Abstract
Branched chain fatty acids (BCFA) have recently been shown to be a major component of the normal human newborn gastrointestinal tract and have long been known to be a component of human milk. Ruminant food products are major sources of fat in the American diet, but there are no studies of milkfat BCFA content in retail milk. We report here the profile and concentrations of BCFA in a representative sampling of retail milk in the 48 contiguous United States (US), and their estimated intake in the American diet. Conventionally produced whole fluid milk samples were obtained from 56 processing plants across the contiguous 48 states. Retail milk samples contain exclusively iso- and anteiso-BCFA with 14–18 carbons. BCFA were 2.05 ± 0.14%, w/w of milkfat fatty acids (mean ± SD), and anteiso-BCFA comprised more than half this total. Based on these data and USDA food availability data, the average per capita BCFA intake of Americans is estimated to be about 220 mg/d from dairy; if current dietary recommendations were followed, BCFA intake would be about 400 mg/d. Adding intake from beef consumption, these estimates rise to approximately 400 and 575 mg/d, respectively. These results indicate that BCFA intake is a substantial fraction of daily fat intake, in amounts exceeding those of many bioactive fatty acids.
Keywords: Branched chain fatty acids, Retail milk, Dietary intake, Milkfat
Introduction
Milk and dairy products are major contributors to fat intake in the American diet. Cow’s milkfat is characterized by high proportions of fatty acids (FA) with 16 carbons or less. Individual FA, such as conjugated linoleic acids (CLA), may have a beneficial effect on health maintenance and prevention of acute and chronic disease [1, 2]. The FA profile of milk in all species, including cow’s milk, is sensitive to dietary fat intake, for instance, concentrate versus pasture versus fish oil supplementation [3-6], thus milk composition may differ depending on production practices. O’Donnell-Megaro et al. [7] recently reported the composition of major FA retail milk in the United States (US). About 3.1% of FA were unidentified and listed as a group under “Other”; branched chain fatty acids (BCFA) are included in this group.
BCFA are primarily saturated FA (SFA) with one or more methyl branches. In human metabolism, they are best studied as components of skin lipids. Overall, several dozen specific BCFA, are found in milk and tissue of ruminants including sheep and goats, presumably synthesized by ruminal organisms that rely on them for membrane lipids [8, 9]. BCFA are conveniently categorized as mono- and di-/multi-methyl BCFA; in monomethyl BCFA, the predominant branching is at the terminal methyl (iso) or next to the terminal methyl (anteiso), as shown in Fig. 1. iso- and anteiso-BCFA are the main BCFA reported in cow’s milk [3, 4, 6, 10, 11]. Terpenoid BCFA, exemplified by internal periodic poly-methyl branching such as phytanic acid (3,7,11,14 tetra-methyl hexadecanoic acid) and its alpha oxidation product pristanic acid (2,6,10,14 tetramethyl pentadecanoic acid) are also reported in cow’s milk [11, 12].
Fig. 1.
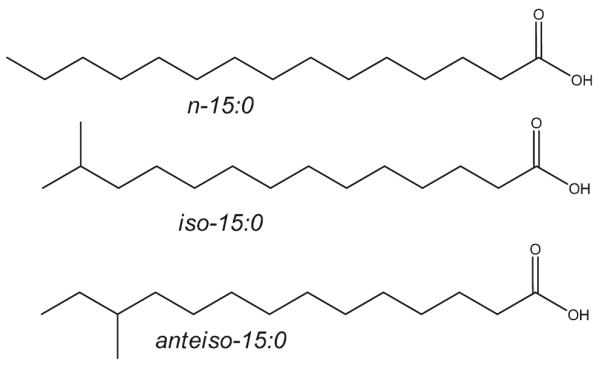
Structures of iso- and anteiso-branched chain fatty acids (BCFA). n-(normal) hydrocarbon chains without branching. iso-BCFA have a bifurcated methyl branch (iso-15:0 is 13-methyl tetradecanoic acid). anteiso-BCFA have a methyl branch on the antepenultimate carbon. anteiso-15:0 is 12-methyl tetradecanoic acid
Information on human intake and metabolism of BCFA is scant, and there are no recommendations for or against dietary intake of BCFA. Ran-Ressler et al. [13] recently showed that BCFA are a major component of the late term fetal and newborn gut contents. They comprise almost one third of the FA in vernix [13], the white fatty film that covers the fetus in utero. Vernix suspended in amniotic fluid is normally swallowed by the fetus, increasingly so as parturition approaches [14], exposing the fetal gut to BCFA from an early age. Moreover, BCFA are also found in human milk [15, 16] where they reportedly comprise 1.5%, w/w. A 1981 report put the concentrations of anteiso-17:0 in colostrum at 0.45%, w/w, higher than the concentrations of docosahexaenoic acid (DHA; 22:6n-3; 0.32%, w/w) and arachidonic acid (20:4n-6; 0.4%, w/w) [16]. A study in rat pups shows that BCFA reduce the incidence of necrotizing enterocolitis (NEC), a devastating intestinal disease affecting premature infants (Ran-Ressler et al. unpublished data). Thus, BCFA are a major component in perinatal nutrition, and although they may have a beneficial role in human health, their presence in the US food chain has been almost completely neglected.
The objective of the present study is to analyze the levels and distribution of BCFA in US retail milk and to estimate the contribution of BCFA to the nutrition of Americans based on estimated intake of milk and other foods produced by ruminant animals such as cheese and beef. In a 1994 survey, dairy products contributed 7.5% of the protein, 4.2% of the total fat, and approximately 8% of the total saturated fat in the diet of adults [17]. Based on NHANES 1999–2004 [18] fluid milk, mostly whole milk, provided 7.5, and 6.4% of energy intake in children age 2–4 and 5–10, respectively. We hypothesized that BCFA are found in retail milk and dairy products in the US and thus are consumed in substantial amounts in the American diet. We report here the first data on the structure and quantitative analysis of BCFA in a representative samples of the US retail milk supply and we show that BCFA intake, per capita, is in excess than that of many bioactive FA.
Experimental Procedures
Sampling
Conventionally produced whole fluid milk samples were obtained from 56 US milk processing plants across the contiguous 48 states. All samples were whole milk obtained in December, 2008. They had been homogenized, pasteurized and packaged for transport to retail stores. Processing plants were selected based on the criteria that they represented at least 50% of the volume of milk produced in that area. Milk was shipped on ice overnight to Cornell University and immediately processed for the analysis of FA composition.
Fatty Acid Analysis
Milk fat extraction was based on the Mojonnier method (AOAC 995.19) as modified by Barbano [19]. Briefly, milkfat was obtained from 10 ml whole fluid milk by a sequence of 3 successive extractions. Ten milliliters of 95% alcohol and 25 ml ethyl ether plus milk was followed by vigorous mixing, then 25 ml petroleum ether was added and followed by vigorous mixing, and decanting of the ether layer. The second and third extractions were similar, except the volume of solvents was reduced to 5 ml of 95% alcohol and 15 ml each of ethyl and petroleum ethers, and the third extraction omitted the 95% alcohol. Ether solutions from the 3 extractions were combined, dried and resuspended in hexane. Methyl esters of the extracted fat were prepared using sodium methoxide as the methylation reagent, according to Christie [20] as modified by Chouinard [21].
Fatty acid methyl ester (FAME) analyses were performed using a Hewlett Packard 5890 Gas Chromatograph (GC) equipped with a split/splitless injector run in splitless mode at 250 °C, and with the flame ionization detector (FID) at 270 °C. A BPX-70 column (25 m × 0.22 mm × 0.25 μm, SGE, Austin, TX) was used for the analysis with H2 as the carrier gas. The oven temperature program was initially 80 °C for one minute, increased by 30 °C per minute to 170 °C and held for 2 min, then increased by 10 °C per minute until a final temperature of 240 °C which was held for 1 min. An equal weight FAME mixture (68A; Nu-Chek Prep, Elysian, MN) was used to calculate response factors. Several pure BCFA were also used as reference standards: iso-14:0, anteiso-15:0; iso-16:0, anteiso-17:0, iso-18:0 and iso-20:0 (Larodan Fine Chemicals AB, Malmo, Sweden).
FAME identities were determined by electron impact ionization mass spectrometry (EI-MS), using a Varian Star 3400 GC coupled to a Varian Saturn 2000 ion trap MS, based on GC retention times and EI mass spectra. The GC–MS column was a BPX-70 (60 m × 0.32 mm × 0.25 μm, SGE, Austin, TX). The following primary characteristic ions were used for saturated and BCFA methyl ester identification: Molecular ion [M], 74 m/z (McLafferty rearrangement), and 87 m/z (formed by cleavage at the fourth carbon). Ions at 88 m/z and 101 m/z were used for the presence of a methyl branch in the second and third carbon, as in the multibranched FA pristanic and phytanic acid, respectively. In addition, the presence, in higher intensities compared to their normal homologues, of the fragments [M-15]+ or [M-43]+ or both indicated the presence of iso-BCFA; while the fragments [M-29]+ or [M-57]+ or both indicated the presence of anteiso-BCFA. The MS spectra of the BCFA were compared to the pure BCFA mixture described above and to an online spectra archive http://www.lipidlibrary.aocs.org/ms/arch_me/index.htm#branch.
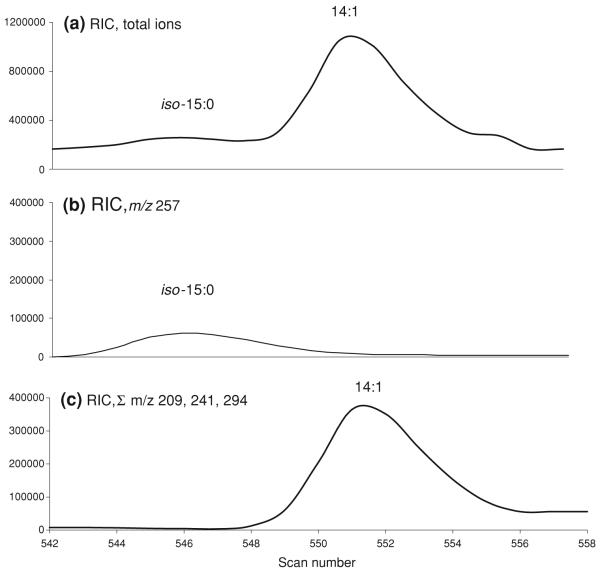
Under the GC-FID conditions described above, two pairs of FAME coelute, iso-15:0/14:1 and iso-17:0/16:1. Coelutions of monoenic and BCFA are unavoidable even on long columns such as CP-Sil-88 [22]. The use of a semipolar column such as the CP-Sil 19 was reported recently to effectively separate BCFA from monounsaturated FA (MUFA) in cheese and fish samples [23]. In the present study, GC with covalent adduct chemical ionization mass spectrometry (CACI-MS) was used to resolve the two sets of overlapping peaks [24]. Solutions of pure 14:1, 15:0, 16:1, and 17:0 FAME (0.5 μg/μl, Nu-Chek Prep Inc., Elysian, MN) were used to determine response factors for ions characteristic of the respective FAME. Selected characteristic ions were plotted to resolve and quantify coeluting FAME. A plot for MH+ was used for the iso-FAME in the milk sample, and a plot of [MH-32]+, MH+, and [M + 54]+ intensities were used for the monounsaturated FAME. An example of GC-CACI-MS selected ion plots used for the resolution and quantification of iso-15:0 and 14:1 is shown in Fig. 2. An area to weight ratio [A/W, arbitrary units/(ug/ul)] was calculated for each of the pure FAME by dividing the area from the corresponding characteristic ions to the known concentrations of the pure FAME. The concentrations (ug/ul) of the sample iso- or the monounsaturated-FAME were then calculated using the ratio between the area under the characteristic ions of the sample FAME and the A/W of the pure corresponding FAME.
Fig. 2.
Resolution of overlapping peaks using GC-CACI-MS. a A total reconstructed ion chromatogram (RIC) showing coelution of iso-15:0 with 14:1. b An RIC of the m/z 257 MH+ used for iso-15:0. c An RIC plot of the sum of signals for m/z 209 [MH-32]+, m/z 241 MH+, and m/z 294 [M + 54]+ for 14:1. See “Experimental Procedures” for further details on quantification
The relative percent contribution of the two interfering FAME was applied to the coeluting peaks to produce yield pure intensities. The percentages of the iso-15:0 and iso-17:0 that were obtained using this method fell within the range reported previously [10, 25].
Results and Discussion
BCFA in Retail Milk
Figure 3 presents in summary form the major classes of FA found in the present retail milk samples. The sum of palmitic acid (16:0) and all other SFA with shorter chain lengths comprised about 51%, w/w (mean). SFA with 17 or more carbons were 12%, MUFA were about 30%, and polyunsaturated FA (PUFA) were 4.7%. These values are similar to those previously found for our similar comprehensive retail milk sampling [7].
Fig. 3.
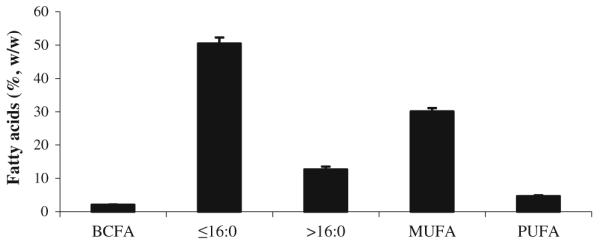
Fatty acid (FA) composition (%, w/w; mean ± SD) of conventional whole milk samples. FA were grouped as follow: Branched chain fatty acids (BCFA); de novo synthesized saturated fatty acids (SFA), including 16:0 (≤16:0); SFA longer than 16:0 (>16:0); monounsaturated fatty acids (MUFA); polyunsaturated fatty acids (PUFA)
BCFA comprised a total of 2.05 ± 0.14% of FA, or most of the 3% of FA listed previously as “other” [7]. The mean BCFA levels reported in the present study fall within the range previously reported in studies that measured cow’s milk FA in response to various diets in small scale experimental studies [3-5, 8, 10].
Table 1 presents the mean proportions of BCFA in retail milk as %, w/w of total FA and as a % of total BCFA (mean ± SD). BCFA with 14–18 carbons and iso- or anteiso-configuration were detected, and no BCFA with multiple branching were found. Four iso-BCFA (iso-C14:0 to iso-C17:0) were detected in all samples; low levels of iso-18:0 were also detected in most but not all of the samples. anteiso-15:0 and anteiso-17:0 were the only anteiso-BCFA found. They were in comparable concentration and constituted more than half of the total BCFA detected.
Table 1.
Branched chain fatty acids (BCFA) in retail milk expressed as %, w/w of total fatty acids and as %, w/w of BCFA (n = 56; mean ± SD)
| BCFAa | Total FAb (%, w/w) | BCFAa (%, w/w) |
|---|---|---|
| iso-14:0 | 0.13 ± 0.04 | 6.4 ± 1.4 |
| iso-15:0 | 0.13 ± 0.01 | 6.6 ± 0.4 |
| anteiso-15:0 | 0.56 ± 0.03 | 27.5 ± 1.3 |
| iso-16:0 | 0.31 ± 0.03 | 14.9 ± 0.9 |
| iso-17:0 | 0.26 ± 0.02 | 12.7 ± 0.7 |
| anteiso-17:0 | 0.61 ± 0.06 | 29.9 ± 2.0 |
| iso-18:0 | 0.04 ± 0.02 | 1.9 ± 0.9 |
BCFA branched chain fatty acids
FA fatty acids
BCFA with fewer than 14 carbons, such as iso- and anteiso-13:0, were not detected in any of the samples. Both iso- and anteiso-13:0 BCFA in cow’s milk have been reported by some [3, 5, 8, 11] but not by others [4, 6, 26]. In the present study, retail milk samples were obtained from processing plants and represent pooled milk from many farms; use of specific feeds on some farms that may support small amounts of ruminal C13 BCFA could be diluted to below detection limits by the milk from other farms [10, 27].
Neither phytanic acid, a product of released phytol from chlorophyll by rumen bacteria [28], nor its peroxisomal oxidation product pristanic acid [29], were detected in the retail milk. As with C13 BCFA, these terpenoid BCFA were reported to be present in bovine milk by some [11,12] but not by others [3-6]. When reported, the variance of these BCFA was high, and it was suggested [12] that differing feed compositions are responsible for variable terpenoid metabolizing ruminal bacteria. As with the case of iso- and anteiso-13:0, it is possible that minor amounts of phytanic and pristanic acid were present in milk from some farms, but diluted to below detection limits when pooled with milk from many farms in the processing plant.
For comparison with bioactive FA found primarily in dairy products, rumenic acid (cis-9, trans-11–18:2), the most concentrated conjugated linoleic acid (CLA) in cow’s milk, was 0.55%, in conventionally produced retail milk. Vaccenic acid (trans-11–18:1), the most concentrated trans monoene in cow’s milk, was 1.48% in US retail milk, and the sum of all trans FA was about 3.1% [7]. Thus, both anteiso- BCFA are at the same levels in milkfat as rumenic acid, and total BCFA are more than half the concentration of total trans-FA.
Estimated Human BCFA Intake from Retail Milk
Based on these data, we can estimate the contribution of BCFA to the nutrition of Americans, using measured and estimated milk intake. One cup (244 g = 8 oz) of whole milk (3.25% milkfat) contains 7.9 g fat [30]. Two percent of 7.9 g yields a total of 158 mg BCFA per cup whole milk. For comparison to intake of bioactive FA in the American diet, this value is greater than the 100 mg average daily consumption of the DHA and eicosapentaenoic acid (EPA) reported in a survey of 8604 Americans between 1999 and 2000 [31] and by women of child bearing age based on NHANES III data [32].
In some population groups, such as small children, milk consumption can be higher. Consumption by small children of 2.6 servings of milk was reported recently, mostly consumed as whole milk [33]. This consumption would thus represent 412 mg BCFA daily, which would provide almost 1% of the total fat intake of children age 2–5 [34]. It thus appears that the absolute daily intake of BCFA from milk—even more so, the amount of BCFA consumed per kilogram body weight—will be higher in some populations, such as in small children.
Apart from milk, other foods produced by ruminant animals, specifically cheese and beef, are expected to be other primary contributors of BCFA to the diet. US cheese BCFA can be estimated from the present measures of milkfat BCFA of about 2% of FA. BCFA averaged about 2%, w/w in Canadian retail beef [35], and similar to Malaysia beef tallow with C13-C20 BCFA with mean concentration 2.3% (mutton tallow had 4.0% BCFA) [36]. We assume American beef has the same BCFA levels as in Canada, and an average 28 and 18% fat content in cheese and in beef, respectively [30]. According to economic disappearance data adjusted for loss, American’s average per capita consumption of cheese is 30 g (1.1 oz), at about 28% fat on average and at about 2% BCFA, cheese contributes 168 mg of BCFA. Similarly, Americans consume 50 g (1.8 oz) of cooked beef per day [37]; at about 18% fat and about 2% BCFA, beef contributes about 180 mg of BCFA per day. Table 2 presents these values summed to estimate total current per capita intake of BCFA of about 400 mg per day from the most common ruminant foods.
Table 2.
Estimated mean daily branched chain fatty acids (BCFA) consumption per capita in the US, based on actual and recommendeda consumption of milk, cheese, and beef
| Food | Actuala |
Recommendeda |
||
|---|---|---|---|---|
| Portion (g) | BCFAb (mg) | Portion (g) | BCFAb (mg) | |
| Milkc | 119 | 54 | 215 | 98 |
| Cheesed | 30 | 168 | 53 | 297 |
| Beefe | 50 | 180 | 50 | 180 |
| Total | 199 | 402 | 318 | 575 |
Intake data estimation based on reference [37]
Actual consumption reflects the current consumption from milk, cheese and beef by Americans, which falls below recommended levels from the milk food group. Recommended consumption represents the levels of BCFA consumption that would obtain if Americans were to consume recommended amounts from the milk food group, keeping the same existing patterns as the actual consumption. For beef, the actual consumption was considered as the recommended one. Thus, the same value for beef was used for both
BCFA branched chain fatty acids
Mean, per capita, milk consumption, based on the proportions currently consumed by Americans of 3.25, 2, and 1% milk
Cheese types included in the analysis are the main cheeses consumed by Americans. An average of 28% fat was used for estimation [30]. Amounts are based on patterns of current consumption of cheese
For cooked beef, an average of 18% fat was used for estimation [30]. Americans consume enough from the meat food group, thus the actual and the recommended consumption were the same
We also consider the implications of dietary guidelines on BCFA consumption [38]. Americans are advised to consume three servings from the milk group, where a serving, for example, is equal to 1 cup of milk or yogurt and 1.5 oz (42 g, 2 slices) of cheese. For simplicity we assume this to be represented by the present average dietary pattern of milk intake in the US, which is 31% whole milk, 39% low fat (2%) milk, 14% lower fat (1%) milk, with the balance nonfat milk [37]. Using these values we estimate a weighted sum of the total BCFA intake from three servings of milk and cheese to be about 400 mg. Beef, cheese and milk together account for an average estimated recommended intake of about 575 mg BCFA per capita per day (Table 2).
Use of 2 cups of whole milk in place of reduced or no fat milks and consuming 1 serving of cheese (42 g, 1.5 oz) while consuming the same amount of beef would bring total BCFA to 731 mg/day (316 mg from milk + 235 mg from cheese + 180 mg from beef). These consumption levels, then, would be higher than the 500 mg/d intake for DHA and EPA for the general population recommended by the American Dietetic Association [39], and more than double the 300 mg/d of DHA and EPA recommended by the World Health Organization in pregnancy and lactation [32].
These estimates show that daily BCFA intake is substantial and that current recommendations promote an increase in present intake, considerably higher than other bioactive FA. Unlike DHA and EPA, for instance, BCFA sources include a greater variety of common food items, principally products of ruminants, regularly consumed by non-vegans. The low level of interest in BCFA nutrition is remarkable considering their long-standing intake.
The American dietary guidelines recommend consumption of low fat dairy products, however studies provide no convincing evidence that increased whole milk consumption is harmful with respect to ischemic heart disease and ischemic strokes [40], or vascular disease and diabetes [41]. Some studies provide evidence that higher consumption of whole fat compared to reduced fat milk was associated with lower body mass index in preschool- and elementary school-age children and less weight gain in adults [33, 42, 43] and with lower incidences of anovulatory infertility [44]. Thus, the emergence of potential benefits of whole milk consumption may have a significant effect on BCFA intake in the US population, increasing BCFA consumption even more.
BCFA are synthesized in large amounts by human skin [45], comprise 29%, w/w, of the FA in vernix and are normal constituents of the healthy newborn gut [13]. A systematic shift in BCFA profile was observed between vernix and meconium of the same infant, implying that the fetal alimentary canal selectively metabolizes BCFA. In very recent work, feeding anoxic rat pups rat milk substitute containing a mixture similar to most BCFA found in the retail milk samples (iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, iso-18:0, and iso-20:0) reduced the incidence of NEC compared to a control group (Ran-Ressler et al. unpublished data) and elevated mRNA levels of the intestinal, anti-inflammatory cytokine IL-10. BCFA are selectively incorporated into phospholipids of rat pup ileum and into human Caco-2 cells [46]. The risk reduction in NEC development may be linked to these observations.
BCFA function in the membranes is similar to cis unsaturated double bonds; both interfere with the ability of saturated hydrocarbon chains to pack tightly forming rigid extended structures. Old data show that an E. coli strain that lost the ability to desaturate saturated FA was restored to wild-type growth rates by the addition of BCFA [47]. Apoptotic properties of BCFA on human breast cancer cells are structure-specific, with iso-16:0 having the highest activity among BCFA of C12-C20 [48]. iso-15:0 inhibited tumor growth in cultured cells and in an in vivo (mouse) models with no obvious deleterious effects [49]. Thus, BCFA of the type found in US retail milk may have a beneficial effect on proper tissue function and on gut development and function.
In conclusion, we document for the first time the profile and amounts of BCFA in retail whole milk in the US. Milk BCFA have chain lengths of 14–18 carbons and include both iso- and anteiso-BCFA. BCFA comprise 2.05%, w/w of the FA in retail whole milk. Estimated BCFA levels in beef and data on per capita milk, cheese, and beef intakes indicate that BCFA intake on average is in excess than that of many bioactive FA. Their importance in the US food supply and bioactivity suggest that they should be more carefully studied for their biological effects.
Acknowledgments
R.R. Ran-Ressler thanks Dr. William Harris Ressler and Peter Lawrence for their valuable comments on the manuscript. Supported by NIH grant R21 HD064604 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH. It was also supported by the Cornell Agricultural Experimental Station and Smith Lever funds from the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture, under Agreement No. NYC-127437. RRR acknowledges support of NIH training grant HD007331, which includes funding from the NICHD and the Office of Dietary Supplements (ODS).
Abbreviations
- BCFA
Branched chain fatty acids
- CACI-MS
Covalent adduct chemical ionization mass spectrometry
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FA
Fatty acids
- FAME
Fatty acid methyl esters
- FID
Flame ionization detector
- GC
Gas chromatograph
- MS
Mass spectrometry
- NEC
Necrotizing enterocolitis
- PUFA
Polyunsaturated fatty acids
- SFA
Saturated fatty acids
References
- 1.Lock AL, Bauman DE. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids. 2004;39:1197–1206. doi: 10.1007/s11745-004-1348-6. [DOI] [PubMed] [Google Scholar]
- 2.Bauman DE, Lock AL, Corl BA, Ip C, Salter AM, Parodi PW. Milk fatty acids and human health: potential role of conjugated linoleic acid and trans fatty acids. In: Sejrsen K, Hvelplund T, Nielson MO, editors. Ruminant physiology: digestion, metabolism, and impact of nutrition on gene expression, immunology, and stress. Wageningen Academic Publishers; Wageningen: 2006. pp. 529–561. [Google Scholar]
- 3.Boeckaert C, Vlaeminck B, Dijkstra J, Issa-Zacharia A, Van Nespen T, Van Straalen W, Fievez V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. J Dairy Sci. 2008;91:4714–4727. doi: 10.3168/jds.2008-1178. [DOI] [PubMed] [Google Scholar]
- 4.Craninx M, Steen A, Van Laar H, Van Nespen T, Martin-Tereso J, De Baets B, Fievez V. Effect of lactation stage on the odd- and branched-chain milk fatty acids of dairy cattle under grazing and indoor conditions. J Dairy Sci. 2008;91:2662–2677. doi: 10.3168/jds.2007-0656. [DOI] [PubMed] [Google Scholar]
- 5.Osborne VR, Radhakrishnan S, Odongo NE, Hill AR, McBride BW. Effects of supplementing fish oil in the drinking water of dairy cows on production performance and milk fatty acid composition. J Anim Sci. 2008;86:720–729. doi: 10.2527/jas.2007-0342. [DOI] [PubMed] [Google Scholar]
- 6.Chilliard Y, Martin C, Rouel J, Doreau M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output. J Dairy Sci. 2009;92:5199–5211. doi: 10.3168/jds.2009-2375. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell-Megaro AM, Barbano DM, Bauman DE. Survey of the fatty acid composition of retail milk in the united states including regional and seasonal variations. J Dairy Sci. 2011;94:59–65. doi: 10.3168/jds.2010-3571. [DOI] [PubMed] [Google Scholar]
- 8.Massart-Leen AM, DePooter H, Decloedt M, Schamp N. Composition and variability of the branched-chain fatty acid fraction in the milk of goats and cows. Lipids. 1981;16:286–292. doi: 10.1007/BF02534951. [DOI] [PubMed] [Google Scholar]
- 9.Duncan WR, Garton GA. Differences in the proportions of branched-chain fatty acids in subcutaneous triacylglycerols of barley-fed ruminants. Br J Nutr. 1978;40:29–33. doi: 10.1079/bjn19780092. [DOI] [PubMed] [Google Scholar]
- 10.Vlaeminck B, Fievez V, Cabrita ARJ, Fonseca AJM, Dewhurst RJ. Factors affecting odd- and branched-chain fatty acids in milk: A review. Animal Feed Science and Technology. 2006;131:389–417. [Google Scholar]
- 11.Vlaeminck B, Lourenco M, Bruinenberg M, Demeyer D, Fievez V. Odd and branched chain fatty acids in rumen contents and milk of dairy cows fed forages from semi-natural grasslands. Commun Agric Appl Biol Sci. 2004;69:337–340. [PubMed] [Google Scholar]
- 12.Lough AK. The chemistry and biochemistry of phytanic, pristanic and related acids. Prog Chem Fats Other Lipids. 1973;14:1–48. doi: 10.1016/0079-6832(75)90001-4. [DOI] [PubMed] [Google Scholar]
- 13.Ran-Ressler RR, Devapatla S, Lawrence P, Brenna JT. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr Res. 2008;64:605–609. doi: 10.1203/PDR.0b013e318184d2e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narendran V, Wickett RR, Pickens WL, Hoath SB. Interaction between pulmonary surfactant and vernix: A potential mechanism for induction of amniotic fluid turbidity. Pediatr Res. 2000;48:120–124. doi: 10.1203/00006450-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Egge H, Murawski U, Ryhage R, Gyorgy P, Chatranon W, Zilliken F. Minor constituents of human milk. Iv. Analysis of the branched chain fatty acids. Chem Phys Lipids. 1972;8:42–55. doi: 10.1016/0009-3084(72)90042-4. [DOI] [PubMed] [Google Scholar]
- 16.Gibson RA, Kneebone GM. Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr. 1981;34:252–257. doi: 10.1093/ajcn/34.2.252. [DOI] [PubMed] [Google Scholar]
- 17.Cotton PA, Subar AF, Friday JE, Cook A. Dietary sources of nutrients among us adults, 1994 to 1996. J Am Diet Assoc. 2004;104:921–930. doi: 10.1016/j.jada.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Wiley AS. Dairy and milk consumption and child growth: Is BMI involved? An analysis of NHANES 1999–2004. Am J Hum Biol. 2010;22:517–525. doi: 10.1002/ajhb.21042. [DOI] [PubMed] [Google Scholar]
- 19.Barbano DM, Clark JL, Dunham CE. Comparison of Babcock and ether extraction methods for determination of fat content of milk: collaborative study. J Assoc Off Anal Chem. 1988;71:898–914. [PubMed] [Google Scholar]
- 20.Christie WW. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res. 1982;23:1072–1075. [PubMed] [Google Scholar]
- 21.Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr. 1999;129:1579–1584. doi: 10.1093/jn/129.8.1579. [DOI] [PubMed] [Google Scholar]
- 22.Dewhurst RJ, Moorby JM, Vlaeminck B, Fievez V. Apparent recovery of duodenal odd- and branched-chain fatty acids in milk of dairy cows. J Dairy Sci. 2007;90:1775–1780. doi: 10.3168/jds.2006-715. [DOI] [PubMed] [Google Scholar]
- 23.Hauff S, Vetter W. Quantification of branched chain fatty acids in polar and neutral lipids of cheese and fish samples. J Agric Food Chem. 2010;58:707–712. doi: 10.1021/jf9034805. [DOI] [PubMed] [Google Scholar]
- 24.Van Pelt CK, Brenna JT. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal Chem. 1999;71:1981–1989. doi: 10.1021/ac981387f. [DOI] [PubMed] [Google Scholar]
- 25.Vlaeminck B, Fievez V, Tamminga S, Dewhurst RJ, van Vuuren A, De Brabander D, Demeyer D. Milk odd- and branchedchain fatty acids in relation to the rumen fermentation pattern. J Dairy Sci. 2006;89:3954–3964. doi: 10.3168/jds.S0022-0302(06)72437-7. [DOI] [PubMed] [Google Scholar]
- 26.Vlaeminck B, Fievez V, van Laar H, Demeyer D. Rumen odd and branched chain fatty acids in relation to in vitro rumen volatile fatty acid productions and dietary characteristics of incubated substrates. J Anim Physiol Anim Nutr (Berl) 2004;88:401–411. doi: 10.1111/j.1439-0396.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 27.Keeney M, Katz I, Allison MJ. On probable origin of some milk fat acids in rumen microbial lipids. J Am Oil Chem Soc. 1962;39:198–201. [Google Scholar]
- 28.Patton S, Benson AA. Phytol metabolism in the bovine. Biochim Biophys Acta. 1966;125:22–32. doi: 10.1016/0005-2760(66)90140-8. [DOI] [PubMed] [Google Scholar]
- 29.Verhoeven NM, Wanders RJA, Poll-The BT, Saudubray JM, Jakobs C. The metabolism of phytanic acid and pristanic acid in man: a review. J Inherit Metab Dis. 1998;21:697–728. doi: 10.1023/a:1005476631419. [DOI] [PubMed] [Google Scholar]
- 30.USDA ARS . National nutrient database for standard reference, release 22. Nutrient data laboratory home page. USDA; Washington, DC: 2009. [updated 2009; cited 2010 22 July]; Updated date: February 1, 2010 [Available from http://www.ars.usda.gov/ba/bhnrc/ndl. [Google Scholar]
- 31.Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data. 2004;348:1–6. [PubMed] [Google Scholar]
- 32.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55:97–122. doi: 10.1159/000228998. [DOI] [PubMed] [Google Scholar]
- 33.Huh SY, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW. Prospective association between milk intake and adiposity in preschool-aged children. J Am Diet Assoc. 2010;110:563–570. doi: 10.1016/j.jada.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.USDA ARS . Nutrient intakes from food: Mean amounts consumed per individual, by gender and age, what we eat in America. NHANES (National Health and Nutrition Examination Survey), 2007–2008. USDA; Washington, DC: 2010. [updated 2010 30 July 2010; cited 2010 4 August]; Available from http://www.ars.usda.gov/ba/bhnrc.fsrg. [Google Scholar]
- 35.Aldai N, Dugan MER, Kramer JKG. Can the trans-18:1 and conjugated linoleic acid profiles in retail ground beef be healthier than steak? Journal of Food Composition and Analysis. 2010;23:326–332. [Google Scholar]
- 36.Chin ST, Man YBC, Tan CP, Hashim DM. Rapid profiling of animal-derived fatty acids using fast gc × gc coupled to time-of-flight mass spectrometry. Journal of the American Oil Chemists Society. 2009;86:949–958. [Google Scholar]
- 37.USDA_Economic_Research_Service_(ERS) Food availability (per capita) data system. USDA; Washington, DC: 2008. [updated 2008; cited 2010 22 July]; Updated date: February 1, 2010 [Available from http://www.ers.usda.gov/Data/FoodConsumption. [Google Scholar]
- 38.USDA . USDA; Washington, DC: 2005. Mypyramid.Gov. [updated 2005; cited 2010 22 July]; Available from http://www.mypyramid.gov/pyramid/index.html. [Google Scholar]
- 39.Kris-Etherton PM, Innis S, American Dietetic Association, Dietitians of Canada Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107:1599–1611. [PubMed] [Google Scholar]
- 40.Elwood PC, Pickering JE, Hughes J, Fehily AM, Ness AR. Milk drinking, ischaemic heart disease and ischaemic stroke ii. Evidence from cohort studies. Eur J Clin Nutr. 2004;58:718–724. doi: 10.1038/sj.ejcn.1601869. [DOI] [PubMed] [Google Scholar]
- 41.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45:925–939. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barba G, Troiano E, Russo P, Venezia A, Siani A. Inverse association between body mass and frequency of milk consumption in children. Br J Nutr. 2005;93:15–19. doi: 10.1079/bjn20041300. [DOI] [PubMed] [Google Scholar]
- 43.Rosell M, Hakansson NN, Wolk A. Association between dairy food consumption and weight change over 9 y in 19,352 perimenopausal women. Am J Clin Nutr. 2006;84:1481–1488. doi: 10.1093/ajcn/84.6.1481. [DOI] [PubMed] [Google Scholar]
- 44.Chavarro JE, Rich-Edwards JW, Rosner B, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod. 2007;22:1340–1347. doi: 10.1093/humrep/dem019. [DOI] [PubMed] [Google Scholar]
- 45.Nicolaides N, Ray T. Skin lipids. 3 Fatty chains in skin lipids. The use of vernix caseosa to differentiate between endogenous and exogenous components in human skin surface lipid. J Am Oil Chem Soc. 1965;42:702–707. doi: 10.1007/BF02540043. [DOI] [PubMed] [Google Scholar]
- 46.Ran-Ressler RR, Kothapalli KS, Glahn R, Brenna JT, editors. Branched chain fatty acids are taken up and metabolized by cultured human intestinal cells (caco-2) Exp Biol (Anahein, Ca) 2010 [Google Scholar]
- 47.Silbert DF, Ladenson RC, Honegger JL. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973;311:349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- 48.Wongtangtintharn S, Oku H, Iwasaki H, Toda T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J Nutr Sci Vitaminol (Tokyo) 2004;50:137–143. doi: 10.3177/jnsv.50.137. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Liu S, Chen X, Chen H, Huang M, Zheng J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000;60:505–509. [PubMed] [Google Scholar]