Abstract
Purpose
Thioredoxin-1 (Trx-1) redox signaling regulates multiple aspects of cell growth and survival, and elevated tumor levels of Trx-1 have been associated with decreased patient survival. PX-12, an inhibitor of Trx-1 currently in clinical development, has been found to decrease tumor levels of the HIF-1α transcription factor. SSAT1 has been reported to bind to HIF-1α and RACK1, resulting in oxygen-independent HIF-1 ubiquitination and degradation. SSAT2, a related protein, stabilizes the interaction of the VHL protein and elongin C with HIF-1 leading to oxygen-dependent HIF-1α ubiquitination and degradation. We investigated the effects of PX-12 and Trx-1 on SSAT1, SSAT2, and inhibition of HIF-1α.
Methods
A panel of cell lines was treated with PX-12 to investigate its effects on SSAT1 and SSAT2 expression, and on HIF-1α protein levels. We also evaluated the regulation of SSAT1 through the Nrf2 and PMF-1, two trans-acting transcription factors.
Results
We found that PX-12 increased nuclear Nrf2 activity and antioxidant response element binding. PX-12 also increased the expression of SSAT1 but not SSAT2 in a PMF-1-dependent manner that was independent of Trx-1. Inhibition of Nrf2 or PMF-1 prevented the increase in SSAT1 caused by PX-12.
Conclusions
The results show that PX-12, acting independently of Trx-1, increases nuclear Nrf2, which interacts with PMF-1 to increase the expression of SSAT1. The degradation of HIF-1α that results from binding with SSAT1 may explain the decrease in HIF-1α caused by PX-12 and could contribute to the antitumor activity of PX-12.
Keywords: PX-12, SSAT1, SSAT2, Nrf2, Trx-1, HIF-1α
Introduction
Thioredoxin-1 (Trx-1) is a small (12 kDa) redox protein that is an important regulator of many aspects of cell proliferation and survival [1]. Thioredoxin reductase, a homodimeric flavoprotein, transfers reducing equivalents from NADPH to Trx-1, which then acts as a redox cofactor and a cellular antioxidant through the transfer of reducing equivalents to peroxiredoxins [2]. Trx-1 also increases the DNA binding of redox-sensitive transcription factors such as activator protein-1 (AP-1) and nuclear factor kappa-B (NF-κB) [3], and binds to and inhibits proapoptotic proteins including apoptosis signal–regulating kinase-1 (Ask-1) [4] and the tumor suppressor PTEN (phosphatase and tensin homolog) [5], which attenuates the activity of the phosphatidylinositol-3-kinase/Akt cell survival pathway in cancer cells. Trx-1 is overexpressed in many human cancers including lung, colon, cervix, stomach, and pancreatic cancer [1], where it is associated with aggressive tumor growth, decreased patient survival [6, 7], and resistance to chemotherapy [8, 9]. We have previously reported that Trx-1 increases the levels of the hypoxia-inducible transcription factor-1alpha (HIF-1α) under both normoxic and hypoxic conditions and increases the expression of HIF-1α downstream targets such as vascular endothelial growth factor (VEGF) leading to increased tumor angiogenesis [10]. We have also suggested that Trx-1 may play a role in the redox regulation of spermidine/spermine N(1)-acetyl transferase 1 (SSAT1) and polyamine homeostasis, which could contribute to the biological effects of Trx-1 [11]. SSAT1 is a polyamine catabolic enzyme that acetylates spermidine and spermine, thus reducing their potency. SSAT1 acts in concert with spermine oxidase and N(1)-acetylpolyamine oxidase to regulate polyamine metabolism [12]. SSAT1 is transcriptionally regulated through the interaction of two trans-acting transcription factors, nuclear factor erythroid-derived 2 (NF-E2)–related factor 2 (Nrf2) and polyamine modulated factor-1 (PMF-1) [13]. SSAT1 binding has been reported to stabilize the interaction between hypoxia-induced HIF-1α, RACK-1 (receptor for activated C kinase-1), and elongin C, thus promoting oxygen-independent HIF-1α ubiquitination and degradation [14]. SSAT2 has 64% similarity and 46% homology to SSAT1 [15] but has a much lower level of acetyltransferase activity toward polyamines and a lower level of expression than SSAT1 [16–18]. SSAT2 has been reported to stabilize the interaction of the von Hippel-Lindau protein (pVHL) and elongin C with HIF-1α and lead to oxygen-dependent HIF-1α ubiquitination and degradation [19].
PX-12, 1-methylpropyl 2-imidazolyl disulfide, is a Trx-1 inhibitor that shows antitumor activity in both in vitro and in vivo [20] and that is currently in clinical development [21]. We have previously reported that PX-12 lowers tumor HIF-1α protein levels and inhibits HIF-1α transactivating activity and the expression of the downstream targets of HIF-1α, including VEGF [21–24]. In the present study, we investigated the regulation of HIF-1α by PX-12 and the associated roles of Trx-1, SSAT1, and SSAT2. We found that PX-12 increases SSAT1 expression and decreases HIF-1α levels independently of Trx-1 and that SSAT2 does not play a role in HIF-1α degradation by PX-12.
Materials and methods
Cells and reagents
Human MiaPaCa-2 pancreatic cancer cells, MCF-7 breast cancer cells, LN-229 glioma cancer cells, and HEK-293 transformed embryonic kidney cells were obtained from American Type Culture Collection (Manassas, VA). Monoclonal antibodies to human HIF-1α, RACK1, and elongin C were purchased from BD Transduction Laboratories (San Diego, CA), and antibodies to Trx-1, Trx-2, Trx-reductase-1 and -2, lamin A/C, β-actin, NAD(P)H dehydrogenase, quinone 1 (NQO1), and Nrf2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PX-12 was synthesized [20], and MG-132 was purchased from Sigma–Aldrich (St. Louis, MO).
Cell culture
MiaPaCa-2, HEK-293, and LN-229 cells were cultured in Dulbecco’s minimum essential medium (Cellgro, Media-tech, Manassas, VA) and 10% fetal calf serum (Hyclone Laboratories, Logan, UT) at 37°C in 5% CO2. MCF-7 cells were cultured in minimum essential medium (Cellgro) and 10% fetal calf serum. All cell lines were genetically verified by the M. D. Anderson Cell Line Characterization Service. For assay, cells were incubated for various times at 37°C in humidified air, 5% CO2 (normoxia), or 1% O2, 5% CO2, 94% N2 (hypoxia) using an Invivo Hypoxia Workstation 400 with Ruskinn hypoxic gas mixer (Ruskinn Technology, Pencoed, UK). Cells (2.5 × 105)) were seeded and grown under normoxic conditions to 70% confluence and then incubated under hypoxic conditions for 16–24 h in the presence or absence of PX-12. Cells cultured under hypoxic conditions were collected under hypoxic conditions.
Western blotting
Cells were grown under normoxic or hypoxic conditions in the presence or absence of 25 μM PX-12. The cells were washed twice in phosphate-buffered saline, and Western blotting was performed as previously described [11]. Blots were quantified using ImageQuant software (Molecular Dynamics/GE Healthcare Biosciences, Pittsburgh, PA). Nuclear and cytoplasmic extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce/Thermo Fisher Scientific, Rockford, IL) in accordance with the manufacturer’s protocols.
Real-time reverse transcription–PCR analysis
Total RNA was isolated from cell lysates using the PARIS kit (Ambion/Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. TaqMan quantitative reverse transcription (RT)–PCR was performed on the ABI 7300 system using the TaqMan one-step reverse transcription–PCR Master Mix kit and predesigned primer/probe pairs for SSAT1, SSAT2, Nrf2, PMF-1, and β2-microglobulin (Applied Biosystems, Foster City, CA). Normalization and analyses were carried out with β2-microglobulin (B2M) using the 2(-delta-delta C(T)) method as the internal reference [25] and with Applied Biosystems GeneAmp 5700 SDS software. All measurements were performed in triplicate.
Small interfering RNA transfection
Small interfering RNA (siRNA) SMARTpool sequences were obtained from Dharmacon/Thermo Fisher Scientific (Waltham, MA), and the cells were transfected with 25 nM siRNA-Trx-1, siRNA-EIF2AK3 (PERK), siRNA-Nrf-2, siRNA-SSAT1, siRNA-SSAT2, siRNA-PMF-1, siRNA-HIF-1α, or a siRNA nontargeting control using Dharma-FECT-1 lipid transfection reagent. The transfection medium was removed after 24 h and replaced with fresh medium, and the cells were grown in 5% CO2 at 37°C for an additional 48–72 h. Western blot analyses were performed to confirm target knockdown by siRNA. Transfected cells were treated with PX-12 and cultured under normoxic or hypoxic conditions for an additional 16–18 h.
DNA-binding assay
Nuclear extracts were obtained using the Nuclear Extract Kit (Active Motif, Carlsbad, CA) in accordance with the manufacturer’s protocol. The Nrf2/antioxidant response element (ARE)–binding assay was conducted using the TransAM Transcription Factor DNA-binding ELISA kit (Active Motif). Nuclear extract DNA was quantified using NanoDrop (Thermo Fisher Scientific).
Statistical analysis
Significance was determined using student’s t test to compare data points with control data. Results at p < 0.05 were considered significant.
Results
PX-12 decreases hypoxia-induced HIF-1α levels and increases SSAT1
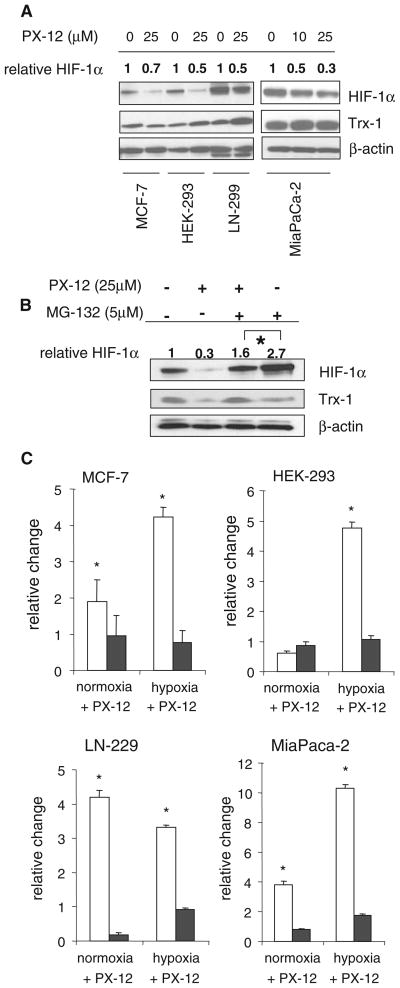
PX-12 decreased hypoxia-induced HIF-1α protein levels in a panel of four cell lines (MiaPaCa-2, MCF-7, HEK-293, and LN-299) confirming earlier observations [22] (Fig. 1a). The proteosomal inhibitor MG-132 was used to determine whether the PX-12–mediated decrease in HIF-1α was the result of increased proteosomal degradation (Fig. 1b). The results show that PX-12 was still able to decrease HIF-1α protein level in the presence of MG-132. PX-12 also increased the expression of SSAT1 under both normoxic and hypoxic conditions in all 4 cell lines (Fig. 1c). SSAT2 expression was not increased by PX-12 under either normoxic or hypoxic conditions.
Fig. 1.
PX-12 inhibits hypoxia-induced HIF-1α protein and induces SSAT expression in cancer cell lines. a Western blot showing hypoxia-induced HIF-1α and Trx-1 protein levels in MCF-7, HEK-293, LN-299, and MiaPaCa-2 cancer cell lines, with or without 25 μM PX-12 for 16 h under hypoxic (1% O2, 5% CO2, 94% N2) conditions. HIF-1α was quantified by densitometry relative to β-actin used as a loading control and expressed relative to the appropriate control without PX-12. b Western blot of HIF-1α and Trx-1 protein levels in MiaPaCa-2 cells with and without 25 μM PX-12 and with and without 5 μM proteasome inhibitor MG-132 for 16 h under hypoxic conditions. HIF-1α levels measured by densitometry were normalized to the β-actin loading control and expressed relative to control without PX-12 or MG-132. c Cancer cell lines were treated with 25 μM PX-12 for 16 h under normoxic (air, 5% CO2) or hypoxic (1% O2, 5% CO2, 94% N2) conditions; total RNA was isolated, and SSAT1 mRNA (open bars) and SSAT2 mRNA (filled bars) were measured by RT–PCR. Levels are expressed relative to cells without PX-12 and are the mean of 3 determinations and bars are SD. *P < 0.05 compared to cells without PX-12
Trx-1 is not required for PX-12 to decrease HIF-1α levels and/or SSAT1 expression
Trx-1 knockdown with siRNA did not decrease hypoxia-induced HIF-1α protein levels although Trx-1 protein levels were markedly decreased (Fig. 2a). We previously reported that transfection of cells with dominant-negative redox-inactive Trx-1 inhibited hypoxia-induced HIF-1α protein levels [10]. The difference with the present results using siRNA-Trx-1 may be due to off target effects of the redox-inactive Trx-1. Furthermore, siRNA knockdown of Trx-1 did not inhibit the increase in SSAT1 caused by PX-12 in either normoxia or hypoxia (Fig. 2b). It should be noted that for unknown reasons, knockdown of Trx-1 actually increased SSAT1 expression. There was no significant effect of Trx-1 knockdown on SSAT2 expression.
Fig. 2.
Trx-1 is not necessary for the effects of PX-12 on HIF-1α and SSAT1. a MiaPaCa-2 cells were transfected with of Trx-1 siRNA (siTrx-1) or the nontargeting siRNA control (siScr) for 72 h under normoxic conditions, cultured for 16 h with or without 25 μM PX-12 under normoxic (air, 5%CO2, open bars), or hypoxic (1% O2, 5% CO2, 94% N2,) conditions, and then subjected to Western blotting for HIF-1α or Trx-1. HIF-1α levels measured by densitometry were normalized to the β-actin loading control and expressed relative to control cells without PX-12. b MiaPaCa-2 cells were transfected with siTrx-1 or the nontargeting siScr for 72 h under normoxic conditions, and then grown for 16 h with 25 μM PX-12 under normoxic or hypoxic conditions. SSAT1 mRNA (open bars) and SSAT2 mRNA expression (filled bars) measured by RT–PCR is expressed relative to changes without PX-12. Values are means of 3 determinations, and bars are SD. *P < 0.05 compared with nontargeting siScr
PX-12 increases nuclear Nrf2 expression and binding to the ARE
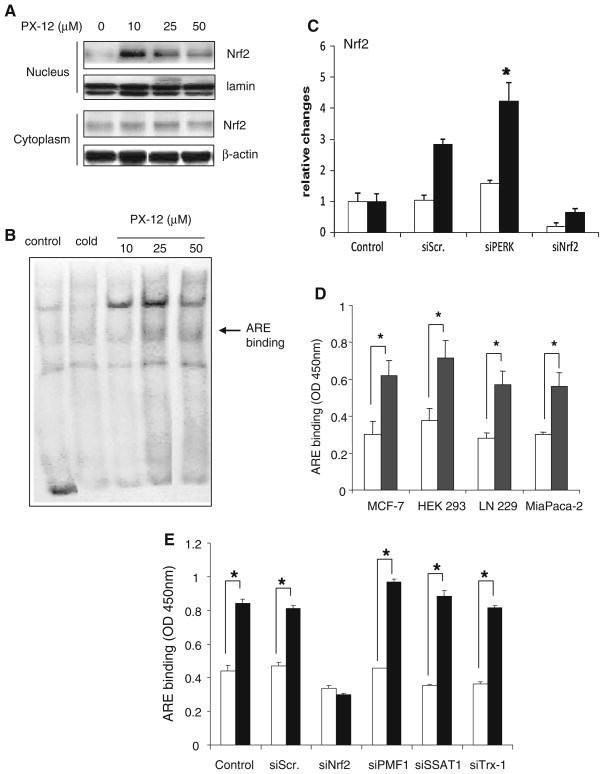
PX-12 was found to increase nuclear Nrf2 protein levels in MiaPaCa-2 cells, but not to affect the levels of Nrf2 protein in the cytoplasm (Fig. 3a). At the same time, Nrf2 binding to the ARE was increased by PX-12 at 10 and 25 μM; however, at 50 μM, PX-12 inhibited ARE binding possibly due to a decrease in nuclear Nrf2 at this high concentration of PX-12 (Fig. 3b). The endoplasmic reticulum stress response acting through PERK can activate Nrf2 [26]. However, PX-12, 25 μM, was able to increase Nrf2 expression under hypoxic conditions even when PERK was inhibited (Fig. 3c). PX-12 significantly increased the binding of Nrf2 to the ARE in the panel of 4 cell lines (Fig. 3d) but failed to increase the binding in siRNA-Nrf2-treated cells, while siRNA-PMF1, siRNA-SSAT1, and siRNA-Trx-1 had no effect on ARE binding (Fig. 3e). PX-12 increased total levels of SSAT1 in hypoxic MiaPaCa-2 and MCF-7 cells but only increased Nrf2 under hypoxic conditions and when Trx-1 was knocked down with siRNA–Trx-1 (Fig. 4a, b).
Fig. 3.
Effect of PX-12 on Nrf2 and its binding to the antioxidant response element (ARE). a Western blot of Nrf2 protein levels in the nucleus and cytoplasm of control MiaPaCa-2 cells and cells treated for 24 h with PX-12 at various concentrations, with lamin A/C and β-actin as loading controls, respectively. b Electrophoretic mobility shift assay of the binding of nuclear extracts to the ARE of MiaPaCa-2 control cells and cells treated for 24 h with PX-12 at various concentrations. Cold is the addition of a 100-fold excess of unlabeled probe. c MiaPaCa-2 cells were transfected for 72 h with different siRNAs and treated without (open bars) or with 25 μM PX-12 (filled bars) for 16 h under hypoxic conditions (1% O2, 5% CO2, 94% N2). Nrf2 expression was measured by RT–PCR and expressed relative to cells without PX-12. Values are the mean of 3 determinations. P < 0.05 compared to cells without PX-12. d Cancer cell lines treated without (open bars) or with 25 μM PX-12 (filled bars) for 16 h under hypoxic conditions. Nrf2 binding to the ARE was measured as relative values, and is shown as the mean of three determinations, with SD as bars. *P < 0.05 compared to control without PX-12. e Nrf2 binding to the ARE is expressed relative values as the mean of three determinations, with bars as SD. *P < 0.05 compared to control without PX-12
Fig. 4.
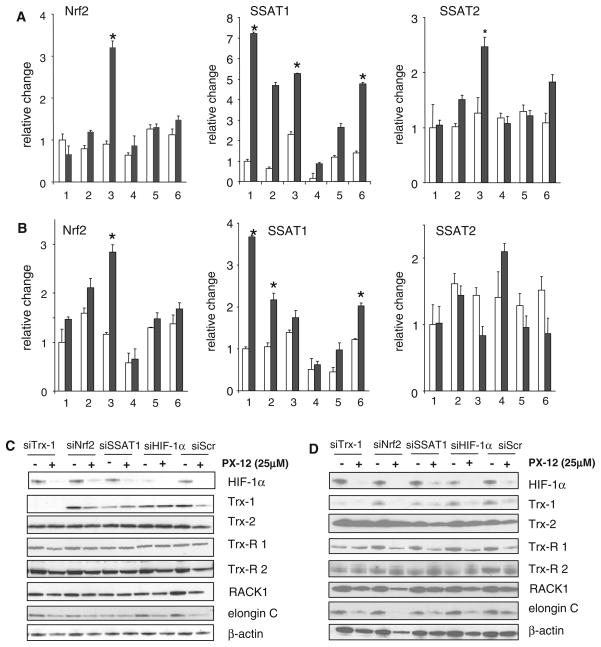
PX-12 induces SSAT1 through Nrf2. a MiaPaCa-2 pancreatic cells and b MCF-7 breast cells were transfected for 72 h with different siRNAs before being exposed to 25 μM PX-12 for 16 h under normoxic conditions (air, 5%CO2, shown in open bars), or hypoxic conditions shown in filled bars). The cell preparations (1% O2, 5% CO2, 94% N2, were: 1, mock transfection (control); 2, transfection with non-targeting siRNA or siRNA; 3, transfection with siRNA-Trx-1; 4, transfection with siRNA-Nrf2; 5, transfection with siRNA-SSAT1; and 6, transfection with siRNA-HIF-1α. After being transfected for 72 h, the cells were grown, and total RNA was isolated. Nrf2, SSAT1, and SSAT2 mRNA were measured by RT–PCR and expressed relative to cells without PX-12. Values are the mean of 3 determinations, and bars are SD. *P < 0.05. C Western blot of MiaPaCa-2 cells; d Western blot of MCF-7 breast cells. Cells in c and d were transfected with the siRNAs for 72 h and exposed to hypoxic conditions for 16 h with and without 25 μM PX-12. Various downstream targets of ARE activation were measured. β-actin was the loading control
PX-12 and downstream targets of the ARE
Although PX-12 increased hypoxia-induced Nrf2 levels in the absence of Trx-1, and increased Nrf2 binding to the ARE in hypoxic MiaPaca-2 cells, it had no effect on the levels of typical ARE downstream targets suggesting that ARE-bound Nrf2 is nonfunctional possibly (Fig. 4c). This could be as a result of modification of Nrf2 by PX-12. However, in hypoxic MCF-7 breast cancer cells, PX-12 caused a decrease in ARE downstream targets, including thioredoxin reductase 1, RACK1, and elongin C (Fig. 4d). As expected, PX-12 decreased HIF-1α and Trx-1 levels in both the MiaPaCa-2 and MCF-7 cell lines. The results suggest a complex effect on antioxidant activity that is not mediated by the ARE or SSAT1 and which is perhaps dependent on the intrinsic oxidant stress state of the cells. Hypoxia is known to increase the production of reactive oxygen species through a process mediated by the mitochondria [25], which could vary between cell lines.
PMF-1 is essential for the induction of SSAT mRNA expression by PX-12
Both Nrf2 and PMF-1 appear to be necessary for hypoxia-induced SSAT1 mRNA expression, and PX-12 does not induce SSAT1 mRNA expression when either Nrf2 or PMF-1 is knocked down (Fig. 5a). SSAT2 mRNA expression was not affected by a loss of Nrf2 or PMF-1 (Fig. 5b). Trx-1 siRNA treatment of MiaPaCa-2 cells showed an increase in PMF-1 in hypoxic conditions, with and without the addition of PX-12 (Fig. 5c).
Fig. 5.
PMF-1 is essential in SSAT1 induction. MiaPaca-2 cells were transfected with various siRNAs or the nontargeting siScr for 72 h under normoxic conditions, and then grown for 16 h with 25 μM PX-12 (filled bar) or without PX-12 (open bar) under hypoxic conditions. a SSAT1; b SSAT2; and c PMF-1 were measured by RT–PCR and expressed relative to changes in cells without PX-12. Values are means of 3 determinations, and bars are SD. *P < 0.05 compared with controls
Discussion
In a previous study, we suggested that Trx-1 might regulate polyamine levels in breast cancer cells through a decrease in SSAT1 expression and this might contribute to the growth-stimulating and antiapoptotic effects of Trx-1 [11]. SSAT1 expression is transcriptionally regulated through the interaction of two trans-acting transcription factors, Nrf2 and PMF-1 [13]. SSAT1 functions in polyamine catabolism and contributes to the regulation of intracellular pools of the polyamines putresceine, spermidine, and spermine. The closely related SSAT2 cannot acetylate polyamines and its likely physiological substrate is thialysine, which is formed by the reaction of L-serine and cysteamine catalyzed by cystathionine-β-synthase [17]. SSAT1 and SSAT2 both promote HIF-1α degradation but by different mechanisms. SSAT1 stabilizes the binding of RACK1 and elongin C with HIF-1α, thus promoting the oxygen-independent ubiquitination and degradation of HIF-1α [14]; SSAT2 stabilizes the binding of pVHL and elongin C with HIF-1α and because pVHL requires oxygen-dependent proline hydroxylation of HIF-1α, this leads to oxygen-dependent HIF-1α ubiquitination and degradation [19]. We anticipated that PX-12 would counteract the effects of Trx-1, resulting in an increase in SSAT1, and thus a decrease in HIF-1α levels. We were therefore surprised to find that PX-12 increased SSAT1 expression and decreased HIF-1α levels independently of Trx-1. We were also interested to see if there might be an increase in SSAT2 finding that its expression level was not increased by PX-12 and that SSAT2 did not play a role in HIF-1α degradation by PX-12.
Nrf2 activates ARE-mediated gene expression and mediates the primary antioxidant response to an oxidative challenge in cells [27, 28]. Interaction of the leucine zipper region of Nrf2 with the c-terminal coiled-coil region of PMF-1 increases the expression of SSAT1 and its response to polyamines [13, 29]. Nrf2 is kept in the cytoplasm in a complex with Kelch-like ECH-associated protein 1 (Keap1), from which it is released by the oxidization of critical cysteine residues. This leads to the nuclear translocation and accumulation of Nrf2 [30]. We found increased levels of nuclear Nrf2 and Nrf2 binding to the ARE in cells treated with PX-12. Nrf2 is also activated by PERK-dependent phosphorylation that leads to dissociation of the Nrf2/Keap1 complex [26]; however, we found that PX-12 increased Nrf2 independently of PERK. We found that PX-12 indirectly increases Nrf2 through its inhibitory effect on Trx-1, but also directly increases Nrf2 expression under hypoxic conditions by a process that is independent of Trx-1. The latter mechanism appears to be the primary means by which PX-12 increases Nrf2. It is noteworthy that the PX-12-induced Nrf2 increase we found in one of the cell lines was not accompanied by increased expression of ARE downstream targets. This suggests a possible cell context–dependent effect for the antioxidant response.
The Sat1 gene encoding SSAT1 contains a positive regulatory element (PRE)–binding site. Along with increases in the levels of Nrf2 and SSAT1 mRNA caused by PX-12, we also found evidence of increased PRE binding suggesting that a Nrf2/PMF-1 mechanism is responsible for the increase in SSAT1 by PX-12. Inhibiting either Nrf2 or PMF-1 prevented the increase in SSAT1 by PX-12. Baek et al. [14] have shown that SSAT1 plays an essential role in the RACK1-mediated ubiquitination of HIF-1α and suggested that this leads to 26S proteasome-dependent HIF-1α degradation. However, we found that the decrease in HIF-1α by PX-12 was insensitive to proteasome inhibition with MG-132, which suggests that another degradation mechanism may be involved.
Thus, the effect of PX-12 in decreasing HIF-1α may occur through multiple mechanisms (Fig. 6). The results of our study show that PX-12 decreases HIF-1α protein levels by an oxygen-independent pathway through an increase in SSAT1 expression mediated by Nrf2 activation and increased ARE binding. Furthermore, we found that this activity of PX-12 is independent of its ability to inhibit Trx-1. Although SSAT2 is essential to the stabilization of pVHL in the oxygen-dependent degradation pathway, PX-12 did not affect SSAT2 expression levels. In conclusion, we have shown that PX-12 is an inhibitor of HIF-1α through Nrf2/PMF-1 complex formation and the induction of SSAT1.
Fig. 6.

Action of PX-12 decreasing HIF-1a by increasing nuclear Nrf2 and SSAT1
Acknowledgments
The authors thank LeeAnn Chastain for editorial assistance. The research was supported by National Institutes of Health grants CA129616, CA109552, and CA077204.
Abbreviations
- ARE
Antioxidant response element
- Ask-1
Apoptosis signal–regulating kinase-1
- HIF-1α
Hypoxia-inducible factor-1alpha
- Keap1
Kelch-like ECH-associated protein 1
- NAD(P)H
Nicotinamide adenine dinucleotide phosphate oxidase
- NQO1
NADPH dehydrogenase, quinone 1
- Nrf2
Nuclear factor erythroid-derived 2(NF-E2)–related factor 2
- PMF-1
Polyamine modulated factor-1
- PRE
Positive regulatory element
- PTEN
Phosphatase and tensin homolog
- pVHL
Von Hippel-Lindau protein
- PX-12
1-Methylpropyl 2-imidazolyl disulfide
- RACK1
Receptor for activated C kinase 1
- RT–PCR
Reverse transcription-polymerase chain reaction
- siRNA
Small interfering RNA
- SSAT1
Spermidine/spermine N(1)-acetyltransferase 1
- SSAT2
Spermidine/spermine N(1)-acetyltransferase 2
- Trx-1
Thioredoxin-1
Footnotes
Conflict of interest GP is a stockholder in Oncothyreon the company that owns PX-12.
Contributor Information
Yon Hui Kim, Email: yhkim@mdanderson.org, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Amy Coon, Arizona Cancer Center, University of Arizona, Tucson, AZ, USA.
Amanda F. Baker, Arizona Cancer Center, University of Arizona, Tucson, AZ, USA
Garth Powis, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
References
- 1.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Kakolyris S, Giatromanolaki A, Koukourakis M, et al. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res. 2001;7:3087–3091. [PubMed] [Google Scholar]
- 7.Raffel J, Bhattacharyya AK, Gallegos A, et al. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 8.Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Nat Acad Sci USA. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawahara N, Tanaka T, Yokomizo A, et al. Enhanced coexpression of thioredoxin and high mobility group protein 1 genes in human hepatocellular carcinoma and the possible association with decreased sensitivity to cisplatin. Cancer Res. 1996;56:5330–5333. [PubMed] [Google Scholar]
- 10.Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 11.Husbeck B, Stringer DE, Gerner EW, Powis G. Increased thioredoxin-1 inhibits SSAT expression in MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2003;306:469–475. doi: 10.1016/s0006-291x(03)00993-8. [DOI] [PubMed] [Google Scholar]
- 12.Pegg AE. Spermidine/spermine-N1-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Devereux W, Stewart TM, Casero RA., Jr Characterization of the interaction between the transcription factors human polyamine modulated factor (PMF-1) and NF-E2-related factor 2 (Nrf-2) in the transcriptional regulation of the spermidine/spermine N1-acetyltransferase (SSAT) gene. Biochem J. 2001;355:45–49. doi: 10.1042/0264-6021:3550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek JH, Liu YV, McDonald KR, Wesley JB, Zhang H, Semenza GL. Spermidine/spermine N(1)-acetyltransferase-1 binds to hypoxia-inducible factor-1alpha (HIF-1alpha) and RACK1 and promotes ubiquitination and degradation of HIF-1alpha. J Biol Chem. 2007;282:33358–33366. doi: 10.1074/jbc.M705627200. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of a second spermidine/spermine N1-acetyltransferase. Biochem J. 2003;373:661–667. doi: 10.1042/BJ20030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Kramer DL, Jell J, Vujcic S, Porter CW. Small interfering RNA suppression of polyamine analog-induced spermidine/spermine n1-acetyltransferase. Mol Pharmacol. 2003;64:1153–1159. doi: 10.1124/mol.64.5.1153. [DOI] [PubMed] [Google Scholar]
- 17.Coleman CS, Stanley BA, Jones AD, Pegg AE. Spermidine/spermine-N1-acetyltransferase-2 (SSAT2) acetylates thialysine and is not involved in polyamine metabolism. Biochem J. 2004;384:139–148. doi: 10.1042/BJ20040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel NL, Boeke M, Ashburner BP. Spermidine/spermine N1-acetyltransferase 2 (SSAT2) functions as a coactivator for NF-kappaB and cooperates with CBP and P/CAF to enhance NF-kappaB-dependent transcription. Biochim Biophys Acta. 2006;1759:470–477. doi: 10.1016/j.bbaexp.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, Liu YV, McDonald KR, et al. Spermidine/spermine-N1-acetyltransferase 2 is an essential component of the ubiquitin ligase complex that regulates hypoxia-inducible factor 1alpha. J Biol Chem. 2007;282:23572–23580. doi: 10.1074/jbc.M703504200. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick DL, Kuperus M, Dowdeswell M, et al. Mechanisms of inhibition of the thioredoxin growth factor system by anti-tumor 2-imidazolyl disulfides. Biochem Pharmacol. 1998;55:987–994. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan RK, Dragovich T, Richards D, Stephenson J, Pestano L, Hiscox A, Leos R, Chow S, Millard J, Kirkpatrick L. Results from phase Ib studies of PX-12, a thioredoxin inhibitor in patients with advanced solid malignancies. J Clin Oncol. 2009;27:15s. (suppl; abstr 2571) [Google Scholar]
- 22.Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- 23.Jordan BF, Runquist M, Raghunand N, et al. The thiore-doxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res. 2005;11:529–536. [PubMed] [Google Scholar]
- 24.Baker AF, Dragovich T, Tate WR, et al. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147:83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Cullinan S, Zhang D, Hannink M, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paddenberg R, Goldenberg A, Faulhammer P, Braun-Dullaeus RC, Kummer W. Mitochondrial complex II is essential for hypoxia-induced ROS generation and vasoconstriction in the pulmonary vasculature. Adv Exp Med Biol. 2003;536:163–169. doi: 10.1007/978-1-4419-9280-2_21. [DOI] [PubMed] [Google Scholar]
- 28.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal AK. Regulation of antioxidant response element-dependent induction of detoxifying enzyme synthesis. Methods Enzymol. 2004;378:221–238. doi: 10.1016/S0076-6879(04)78018-0. [DOI] [PubMed] [Google Scholar]
- 30.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]