Abstract
Vigorous activity after diagnosis was recently reported to be inversely associated with prostate cancer-specific mortality. However, men with metastatic disease may decrease their activity due to their disease; thus a causal interpretation is uncertain. We therefore prospectively examined vigorous activity and brisk walking after diagnosis in relation to risk of prostate cancer progression, an outcome less susceptible to reverse causation, among 1,455 men diagnosed with clinically localized prostate cancer. Cox proportional hazards regression was used to examine vigorous activity, non-vigorous activity, walking duration, and walking pace after diagnosis and risk of prostate cancer progression. We observed 117 events (45 biochemical recurrences, 66 secondary treatments, 3 bone metastases, 3 prostate cancer deaths) during 2,750 person-years. Walking accounted for nearly half of all activity. Men who walked briskly for ≥3 hours/week had a 57% lower rate of progression compared to men who walked at an easy pace for <3 hours/week (hazard ratio (HR): 0.43; 95% confidence interval (CI): 0.21, 0.91; p-value: 0.03). Walking pace was associated with decreased risk of progression independent of duration (HR brisk vs. easy pace: 0.52; 95% CI: 0.29, 0.91; p-trend: 0.01). Few men engaged in vigorous activity, but there was a suggestive inverse association (HR ≥3 hours per week vs. none: 0.63; 95% CI: 0.32, 1.23; p-trend: 0.17). Walking duration and total non-vigorous activity were not associated with risk of progression independent of pace or vigorous activity respectively. Brisk walking after diagnosis may inhibit or delay prostate cancer progression among men diagnosed with clinically localized prostate cancer.
Keywords: Prostate cancer, epidemiology, physical activity, survivorship, prognosis
INTRODUCTION
Over 2.2 million men currently live with prostate cancer in the United States (U.S.) and approximately 217,000 new cases were diagnosed in 2010. The vast majority of new cases (92%) are diagnosed in the localized or regional stage and have a five year disease-specific survival approaching 100%; yet prostate cancer remains the second leading cause of cancer death among men in the U.S. (1). Little is known regarding the associations between modifiable lifestyle factors after diagnosis and risk of prostate cancer progression from localized to lethal disease.
Our group was the first to report that post-diagnostic vigorous activity was associated with a statistically significant 61% reduction in risk of prostate cancer-specific mortality among men diagnosed with non-metastatic prostate cancer (2). There is some concern that this association reflected a reduction in physical activity among men with metastatic disease. Thus, in an independent study population, we examined whether post-diagnostic vigorous activity and brisk walking were associated with reduced risk of prostate cancer progression, defined primarily by biochemical recurrence (e.g. prostate specific antigen (PSA) rise). Biochemical recurrence is an indicator of disease progression that manifests prior to the development of physical symptoms, thus avoiding potential bias due to reverse causation.
Vigorous activity is associated with lower levels of insulin, bio-available insulin-like growth factor 1 (IGF1), and inflammatory cytokines, leading to a milieu that may inhibit proliferation and promote apoptosis of prostate cancer cells (3–7). Although brisk walking is classified as a moderate-intensity activity (8), it has also been associated with reductions in these factors (9, 10), as well as decreased risk of diseases affected by this milieu (e.g. total mortality (11), type II diabetes (12), coronary heart disease (13)). Moreover, in our previous report, we observed a suggestive inverse association between brisk walking and risk of prostate cancer-specific mortality (2).
Based on our previous findings and the potential biologic mechanisms, we hypothesized that both vigorous activity and brisk walking after diagnosis would inhibit prostate cancer progression among men diagnosed with localized disease. Thus, in the current study, we prospectively examined vigorous activity, non-vigorous activity, walking duration, and walking pace after diagnosis and risk of prostate cancer progression among men diagnosed with clinically localized prostate cancer and participating in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE ).
MATERIALS AND METHODS
Study population
This analysis was based on a sub-study of CaPSURE (14, 15). Starting in 1995, 40 urology clinics throughout the U.S. enrolled men with biopsy-verified prostate cancer. Participants completed a questionnaire on sociodemographics, medical symptoms, and use of health services at enrollment and every six months thereafter. Urologists reported clinical data at enrollment and subsequent clinic visits. During 2004–2005, 2,134 CaPSURE participants completed physical activity and dietary questionnaires; these men constitute the base population for this analysis. The Institutional Review Board of the University of California, San Francisco approved this study.
Physical activity assessment
Participants were asked how often (never to ≥11 hours per week) on average over the past year they participated in walking or hiking; jogging (>10 minutes/mile); running (≤10 minutes/mile); calisthenics, aerobics, rowing, Nordic track; bicycling; tennis, squash, racquetball; lap swimming; weightlifting; and other aerobic exercise (e.g. heavy outdoor work), as well as their usual walking pace and flights of stairs climbed daily. Our questionnaire was based on a well-validated questionnaire; the correlation between the average of four one-week seasonal activity diaries and the original questionnaire was 0.58 for vigorous activity and 0.28 for non-vigorous activity (16).
A metabolic equivalent task (MET) value was assigned to each activity based on the energy required by that activity relative to the resting metabolic rate (17). Activities were classified as vigorous if they required six or more METs (8). Physical activity was categorized based on the distribution in the study population (Table 1): vigorous (0, 0.1–1.24, 1.25 –2.9, ≥3 hours/week), non-vigorous (<1, 1–2.9, 3–4.9, 5–9.9, ≥10 hours/week), walking duration (<0.5, 0.5–1.4, 1.5–3.9, 4.0–6.9, ≥7 hours/week), and walking pace (<2, 2–2.9, ≥3 mph). We also classified men according to their usual walking pace (≥3 vs. <3 mph) and duration (≥3 vs. <3 hours/week); three hours per week was the average walking duration in this population.
Table 1.
Distribution of ten leisure-time physical activities among 1,455 men diagnosed with clinically localized prostate cancer.
| MET value | MET–hours/week (%) *† | Duration (%)† | |
|---|---|---|---|
|
Vigorous activities (≥ 6 METs)
| |||
| Running (≤ 10 minutes/mile) | 12.0 | 2.8 | 1.2 |
| Jogging (> 10 minutes/mile) | 7.0 | 2.2 | 1.6 |
| Lap swimming | 7.0 | 1.7 | 1.2 |
| Bicycling (including stationary machine) | 7.0 | 8.6 | 6.0 |
| Tennis, squash, or racquetball | 7.0 | 3.7 | 2.5 |
| Calisthenics, aerobics, rowing, Nordic track | 6.0 | 7.2 | 5.9 |
|
| |||
|
Non–vigorous activities (< 6 METs)
| |||
| Other aerobic exercise (e.g. heavy outdoor work, raking, mowing) | 5.5 | 37.5 | 33.3 |
| Weightlifting or Nautilus | 4.5 | 4.5 | 4.9 |
| Walking or hiking (including walking for transportation or at golf) ‡ | 3.0 | 30.5 | 43.4 |
| Flights of stairs § | 0.11 | 1.2 | |
Abbreviations: MET, metabolic equivalent task.
MET–hours/week calculated by multiplying the MET value assigned to an activity by the time spent engaged in that activity.
Percentages may not add up to 100% due to rounding.
METs assigned to walking depended on walking pace. Normal pace (2–2.9 mph) was assigned 3 METs.
Duration of flights of stairs climbed per week was assumed negligible.
Clinical follow–up and outcome assessment
Urologists collected clinical data throughout follow-up and participants reported treatments, medications, and hospitalizations every six months. If a hospitalization was reported, we obtained all relevant medical records. We abstracted prostate specific antigen (PSA) levels, clinical stage, biopsy Gleason sum, treatment, and occurrence of metastases from urologists’ reports and medical records. Mortality data were obtained via the National Death Index and Bureau of Vital Statistics.
The primary outcome was prostate cancer progression defined as: prostate cancer death, bone metastases from prostate cancer, biochemical recurrence, or secondary treatment. A death was attributed to prostate cancer if prostate cancer was listed as the primary, secondary, or tertiary cause of death on the death certificate and no other malignancy was listed as a higher order cause. Bone metastases included urologist report of: 1) prostate cancer progression to bone, 2) positive bone scan, 3) radiation for metastasis to a bone, or 4) TNM stage M1b. Biochemical recurrence was defined as two consecutive PSA tests ≥0.2 ng/ml at least eight weeks after radical prostatectomy or a rise of 2.0 ng/ml or more above post-radiation nadir (18). Secondary treatments included any treatment initiated at least six months after primary treatment ended. Among men who had primary treatment, initiation of secondary treatment is indicative of biochemical or clinical evidence of disease progression (19, 20). The date of prostate cancer progression was the first of the following: prostate cancer death, diagnosis of bone metastases from prostate cancer, second PSA test ≥0.2 ng/ml after radical prostatectomy, first PSA test ≥2.0 ng/ml over post-radiation nadir, or initiation of secondary treatment.
Inclusion criteria
The base population for this study included 2,134 men who completed the activity questionnaire in 2004–2005. We excluded men with non-localized disease at diagnosis (i.e. clinical T-stage T3+), men whose disease progressed prior to the questionnaire, and men who had not completed primary treatment prior to the questionnaire (n = 548). Men who reported an energy intake outside 800–4200 kcal/day (n = 67) were excluded because their dietary information was considered unreliable and use of their dietary data could lead to incomplete adjustment for potential dietary confounders. We also excluded men missing physical activity data (n = 11) or clinical information at diagnosis (n = 53), leaving 1,455 men for analysis.
Statistical analysis
We used Cox proportional hazards regression to examine post-diagnostic vigorous activity, non-vigorous activity, walking duration, and walking pace in relation to risk of prostate cancer progression. Person-time was calculated from date of the questionnaire to date of progression, death from another cause, last contact, or August 21, 2009, whichever occurred first. Categories of physical activity were modeled using indicator variables, and we performed tests of trend by modeling the median of each exposure category continuously.
Our basic model (Model 1) included age at diagnosis (continuous) and days from diagnosis to the questionnaire (continuous). Model 2 was additionally adjusted for biopsy Gleason sum (<7, 7, >7), PSA at diagnosis (tertiles), and primary treatment (radical prostatectomy, radiation therapy, other/watchful waiting, hormone therapy). We modeled PSA and treatment using indicator variables and biopsy Gleason sum using an ordinal variable. We adjusted vigorous and non-vigorous activity for each other, adjusted walking pace and duration for each other, and examined non-vigorous activity, walking duration, and walking pace in relation to risk of progression among men who did not engage in vigorous activity. Adjustment for other risk factors for prostate cancer progression, including: race, prostate cancer family history, smoking, diabetes, education, income, and intakes of tomato products, poultry with skin, dairy, cruciferous vegetables, and eggs, did not change our results (21–26).
Body mass index (BMI) may mediate the association between physical activity and prostate cancer progression. Therefore, we examined a third model adjusted for BMI (Model 3). Additionally, we used likelihood ratio tests to examine whether biopsy Gleason sum (<7 versus ≥7), age at diagnosis (continuous), BMI (<25 versus ≥25 kg/m2), or time from diagnosis to the questionnaire (continuous) modified the relation between physical activity and prostate cancer progression.
To examine whether men who had decreased their physical activity as a result of progression of their disease were influencing our results, we performed a sensitivity analysis with a one-year lag. We were also concerned some men may undergo secondary treatment due to anxiety rather than a biologic change in their disease, hence we performed analyses excluding watchful waiters (n = 44) or men who underwent secondary treatment without evidence of a preceding PSA rise (n = 33). Men treated close in time to the activity questionnaire may not have returned to their normal activity level; hence we performed an analysis excluding men treated within six months prior to the questionnaire (n = 204). We also examined the results restricted to men diagnosed in 2000 or after (n = 1166) and men who had radical prostatectomy as their primary treatment (n = 922).
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). P-values were two-sided and significant at P ≤ 0.05.
RESULTS
We observed 117 events (45 biochemical progressions, 66 secondary treatments, three bone metastases, three prostate cancer deaths) among 1,455 men during 2,750 person-years. The median time from diagnosis to the questionnaire was 27 months (interquartile range (IQR): 15 to 46 months) and median follow-up after the questionnaire was 22 months (IQR: 9 to 31 months). Approximately 24% of participants (n=347) withdrew from the study, were lost to follow-up, or declined clinical follow-up prior to the end of the study in August 21, 2009. These men did not differ materially from the remaining participants in terms of age at diagnosis, BMI, biopsy Gleason sum, primary treatment, clinical T-stage at diagnosis, vigorous activity, or usual walking pace.
Men who engaged in more vigorous activity or who walked at a brisk pace were younger, leaner, and less likely to be current smokers than the least active men (Table 2). In addition, more vigorously active men were more likely to have moderately differentiated disease (biopsy Gleason sum = 7) and less likely to have “other” treatment than less vigorously active men. Men who reported walking at a brisk pace were less likely to have a PSA ≥10 ng/ml at diagnosis, more likely to have radical prostatectomy as their primary treatment, and less likely to have radiation therapy as their primary treatment compared to men who reported walking at an easy pace.
Table 2.
Age–standardized characteristics of 1,455 men with clinically localized prostate cancer, by vigorous activity, usual walking pace, and overall.
| Vigorous activity (hours/week) | Walking pace (MPH) | Total Mean (SD) or No. (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | ≥3 | P–trend * | < 2 | ≥3 | P–trend * | ||
| No. of participants | 722 | 189 | 211 | 502 | 1264 | ||
| Age at diagnosis, mean (SD), y | 65 (8) | 64 (8) | 0.007 | 69 (7) | 62 (8) | <0.001 | 65 (8) |
| Body mass index, kg/m2, (%) † | |||||||
| <25 | 24 | 39 | <0.001 | 22 | 35 | <0.001 | 379 (27) |
| 25–29.9 | 52 | 50 | 0.88 | 45 | 53 | 0.48 | 750 (53) |
| ≥30 | 24 | 11 | <0.001 | 32 | 13 | <0.001 | 283 (20) |
| Current smokers, (%) | 7 | 4 | 0.05 | 9 | 4 | 0.02 | 90 (6) |
| PSA at diagnosis ≥10 ng/mL, (%) | 16 | 12 | 0.09 | 23 | 13 | 0.03 | 214 (15) |
| Clinical stage, (%)† | |||||||
| T1 | 56 | 49 | 0.36 | 52 | 57 | 0.31 | 801 (55) |
| T2 | 44 | 51 | 0.36 | 48 | 43 | 0.31 | 654 (45) |
| Biopsy Gleason sum, (%) † | |||||||
| 2–6 | 74 | 68 | 0.02 | 73 | 69 | 0.35 | 1034 (71) |
| 7 | 20 | 28 | 0.004 | 20 | 25 | 0.28 | 347 (24) |
| 8–10 | 6 | 4 | 0.32 | 7 | 6 | 0.90 | 74 (5) |
| Primary treatment, (%) † | |||||||
| Radical prostatectomy | 62 | 64 | 0.42 | 54 | 67 | 0.01 | 922 (63) |
| Radiation therapy | 24 | 24 | 0.97 | 29 | 20 | 0.04 | 351 (24) |
| Hormonal therapy | 5 | 6 | 0.34 | 8 | 5 | 0.23 | 110 (8) |
| Other/watchful waiting | 9 | 5 | 0.04 | 9 | 8 | 0.69 | 72 (5) |
Abbreviations: MPH, miles per hour; PSA, prostate specific antigen
Calculated using the median of each category as a continuous term.
Percentages may not add to 100% due to rounding.
Walking and other aerobic exercise (e.g. heavy outdoor work) each accounted for approximately one-third of energy expended by reported leisure-time physical activity, and together accounted for more than 75% of all time engaged in reported leisure-time activity in this population (Table 1). Vigorous activities accounted for approximately 26% of energy expended by reported leisure-time activity and 18% of total time spent in reported leisure-time activity.
Men who walked three or more hours per week at a brisk pace had a statistically significant 57% reduced rate of prostate cancer progression compared to men who walked less than three hours per week at a less than brisk pace (HR: 0.43; 95% CI: 0.21, 0.91; p-value: 0.03) (Figure 1). These findings persisted after adjustment for BMI (HR: 0.48; 95% CI: 0.23, 1.01; p-value: 0.05) and when restricting to the 722 men who did not engage in vigorous activity (HR: 0.37; 95% CI: 0.11, 1.22; p-value: 0.10), although the confidence intervals widened. Moreover, walking pace was associated with a statistically significant reduced risk of prostate cancer progression independent of walking duration (HR: 0.52; 95% CI: 0.29, 0.91; p–trend: 0.01) (Table 3). Adjustment for BMI did not material change the results (HR: 0.56; 95% CI: 0.32, 1.00; p–trend: 0.02). Walking duration and total non-vigorous physical activity were not associated with risk of prostate cancer progression independent of walking pace and vigorous activity, respectively.
Figure 1.
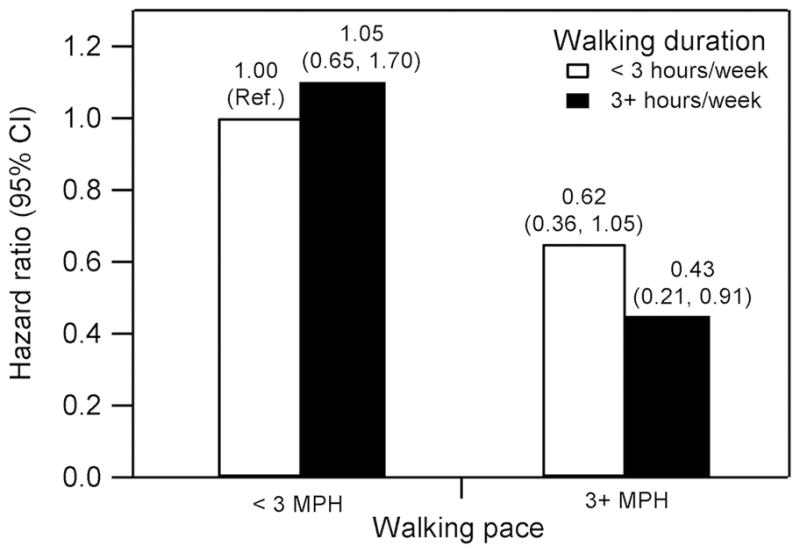
Post–diagnostic walking duration, walking pace, and risk of prostate cancer progression among 1,455 men diagnosed with clinically localized prostate cancer. Abbreviations: CI, confidence interval; MPH, miles per hour. Adjusted for age at diagnosis (continuous), days from diagnosis to questionnaire (continuous), primary treatment (radical prostatectomy, radiation, other/watchful waiting, hormone), biopsy Gleason sum (<7, 7, >7), and prostate–specific antigen at diagnosis (tertiles). Events/person-years: <3 MPH, <3 hours/week = 66/1289; <3 MPH, 3+ hours/week = 23/471; 3+ MPH, <3 hours/week = 18/569; 3+ MPH, 3+ hours/week = 8/396. Ten participants (0.7%) who reported being unable to walk and reported no time spent walking were excluded.
Table 3.
Post–diagnostic non–vigorous activity, vigorous activity, walking duration, and walking pace in relation to risk of prostate cancer progression among 1,455 men diagnosed with clinically localized prostate cancer.
| Non–vigorous (hours/week) | 0–0.9 | 1.0–2.9 | 3.0–4.9 | 5.0–9.9 | ≥10.0 | P–trend * |
|---|---|---|---|---|---|---|
| Events/person–y | 22/456 | 34/774 | 13/269 | 23/697 | 25/554 | |
| Model 1HR (95% CI) † | 1.0 | 0.91 (0.53, 1.55) | 0.97 (0.49, 1.93) | 0.73 (0.40, 1.31) | 0.96 (0.54, 1.70) | 0.89 |
| Model 2HR (95% CI) ‡ | 1.0 | 0.78 (0.45, 1.33) | 0.84 (0.42, 1.69) | 0.61 (0.34, 1.11) | 0.87 (0.49, 1.56) | 0.98 |
| Model 3HR (95% CI) § | 1.0 | 0.80 (0.45, 1.40) | 0.90 (0.44, 1.83) | 0.71 (0.38, 1.32) | 0.96 (0.52, 1.76) | 0.76 |
|
| ||||||
| Vigorous (hours/week) | 0.0 | 0.1–1.24 | 1.25–2.9 | ≥3.0 | P–trend * | |
| Events/person–y | 66/1389 | 25/643 | 16/384 | 10/334 | ||
| Model 1HR (95% CI) † | 1.0 | 0.81 (0.51, 1.28) | 0.93 (0.54, 1.60) | 0.62 (0.32, 1.21) | 0.19 | |
| Model 2HR (95% CI) ‡ | 1.0 | 0.88 (0.55, 1.39) | 0.84 (0.48, 1.45) | 0.63 (0.32, 1.23) | 0.17 | |
| Model 3HR (95% CI) § | 1.0 | 0.88 (0.55, 1.41) | 0.71 (0.40, 1.28) | 0.69 (0.35, 1.36) | 0.19 | |
|
| ||||||
| Walking duration (hours/week) | 0–0.4 | 0.5–1.4 | 1.5–3.9 | 4.0–6.9 | ≥7.0 | P–trend * |
| Events/person–y | 25/515 | 38/851 | 23/507 | 16/475 | 15/402 | |
| Model 1HR (95% CI) † | 1.0 | 0.95 (0.57, 1.58) | 0.98 (0.55, 1.73) | 0.79 (0.42, 1.48) | 0.89 (0.47, 1.68) | 0.95 |
| Model 2HR (95% CI)‡,|| | 1.0 | 0.91 (0.53, 1.54) | 1.12 (0.62, 2.05) | 0.91 (0.46, 1.77) | 0.98 (0.50, 1.92) | 0.99 |
| Model 3HR (95% CI) §,|| | 1.0 | 0.81 (0.47, 1.40) | 0.94 (0.51, 1.75) | 0.74 (0.38, 1.45) | 0.80 (0.41, 1.58) | 0.86 |
|
| ||||||
| Walking pace (MPH) || | Easy (< 2.0) | Normal (2.0–2.9) | Brisk (≥3.0) | P–trend * | ||
| Events/person–y | 27/417 | 62/1348 | 26/971 | |||
| Model 1HR (95% CI) † | 1.0 | 0.80 (0.50, 1.26) | 0.50 (0.28, 0.86) | 0.01 | ||
| Model 2HR (95% CI) ‡ | 1.0 | 0.94 (0.58, 1.50) | 0.52 (0.29, 0.91) | 0.01 | ||
| Model 3HR (95% CI) § | 1.0 | 0.96 (0.60, 1.56) | 0.56 (0.32, 1.00) | 0.02 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; MPH, miles per hour.
Calculated using the median of each category as a continuous term.
Model 1 adjusted for age at diagnosis (continuous) and time from diagnosis to questionnaire (continuous).
Model 2 adjusted for covariates in Model 1 plus primary treatment (radical prostatectomy, radiation, other/watchful waiting, hormone), biopsy Gleason sum (<7, 7, >7), and prostate–specific antigen at diagnosis (tertiles). Vigorous and non–vigorous activities were adjusted for each other and walking duration and walking pace were adjusted for each other.
Model 3 adjusted for covariates in Model 2 plus body mass index (<25, 25–29.9, ≥30 kg/m2). 43 participants (3%) with missing body mass index were not included.
Ten participants (0.7%) who reported being unable to walk and reported no time spent walking were excluded from models with walking pace.
We observed a non-statistically significant inverse association between vigorous activity and risk of prostate cancer progression. Men who engaged in three or more hours per week of vigorous activity had a 37% decreased risk of progression compared to men who engaged in no vigorous activity (HR: 0.63; 95% CI: 0.32, 1.23; p-trend: 0.17). Few men engaged in vigorous activity in this population, which may explain the lack of statistical significance.
We found no evidence of effect modification by time from diagnosis to questionnaire, age at diagnosis, or BMI. However, there was a statistically significant interaction between biopsy Gleason sum and walking duration (interaction p-value = 0.006) and non-vigorous activity (interaction p-value = 0.03). For example, among men with biopsy Gleason sum <7 (n = 1034), walking seven or more hours per week was associated with a 61% reduction in risk of prostate cancer progression compared to walking less than half an hour per week (HR: 0.39; 95% CI: 0.11, 1.41). There was no reduction in risk among men with biopsy Gleason sum ≥7 (n = 421; HR: 1.33; 95% CI: 0.54, 3.29) (data not shown in tables).
Our results did not materially change after excluding watchful waiters, events defined by secondary treatment without a preceding PSA rise, or men who completed their primary treatment within six months prior to the questionnaire. Our results were also robust in analyses that included a one-year lag, or when restricting to men diagnosed since 2000 or men who had radical prostatectomy as their primary treatment.
DISCUSSION
In this prospective study of physical activity among prostate cancer patients, we observed a strong inverse relation between walking pace after diagnosis and risk of prostate cancer progression. Men who walked briskly for three or more hours per week had the lowest risk of progression. There was also a suggestion of an inverse association for vigorous activity, but few men engaged in vigorous activity in this study population and this result was not statistically significant.
To our knowledge, only one other study has examined post-diagnostic physical activity in relation to clinical outcomes in prostate cancer survivors (2). In that study, men who engaged in three or more hours per week of vigorous activity experienced a 61% reduced rate of prostate cancer–specific mortality compared to men who engaged in less than one hour per week (HR: 0.39; 95% CI: 0.18, 0.84; p-trend: 0.03). Additionally, men who walked briskly after diagnosis experienced a 48% reduction in all-cause mortality (HR: 0.52; 95% CI: 0.39, 0.70, p-trend: <0.001) and a non–statistically significant reduction in prostate cancer–specific mortality (HR: 0.66; 95% CI: 0.34, 1.29) compared to men who walked at an easy pace. Reverse causation is a concern when examining the relation between physical activity and prostate cancer-specific mortality, in that men with metastatic disease may reduce their physical activity as a result of their disease, creating a spurious association between decreased activity and poor prognosis. Thus, a particular strength of the current study is our outcome of prostate cancer progression, as this endpoint is far less susceptible to reverse causation given that the early indicators of progression occur prior to any symptoms.
Brisk walking may affect prostate cancer progression by reducing insulin resistance, decreasing bioavailable IGF-1, and increasing adiponectin levels. Circulating levels of insulin, bio–available IGF1, and adiponectin affect proliferation and apoptosis of prostate cancer cells in vitro (5, 6, 27) and in vivo (5), and have been associated with risk of advanced or fatal prostate cancer (28–30). In the Physician’s Health Study, men in the highest quartile of pre-diagnostic C-peptide levels, a marker of insulin secretion, had a 2.38-fold increased risk of prostate cancer-specific mortality compared to men in the lowest quartile (HR: 2.38; 95% CI: 1.31, 4.30; p-trend: 0.008) (28). Among men with prostate cancer, men in the highest quintile of pre-diagnostic adiponectin had a 61% reduced risk of dying from prostate cancer compared to men in the lowest quintile (HR: 0.39; 95% CI: 0.17, 0.85; p-trend: 0.02) (30).
Further support for this mechanism comes from studies demonstrating reduced cell growth and increased apoptosis of prostate cancer cells cultured in serum from healthy men who engaged in regular aerobic exercise. Serum from exercising men had lower insulin and IGF1 and higher IGF binding protein-1 values compared to men who did not exercise; and addition of IGF1 to the exercisers’ serum removed its anti-proliferative, pro-apoptotic effect (7, 31, 32).
Brisk walking may also affect prostate cancer progression by reducing inflammation. In a 12-month randomized controlled trial among elderly persons, walking “somewhat hard” was associated with lower circulating interleukin-6 (IL-6) (33). IL-6 promotes cell proliferation and inhibits apoptosis of prostate cancer cells in vitro (3), and high levels of IL-6 predicted a 73% increased risk of dying from prostate cancer among normal weight men (34).
We acknowledge that our study has several limitations. First, we had limited power due to a small number of events, including only three prostate cancer deaths, and low participation in vigorous activity. However, our progression-based outcome is less susceptible to reverse causation compared to prostate cancer-specific mortality, as physical symptoms of prostate cancer progression that may cause a decrease in physical activity are unlikely to precede biochemical recurrence. Furthermore, many elderly prostate cancer patients are not capable of performing vigorous activities, and thus our findings for brisk walking are particularly relevant for designing future intervention studies.
Second, we cannot eliminate non-differential measurement error in our prospective physical activity assessment. Vigorous activities occur infrequently or sporadically in older persons and may be less accurately recalled than usual walking pace, which could partially explain the lack of a statistically significant association for vigorous activity. Third, we had no data on pre-diagnostic physical activity; however data from the Health Professionals’ Follow–up Study support an association between post-diagnostic activity and prostate cancer-specific mortality independent of pre-diagnostic activity (2). Fourth, 24% of the men who completed the physical activity questionnaire were lost to follow-up. These men did not differ from the remaining men in terms of their clinical prognostic factors, age at diagnosis, BMI, vigorous activity, or usual walking pace; therefore, although loss of these men reduced our statistical power, it is unlikely to have biased our results. Lastly, the participants in our study were volunteers from a large population-based prostate cancer registry. The men who volunteered were younger at diagnosis, more likely to be white, and had better prognostic risk disease compared to the general CaPSURE population. Thus, our results may not be generalizable to non-Caucasian populations or populations with a different distribution of clinical prognostic factors.
In conclusion, we observed a statistically significant inverse association between brisk walking after diagnosis and risk of prostate cancer progression in men diagnosed with clinically localized prostate cancer. These results were based on a relatively small number of events among brisk walkers and thus further study is needed. However, our results are consistent with the only other study of physical activity after diagnosis and clinical outcomes in prostate cancer survivors, and suggest significant clinical benefits of brisk walking for men with prostate cancer.
Acknowledgments
Financial support: Department of Defense (W81XWH–04–1–0850); National Institutes of Health training grant (R25 CA098566); Prostate Cancer Foundation; Abbott Labs, Abbott Park, IL.
We thank the participants and staff of CaPSURE for their valuable contributions, and Andrew Van Blarigan for his assistance in creating Figure 1.
Footnotes
Potential conflicts of interest: None disclosed
References
- 1.Prostate. SEER Stat Fact Sheets 2010. 2009 November; [cited 2011 March 14]; Available from: http://seer.cancer.gov/statfacts/html/prost.html.
- 2.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011 Feb 20;29(6):726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haverkamp J, Charbonneau B, Ratliff TL. Prostate inflammation and its potential impact on prostate cancer: a current review. J Cell Biochem. 2008 Apr 1;103(5):1344–53. doi: 10.1002/jcb.21536. [DOI] [PubMed] [Google Scholar]
- 4.Lee I, Blair S, Manson J, Paffenbarker RSJ, editors. Epidemiologic Methods in Physical Activity Studies. New York: Oxford University Press; 2009. [Google Scholar]
- 5.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008 Feb;114(1):23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 6.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007 Sep;86(3):s858–66. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 7.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003 Aug 1;56(3):201–6. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 8.Physical Activity Guidelines Advisory Committee. Physical Activity Advisory Committee Report, 2008. Washington, DC: Department of Health and Human Services; 2008. [Google Scholar]
- 9.Hamer M, Steptoe A. Walking, vigorous physical activity, and markers of hemostasis and inflammation in healthy men and women. Scand J Med Sci Sports. 2008 Dec;18(6):736–41. doi: 10.1111/j.1600-0838.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- 10.Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009 Jan;18(1):306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001 Jun 25;161(12):1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 13.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002 Oct 23–30;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004 Apr;171(4):1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 15.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996 Nov;48(5):773–7. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 16.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996 Jan;7(1):81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009 Nov;182(5):2232–41. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 19.Grossfeld GD, Li YP, DP PL, Carroll PR. Patterns of failure after primary local therapy for prostate cancer and rationale for secondary therapy. Urology. 2002 Sep;60(3 Suppl 1):57–62. doi: 10.1016/s0090-4295(02)01574-1. discussion -3. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Cancer of the Prostate Strategic Urological Research E. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008 Jan 15;112(2):307–14. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 21.Mucci LA, Signorello LB, Adami HO. Prostate Cancer. In: Adami HO, Hunter DJ, Trichopoulos D, editors. Textbook of Cancer Epidemiology. 2. New York: Oxford University Press; 2008. pp. 517–42. [Google Scholar]
- 22.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121(7):1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. Am J Clin Nutr. 2010 Mar;91(3):712–21. doi: 10.3945/ajcn.2009.28474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Causes Control. 2006 Mar;17(2):199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 25.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005 Nov 10;23(32):8152–60. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 26.Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009 Apr 15;169(8):937–45. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila Pa) 2008 Oct;1(5):369–75. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008 Nov;9(11):1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009 May 15;124(10):2416–29. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Stampfer MJ, Mucci L, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010 Jan;56(1):34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung PS, Aronson WJ, Ngo TH, Golding LA, Barnard RJ. Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol. 2004 Feb;96(2):450–4. doi: 10.1152/japplphysiol.00871.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003 Jun;144(6):2319–24. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008 Nov;56(11):2045–52. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark JR, Li H, Kraft P, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009 Jun 1;124(11):2683–9. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]


