Abstract
Markers that reliably identify cancer stem cells (CSC) in ovarian cancer could assist prognosis and improve strategies for therapy. CD133 is a reported marker of ovarian CSC. Aldehyde dehydrogenase (ALDH) activity is a reported CSC marker in several solid tumors but it has not been studied in ovarian CSC. Here we report that dual positivity of CD133 and ALDH defines a compelling marker set in ovarian CSC. All human ovarian tumors and cell lines displayed ALDH activity. ALDH+ cells isolated from ovarian cancer cell lines were chemoresistant and preferentially grew tumors compared to ALDH− cells, validating ALDH as a marker of ovarian CSC in cell lines. Notably, as few as 1000 ALDH+ cells isolated directly from CD133(−) human ovarian tumors were sufficient to generate tumors in immunocompromised mice, whereas 50,000 ALDH− cells were unable to initiate tumors. Using ALDH in combination with CD133 to analyze ovarian cancer cell lines we observed even greater growth in the ALDH+CD133+ cells compared to ALDH+CD133− cells, suggesting a further enrichment of ovarian CSC in ALDH+CD133+ cells. Strikingly, as few as 11 ALDH+CD133+ cells isolated directly from human tumors were sufficient to initiate tumors in mice. Like other CSC, ovarian CSC exhibited increased angiogenic capacity compared to bulk tumor cells. Lastly, the presence of ALDH+CD133+cells in debulked primary tumor specimens correlated with reduced disease-free and overall survival in ovarian cancer patients. Taken together, our findings define ALDH and CD133 as a functionally significant set of markers to identify ovarian CSCs.
Keywords: Cancer stem cells, Ovarian cancer, Angiogenesis, CD133, Aldehyde-dehydrogenase
Introduction
Some tumors follow a cancer stem cell (CSC) model, while others do not. In tumors that follow a CSC model, CSC are believed to make up a limited percentage of the tumor cells, yet be the driving force behind cancer growth (1, 2). The presence of such cells could explain why cancer often relapses despite a complete clinical remission with initial therapy; with time a few residual treatment resistant stem cells could repopulate the tumor.
Ovarian cancer is a tumor which commonly shows a complete remission in response to chemotherapy, yet the majority of patients relapse. This suggests a cancer stem cell model may be relevant in ovarian cancer. Supporting the idea of ovarian cancer stem cells, Nephew and colleagues used a primary human ovarian tumor specimen to generate in vitro tumor ‘spheroids’ (3). 100 isolated spheroid cells were capable of generating tumors in mice reminiscent of the primary tumor. Tumor spheroids had enriched expression of the stem cell markers CD117 and CD44 suggesting these were possible markers of ovarian CSC. CD133 has also been reported as a marker of ovarian CSC. Limited numbers of CD133+ cells from ovarian cancer cells lines generated large tumors more rapidly than CD133− cells, and CD133+ cells produced tumors in mice with CD133+ and CD133− cells (4). Furthermore a second group reported that the CD133+ cell population in the primary human tumor xenografts in mice was primarily responsible for serial tumor passage (5). Unfortunately, CD133 is only expressed in ~40% of ovarian cancer cell lines ~30% of primary ovarian tumors, thus CD133 may not be a useful CSC marker for a majority of ovarian cancers (4). Finally, CD24 alone, and in combination with CD44 and EpCAM, was reported to mark cells with ovarian CSC activity in various cancer cell lines (6, 7). Interestingly a proportion of these cells also expressed CD133.
Despite these studies of ovarian CSC, several issues remain. To date, no study has isolated an ovarian cancer stem cell population directly from human tumors to initiate tumors in mice; all used cell lines, or stem cells isolated from primary cells following in vitro or in vivo passage. This limits the ability to isolate and characterize ovarian CSC without concerns for genetic and phenotypic changes associated with the passage of cells.
Recent studies in other solid tumors identified aldehyde dehydrogenase enzymatic activity (ALDH) as a potential maker for CSC. In a study of breast cancer, ALDH+ cells were present in a majority of tumors and capable of directly generating tumors in vivo (8). Independent studies in colon cancer suggest ALDH identifies colon cancer stem cells; as few as 25 ALDH+ cells could generate tumors while ALDH− cells could not (9, 10). Similarly, ALDH has been proposed as a marker of CSC in leukemia, head and neck, lung and pancreatic cancers (11–14). Interestingly, ALDH has been proposed, together with CD133, to identify CSC population in hepatocellular carcinoma (15). Based upon the evidence for ALDH as a stem cell marker in solid tumors, we performed an extensive analysis of ALDH activity alone, and in combination with CD133, as a marker of ovarian CSC.
Materials/Subjects & Methods
Tumor Processing
Informed consent was obtained from all patients prior to tissue procurement. All studies were performed with the approval of the Institutional Review Board of the University of Michigan. All tumors were stage III or IV epithelial ovarian or primary peritoneal cancer. Tumors were mechanically dissected into single cell suspensions and isolated on a ficol gradient as previously described (16). For ascites studies cell pellets were collected by centrifugation, and red cells lysed using ACK buffer, washed, passed through a 40μm filter, then passed 4x through a Standard Hub Pipetting needle to isolate single cells.
Sphere formation
Sphere culture was performed as previously described (8, 17). Briefly, FACS isolated ALDH+/−CD133+/− cell populations were plated in triplicate in ultra-low attachment plates in serum-free MEBM-2 (Lonza). Cells were plated at the indicated density and from 1,000–10,000 cells/ml in subsequent passage. Sphere formation was assessed 2 weeks after seeding the cells.
Flow cytometric analysis and Fluorescence-activated cell sorting (FACS)
Primary ovarian tumor/ascites or cell lines single cell suspensions were counted and incubated with primary antibodies (antibody information for all experiments provided in supplemental Table 1). ALDH+ enzymatic activity was defined using the ALDEFLUOR kit per protocol (Stem Cell Technologies, Vancouver, BC, Canada). For each sample ½ of cell/substrate mixture was treated with 50 mmol/L diethylaminobenzaldehyde (DEAB). Cells were incubated for 45 min. Gating was established using Propidium Iodide (PI)-exclusion for viability and ALDEFLUOR/DEAB treated cells were used to define negative gates. FACS was performed with ≥1×105 cells using the BD FACSCanto II (Becton Dickinson, San Diego, USA) or FACSAria (Becton Dickinson) under low pressure in the absence of UV light.
Chemotherapy Resistance of ALDH+ cells
1×106th SKOV3 cells were plated in triplicate in DMEM-10% FBS overnight. Cells were treated with Cisplatin (0.1–3μg/ml SICOR pharmaceuticals Inc., Irvine, CA). After 72 hours cells were harvested and FACS analyzed with PI/ALDEFLUOR assay as described. Alternatively, ALDH+ and ALDH− SKOV3 cells were FACS isolated, allowed to recover for ~48 hours, counted, and then ~200,000 cells were plated in replicate. Cells were treated with 1.5μg/ml of cisplatin or media alone for 72 hours. Number of viable cells was then determined using the Countess automated cell counter (Invitrogen, Carlsbad, CA, USA) 3, 7, and 14 days after cisplatin therapy and plotted as a percentage of initial cell input.
Human tumor xenotransplants
Animals were housed in the University of Michigan Unit for Laboratory Animal Medicine and protocols were approved under the University Committee on the Use and Care of Animals. Tumor cells were FACS isolated, resuspended (1:2) in PBS:Matrigel (BD Biosciences, San Jose, CA, USA) and implanted subcutaneously into the axillas of NOD/SCID/IL-2Rγnull (NOG) mice (18–20). Animals were euthanized when the tumors were approximately 0.5–1.0 cm in the largest diameter unless otherwise indicated. Tumors were resected, weighed, and a portion of each tumor was snap frozen for histological analysis. The remaining portion was processed into a single cell suspension as described. Tumor volumes were calculated using the LxWxW/2 formula. Tumor weights were compared using a student’s T-test. Tumor growth curves were compared using ANOVA and a student’s T-test.
For in vivo serial passaging, single cell suspensions of ALDH+ and ALDH− cell initiated tumors (SKOV3-1000 cells and Hey-1 100 cells) were sorted into ALDH− and ALDH+ fractions and re-injected into mice. n=4 for each sorted cell subtype. Four passages were performed.
A2780, Ovcar8, and PEO4 cell lines were provided by Susan Murphy (Duke University). All others were provided by Rebecca Liu (University of Michigan).
For passaging of human tumor xenografts, tumors were resected and processed as above. 5000 ALDH+ or ALDH− cells were FACS isolated and then re-implanted into NOG mice as described. For ALDH+CD133+ cell derived tumors, ~1000–2000 cells for each ALDH+/−CD133+/− population were reimplanted into NOG mice.
Immunohistochemistry (IHC) and microvascular density assessment
Fresh tumors were harvested in linear growth phase (~500 mm3) embedded in OCT-Compound (Tissue-Tek, CA, USA) and snap frozen. 7 μm cryosections were processed as previously described (16). Primary anti-mouse CD31 (1:600) and anti-mouse CD105 (1:200) (supplemental Table 1) were incubated for 2 hours at 20°C. Slides were processed using the Envision system (Dako) per protocol. CD31 and CD105 IHC were performed on 7–11 independent sections of 4 independent ALDH+ or ALDH− cell derived tumors (Hey-1, SKOV3), or ALDH−CD133+, ALDH+CD133+, ALDH+CD133− or ALDH−CD133− cell derived tumors (A2780-DK). Microvascular density assessment was then performed as previously described (16). Images were captured on an Olympus BX41 (Pennsylvania, USA) fluorescent microscope with a 12 MB digital camera at 16 bit depth/300 dpi. Total stain area/low power field (100X) as defined by pixel area (X:Y 1:1) and hue, was assessed using Olympus Microsuite Biological Suite software and compared between groups using a two-sided student’s t-test.
ALDH+CD133+ Expression in the ovarian tissue microarray
Confirmed, formalin-fixed, paraffin-embedded ovarian epithelial tumors were obtained from the Department of Pathology, University of Michigan. Cores were obtained from the most viable/non-necrotic areas of the tumor. Each tumor had 3–7 independent cores. A TMA was constructed from 56 ovarian cancer patients, with staging surgery between 1995 and 2002 (7 (12.5%), 6 (10.7%), 37 (66.1%), and 6 (10.7%) patients with stage I, II, III, and IV disease, respectively), median age 58 years (minimum: 30, maximum: 84).
TMA sections were subjected to microwave epitope retrieval in 1 mM EDTA, pH8, washed and blocked with Background-Sniper (BioCare Medical, Concord, CA) for 30 minutes. Primary antibody (anti-Aldehyde dehydrogenase (1:100) and anti-CD133 (1:100)) was incubated overnight at 4°C. Slides were then washed and incubated with goat anti-rabbit IgG (1:200) and goat anti-mouse IgG (1:200) in background-sniper for 60 minutes at 20°C. The slides were washed, stained with 4′,6-diaminodo-2-phenylindole and mounted (ProLong Gold, Molecular probes, Carpinteria, CA). Monochrome images of each TMA core were captured with an Olympus BX51 microscope at 3 different extinction/emission wavelengths (DAPI, AF488 and AF648), artificially colored and combined to form images.
Tumors were scored as ALDH−CD133−, ALDH+CD133−, ALDH+CD133+, or ALDH-CD133+ (see below). Only cells associated with tumor islets were scored; stromal and vascular staining were not evaluated. The product limit method of Kaplan and Meier was used to estimate overall and recurrence-free survival. Follow-up time was calculated from the date of diagnosis (staging surgery) until the date of death or first disease recurrence for the endpoints, respectively. Patients not reaching endpoint/s were censored on their last known follow-up date. Comparisons in the estimated overall or recurrence-free survival by CD133 and ALDH expression groups were conducted using the Log-rank test statistic, p-values ≤ 5% suggest meaningful differences between groups.
Results
Characterization of ALDH Activity in Human Ovarian Tumors and Ovarian Tumor Cell Lines
Previous studies reported that CD117/CD44, CD133, CD90, CD24, CD44 and ALDH may identify cancer stem cells in ovarian or other solid tumors (3–5, 21) (8, 22–28). We analyzed more than 13 primary human ovarian tumors and 5 ascites specimens for these tumor markers. ALDH was the only marker detectable in all primary tumor and ascites (Table 1, representative gating provided in Supplemental Figure 1). CD133 expression was detected in 9/13 primary tumors. Higher levels of CD133+ cells were present in ovarian tumor ascites. CD117 was present in only 5/11 tumors tested. In contrast, CD44 was expressed at very high levels in all of the tumors tested. CD24 and CD90 demonstrated variable expression.
Table 1.
Expression of putative CSC markers in human ovarian tumor samples and cell lines.
| Primary Tumor | % ALDH+ | % CD133+ | % CD44+ | % CD117+ | % CD90+ | % CD24+ |
|---|---|---|---|---|---|---|
| Pt 32 | 7.78 | 5.49 | 96.30 | NA | NA | 6.04 |
| Pt 55 | 5.17 | 1.41 | 97.60 | 0.65 | 4.61 | 3.70 |
| Pt 63 | 2.59 | 6.35 | NA | NA | NA | NA |
| Pt 75 | 2.81 | 5.62 | 92.90 | 0.23 | 86.00 | 4.16 |
| Pt 87 | 4.64 | 6.01 | 78.70 | 4.28 | 97.40 | NA |
| Pt 91 | 0.25 | 0 | 10.00 | 0 | 0.93 | 38.30 |
| Pt 94 | 2.23 | 3.60 | 91.50 | 1.40 | 95.40 | NA |
| Pt 106 | 4.31 | 16.60 | 22.51 | 6.37 | 1.98 | 28.08 |
| Pt 107 | 0.40 | 0 | 77.11 | 0 | 1.56 | 3.11 |
| Pt 109 | 2.08 | 21.10 | 70.30 | 0 | NA | 20.91 |
| Pt 111 | 5.38 | 1.00 | 48.30 | 0 | 0 | 33.70 |
| Pt 112 | 1.45 | 0 | 39.50 | 0 | 37.10 | 53.70 |
| Pt 118 | 3.47 | 0 | 72.70 | 0 | 0 | 4.07 |
| Ascites | ||||||
|
| ||||||
| Pt 32 | 5.70 | 39.30 | 17.60 | 0 | 4.00 | 7.00 |
| Pt 55 | 6.79 | 51.60 | 32.30 | 0 | 1.73 | 9.00 |
| Pt 94 | 1.50 | 1.38 | 41.00 | 6.89 | 7.40 | 16.70 |
| Pt 118 | 5.70 | 1.40 | 40.00 | NA | 0 | 5.20 |
| Pt 122 | 3.92 | 4.15 | 1.06 | NA | 1.92 | 9.21 |
| Cell lines | ||||||
|
| ||||||
| A2008 | 1.47 | 0 | 90.00 | 0 | 0 | 74.00 |
| SKOV3 | 4.19 | 0.33 | 90.00 | 0 | 6.80 | 6.60 |
| Hey-1 | 6.51 | 0 | 99.70 | 0 | 0 | 4.80 |
| A2780 | 9.00 | 10.01 | 0 | 78.43 | 80.30 | 81.02 |
| OVCAR8 | 1.90 | 40.15 | 7.80 | 40.76 | 34.32 | 49.30 |
| OVCAR3 | 4.91 | 0 | 99.30 | 0 | 0 | 5.30 |
| OVCAR432 | 1.49 | 0 | 5.25 | 0 | 93.00 | 0 |
We also characterized the expression of these CSC markers in human ovarian cancer cell lines. Once again ALDH was the only marker present in all cell lines in a limited sub-population of cells (Table 1). At least one cell line expressed very high levels (≥40%) of each of the other putative stem cell makers. This may be due to the fact that cell lines may have increased ‘stemness’ associated with in vitro growth selection and passage necessary for the generation of cell lines.
ALDH Activity Identifies CSC Like Cells in Ovarian Tumor Cell Lines
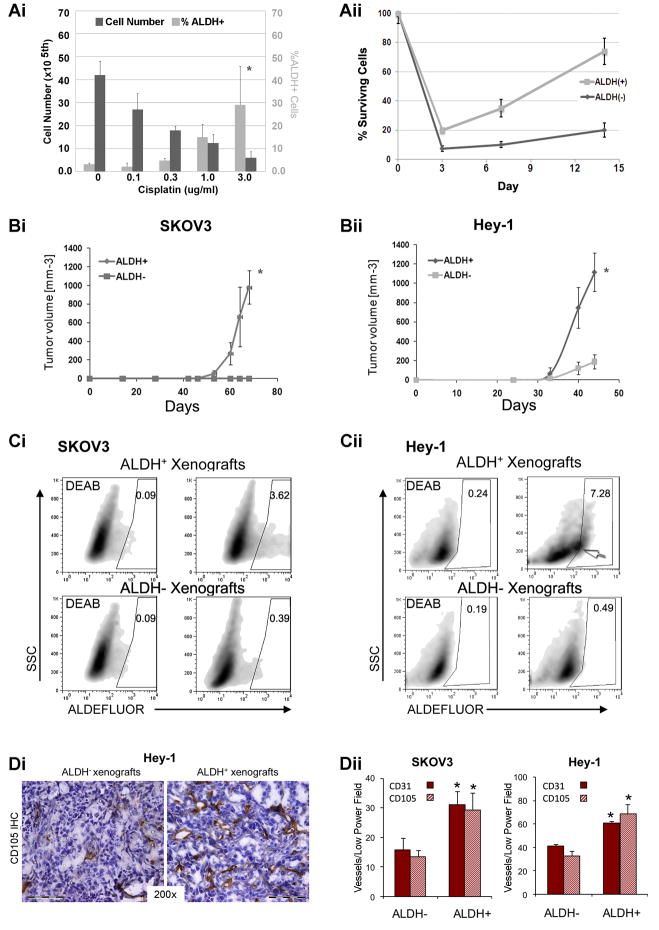
From our studies, ALDH was the only potential stem cell marker expressed in all primary tumor specimens and detected in limited cellular sub-population of human primary tumor cells (0.25–7.78%, Table 1). This suggests that ALDH may be a useful CSC marker in ovarian cancer. One of the proposed characteristics of cancer stem cells is chemo-resistance. We therefore assayed the percentage of ALDH+ cells after treatment with increasing doses of cisplatin. While there was a clear, dose dependent decrease in the total number of viable cells, we observed a significant increase in the percentage of ALDH+ cells (Figure 1Ai), suggesting chemo-resistance of ALDH+ cells and/or the induction of ALDH by cisplatin. We next treated FACS isolated ALDH+ and ALDH− SKOV3 cells with PBS or cisplatin. We observed no differences in the growth of PBS treated ALDH+ or ALDH− cells (data not shown). Cisplatin treatment induced cell death in both ALDH+ and ALDH− cells cell populations. However ALDH+ cells demonstrated greater viability and recovered from cisplatin treatment more rapidly than ALDH− cells, suggesting relative chemo-resistance ALDH+ vs. ALDH− cells (Figure 1Aii).
Figure 1. In vitro and in vivo outgrowth of ALDH+ cells human ovarian cancer cell lines.
(Ai) Absolute cell number and %ALDH+ SKOV3 cells following treatment with indicated concentrations of Cisplatin. (Aii) Percent viable FACS sorted ALDH− and ALDH+ cells prior to and 3, 7, or 14 days following treatment with 1.5 μg/ml Cisplatin. (Bi and ii) Tumor growth curves of 100 FACS isolated ALDH+ and ALDH− SKOV3 and Hey1 cells. (Ci and ii) ALDEFLUOR staining of ALDH+ and ALDH− SKOV3 and Hey1 tumor xenografts with DEAB controls. (D) CD105 IHC from Hey-1 ALDH− and ALDH+ xenografts tumors. (E) Quantification of CD31+ and CD105+ microvascular density in the indicated tumor xenografts. Scale bar indicates 100μm. All data is representative of at least 2 independent experiments (n=5 tumors/experiment). * Indicates p<0.01 versus controls.
A primary characteristic of CSC is the ability to initiate tumors with limited numbers of cells. We next tested the cancer initiating capability of ALDH+ cells in mice. In 3 cell lines tested (SKOV3, HEY1--Figure 1B, and OVCAR8 data not shown), ALDH+ cells generated larger tumors at a significantly faster rate than equal numbers of ALDH− cells. As few as 100 ALDH+ SKOV3 or HEY1 cells formed tumors while similar numbers of ALDH− cells did not, or rarely formed tumors respectively (Table 2). FACS analysis of 1000 ALDH+ vs. ALDH− cells derived tumors demonstrated that tumors derived from ALDH+ cells had both ALDH+ and ALDH− cells in the same ratios as observed in the original tumor cell line. Tumors derived from 1000 ALDH− cells had 10–20 fold reduction in the number or ALDH+ cells (Figure 1C). Both SKOV3 and HEY1 ALDH+ cells successfully generated tumors over four consecutive serial passages, whereas ALDH− tumors were only able to passage twice.
Table 2.
Tumor initiating capacity of limiting dilutions of ALDH+ and ALDH− cells from the SKOV3 and Hey1 ovarian cancer cell lines and nine primary human ovarian tumors.
| Cell | SKOV3 Cell Line | Hey1 Cell Line | Primary Human Tumors | |||
|---|---|---|---|---|---|---|
| Cell type | ALDH+ | ALDH− | ALDH+ | ALDH− | ALDH+ | ALDH− |
| # Cells Injected | Tumors Formed | Tumors Formed | Tumors Formed | |||
| 100 | 4/4 | 0/4 | 4/5 | 1/5 | 0/5 | 0/5 |
| 300 | 4/5 | 0/10 | 10/10 | 4/10 | ND | ND |
| 1000 | 10/10 | 4/10 | 10/10 | 8/10 | 2/9 | 0/9 |
| 50000 | ND | ND | ND | ND | ND | 0/9 |
Finally, CSC are reported to have increased angiogenic capacity (29, 30). Immunohistochemical analysis of the ALDH+ derived tumors, compared to ALDH− derived tumors, demonstrated significantly (2 fold) increased microvascular density. This was true for both the pan-endothelial marker CD31 or the angiogenic endothelial marker CD105 (31) (Fig 1D). FACS analysis of ALDH+ and ALDH− cell derived tumors revealed a similar increase in the numbers of CD31+ and CD105+ cells (data not shown). Taken together our data in ovarian cancer cell lines suggest ALDH identifies a population of cells enriched for a CSC phenotype; ALDH+ cells are chemoresistant, angiogenic cells with significant tumor initiating capacity.
ALDH+ Cells from Human Ovarian Cancers Initiate Tumors in Mice
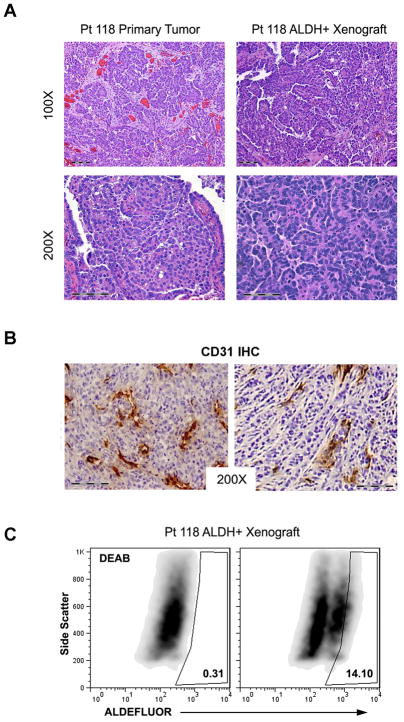
Given our findings in cell lines, we tested the ability of ALDH+ cells isolated from human tumors to generate tumors in mice; the ultimate proof of a putative human CSC. While 50,000 ALDH− cells did not grow tumors, 1000 ALDH+ cells generated tumors from 2/9 primary tumors (Table 2). Tumor generation required approximately ~9 months. Histological analysis of the xenograft tumors revealed well differentiated papillary-serous tumors identical to that seen in the primary tumor (Figure 2A). FACS analysis of tumor xenografts revealed robust ALDH+ and ALDH− cell populations (Figure 2C). Similar to cell line studies ALDH+ cell derived tumors were highly vascular (Figure 2B). We have successfully passaged ALDH+ cells from the Pt118 tumor three times. These data suggest ALDH activity identifies a population of ovarian cancer cells enriched for CSC activity.
Figure 2. In vivo tumor generation from FACS isolated ALDH+ primary human epithelial ovarian tumor cells.
(A) Histology of primary tumor and tumor xenograft generated from ALDH+ cells isolated from Pt118’s tumor. (B) CD31 IHC analysis of primary ovarian ALDH+ cell derived tumor xenografts. (C) FACS analysis demonstrating DEAB control (left) and ALDEFLUOR activity (right) from Pt 118 ALDH+ cell tumor xenograft second passage.
Tumorigenicity of Cell Lines Co-Expressing ALDH and CD133
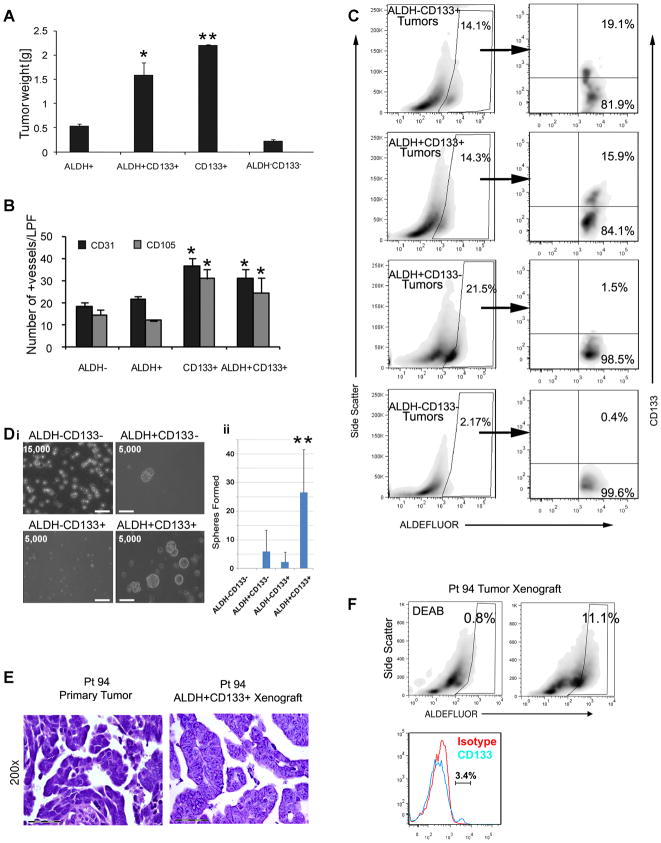
In the hematopoietic system ALDH used in combination with CD133 improves the identification of stem cells. We next examined the role ALDH in combination with CD133in ovarian tumors. FACS confirmed the presence of rare ALDH+CD133+ cells in several cell lines (Supplemental Table 2). Using limiting dilution studies of A2780 cells we observed similar tumor initiation capacity of both ALDH+CD133+ and ALDH−CD133+ cells. In contrast, ALDH+CD133− and ALDH−CD133− cells had limited tumor initiation capacity at the lowest cell numbers (Table 3). Interestingly, the ALDH−CD133+ cells generated larger tumors faster than ALDH+CD133+ cells, and ALDH+CD133+ cell derived tumors were significantly larger than ALDH+CD133− and ALDH−CD133− cell tumors (Figure 3A). ALDH−CD133+ and ALDH+CD133+ cell tumors both demonstrated increased microvascular density compared to ALDH+CD133− and ALDH−CD133− tumors (Figure 3B and supplemental Figure 2). FACS analysis of both ALDH−CD133+ and ALDH+CD133+ cell derived tumors recapitulated the cellular populations observed in the primary cell line giving rise to all four CD133+/−ALDH+/−cell populations. In contrast, ALDH+CD133− or ALDH−CD133− cell xenografts had little or no capacity to generate CD133+ cells (Figure 3C). This observation indicates that ALDH+CD133− cells and ALDH−CD133− cells must be downstream of CD133+ expressing cells in a differentiation pathway. Similar results were obtained with the OVCAR-8 cell line (data not shown). These studies suggest a potential hierarchy of angiogenic ovarian cancer stem/progenitor cells based on ALDH activity and CD133 expression.
Table 3.
Tumor initiating capacity of limiting dilutions of ALDH+/−CD133+/− cells from the A2780 ovarian cancer cell line and nine primary human ovarian tumors.
| A2780 Cell Line | ||||
|---|---|---|---|---|
| Cell Type | ALDH−CD133+ | ALDH+CD133+ | ALDH+CD133− | ALDH−CD133− |
| # Cell Injected | Tumors Formed | |||
| 30 | 4/6 | 4/6 | 0/5 | 0/5 |
| 100 | 5/5 | 5/5 | 3/6 | 0/5 |
| 1000 | 10/10 | 10/10 | 10/10 | 7/10 |
| Primary Human Tumor Samples | ||||
| Cell Type | ALDH−CD133+ | ALDH+CD133+ | ALDH+CD133− | ALDH−CD133− |
| Tumors Formed | ||||
| # Cell Injected | ||||
| 10–500 | 0/9 | 4/9 | 0/9 | ND |
| 5000 | ND | ND | 0/5 | 0/9 |
| 50000 | ND | ND | ND | 0/9 |
Figure 3. Characterization of ALDH+CD133+ cells as CSC in ovarian tumor cell lines and primary human epithelial ovarian tumors.
(A) Tumor weights from 1000 cells of the indicated FACS isolated A2780 tumor cell populations (n=5/group). (B) Quantification of CD31+ and CD105+ microvascular density (vessels/low power field) of the indicated A2780 cell line populations. (C) FACS analysis of indicated A2780 xenografts for expression ALDH, and CD133 in ALDH+ gate. (Di) Spheres generated from FACS isolated ALDH−/+CD133+/− cells from a primary ovarian tumor sample. Cell numbers plated at the initiation of the sphere assay are indicated. Scale bar (lower left) indicates 100μm. (ii) Average sphere formation in the indicated cell population from 8 different patients. (E) Histology of Pt94 primary tumor and tumor xenograft generated from ALDH+CD133+ isolated from Pt94. (F) FACS analysis of Pt 94 tumor, left panel demonstrates DEAB control, middle demonstrates ALDEFLUOR activity in Pt 94 xenograft, right demonstrates histogram for CD133 with isotype control. *indicates p<0.05 compared to ALDH+CD133− and ALDH−CD133− groups, ** indicates p<0.05 versus all other groups.
ALDH+CD133+ Cells from Human Ovarian Cancers have a CSC Phenotype and have an Increased Ability to Initiate Tumors in Mice
Given these findings, we assessed if the combination of ALDH activity and CD133 expression in primary human tumors could improve the isolation of ovarian CSC. Analysis of CD133+ primary human ovarian tumors demonstrated a small percentage of cells which co-expressed ALDH activity and CD133 (~0.1–0.01%, Supplemental Table 3). We used the tumor sphere assay to analyze the phenotype of the primary ALDH+/−CD133+/− cell populations from 8 patients. When ~5000 cells were analyzed, from 6/8 patients we observed significantly greater numbers of spheres in the ALDH+CD133+ cell population (Figure 3D and supplemental Figure 3). In the other two patient samples we observed preferential sphere formation in the ALDH+CD133− cells and the CD133+ALDH− cells respectively (supplemental Figure 3). ALDH−CD133− negative cells were only able to generate spheres when 100,000 cells were assayed. These spheres were unable to passage suggesting these were adhesive cell clusters rather than true spheres.
We next tested the in vivo cancer initiating ability of ALDH+/−CD133+/− cells from primary human ovarian tumors. From 9 primary tumors tested, we were able to successfully generate tumors from 11, 500, 500 and 500 ALDH+CD133+ cells from four patients (Pt 32, Pt55, Pt94, and Pt171 respectively, Table 3). All four of these patient samples demonstrated greatest tumor sphere generation in the ALDH+CD133+ cell populations (supplemental Figure 3). Tumor growth required 4–7 months. Tumors were not obtained from similar numbers of ALDH−CD133+ cells, 5,000 ALDH+CD133−, or 50,000 ALDH−CD133− cells isolated simultaneously from the same tumor samples.
Histological analysis of the xenografts revealed highly vascular, high grade papillary-serous carcinomas which mirrored that of the primary tumor (Figure 3E and data not shown). FACS analysis of these tumors revealed that, ALDH+CD133+ cells were able to generate tumors with ALDH+/−CD133+/− cells (Figure 3F). ALDH+CD133+ cell derived tumors from two patient’s samples have successfully passaged 4 times. A third is currently in first passage. These studies indicate that in CD133 positive human ovarian tumors, cancer stem cells may be identified by the dual expression of ALDH and CD133.
The Presence of ALDH+CD133+ cells predicts clinical outcome
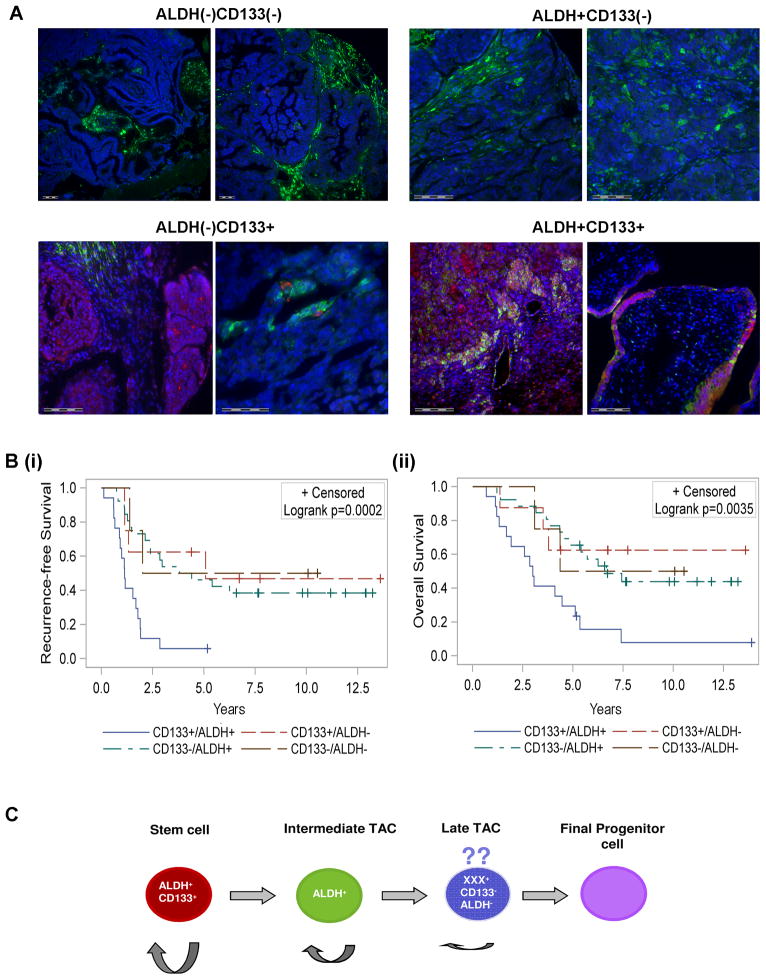
As ALDH+CD133+ cells demonstrated greater sphere forming potential, greater engraftment potential, and more rapid tumor generation than ALDH+CD133− tumors, we speculated ALDH+CD133+ cells may identify a more aggressive tumor phenotype. We therefore scored a panel of 56 ovarian tumors for the presence of ALDH and CD133 expressing cells using immunofluorescence. Tumors were scored as (1) ALDH−CD133− (no ALDH or CD133 expression detectable in tumor islets), (2) ALDH+CD133− (ALDH expression but no CD133 expression in tumor islets), (3) ALDH+CD133+ (any tumor with more than one ALDH+CD133+ cell/section) or (4) ALDH−CD133+ (any tumor without ALDH+CD133+ cells in the tumor islet, and at least 1 ALDH−CD133+ tumor cell) (Figure 4A). Stromal and vascular staining were excluded. While we did not observe any correlation with tumor stage, grade, or platinum resistance, Kaplan-Meier analysis demonstrated that patients with ALDH+CD133+ tumor cells have worse progression free and overall survival compared to the other groups individually or combined (Figure 4B).
Figure 4. ALDH+CD133+ cells identify poor risk tumors.
(A) Representative Immunofluorescent classification of ovarian tumors as ALDH+/−CD133+/−; ALDH is stained in green and CD133 in red. Scale bars indicate 100 micrometers. (Bi and ii) Kaplan-Meier analysis of overall and recurrence free survival in 56 patients whose tumors were scored as either ALDH−CD133− (n=4), ALDH+CD133− (n=26), ALDH−CD133+ (n=8), or ALDH+CD133+ (n=18). See methods for scoring methodology. (C) Proposed model for ovarian cancer stem cell differentiation. We speculate an ALDH+CD133+ CSC gives rise to an ALDH+CD133− transient amplifying cell (TAC). This cell subsequently gives rise to an ALDH−CD133− late TAC. The ability to self replicate is indicated by semi-circular arrow, with cells on the left having greatest self-renewal capacity and cells to the right having minimal self-renewal capacity.
Discussion
ALDH as a Marker of Ovarian CSC
We present here a thorough analysis of ALDH activity, alone and in combination with CD133, as an ovarian CSC marker. Consistent with the cancer stem cell hypothesis, (1) ALDH activity is present in a small percentage of ovarian tumor cells, (2) small numbers of ALDH+ cells and ALDH+CD133+ cells are capable of tumor initiation and propagation, and (3) these cells generate tumors which recapitulate the original tumor cell composition. In addition these cells demonstrate resistance to chemotherapy and increased angiogenic capacity. Importantly ALDH+ cells were identified in all tumor cell lines and primary human tumor samples analyzed. While CD133 appears to be in an important ovarian CSC marker, it is undetectable in approximately half of ovarian cancers. ALDH offers a means to isolate ovarian CSC in CD133− tumors.
This study is the first in ovarian cancer to successfully generate in vivo tumors from a putative stem cell population directly isolated from human tumors. Other studies of ovarian CSC studied cell lines, used in vitro culture prior to isolation and growth in vivo, or injected total tumor cell suspensions for growth in vivo followed by isolation of stem cells from primary xenografts (4, 5, 32, 33). While representing important tools, these manipulations could lead to genetic/phenotypic changes in the CSC population.
Using ALDH and CD133 to Define a Hierarchy of Ovarian CSC
In CD133+ tumors, the combination of ALDH and CD133 appears to enhance ovarian CSC isolation. As few as 11 primary human ALDH+CD133+ cells generated tumors in vivo. In addition we observed an increased tumor engraftment rate of ALDH+CD133+ compared to ALDH+CD133− cells. ALDH+CD133+ cell derived tumors, in both patient samples and cell lines, were able to generate all ALDH+/−CD133+/− cellular populations. In contrast ALDH+CD133− cell derived tumors from both patients and cell lines did not generate CD133+ cells. This implies that CD133+ cells are ‘upstream’ of CD133− cells in a potential differentiation pathway.
Taking all of our observations together we hypothesize a hierarchical model of ovarian CSC differentiation (Figure 4C). In this model ALDH+CD133+ cells with self-renewal capacity give rise to ALDH+CD133− cells with limited self renewal capacity, and ultimately give rise to the differentiated ALDH−CD133− cells. We propose that ovarian CSC can arise from any of the cellular populations with self-renewal capacity. Given that we observed some tumor generation in the CD133−ALDH− cellular compartment of cell lines, we postulate that there may an ovarian CSC/progenitor which lacks appreciable expression of either CD133 or ALDH. Alternatively, this may be an artifact of tumor cell lines, or due to to trace ALDH+CD133+ cellular contamination of the ALDH−CD133− cells.
The combination of ALDH and CD133 to enhance stem cell identification is parallel to the hematopoietic system (34–39). Our model is consistent with hematologic malignancies where partially differentiated progenitor cells with self-renewal capacity can manifest stem cell properties (40–42). Such a model accounts for the variability observed in patients and ovarian cancer cell lines. The expression of other reported ovarian CSC markers, including CD24, CD44, and CD117 in relation to ALDH and CD133 remains to be determined. It seems likely given the heterogeneity of ovarian tumors, that there may be other distinct stem cell populations.
Based on our model we would predict that tumors derived from early stem cells (i.e. ALDH+CD133+ cells) would portend a poorer prognosis, while tumors driven by stem cells derived from more differentiated cells (ALDH+CD133−) would have a better outcome. In our study, while the presence of ALDH+CD133+ cells did not correlate with tumor grade, the presence of ALDH+CD133+ cells strongly correlated with poor outcome. Consistent with our model, increased ALDH expression in ovarian tumor islets, has been associated with improved prognosis (43). However, the role of ALDH alone as a prognostic factor is controversial with a new report suggesting increased ALDH expression indicates poor prognosis in ovarian cancer (44). It is possible that stratification of ALDH expression based on CD133 co-expression could explain this discrepancy.
ALDH−CD133+ in Ovarian Cancer
The role of ALDH−CD133+ cells remains uncertain. In primary specimens, ALDH−CD133+ cells did not generate significant numbers of spheres and did not generate tumors. In cell lines ALDH−CD133+ cells grew more rapidly than ALDH+CD133+ cells, and had similar tumor initiating capacity as the ALDH+CD133+ cells at low cellular concentrations. ALDH−CD133+ cell derived tumors generated all ALDH+/−CD133+/− cellular populations. Insensitivity of the ALDEFLUOR assay and conservative ALDH+ gating may allow for ALDH ‘dim’ cells within the ALDH−CD133+ population of cells may explain these observations. However ALDH−CD133+ cells were clearly observed with immunofluorescent analysis. It is possible that ALDH−CD133+ cells isolated from human tumors may not tolerate the stressful conditions associated with FACS isolation. Finally, ALDH−CD133+ cells isolated from whole tumor cellular suspensions may be predominantly non-tumor cells such as endothelial progenitors, diluting the number of ALDH−CD133+ tumor cells. Cell line studies would suggest a close relationship between ALDH−CD133+ and ALDH+CD133+ cells. Further studies are necessary to clarify the role of ALDH−CD133+ cells.
Improving Ovarian CSC Growth in vivo
Our study is the first to demonstrate the generation of tumor xenografts from CSC directly isolated from primary ovarian carcinoma. However, the tumor generation rate for cells isolated from primary human ovarian tumors was low (20–40%). This could be secondary to FACS induced cellular toxicity. Alternatively, the subcutaneous tumor inoculation may be an inferior location for tumor growth; only 50% of glioblastoma stem cells engrafted when implanted subcutaneously, while 100% engraftment was obtained when these cells were orthotopically implanted into the cranium (45). Likewise, increased tumorigenesis was obtained when pancreatic cancer stem cells were injected into the pancreas as opposed to a subcutaneous site (46). Finally, use of a ‘humanized’ microenvironment significantly enhanced engraftment of breast CSC (27). Thus future studies using orthotopic CSC injection or a humanized microenvironment for ovarian CSC may increase engraftment rates.
Conclusion
Collectively, our data indicates that ALDH is a marker of CSC in some ovarian tumors which lack CD133 expression. In tumors which express CD133, the combination of ALDH and CD133 can be used to identify a more aggressive CSC suggesting a potential hierarchy of cells with distinct cancer growth potential. Based upon this we propose a model of ovarian cancer stem cell differentiation similar to that observed for hematopoietic cells.
Supplementary Material
Acknowledgments
We thank the members of the Tissue Procurement, Flow Cytometry, and Microarray cores. The A2780 and OVCAR8 cell lines were provided by Dr S. Murphy (Duke University). This work was generously supported by the Damon Runyon Cancer Research Foundation Clinical Investigator Award, and the NIH New Investigator Innovator Award grant#00440377.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report
References
- 1.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. Journal of Clinical Oncology. 2008;26:2795–9. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Balch C, Chan MW, Lai H-C, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Research. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–18. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 5.Curley MD, Therrien VA, CL C. CD133 Expression Defines a Tumor Initiating Cell Population in Primary Human Ovarian Cancer. Stem Cells. 2009 doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 6.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH, Gao MQ, et al. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 29:2672–80. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Dombkowski D, Meirelles K, Pieretti-Vanmarcke R, Szotek PP, Chang HL, et al. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci U S A. 2010;107:18874–9. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ran D, Schubert M, Pietsch L, Taubert I, Wuchter P, Eckstein V, et al. Aldehyde dehydrogenase activity among primary leukemia cells is associated with stem cell features and correlates with adverse clinical outcomes. Experimental Hematology. 2009;37:1423–34. doi: 10.1016/j.exphem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Molecular Cancer Research: MCR. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y-C, Chen Y-W, Hsu H-S, Tseng L-M, Huang P-I, Lu K-H, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochemical & Biophysical Research Communications. 2009;385:307–13. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma S, Chan KW, Lee TK-W, Tang KH, Wo JY-H, Zheng B-J, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Molecular Cancer Research: MCR. 2008;6:1146–53. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 16.Pulaski HL, Spahlinger G, Silva IA, McLean K, Kueck AS, Reynolds RK, et al. Identifying alemtuzumab as an anti-myeloid cell antiangiogenic therapy for the treatment of ovarian cancer. Journal of Translational Medicine. 2009;7:49. doi: 10.1186/1479-5876-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topley P, Jenkins DC, Jessup EA, Stables JN. Effect of reconstituted basement membrane components on the growth of a panel of human tumour cell lines in nude mice. Br J Cancer. 1993;67:953–8. doi: 10.1038/bjc.1993.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM. Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer. 1993;68:909–15. doi: 10.1038/bjc.1993.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–8. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- 21.Caunt M, Mak J, Liang W-C, Stawicki S, Pan Q, Tong RK, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–42. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE [Electronic Resource] :e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69:991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–9. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 26.Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW, Zoeller M. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008;8:784–804. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Research. 2009;69:8208–15. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Research. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931–7. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 32.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 33.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE. Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the ALDH-bright cell population of cryopreserved, banked UC blood. Cytotherapy. 2007;9:569–76. doi: 10.1080/14653240701466347. [DOI] [PubMed] [Google Scholar]
- 35.Christ O, Lucke K, Imren S, Leung K, Hamilton M, Eaves A, et al. Improved purification of hematopoietic stem cells based on their elevated aldehyde dehydrogenase activity. Haematologica. 2007;92:1165–72. doi: 10.3324/haematol.11366. [DOI] [PubMed] [Google Scholar]
- 36.Pearce DJ, Bonnet D. The combined use of Hoechst efflux ability and aldehyde dehydrogenase activity to identify murine and human hematopoietic stem cells. Exp Hematol. 2007;35:1437–46. doi: 10.1016/j.exphem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Storms RW, Green PD, Safford KM, Niedzwiecki D, Cogle CR, Colvin OM, et al. Distinct hematopoietic progenitor compartments are delineated by the expression of aldehyde dehydrogenase and CD34. Blood. 2005;106:95–102. doi: 10.1182/blood-2004-09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75:1947–50. [PubMed] [Google Scholar]
- 39.Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–60. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 40.Harris MA, Yang H, Low BE, Mukherjee J, Guha A, Bronson RT, et al. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68:10051–9. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–2. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 42.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Modern Pathology. 2009;22:817–23. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–99. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. Journal of Cellular Biochemistry. 2007;101:805–15. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.