Abstract
The phenomenon of multidrug resistance (MDR) has decreased the hope for successful cancer chemotherapy. The ATP-binding cassette (ABC) transporter super-family is the largest transmembrane family. The overexpression of ABC transporters is a major determinant of MDR in cancer cells both in vitro and in vivo. Unfortunately, until recently, most of the strategies used to surmount ABC transporter-mediated MDR have had limited success. An ideal modulator of MDR would be one that has a low liability to induce toxicity and alter the pharmacokinetic profile of antineoplastic drugs. Sildenafil (Viagra®), an inhibitor of cGMP-specific phosphodiesterase types 5 (PDE5) was found to significantly reverse ABC-transporters-mediated MDR. Our results indicated that sildenafil had differential inhibitory effects on ABC transporters; it significantly decreased the efflux activity of ABCB1 and ABCG2, but had no significant effects on ABCC1. Emerging evidence indicates that sildenafil and other PDE5 inhibitors may enhance the sensitivity of certain types of cancer to standard chemotherapeutic drugs.
Keywords: Sildenafil, PDE5 inhibitors, multidrug resistance, cancer chemotherapy, ABC transporters
Background
Multidrug resistance (MDR) in cancer cells is the primary cause of cancer chemotherapy failure. One of the major determinants of the MDR phenotype is the overexpression of ATP-binding cassette (ABC) transporters, which include ABCB1 (P-glycoprotein/MDR1), ABCCs (MRPs) and ABCG2 (BCRP/MXR/ABCP) (1). Membrane proteins in this superfamily share the ability to actively transport a wide variety of substrates across the cellular membrane, ranging from ions, sugars, amino acids, vitamins, lipids and drugs to larger molecules, although each transporter has its own substrates specificity (1). When these transporters are overexpressed in cancer cells, they pump out or extrude structurally and mechanistically different chemotherapeutic drugs, thereby lowering intracellular drug concentration, leading to an attenuated chemotherapeutic effect (2). Accumulating evidence shows that ABC transporters, besides contributing to drug resistance, also play the important role in tumorigeneis (3). The expression of specific ABC transporters is associated with tumour-initiating cells or cancer stem cells in several types of cancer, and their clinical relevance is now in a wide of researches (3). Ideally, inhibition of these transporters should lead to re-sensitization of MDR cancer cells or cancer stem cells to chemotherapeutic drugs and may allow for a more efficacious treatment of cancer patients. For over 30 years, a significant effort has been made to develop specific inhibitors/modulators that can reverse MDR in cancer cells and typically these compounds have been classified as first, second or third generation inhibitors/modulators. The first-generation inhibitors/modulators are drugs used clinically with well established pharmacological profiles, such as verapamil (4) and cyclosporine A (5). However, both of these were ineffective in clinical trials as a result of serious adverse effects produced by the dose required to significantly reverse MDR (6, 7). Subsequently, second-generation inhibitors/modulators, the derivatives of the first-generation inhibitors/modulators with more potent inhibitory activity, (e.g. SDZ PSC833, valspodar), were developed and tested (8). Unfortunately, data from clinical trials indicated that SDZ PSC833 inhibited the metabolism and elimination of certain clinically used anticancer drugs, thereby increasing their plasma levels, producing toxicity (9). The third-generation inhibitors/modulators were derived from the second-generation lead compounds based on the pharmacophores by chemical modification using structure-activity relationships. Subsequent studies indicated that these compounds, such as LY335979 (Zosuquidar) (10), GF120918 (Elacridar) (11), MS-209 (Dofequidar) (12) had high affinity (nM) for ABC transporters. Although they showed promising activity in preclinical studies and in early clinical trials as potent and non-toxic inhibitors/modulators, most of them were found to lack significant efficacy in late phase clinical trials (13). Based on the aforementioned results, it is clear that there is still the need for the development and testing of efficacious and non-toxic inhibitors/modulators.
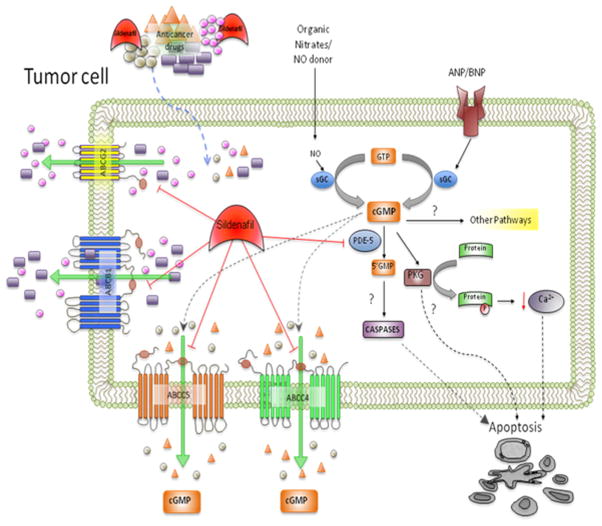
Sildenafil (Viagra®), an inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5), is clinically used to treat erectile dysfunction (ED) and pulmonary arterial hypertension (PAH) (WHO Class I). Sildenafil binds to PDE5 and prevents the biotransformation of second messenger 3′,5′-cGMP to 5′-GMP thus increasing intracellular cGMP levels. It has been reported that high cGMP levels are associated with increased blood flow as a result of vascular smooth muscle vasodilation (14). Previously, cyclic nucleotide second messengers such as cGMP and cAMP were shown to be substrates with low micromolar affinity for ABCC4/MRP4 and ABCC5/MRP5. Furthermore, sildenafil significantly inhibits the efflux activity of ABCC4 and ABCC5 (15, 16). Our recent studies have shown that sildenafil also inhibits the activity of ABC transporters such as ABCB1 and ABCG2 and reverses MDR in cancer cells mediated by these transporters (17) (Figure 1)
Figure 1. Proposed mechanisms for sildenafil-induced augmentation of the efficacy of antineoplastic drugs - A schematic model.
Sildenafil may enhance the therapeutic effect of antineoplastic drugs by 1) blocking the substrate efflux function of multidrug resistant transporters such as ABCC4/MRP4 (16), ABCC5/MRP5 (15), ABCB1/P-gp and ABCG2/BCRP (17) and/or 2) increasing cGMP levels via its inhibition of PDE5, ABCC4- and ABCC5-mediated efflux of cGMP. In addition, it has been proposed that in tumor cells, the elevation of cGMP levels by inhibition of PDE5 may activate PKG, producing growth suppression or apoptosis (25, 26).
PDE5, Phosphodiesterase type 5; PKG, Protein kinase G; cGMP, cyclic guanosine monophosphate
Key Findings
In our study, we examined the in vitro effect of sildenafil on ABCB1-, ABCC1- and ABCG2-mediated MDR in cancer cells. Our data indicated that sildenafil has differential effects on these three transporters. Cytotoxicity assays showed that sildenafil significantly sensitized ABCB1 overexpressing cells to the ABCB1 substrates colchicine, vinblastine and paclitaxel. In addition, sildenafil sensitized wild-type or mutant ABCG2-overexpressing cells to the ABCG2 substrates flavopiridol, mitoxantrone and SN-38. However, sildenafil did not significantly sensitize ABCC1-overexpressing cells to its substrate vincristine. In addition, sildenafil did not have any significant effect on the sensitivity of any of the parental cell lines to the aforementioned antineoplastic drugs. Consistent with the cytotoxicity data, the results of the drug accumulation study showed that sildenafil significantly enhanced the intracellular accumulation of paclitaxel in the ABCB1 overexpressing cells, as well as mitoxantrone and BODIPY-prazosin in either wild-type or mutant ABCG2 overexpressing cells. In addition, the results of membrane vesicles transport experiments demonstrated that sildenafil directly inhibited the ABCG2-mediated transport of E217βG and methotrexate. Sildenafil signfiicantly stimulated ABCB1 and ABCG2 ATPase activity, whereas it inhibited photolabeling of ABCB1 and ABCG2 with [3H]IAAP. We also predicted the binding conformation of sildenafil within the large cavity of the transmembrane region of ABCB1 based on the homology model (17).
We also examined the effect of another PDE5 inhibitor, vardenafil (Levitra), a structural analogue of sildenafil, on ABC transporter-mediated MDR in cancer cells. The results showed that vardenafil significantly sensitized ABCB1 overexpressing cells to the ABCB1 substrates vinblastine and paclitaxel, increased the intracellular accumulation of paclitaxel in the ABCB1 overexpressing cells, significantly stimulated the ATPase activity of ABCB1 and inhibited photolabeling of ABCB1 with IAAP. In contrast, vardenafil had no significant effect on any of the parental cells or on reversing ABCC1- and ABCG2-mediated MDR (unpublished data).
Implications
Recently, it has been reported that increased PDE5 expression occurs in multiple human carcinomas including urinary bladder cancers (18), metastatic breast cancers (19) and non-small cell lung cancers (20). These findings suggest that PDE5 may play a role in tumorigenesis. Therefore, inhibiting PDE5 activity may have antineoplastic effects. Several groups have evaluated the effect of sildenafil and other PDE5 inhibitors on cancer treatment. Sildenafil and vardenafil suppress tumor cell growth and induce caspase-dependent apoptosis of B-cell chronic lymphocytic leukemia cells in vitro (21). In a rat brain tumor model, the PDE5 inhibitors sildenafil and vardenafil increased the transport of doxorubicin across blood-brain tumor barrier and enhanced the efficacy of chemotherapy (22). It has been demonstrated that sildenafil reverses tumor-induced immunosuppression and enhances the antitumor response by reducing myeloid-derived suppressor cell function, leading to delay in tumor growth (23). Moreover, sildenafil was reported to enhance sensitivity of breast cancer cells to doxorubicin without exacerbating its toxicity to either bone marrow cells or macrophages (24). Sildenafil also increased the chemotherapeutic efficacy of doxorubicin in prostate cancer in vivo and ameliorated cardiac dysfunction (25). Another PDE5 inhibitor sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by elevation of cGMP, and activation of protein kinase G (26). Based on these studies and our data, it is reasonable to hypothesize that sildenafil may enhance anticancer drugs sensitivity and potentially improve the chemotherapeutic outcome in cancer patients due to its inhibitory effect on PDE5, ABCB1 and ABCG2. Future studies examining the combined use of sildenafil with anticancer drugs need to consider several issues. First, it is important to examine the expression levels of PDE5, ABCB1 and ABCG2 in cancer tissues. In addition to overexpression of ABC transporters, other drug resistance determinants in cancer cells include changes in metabolizing and detoxifying systems, such as DNA repair and the cytochrome P450 oxidases and drug-induced alterations in apoptosis (1). Hence, the expression levels of sildenafil target proteins such as PDE5, ABCB1 and ABCG2 would significantly determine the efficiency of sildenafil. Second, determining the concentrations that would be effective in vivo would definitely improve the outcome of the combined use of sildenafil with anticancer drugs. The maximum observed plasma concentration (Cmax) of a single oral dose of 25–200 mg of sildenafil in healthy male subjects is 127–1150 ng/ml (0.2–2 μM) (27), which is slightly lower than the concentration that we observed for MDR reversal (17). Therefore, concentrations of sildenafil exceeding those required for PDE5 inhibition seem to be required to enhance the effects of chemotherapeutic drugs. Third, the pharmacokinetic profile of sildenafil and anticancer drugs may be affected by each other, potentially resulting in an increased therapeutic response but also in adverse effects. This is possible as ABCB1 and ABCG2 are highly expressed in several normal tissues (1), where the concentration and distribution of sildenafil and anticancer drugs may be altered if used in combination. Finally, sildenafil is primarily metabolized by the cytochrome P450 (CYP) isoenzyme CYP3A4 (28) and the substrates of CYP3A4 considerably overlap with those of ABCB1 (29). Consequently, the metabolism and elimination of sildenafil as well as the anticancer drugs, some of which are substrates of both CYP3A4 and ABCB1 (29), may be affected when these drugs are used in combination.
Future Directions
Our studies demonstrated for the first time that two imidazotriazinone compounds, sildenafil and vardenifil, which are PDE5/PDE6 inhibitors, can reverse ABCB1- or ABCG2-mediated MDR in cancer cells by directly blocking their drug efflux function. In addition, our results suggest that imidazotriazinone compounds may be a novel class of ABC transporters inhibitors. The effects of additional imidazotriazinone compounds on ABC transporter function will be investigated in the future. If the structure-activity relationship (SAR) for sildenafil to inhibit cGMP hydrolase and ABC transporters is elucidated, this may allow for the synthesis of sildenafil analogues that are more effacacious in reversing MDR without affecting the cGMP hydrolase activity of PDE5. Clearly, further in vivo studies will be required to determine if sildenafil can be used as a modulator of ABCB1 and ABCG2 to reverse MDR in cancer cells. Currently, it is unknown as to whether sildenafil can reverse MDR in human cancers mediated by ABCB1 and ABCG2. Furthermore, it may be useful to evaluate the efficacy of chemotherapy in cancer patients who are being treated with sildenafil for other clinical indications.
Acknowledgments
Grant Support
This work was supported by funds from NIH R15 No. 1R15CA143701 (Z.S. Chen), St. John’s University Seed grant No. 579-1110 (Z.S. Chen) and the Chinese National Natural Science Foundation No.81061160507 (L.W. Fu). Z. Shi is a recipient of Kaisi fellowship for overseas study at St. John’s University from Sun Yat-Sen University.
We thank Dr. C.R. Ashby Jr. and members in the Chen lab (St. John’s University), Dr. S.V. Ambudkar (NCI, NIH) for helpful discussions and review of the manuscript.
Abbreviations
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- PDE5
phosphodiesterase type 5
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–85. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 4.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–72. [PubMed] [Google Scholar]
- 5.Twentyman PR, Fox NE, White DJ. Cyclosporin A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987;56:55–7. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, Trump DL, Koeller JM, Egorin MI, Olman EA, Witte RS, et al. Phase I study of vinblastine and verapamil given by concurrent iv infusion. Cancer Treat Rep. 1985;69:795–9. [PubMed] [Google Scholar]
- 7.Bartlett NL, Lum BL, Fisher GA, Brophy NA, Ehsan MN, Halsey J, et al. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1994;12:835–42. doi: 10.1200/JCO.1994.12.4.835. [DOI] [PubMed] [Google Scholar]
- 8.Twentyman PR, Bleehen NM. Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporin [corrected] Eur J Cancer. 1991;27:1639–42. doi: 10.1016/0277-5379(91)90435-g. [DOI] [PubMed] [Google Scholar]
- 9.Bates S, Kang M, Meadows B, Bakke S, Choyke P, Merino M, et al. A Phase I study of infusional vinblastine in combination with the P-glycoprotein antagonist PSC 833 (valspodar) Cancer. 2001;92:1577–90. doi: 10.1002/1097-0142(20010915)92:6<1577::aid-cncr1484>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48:708–15. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- 11.Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–85. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 12.Saeki T, Nomizu T, Toi M, Ito Y, Noguchi S, Kobayashi T, et al. Dofequidar fumarate (MS-209) in combination with cyclophosphamide, doxorubicin, and fluorouracil for patients with advanced or recurrent breast cancer. J Clin Oncol. 2007;25:411–7. doi: 10.1200/JCO.2006.08.1646. [DOI] [PubMed] [Google Scholar]
- 13.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 15.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–74. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–54. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 17.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-3820. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–8. [PubMed] [Google Scholar]
- 19.Pusztai L, Zhen JH, Arun B, Rivera E, Whitehead C, Thompson WJ, et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21:3454–61. doi: 10.1200/JCO.2003.02.114. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–88. [PubMed] [Google Scholar]
- 21.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–9. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 22.Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL, Jr, et al. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0765-7. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–7. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–40. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53 (Suppl 1):5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warrington JS, Shader RI, von Moltke LL, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra): identification of human cytochromes and potential drug interactions. Drug Metab Dispos. 2000;28:392–7. [PubMed] [Google Scholar]
- 29.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–22. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]