs.02840. [DOI] [PubMed] [Google Scholar]
- 40.Ji RC, Kurihara K, Kato S. Anat Sci Int. 2006;81:201. doi: 10.1111/j.1447-073X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 41.Bearman RM, Bensch KG, Levine GD. Anat Rec. 1975;183:485. doi: 10.1002/ar.1091830402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.