Abstract
Twist1, a bHLH transcription factor, promotes breast tumor cell epithelial-mesenchymal transition (EMT), invasiveness and metastasis. However, the mechanisms responsible for regulating Twist1 stability are unknown in these cells. We identified the serine 68 (S68) as a major phosphorylation site of Twist1 by mass spectrometry and with specific antibodies. This S68 is phosphorylated by p38, JNK and ERK1/2 in vitro, and its phosphorylation levels positively correlate with Twist1 protein levels in HEK293 and breast cancer cells. Prevention of S68 phosphorylation by an alanine (A) mutation (S68A) dramatically accelerates Twist1 ubiquitination and degradation. Furthermore, activation of MAPKs by an active Ras protein or TGF-β treatment significantly increases S68 phosphorylation and Twist1 protein levels without altering Twist1 mRNA expression, while blocking of MAPK activities by either specific inhibitors or dominant negative inhibitory mutants effectively reduces the levels of both induced and un-induced S68 phosphorylation and Twist protein. Accordingly, the mammary epithelial cells expressing Twist1 exhibit much higher degrees of EMT and invasiveness upon stimulation with TGF-β or the active Ras as well as taxol resistance compared to same cells expressing the S68A-Twist1 mutant. Importantly, the levels of S68 phosphorylation in the invasive human breast ductal carcinomas positively correlate with the levels of Twist1 protein and JNK activity and are significantly higher in progesterone receptor-negative and HER2-positive breast cancers. These findings suggest that activation of MAPKs by tyrosine kinase receptors and Ras signaling pathways may substantially promote breast tumor cell EMT and metastasis via phoshorylation and stabilization of Twist1.
Introduction
Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) and p38 isoforms (p38-α and -β), are serine (Ser)/threonine (Thr)-specific protein kinases. These MAPKs respond to extracellular stimuli and regulate various cellular activities (1). In human breast cancer, activation of MAPK signaling cascades increases cell proliferation, inhibits cell apoptosis and promotes metastasis (2, 3). The increased JNK activity positively correlates with the tamoxifen resistance in breast cancer (4, 5). JNK activity is also involved in the activation of AP-1 and NF-κB transcription factors in glucocorticoid-induced mammary epithelial acinar formation in 3D culture (6).
The active Ras drives the activation of MAPKs in breast cancer (7-9). Overexpression of H-Ras or expression of constitutively active H-RasV12 mutant, which activates MAPKs, is fully capable of inducing metastatic mammary tumors in mice or of transforming fibroblasts into metastatic tumor-forming cells (9, 10). Expression of H-ras also induces human MCF-10A mammary epithelial cells to form ductal carcinoma in situ, followed by development of invasive ductal carcinoma in mouse mammary glands (11). MAPKs activate a number of transcription factors important for tumor cell initiation, growth and survival, such as the estrogen receptor (ERα), AP-1, NF-κB and Ets family transcription factors (12-15). A recent study also demonstrated that ERK2 is responsible for mediating Ras-induced epithelial-mesenchymal transition (EMT) in MCF-10A cells (16). However, it remains unclear how MAPKs regulate transcription factors such as Twist1 that promotes breast tumor cell EMT, invasiveness and metastasis.
Twist1 is a member of the basic helix-loop-helix (bHLH) transcription factor family. In breast cancer, Twist1 expression is increased in patients associated with poor survival and metastasis, while knockdown of Twist1 reduces breast cancer cell invasiveness and metastasis (17). Twist1 mainly enhances cancer metastasis by repressing E-cadherin expression and promoting EMT, cell migration and cell invasion (18-20). Our recent study reported that Twist1 interacts with several components of the NuRD complex including MTA2, RbAp46, Mi-2 and HDAC2 and recruits this complex to the E-cadherin promoter to repress E-cadherin expression and promote cancer cell EMT and metastasis. Knockdown of Twist1, MTA2 or RbAp46 in 4T1 and MDA-MB-435 cells inhibits their metastasis in vivo (21). Another study also reported that Twist1-induced EMT could generate cells that share properties with cancer stem cells (22). In addition, Twist1 can also work cooperatively with oncogenic proteins such as Ras or ErbB2 to induce complete EMT by overriding oncogene-induced premature senescence (23).
Here, we report that Twist1 is phosphorylated at Ser 68 by Ras-activated JNK, ERK and p38 MAPKs, and this posttranslational modification is required to maintain Twist stability and its stability-dependent functions in controlling EMT and cell invasion. Furthermore, the levels of Twist1 phosphorylation at Ser 68 in human Her2-positive ductal carcinomas correlate positively with the levels of Twist1 protein and JNK activities but negatively with progesterone receptor (PR) expression. These findings suggest that MAPK-mediated Twist1 phosphorylation and stabilization play an important role in breast cancer cell EMT and invasion.
Materials and Methods
The inducible HEK293 cell lines expressing Flag (F) or F-tagged Twist1 (F-Twist1) were generated as previously described (21). Both types of cells were treated with 0.1 μg/ml of doxicyclin (DOX) for 6 hours to induce F and F-Twist1 expression. Clear cell lysates were prepared in the presence of protease inhibitor cocktail and the NaVO3 phosphotase inhibitor and subjected to immunoprecipitation using the anti-Flag M2 agarose beads (Sigma). After being washed thoroughly, the bound proteins were eluted by 3×Flag peptide solution (Sigma), separated in a SDS-PAGE gel and stained with Coomassie Blue. The F-Twist1 band was excised, digested in trypsin solution and analyzed by mass spectrometry to identify phosphorylation site as described previously (24).
The experimental procedures of immunoblotting, phosphorylation, protein stability, ubiquitination, RT-PCR, cell invasion and human breast tumor immunostaining were described in the Supplementary Material due to the limited space.
Results
Twist1 is phosphorylated on serine 68
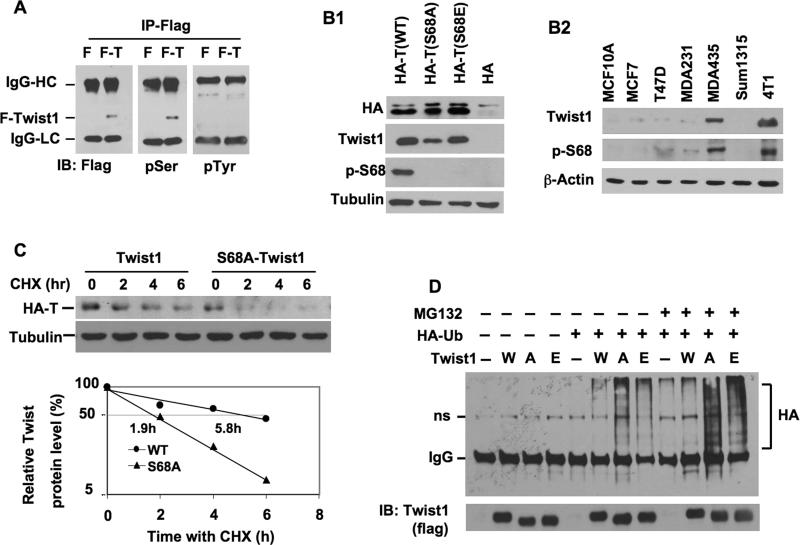
To study Twist1 phosphorylation, we generated DOX-inducible 293 cell lines expressing either F or F-Twist1 and immunopurified F and F-Twist1 from these cells. Western blot analyses confirmed that F-Twist1 protein was produced in F-Twist1 293 cells but not in F 293 cells (Fig. 1A). The apparent molecular weight of F-Twist1 was slightly reduced by active λ-PPase treatment but not by heat-inactivated λ-PPase (Suppl. Fig. S1A), suggesting that F-Twist1 is a phosphorylated protein. Furthermore, F-Twist1 positively reacted with pSer antibody but not pTyr antibody, indicating that F-Twist1 contains phosphorylated serine residue(s) (Fig. 1A).
Fig. 1. Twist1 expression, purification, phosphorylation and stability assays.
A. Immunoprecipitated F-Twist1 (F-T) was analyzed by immunoblotting (IB) with antibodies against Flag, p-Serine (pSer) and p-Tyrosine (pTyr). Immunoprecipitation from F cells served as a negative control. IgG-HC and IgG-LC, IgG heavy and light chains. B1. 293 cells were transfected with the indicated plasmids. Cell lysates were assayed by IB with the indicated antibodies. HA-T, HA-tagged Twist1; p-S68, pS68-Twist1. B2. The cell lysates were prepared from the indicated cell lines and analyzed by IB with antibodies against Twist1, pS68-Twist and β-actin. C. 293 cells were transfected with HA-Twist1 or HA-S68A-Twist1 plasmids. After 12 hours, cells were treated with cycloheximide for time periods as indicated. IB was performed with HA and tubulin antibodies. Densitometric values were determined and presented. The half lives (50%) of HA-Twist1 and HA-S68A-Twist1 are indicated. D. F (-), F-Twist1 (W), F-S68A-Twist1 (A) and F-S68E-Twist1 (E) inducible 293 cells were transfected with mock plasmids or HA-ubiquitin expression plasmids as indicated. After 12 hours of transfection, cells were treated with Dox for 6 hours before cells were treated with a vehicle or MG132 for another 6 hours. Immunoprecipitation was performed with Flag antibody, followed by IB with HA and Flag antibodies as indicated. ns, non-specific band.
To map the phosphorylation site(s), the F-Twist1 band was excised from the gel, digested by trypsin, and subjected to mass spectrometry analysis. This unbiased approach identified only Ser 68 as the phosphorylated residue in F-Twist1 (Suppl. Fig. S2). This assay was performed twice with two batches of purified F-Twist1; the same results were uniform across all trials. To evaluate the effects of pS68 on F-Twist1 molecular features, we mutated Ser 68 to alanine (S68A) and glutamine (S68E) and expressed these mutants in inducible 293 cell lines. Both mutant proteins showed slightly reduced apparent molecular weights when compared to wild type F-Twist1 and had no detectable phosphoserine residue (Suppl. Fig. S1B). These results demonstrate that Ser 68 is the major phosphorylation site of F-Twist1 in 293 cells.
A short Twist1 peptide containing pS68 was used to generate rabbit antiserum. From the antiserum, the pS68-Twist1-specific and pS68-insensitive Twist antibodies were purified. As expected, the pS68-Twist1 antibody specifically recognized the HA-Twist1 with Ser 68 but not the HA-Twist1-S68A and HA-Twist1-S68E mutants, while pS68-Twist1 insensitive antibody recognized all three proteins (Fig. 1B1). Using these antibodies, we measured the levels of total Twist1 and pS68-Twist1 in several cell lines. The Twist1 level is high in MDA-MB-435 and 4T1 metastatic breast cancer cells and low in MCF-10A mammary epithelial cells, non-metastatic ERα-positive MCF-7 and T47D breast cancer cells, and moderately invasive ERα-negative MDA-MB-231 and Sum1315 breast cancer cells. Interestingly, the pS68-Twist1 levels positively correlate with total Twist1 levels in these cells (Fig. 1B2). These results suggest a potential link that relates pS68-Twist1, Twist1 concentration, and breast cancer metastasis.
pS68 stabilizes Twist1 by protecting Twist1 from ubiquitination
We expressed Twist1, Twist1-S68A and Twist1-S68E as GFP fusion proteins in HeLa cells and examined their subcellular localization by fluorescence microscopy. All three fusion proteins were observed predominantly in the nucleus (Suppl. Fig. S3A), while the GFP control protein was observed in both the cytoplasm and nucleus (data not shown). These results indicate that pS68 is not required for Twist1 nuclear localization. Next, since Twist1 forms a heterodimer with E12 (25), we compared the heterodimerization function of Twist1 to that of Twist1-S68A by fusing E12 to the Gal4 DNA-binding domain, and Twist1 and Twist1-S68A to the VP16 transcriptional activation domain in a mammalian two-hybrid system using UAS-tk-luciferase reporter. However, no difference in reporter activity was found in these assays (Suppl. Fig. S3B), suggesting that pS68 is not required for the Twist dimerization function. Finally, we assessed the stability of Twist1 and Twist1-S68A proteins after protein synthesis was blocked in CHX-treated 293 cells. The half-lives of HA-Twist1 and HA-Twist1-S68A in the transfected 293 cells were 5.8 and 1.9 hours (Fig. 1C). Similar results were obtained from inducible 293 cell lines expressing F-Twist1 and F-Twist1-S68A, in which S68A mutation decreased the half-life of Twist1 from 6.2 to 1.8 hours (Suppl. Fig. S1C and D).
To assess the molecular mechanism underlying pS68-promoted Twist1 stability, we expressed HA-ubiquitin in the inducible F-Twist1, F-Twist1-S68A and F-Twist1-S68E cell lines and immunoprecipitated these F-tagged proteins for ubiquitination assay. The association of Twist1 with HA-ubiquitin appeared as typical high molecular weight ladders. Blocking of proteasomal activity by inhibitor MG132 resulted in slightly higher accumulations of Twist1-ubiquitin complexes, suggesting that Twist1 protein was polyubiquitinated before proteasome-mediated degradation (Fig. 1D). Importantly, coexpression of HA-ubiquitin with F-Twist1-S68A or F-Twist1-S68E robustly increased the degrees of ubiquitination of these proteins when compared to the F-Twist1 protein. The increase in ubiquitination of these mutant Twist1 proteins was particularly evident in the presence of MG132 (Fig. 1D). These results indicate that phosphorylation of S68 protects Twist1 from ubiquitination and degradation.
Ser 68 in Twist1 is phosphorylated by MAPKs
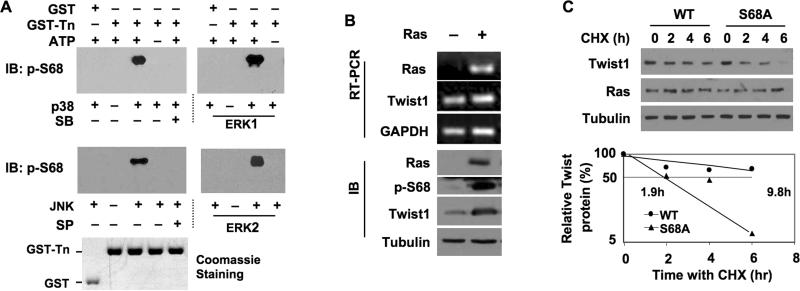
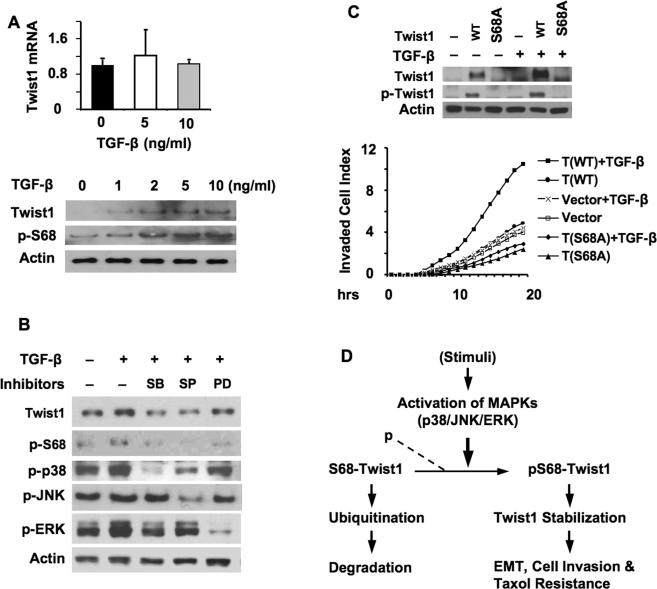
Sequence analysis suggested that S68 in Twist1 lies in a Ser/Thr-Pro consensus phosphorylation site of MAPKs (26). Therefore, we generated and purified recombinant GST protein and GST fusion protein containing the N-terminal 112 a.a. (Twist1-N) and performed in vitro phosphorylation assays with members of the MAPK family including p38, JNK, ERK1 and ERK2. The assays demonstrated that all four MAPKs efficiently phosphorylated Ser 68 in Twist1-N, as detected by the pS68-specific antibody. The GST control did not show any phosphorylation in the same assay. The p38- and JNK-mediated S68 phosphorylations were blocked by their respective specific kinase inhibitors, SB203580 and SP60125 (Fig. 2A). Thus, the S68 in Twist1 is an ideal target site for MAPKs.
Fig. 2. MAPKs phosphorylate Twist1 at Sre68 in vitro and Ras activation stabilizes Twist1 in HEK293 cells.
A. GST control and GST-Twist1-N Proteins were incubated with MAPKs as indicated for in vitro phosphorylation assays. Immunoblotting (IB) was performed with pS68-Twist1 antibody. Coomassie blue staining of GST and GST-Twist1-N Proteins served as the loading control. SB203580, a p38 MAPK inhibitor; SP60125, a JNK inhibitor. B. 293 cells were transfected with HA-Twist1 plasmids in combination with H-RasV12 (+) plasmids or its mock vector (-). H-Ras, Twist1 and GAPDH mRNAs were measured by RT-PCR. H-Ras, pS68-Twist1 and tubulin proteins were measured by IB. C. 293 cells were transfected with H-RasV12 plasmids in combination with HA-Twist1 or HA-S68A-Twist1 plasmids. After 12 hours, cells were treated with cycloheximide as indicated. IB was performed with antibodies against HA (for Twist1), H-Ras and tubulin. Densitometric values were determined and plotted.
We further investigated the signaling pathway that regulates pS68 in Twist1. It has been shown that Twist1-promoted EMT can be further enhanced by Ras or ErbB2 oncoproteins (23). To investigate whether the Ras-activated MAPKs phosphorylate Twist1 on S68 in vivo, we co-expressed HA-Twist1 with either H-RasV12, a constitutively active form of human H-Ras, or a control plasmid in 293 cells. The Twist1 mRNA levels were comparable across cells with and without H-RasV12 expression. However, H-RasV12 expression robustly increased the level of pS68, which was accompanied by increased total Twist1 protein (Fig. 2B). To determine whether H-RasV12-stimulated pS68 increases the stability of Twist1, we co-expressed either Twist1 or Twist1-S68A with H-RasV12 and performed a CHX-based protein chase experiment. HRasV12 expression extended the half life of Twist1 from 5.8 to 9.8 hours, while it did not significantly change the half life of Twist1-S68A, which was 1.9 hours in both the presence and absence of H-RasV12 (Compare Fig. 2C to Fig. 1C). To extrapolate our observations to breast epithelial cells, we generated a series of MCF-10A stable cell lines using retroviruses that mediate the expression of Twist1, Twist1-S68A and H-RasV12 in combination. Again, HRasV12 selectively increased the Twist1 level in MCF-10A cells without stabilizing the Twist1-S68A mutant and had little effect on their mRNA levels (Suppl. Fig. S4). These results demonstrate that activation of the Ras pathway in cells dramatically enhances Twist1 stability through stimulating S68 phophorylation.
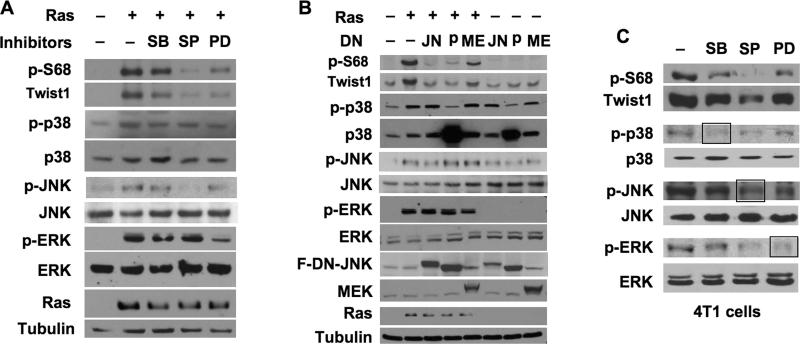
Ras activates multiple MAPKs (27-29). To define the Ras-activated MAPKs that phosphorylate S68 of Twist1, we treated Twist1 and H-RasV12-transfected 293 cells with specific MAPK inhibitors and coexpressed specific MAPK-dominant negative inhibitory mutants (DNIM) in these cells to inhibit individual p38, JNK and ERK kinases. SB203580 treatment slightly and expression of the p38 DNIM (30) significantly reduced the active form of p38, p-p38, which associated accordingly with a moderate and a more dramatic reduction in pS68-Twist1 and Twist1 proteins, respectively (Fig. 3A and B). SP60125 treatment effectively reduced p-JNK levels and also reduced pS68-Twist1 and total Twist1 proteins (Fig. 3A). The JNK DNIM inhibits JNK activity by competing with wild type JNK for activation and substrate binding (31). Expression of JNK DNIM efficiently decreased the levels of pS68-Twist1 and total Twist1, although it only partially reduced p-JNK levels (Fig. 3B). Inhibition of ERK activity by PD98059 treatment or MEK DNIM (32) expression similarly reduced pS68-Twist1 and Twist1 protein levels (Fig. 3A and B). Furthermore, inhibition of p38, JNK or ERK kinases in metastatic 4T1 breast cancer cells expressing high endogenous Twist1 also decreased pS68-Twist and total Twist1 proteins with variable efficacies. Among them, the JNK inhibitor treatment reduced both p-JNK and p-ERK levels, and thereby was particularly potent in reducing both the pS68-Twist1 and total Twist1 levels (Fig. 3C). These results indicate that all three subfamily members of MAPKs are capable to phosphorylate and stabilize Twist1 protein in both 293 and breast tumor cells.
Fig. 3. Inhibition of MAPKs reduces pS68-Twist1 and Twist1 proteins.
A. 293 cells were transfected with Twist1 plasmids and H-RasV12 or mock plasmids as indicated. After 12 hours, cells were treated with a vehicle, SB203580, SP60125 or PD98059. Immunoblotting was performed with the indicated antibodies. B. 293 cells were transfected with Twist1, in combination with mock vectors (-), H-RasV12 expression plasmids (+) and dominant negative (DN) forms of Flag-DN-JNK (JN), Flag-DN-p38 MAPK (p) or DN-MEK (ME) as indicated. Immunoblotting was performed with the indicated antibodies. Note that the p38 antibody recognized both endogenous and DN-p38. The anti-Flag antibody recognized both Flag-DN-JNK and Flag-DN-p38. C. 4T1 cells were treated with a vehicle or different MAPK inhibitors for 8 hours. Immunoblotting was performed with the antibodies indicated to detect the indicated endogenous proteins.
Ser 68 phosphorylation is required for Twist1-promoted EMT and breast cancer cell invasion
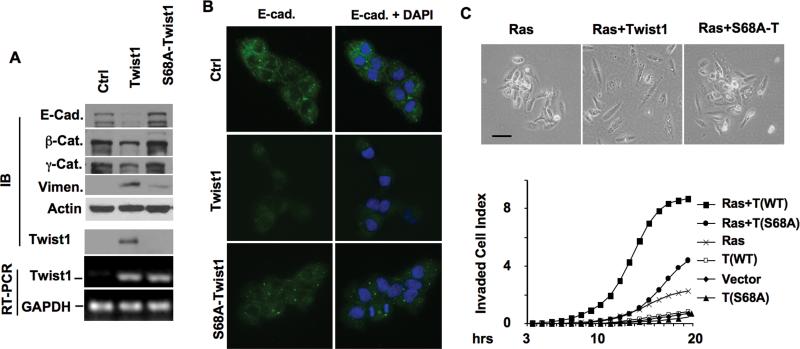
To understand the role of pS68 in Twist1 function, we first examined how Twist1 phosphorylation contributed to Twist1-promoted EMT. Among three stable MCF-10A human mammary epithelial cell lines expressing F tag (control), F-Twist1 or F-Twist1-S68A, F-Twist1 protein was steadily detected, while Twist1-S68A protein was barely detected due to its instability, even though both cell lines expressed comparable Twist1 and Twist1-S68A mRNAs (Fig. 4A). As expected from previous studies (17), Twist1 expression inhibited the expression of epithelial markers including E-cadherin, β-catenin and γ-catenin, but promoted the expression of mesenchymal marker vimentin in MCF-10A cells. However, Twist1-S68A expression failed to inhibit these epithelial markers and only slightly promoted vimentin expression (Fig. 4A). Similar results were also obtained from E-cadherin immunostaining, showing higher E-cadherin signals in Twist-S68A cells versus Twist1 cells (Fig. 4B). These results suggest that S68 phosphorylation is required for Twist1-promoted EMT.
Fig. 4. Ser 68 phosphorylation is required for Ras-stimulated and Twist1-promoted breast cancer cell invasion.
A. Stable MCF-10A cell lines with mock control, Twist1 expression or Twist1-S68A expression were assayed by immunoblotting (IB) with antibodies indicated. Twist1, S68A-Twist1 and GAPDH mRNAs were measured by RT-PCR. B. The above cells were immunostained by the E-cadherin antibody (green color in all panels). Cell nuclei were stained by DAPI (right panels). C. Morphologies of MCF-10A cell lines expressing H-RasV12 and Twist1, S68A-Twist1 or a mock control as indicated (upper panel). Real-time cell invasion assays (lower panel) were performed with the 3 cell lines in the upper panel and the 3 cell lines in panel A.
Ras can transform MCF-10A cells (33) and work with Twist1 in MDCK cells to achieve a complete EMT phenotype (23). To examine the specific role of pS68 as an effector in the Ras signaling, we generated MCF-10A cell lines expressing H-RasV12 and either Twist1 or Twist1-S68A. Either H-RasV12 expression alone or accompanied by Twist1 or Twist1-S68A diminished E-cadherin expression, suggesting that H-RasV12 alone is sufficient to induce EMT in MCF-10A cells under our experimental conditions (data not shown). However, cells expressing H-RasV12 and Twist1 showed morphology closer to that of fibroblasts (Fig. 4C). More importantly, MCF-10A cells expressing Twist1 or Twist1-S68A alone showed little change in cell invasion through a Matrigel layer when compared to control MCF-10A cells, while co-expression of Twist1 and H-RasV12 robustly increased cell invasiveness. However, expression of H-RasV12 alone only slightly increased cell invasiveness, and co-expression of Twist-S68A and H-RasV12 only moderately increased cell invasiveness (Fig. 4C). These results demonstrate that S68 phosphorylation is required for Ras-stimulated and Twist1-promoted cell invasiveness.
TGF-β activates Ras/MAPK pathways such as p38 MAPK and regulates gene expression to promote EMT and cancer metastasis (34-36). Thus, we examined whether TGF-β could induce Twist1 phosphorylation on Ser 68 and increase Twist1 in MDA-MB-231 human breast cancer cells with a relatively low level of Twist1 protein. Indeed, TGF-β treatment increased pS68-Twist1 and total Twist1 protein in a dose dependent manner without changing Twist1 mRNA levels (Fig. 5A). Inhibition of p38 with SB203580 and inhibition of JNK with SP60125 both blocked TGF-β-induced increases in pS68-Twist1 and total Twist1 protein levels, while inhibition of ERK kinases with PD98059 showed lesser effects (Fig. 5B). To confirm that TGF-β indeed targets Ser 68 to stabilize Twist1, we treated MCF-10A cell lines expressing either Twist1 or Twist1-S68A with TGF-β. TGF-β treatment significantly increased pS68-Twist1 and total Twist1 protein levels in Twist1-expressing cells, but failed to increase the Twist1-S68A protein in Twist1-S68A-expressing cells. In fact, Twist1-S68A was difficult to detect due to its instability, regardless of TGF-β treatment (Fig. 5C). These results indicate that activation of the TGF-β/MAPK signaling pathways stabilizes Twist1 protein through induction of Twist1 phosphorylation on Ser 68.
Fig. 5. TGF-β induces pS68-Twist1 and Twist1 proteins and cell invasion.
A. MDA-MB-231 cells were treated with TGF-β as indicated. Twist1 mRNA levels were measured by real time RT-PCR and normalized to 18S RNA levels (upper panel). Lysates were prepared from TGF-β-treated MDA-MB-231 cells and subjected to immunoblotting with antibodies against Twist1, pS68-Twist1 and β-actin (lower panel). B. MDA-MB-231 cells were treated with TGF-β and/or different MAPK inhibitors. Cell lysates were analyzed by immunoblotting with the indicated antibodies. C. MCF-10A cells with mock vector (-), Twist1 (WT) expression or S68A-Twist1 (S68A) expression were treated with a vehicle (-) or TGF-β as indicated. Immunoblotting was performed using antibodies against Twist1, pS68-Twist1 and β-actin (upper panel). Cell invasion assays were performed in the absence or presence of TGF-β as indicated. D. Summary diagram of working model. If S68-Twist is not phosphorylated by MAPKs, it will subject to ubiquitination and degradation. If phosphorylated, the pS68-Twist1 is resistant to ubiquitination and degradation and becomes stabilized, so its function for promotion of EMT, cell invasion and cell survival will be enhanced.
To estimate the role of Ser 68 phosphorylation in TGF-β-induced cell invasion, we performed cell invasion assays in the presence or absence of TGF-β with MCF-10A cell lines expressing Twist1, Twist1-S68A or mock control. The vehicle-treated mock, Twist1, and Twist1-S68A-expressing cells and TGF-β treated mock and Twist1-S68A-expressing cells showed discernible, but insignificant, changes in their invasion capabilities. However, TGF-β-treated Twist1-expressing cells showed a robust increase in cell invasion capability (Fig. 5C). These results suggest that Ser 68 phosphorylation plays an important role in mediating TGF-β-induced and Twist1-promoted cell invasion.
Twist1 is known to promote cell survival and increases cellular resistance to taxol-induced apoptosis (37). Upon taxol treatment, the MCF-10A cells expressing Twist-S68A exhibited a slightly increased viability than MCF-10A cells with the mock vector, but a remarkedly decreased viability than MCF-10A cells expressing wild type Twist1 (Suppl. Fig. S5). These results suggest that prevention of Twist1 from S68 phosphorylation reduces its function to enhance taxol resistance.
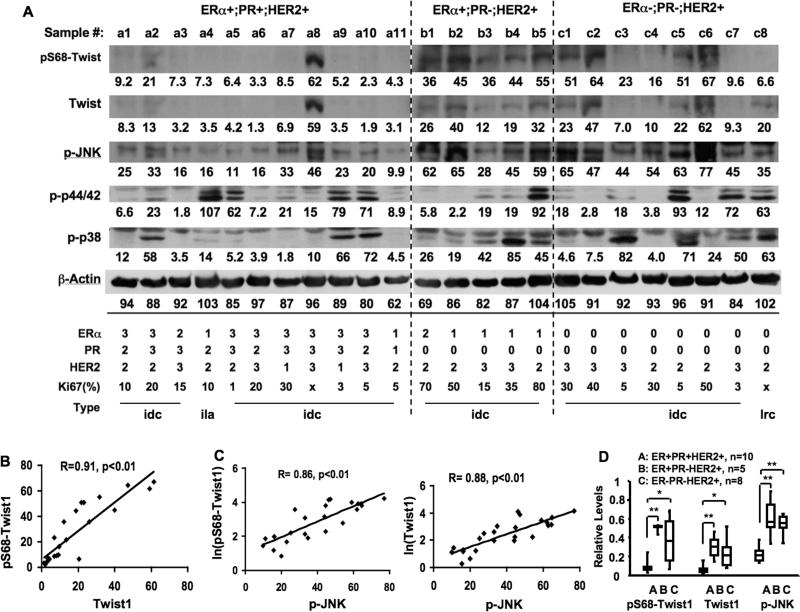
The Twist1 levels in invasive breast ductal carcinomas correlate positively with p68-Twist1 and active p-JNK and negatively with PR levels
To explore the clinical implications of pS68-Twist1, we investigated the correlations among pS68-Twist1, Twist1 and active forms of MAPKs in 24 invasive human breast tumor samples by Western blot (Fig. 6A). Among these tumor samples, levels of pS68-Twist1 and Twist1 proteins were found to be closely associated (Fig. 6B), supporting the finding that phosphorylation of Ser 68 stabilizes Twist1 protein not only in breast cancer cell lines but also in human breast tumors. Both pS68-Twist1 and Twist1 levels correlated positively and closely with active p-JNK levels (Fig. 6C). However, levels of pS68-Twist1 and Twist1 proteins showed no obvious correlations with active forms of ERK1/2 and p38 MAPKs (Fig. 6A). These results suggest that JNK may play a role more dominant than other MAPKs in phosphorylating S68 of Twist1 in invasive breast cancers.
Fig. 6. Analyses of pS68-Twist1, Twist1 and MAPK levels in human primary breast tumors.
A. Immunoblotting analyses of breast tumors using antibodies against pS68-Twist1, Twist1, p-JNK, p-p44/42 ERKs, p-p38 MAPK and β-actin. The values of band intensity measured by densitometry are listed. The tumor types are indicated as invasive ductal carcinoma (idc), invasive lobular andenocarcinoma (ila) and lipid-rich epithelia carcinoma (lrc). Tumors were divided into 3 groups according to the immunostaining results of ERα, PR and HER2. The percentage of Ki67-positive cell numbers is listed. For ERα and PR, the staining intensities were graded as 0 (negative), 1 (weak staining), 2 (medium staining) and 3 (strong staining), and the ratios of positive cells were graded as 1 (0-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%). The final scores indicated here were determined according to the sum of staining intensity and positive cell ratio grades: 0, ≤ 2 sum points; 1, 3-4 sum points; 2, 5-6 sum points; and 3, 7 sum points. For HER2, score 0 was designated as negative staining or <10% of tumor cells with weak membrane staining; score 1 was designated as >10% of tumor cells with weak staining and partial membrane staining; score 2 was designated as >10% of tumor cells with weak to medium membrane staining; and score 3 was designated as >10% of tumor cells with strong membrane staining. B. The correlation relationship between Twist1 and pS68-Twist1 levels in breast tumors assayed in panel A. The correlation was statistically calculated by Spearman rank correlation analysis. C. The correlation relationships between p-JNK and pS68-Twist1 or Twist1 levels in breast tumors assayed in panel A. D. The relative levels of pS68-Twist1, Twist1 and p-JNK in ERα+;PR+;HER2+, ERα+;PR-;HER2+, and ERa-;PR-;HER2+ breast tumors assayed in panel A. The data are presented in a box plot, where the upper limit of the rectangle is the third quartile and the bottom limit is the first quartile. *, p < 0.01; **, p< 0.001 by t test.
Among the 24 tumors, there were 11 ERα+;PR+;HER2+ tumors in Group A, 5 ERα+;PR-;HER2+ tumors in Group B, and 8 ERα-;PR-;HER2+ tumors in Group C. According to clinical diagnosis, 22 tumors were invasive ductal carcinomas, and the remaining two were an invasive lobular adenocarcinoma in Group A (#a4) and a lipid-rich epithelial carcinoma in Group C (#c8) (Fig. 6A). High-level pS68-Twist1 and Twist1 proteins (band intensity > 10) were detected in 2 of 11 (18%) tumors in Group A, all tumors in Group B, and 6 of 8 (75%) tumors in Group C (Fig. 6A). The average abundances of pS68-Twist1 and Twist1 in Groups B and C were significantly higher than that of Group A (Fig. 6D). Similarly, high-level p-JNK (band intensity > 34) was found in 1 of 11 (9%) tumors in Group A, 4 of 5 (80%) tumors in Group B, and all 8 tumors in Group C (Fig. 6A). The average levels of p-JNK in tumors of Groups B and C were also significantly higher than that of the Group A tumors (Fig. 6D).
Tumors with high levels of pS68-Twist1, Twist1 and p-JNK also exhibited higher rates of cell proliferation as detected by Ki67 immunostaining (Fig. 6A). Among the 22 tumors stained by Ki67 antibody, the 12 tumors with low pS68-Twist1 or Twist1 (either band intensity < 10 in Fig. 6A) showed an average of 10.6 ± 8.5 (%) Ki67-positive cells, while the 10 tumors with high pS68-Twist and Twist1 (both band intensities > 10 in Fig. 6A) showed a significantly higher average of 40.5 ± 22 (%) Ki67-positive cells (p < 0.001 by unpaired t-test).
Discussion
Similar to other signaling proteins, transcription factors are commonly regulated by phosphorylation in response to various cellular signals. The transcriptional activities of several bHLH transcription factors, such as E47, Id3 and Twist1, are modulated by phosphorylation (38-40). Twist1 dimerization and DNA binding are also enhanced by PKA-mediated phosphorylation at Thr 125 and Ser 127 but inhibited by PP2A-mediated de-phosphorylation of these residues (40, 41). Furthermore, the phosphorylation-regulated dimerization of Twist1 and Hank2 plays an essential role in limb development in both mice and chicks (42). In addition, Twist1 is phosphorylated at Ser 42 by Akt, and this modification is required for Twist1 to inhibit p53-mediated cell apoptosis in response to DNA damage (43).
In this study, we found that Ser 68 is a major phosphorylation site in Twist1 expressed in 293 cells; it was the only site detected by the mass spectrometry, and the single S68A mutation diminished overall serine phosphorylation in the entire Twist1 protein. Our data demonstrated that Ser 68 phosphorylation is not required for Twist1 nuclear localization and dimerization, indicating that Ser 68 phosphorylation has a function distinct from Thr 125 and Ser 127 phosphorylation. However, the relationship between Ser 68 phosphorylation and Ser 42 phosphorylation in p53 function is currently unknown.
MAPK-mediated phosphorylation plays crucial roles in stabilizing important transcription factors. For example, JNK-mediated phosphorylation increases p53 stability and transcriptional activity in response to stress (44). The Ras-ERK MAPK cascade protects GATA3 in Th2 cell differentiation and Myc in cell growth and oncogenesis from degradation by the ubiquitin-proteasome pathway (45, 46). Considering the importance of Twist1 in development and cancer, the cellular amount and function of Twist1 must be strictly controlled. It has been shown that a truncated Twist1 mutant associated with SCS is unstable (25), and that Twist1-mediated inhibition of bone morphogenetic protein (BMP) signaling is counter-regulated by E47 interaction-enhanced and Id1 interaction-reduced Twist1 stability (47). In this study, we have shown that S68A mutation significantly decreases Twist1 stability in both transiently transfected cells and stable cell lines. On the base of a previous study showing Twist1 degradation by the ubiquitination-proteasome pathway (48), this study further demonstrated that prevention of Ser 68 phosphorylation in Twist1-S68A accelerates Twist1 polyubiquitination, which is consistent with its rapid degradation. However, the Twist1-S68E mutation failed to mimic the role of S68 phosphorylation. Although the reason is unclear, phosphoserine residue has 2 units of negative charges but the E residue has only one, and the side chain of phosphoserine is also more bulky than the E side chain. These differences may allow phosphoserine and E to have different capabilities for protein-protein interaction, and thereby different stabilities.
Furthermore, we have provided compelling in vitro and in vivo evidence showing that Twist1 is phosphorylated at Ser 68 by MAPKs. Interestingly, the Ser 68 can be phosphorylated by all p38, JNK and ERK1/2 MAPKs in vitro and in 293 cells expressing an active H-RasV12. In 4T1 cells, all inhibitors for p38, JNK or ERK reduced Twist1 phosphorylation and protein levels. Among the used inhibitors, the SP60125 treatment caused reduction of both active forms of p-JNK and p-ERK, resulting an additive suppression of Twist1 phosphorylation and total protein levels. These results indicate that MAPK-mediated Ser 68 phosphorylation inhibits Twist1 degradation by the ubiquitin-proteasome pathway. These findings make Twist1 a direct MAPK target important for EMT and metastasis. However, although all three subfamily members of MAPKs can phosphorylate Twist1 at Ser 68, the specific MAPK that ultimately plays the dominant role may depend on cellular context.
Twist1 accumulation caused by Ras-MAPK signaling more effectively enhances Twist1-mediated EMT and cell invasion, as demonstrated by the more obvious changes in EMT markers in Twist1-expressing MCF-10A cells as compared to Twist1-S68A-expressing cells. TGF-β activates p38 MAPK and induces EMT and invasiveness in breast tumor cells. In our experiment, TGF-β predominantly activated p38 MAPK in human breast cancer cells, resulting in a clear increase in Twist1 protein that was sensitive to the inhibitor of p38 MAPK. Moreover, the Twist1-promoted MCF-10A cell invasion was tremendously enhanced by both active Ras and TGF-β stimulation when compared to Twist1-S68A-expressing MCF-10A cells that received the same treatments. These results suggest that MAPK-mediated Twist phosphorylation at Ser 68 may carry out an important function in the promotion of breast cancer cell EMT and invasion. Taken together, our findings can be summarized in Fig. 5D.
A recent study has shown that Ras-ERK2 signaling upregulates Fra1 to induce ZEB1/2 expression, which subsequently promotes cell EMT, migration and invasion (16). This pathway runs parallel to the pathway defined in this study, which shows that Ras-activated MAPKs phosphorylate and stabilize Twist1 to promote breast tumor cell EMT and invasion. Thus, multiple downstream molecular effectors are responsible for mediating the effects of MAPKs on breast tumor cell EMT, migration, invasion and metastasis. These findings may explain why super activation of Ras can induce a full EMT phenotype while overexpression of Twist1 induces only a partial EMT phenotype in non-cancerous mammary epithelial cells such as MCF-10A and epithelial-like/non-metastatic breast cancer cells such as MCF-7 cells (data not shown).
In HER2-positive human breast tumors, we found that the levels of Twist1 protein positively correlate with levels of Ser 68 phosphorylation and active JNK, but not with those of active ERK1/2 and p38 MAPKs. These observations suggest that JNK activation could be the primary MAPK in stabilizing Twist1 in these tumors. In drosophila, JNK controls border cell cluster integrity and collective cell migration and also induces pseudo-EMT and cell mobility during imaginal disc eversion (49, 50). In mouse keratinocytes, JNK can mediate TGFβ induced EMT (51). In human MCF-7 breast cancer cells, TNFα-can induces JNK and enhances cell invasiveness, while it does not do so in immortalized mammary epithelial cells (52). Taking into account our data demonstrating that JNK phosphorylates Twist1 in vitro and in multiple cell lines, and that Twist1 phosphorylation and protein levels correlate positively with JNK activity in invasive breast ductal carcinomas, we propose a molecular pathway that connects active JNK to Twist1 phosphorylation and accumulation, and to Twist1-promoted EMT and metastasis in human breast cancer.
Finally, our data showed that high levels of total Twist1, phosphorylated Twist1 and active JNK associate with PR-negative status in breast tumors with or without ERα. It has been shown that PR-negative status is associated with loss of responsiveness to endocrine therapy (53) and that the PR-/ER+ breast cancer is associated with bone metastasis (54). Our data therefore suggest a possible link between Twist1 elevation and endocrine therapy resistance and bone metastasis. These findings also suggest that JNK and Twist1 may be potential therapeutic targets in ERα+/PR- and ERα-/PR- breast tumors.
Supplementary Material
Acknowledgments
We thank Fred Miller for 4T1 cell, Roger Davis for the dominant negative p38 and JNK plasmids, Julian Downward for the H-RasV12 and dominant negative MEK plasmids, Ray Wu for the HA-Ubiquitin plasmid and James T. Kadonaga for the E12 plasmid. This work is partially funded by NIH grants CA112403, DK058242 and CA119689.
References
- 1.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 2.Haagenson KK, Wu GS. The role of MAP kinases and MAP kinase phosphatase-1 in resistance to breast cancer treatment. Cancer Metastasis Rev. 2010;29:143–9. doi: 10.1007/s10555-010-9208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salh B, Marotta A, Matthewson C, Ahluwalia M, Flint J, Owen D, et al. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 1999;19:731–40. [PubMed] [Google Scholar]
- 4.Johnston SR, Lu B, Scott GK, Kushner PJ, Smith IE, Dowsett M, et al. Increased activator protein-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin Cancer Res. 1999;5:251–6. [PubMed] [Google Scholar]
- 5.Schiff R, Reddy P, Ahotupa M, Coronado-Heinsohn E, Grim M, Hilsenbeck SG, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst. 2000;92:1926–34. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 6.Murtagh J, McArdle E, Gilligan E, Thornton L, Furlong F, Martin F. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J Cell Biol. 2004;166:133–43. doi: 10.1083/jcb.200403020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–20. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malaney S, Daly RJ. The ras signaling pathway in mammary tumorigenesis and metastasis. J Mammary Gland Biol Neoplasia. 2001;6:101–13. doi: 10.1023/a:1009572700317. [DOI] [PubMed] [Google Scholar]
- 9.Webb CP, Van Aelst L, Wigler MH, Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci U S A. 1998;95:8773–8. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49:465–75. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 11.Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009;11:R66. doi: 10.1186/bcr2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold SF, Obourn JD, Jaffe H, Notides AC. Phosphorylation of the human estrogen receptor by mitogen-activated protein kinase and casein kinase II: consequence on DNA binding. J Steroid Biochem Mol Biol. 1995;55:163–72. doi: 10.1016/0960-0760(95)00177-2. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol. 2004;24:10954–64. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–27. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–76. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 19.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–27. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2012;21:275–89. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelialmesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Herring CJ, Romano PR, Szczepanowska J, Brzeska H, Hinnebusch AG, et al. Identification of phosphorylation sites in proteins separated by polyacrylamide gel electrophoresis. Anal Chem. 1998;70:2050–9. doi: 10.1021/ac971207m. [DOI] [PubMed] [Google Scholar]
- 25.El Ghouzzi V, Legeai-Mallet L, Aresta S, Benoist C, Munnich A, de Gunzburg J, et al. Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet. 2000;9:813–9. doi: 10.1093/hmg/9.5.813. [DOI] [PubMed] [Google Scholar]
- 26.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Li YS, Shyy JY, Li S, Lee J, Su B, Karin M, et al. The Ras-JNK pathway is involved in shear-induced gene expression. Mol Cell Biol. 1996;16:5947–54. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T, Yokote K, Tamura K, Takemoto M, Ueno H, Saito Y, et al. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J Biol Chem. 1999;274:13954–60. doi: 10.1074/jbc.274.20.13954. [DOI] [PubMed] [Google Scholar]
- 29.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–54. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 31.Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, et al. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–6. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 32.Pages G, Brunet A, L'Allemain G, Pouyssegur J. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1). Embo J. 1994;13:3003–10. doi: 10.1002/j.1460-2075.1994.tb06599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, et al. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- 34.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a smad-dependent pathway. J Biol Chem. 2000;275:35656. [PubMed] [Google Scholar]
- 36.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–59. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Ling MT, Guan XY, Tsao SW, Cheung HW, Lee DT, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene. 2004;23:474–82. doi: 10.1038/sj.onc.1207128. [DOI] [PubMed] [Google Scholar]
- 38.Lluis F, Ballestar E, Suelves M, Esteller M, Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–84. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrest ST, Taylor AM, Sarembock IJ, Perlegas D, McNamara CA. Phosphorylation regulates Id3 function in vascular smooth muscle cells. Circ Res. 2004;95:557–9. doi: 10.1161/01.RES.0000142735.67542.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, et al. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–81. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firulli BA, Redick BA, Conway SJ, Firulli AB. Mutations within helix I of Twist1 result in distinct limb defects and variation of DNA binding affinities. J Biol Chem. 2007;282:27536–46. doi: 10.1074/jbc.M702613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firulli AB, Conway SJ. Phosphoregulation of Twist1 provides a mechanism of cell fate control. Curr Med Chem. 2008;15:2641–7. doi: 10.2174/092986708785908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vichalkovski A, Gresko E, Hess D, Restuccia DF, Hemmings BA. PKB/AKT phosphorylation of the transcription factor Twist-1 at Ser42 inhibits p53 activity in response to DNA damage. Oncogene. 2010 doi: 10.1038/onc.2010.115. [DOI] [PubMed] [Google Scholar]
- 44.Buschmann T, Potapova O, Bar-Shira A, Ivanov VN, Fuchs SY, Henderson S, et al. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol Cell Biol. 2001;21:2743–54. doi: 10.1128/MCB.21.8.2743-2754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280:29409–19. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 46.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–79. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi M, Nimura K, Kashiwagi K, Harada T, Takaoka K, Kato H, et al. Comparative roles of Twist-1 and Id1 in transcriptional regulation by BMP signaling. J Cell Sci. 2007;120:1350–7. doi: 10.1242/jcs.000067. [DOI] [PubMed] [Google Scholar]
- 48.Demontis S, Rigo C, Piccinin S, Mizzau M, Sonego M, Fabris M, et al. Twist is substrate for caspase cleavage and proteasome-mediated degradation. Cell Death Differ. 2006;13:335–45. doi: 10.1038/sj.cdd.4401744. [DOI] [PubMed] [Google Scholar]
- 49.Llense F, Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 2008;18:538–44. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 50.Pastor-Pareja JC, Grawe F, Martin-Blanco E, Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–99. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Santibanez JF. JNK mediates TGF-beta1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett. 2006;580:5385–91. doi: 10.1016/j.febslet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 53.Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14:458–65. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Wei B, Wang J, Bourne P, Yang Q, Hicks D, Bu H, et al. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol. 2008;39:1809–15. doi: 10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.