Abstract
Mutated BRAF is detected in ~45% of papillary thyroid cancers (PTC). To model PTC, we bred mice with adult-onset, thyrocyte-specific expression of BRAFV600E. One month following BRAFV600E expression, mice displayed increased thyroid size, widespread alterations in thyroid architecture and dramatic hypothyroidism. Over 1 year, without any deliberate manipulation of tumor suppressor genes, all mice developed PTC displaying nuclear atypia and marker expression characteristic of the human disease. Pharmacological inhibition of MEK1/2 led to decreased thyroid size, restoration of thyroid form and function and inhibition of tumorigenesis. Mice with BRAFV600E-induced PTC will provide an excellent system to study thyroid tumor initiation and progression and the evaluation of inhibitors of oncogenic BRAF signaling.
INTRODUCTION
Thyroid malignancies are the most common tumors of the endocrine system with ~45,000 newly diagnosed cases estimated in USA in 2010 (1). Although thyroid cancer is often indolent, there is concern about its rapidly increasing incidence, especially amongst women (2). Of the various histological sub-types, Papillary Thyroid Carcinoma (PTC) represents ~80% of all cases. Although surgery combined with radioiodine therapy is often curative, a better understanding of how thyroid cancer genetics influences the pathophysiology and therapy of this disease is required.
Of the somatic genetic alterations detected in PTC, mutational activation of BRAF is most common (~45%) and often associated with more aggressive disease (3). As observed in melanoma and colon cancer, the most common mutation is a T1799→A tranversion in exon 15 that encodes BRAFV600E (4). Once mutationally activated, the BRAFV600E→MEK→ERK MAP kinase signaling pathway elicits alterations in gene expression that contribute to the aberrant behavior of the cancer cell. Moreover, recent data suggest BRAFV600E is required for PTC maintenance since pharmacological inhibition of BRAFV600E by PLX-4032 in thyroid cancer patients led to tumor regression (5).
We have previously described the utility of BRafCA mice carrying a Cre-activated allele of BRaf to model lung cancer (6) and melanoma (7). Using mice with thyrocyte-specific expression of a conditional Cre recombinase (CreERT2) under the control of the Thyroglobulin promoter (Thyro::CreERT2), we explored the consequences of induced BRAFV600E expression in adult thyroid. Shortly after BRAFV600E expression, mice displayed signs of hypothyroidism accompanied by striking alterations in the size and architecture of thyroid follicles. Over a 6–9 month period all of these mice developed PTC displaying phenotypic characteristics of the cognate human disease. Moreover, treatment of the mice with a pharmacological MEK inhibitor elicited a striking reduction in thyroid size, restoration of thyroid hormone production and inhibition of tumorigenesis. Importantly, due to leaky activity of CreERT2, untreated Thyro::CreERT2; BRafCA mice developed PTC without displaying hypothyroidism, albeit with delayed kinetics compared to tamoxifen treated mice. These data suggest that, unlike in the lung and skin where BRAFV600E induces a clearly defined stage of benign tumorigenesis, BRAFV600E can promote thyroid cancer progression without deliberate manipulation of tumor suppressor genes. Moreover, this system demonstrates utility in modeling the response of PTC to pharmacological inhibition BRAFV600E→MEK→ERK signaling.
MATERIALS AND METHODS
Mouse breeding and manipulation
BRafCA mice were described previously (6, 7). Thyroglobulin::CreERT2 (Thyro::CreER) mice were generated by conventional transgenic technology and will be described in full elsewhere (Amendola et al., Manuscript in preparation). Thyrocyte specific activation of CreERT2 activity was achieved by intraperitoneal injection of 100μl of a 10mg/ml stock of Tamoxifen dissolved in peanut oil into adult (~30 days old) mice.
Histology and Immunofluorescence of mouse thyroid tissue sections
Mice were euthanized by aortic dissection and thyroids removed, rinsed in ice cold PBS and fixed for 4 hours in Z-Fix (Anatech, MI, USA). 4–5μm sections of formalin fixed, paraffin embedded tissues were stained with Hematoxilin & Eosin or processed for immunofluorescence with epitope unmasking performed by boiling slides for 10 minutes (10mM Tris, 0.5mM EGTA pH 9.0). Primary antibodies were obtained from the listed commercial sources: α-TTF-1 (1:200, Santa Cruz), α-Ki67 (1:300, Abcam), α-CK19 (1:300, TROMA-III, Hybridoma bank, University of Iowa) and α-Galectin-3 (1:200, Abcam), α-HMGA2 (1:700, Biocheck, CA). Primary antibody binding was detected using either goat α-rabbit Alexa-488 (1:500) or goat α-rat Alexa-488 (1:500) (Molecular Probes) and then counter stained with DAPI.
Immunoblotting
Snap frozen thyroid specimens were extracted using a TissueLyser (Qiagen) in 1%(v/v) Triton-X, 20mM Hepes pH=9.0, 150mM NaCl, 10% (v/v) Glycerol, 1mM EDTA, 1mM EGTA buffer supplemented with Halt protease/phosphatase inhibitor cocktail (Pierce). Western blots of cell extracts were probed with α-pERK1/2 or α-total ERK1/2 (Cell Signaling Technology). Primary antibody binding was detected using goat α-rabbit IR800 or goat α-mouse IR680 1:15,000 (Li-Cor Bioscience) and imaged using a LI-COR Odyssey FC imager.
Serum TSH and T4 measurements
Mouse serum (0.5–1ml) was collected from retro-orbital bleeds at the time of euthanasia. Serum TSH and T4 was measured using specific radioimmune assays (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA).
Drug administrations
A suspension of the MEK1/2 inhibitor, PD0325901, was prepared by sonication in 0.5%(w/v) Hydroxy-Propyl-Methylcellulose (Sigma), 0.2%(v/v) Tween-80 that was prepared fresh every week. PD0325901 was administered to mice daily by oral gavage at 12.5mg/kg for 4 weeks. Triiodothyronine (T3 - Sigma) was supplemented into drinking water at 100ng/ml with 1%(w/v) sucrose. Effective daily dose was estimated at 100–200ng/mouse/day based on mouse water consumption of 1–2ml/day/mouse.
Ultrasound imaging
Mice were anesthetized using 2%(v/v) isofluorane and fur around the neck was removed using Veet® depilatory cream. Ultrasound images were collected weekly using the Vevo770 system from VisualSonic. Thyroid size was assessed by counting pixels at the largest diameter of the thyroid using ImageJ (NIH) and converting pixel count into area (mm2) using scaling software internal to the device. Differences in thyroid size were plotted in relation to the area of the largest diameter of the thyroid at the start of the drug administration period (d0) compared to the end (d32).
RESULTS
Expression of BRAFV600E, but not KRASG12D, induces PTC in adult mice
To assess the consequences of BRAFV600E expression in adult mouse thyrocytes, we generated compound Thyroid Peroxidase (TPO)::Cre; BRafCA mice such that expression of BRAFV600E is initiated during thyroid development under the influence of the TPO::Cre transgene (8). Although TPO::Cre; BRafCA mice were born at normal Mendelian frequency, they displayed low birth weight and a general failure to thrive suggesting that BRAFV600E expression had deleterious developmental effects during mouse embryogenesis. Consequently, we discontinued these studies.
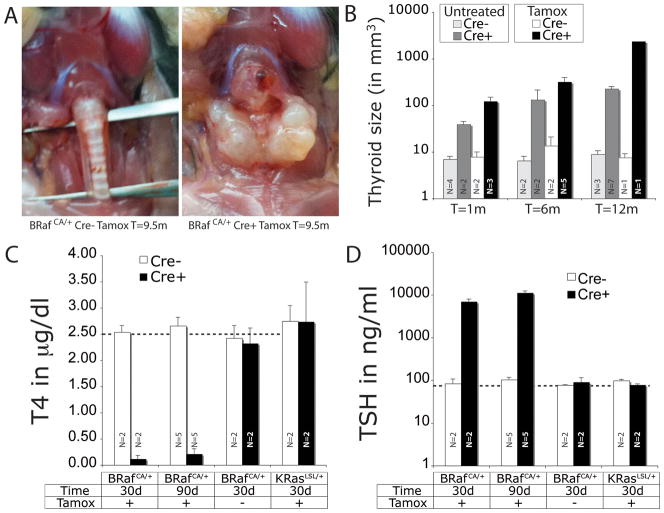
To elicit oncogene expression in the adult thyroid, we utilized Thyro::CreERT2 mice in which expression of CreERT2 is controlled by the thyroid-specific thyroglobulin promoter. Since mutationally activated RAS genes are also detected in 10–15% of PTC, we generated cohorts of Thyro::CreERT2 mice carrying either our Cre activated BRafCA allele or a Cre-activated KRasLSL allele so that we could compare and contrast the effects of oncogenic BRAFV600E to KRASG12D on thyrocytes (9). One-month-old mice of each genotype were administered tamoxifen to induce oncogene expression and then dissected at different times from 1 week to 12 months thereafter. Consistent with previous data, we observed no effect of KRASG12D expression on thyroid size in Thyro::CreERT2; KRasLSL mice at any time, even after repeated tamoxifen administration (Data not shown) (10). By contrast, tamoxifen treated Thyro::CreERT2; BRafCA mice developed a dramatically enlarged, goiterous, hypercellular thyroid that was up to 10 times larger than controls one month and up to 300 times larger 12 months after BRAFV600E expression (Figs. 1A & 1B). Interestingly, untreated Thyro::CreERT2; BRafCA mice also displayed increased thyroid volume, which may be a consequence of stochastic BRAFV600E expression due to leaky CreER activity.
Figure 1. BRAFV600E expression induces goiter which progresses to PTC.
A. Representative images of the thyroid gland from control BRafCA/+ (left) or Thyro:CreERT2; BRafCA/+ mice (right) 9.5 months after tamoxifen administration.
B. Quantification of average thyroid volume of mouse of the indicated genotype either untreated or treated with tamoxifen as indicated. N=number of mice in each group.
C & D. Serum concentration of T4 (C) or TSH (D) in Thyro::CreER mice that also carry either a Cre-activated BRafCA or KRasLSL allele as indicated. Tamoxifen treated (+Tamox) or control (−Tamox) mice were euthanized at 30 or 90 days as indicated and serum samples prepared. Serum T4 or TSH were measured by radioimmune assay as described in Materials and Methods. Dotted lines indicate the concentration of T4 (μg/dl) or TSH (ng/ml) in normal mice. N=number of mice in each group.
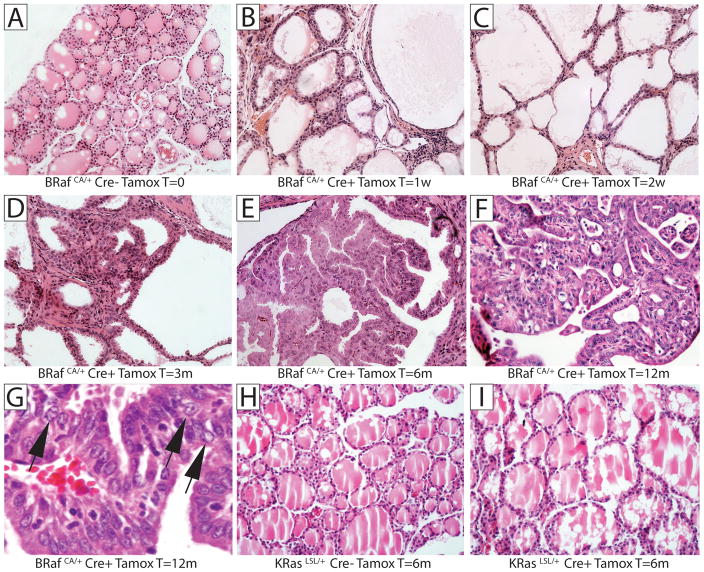
Histological analysis of untreated BRafCA mice revealed normal thyroid architecture with spherical follicles full of eosinophilic colloid similar to human thyroid histology (Fig. 2A). However, within 7 days after tamoxifen treatment, Thyro::CreERT2; BRafCA mice displayed dramatically enlarged follicles throughout the entire gland (Fig. 2B). 14 days after BRAFV600E expression, thyrocytes displayed a squamous morphology accompanied by a large increase in follicle size and a loss of colloid (Fig. 2C). Consistent with the high frequency of LacZ expression in thyrocytes in tamoxifen treated Thyro::CreERT2; Rosa26R mice (data not shown), it is likely that the BRafCA allele was rearranged to encode BRAFV600E in the majority of thyrocytes. Despite the remarkably rapid onset of changes to thyroid architecture, it was unclear exactly when thyrocytes assumed malignant characteristics. At 3 months, occasional foci of hyperplastic tall cells were detected (Fig. 2D). However, the first clear indication of thyroid malignancy was detected 6 months after BRAFV600E expression (Fig. 2E) when nodules of tumor cells displaying a characteristic papillary structure were readily apparent. By 12 months we detected evidence of extensive PTC (Fig. 2F) displaying abnormal cytology including grooved nuclei, nuclear inclusions and cleared nuclei (Fig. 2G, arrowed from left to right respectively), all of which are characteristic features of human PTC. Indeed, at late times following BRAFV600E expression (9.5 to 12 months), mice displayed confluent PTC that involved the majority of the gland. Although we occasionally detected evidence of localized tumor invasion into the surrounding muscle, we did not observe frank metastasis to lymph nodes or distant organs. Moreover, 13 months following BRAFV600E expression, although the mice had readily palpable thyroid tumors, they were not overtly sick and did not require euthanasia. Consistent with the lack of effect of KRASG12D on thyroid size, Thyro::CreER; KRasLSL mice displayed no evidence of histological abnormalities 6 months after tamoxifen treatment (Figs. 2H & 2I), which serves as an ideal control for the specificity of the effects of BRAFV600E in this experiment.
Figure 2. Histological analysis of thyroid tissue.
Control (Cre−) or Thyro::CreER (Cre+) mice carrying either a Cre-activated BRafCA or KRasLSL alleles were either untreated or treated with tamoxifen as indicated. At different times thereafter (1 week – 12 months), mice were euthanized for analysis of formaldehyde fixed, paraffin embedded (FFPE) thyroid tissue by Hematoxylin and Eosin (H&E) staining. A: Control BRafCA mice, B–G: Tamoxifen treated Thyro::CreER; BRafCA mice analyzed: 1 week (B); 2 weeks (C); 3 months (D); 6 months (E) or; 12 months (F & G) after tamoxifen treatment. H: Control KRasLSL mice. I: Thyro::CreER; KRasLSL mice analyzed 6 months after tamoxifen treatment. Magnification is 200x with the exception of G, which is 400x
These data indicate that, whereas KRASG12D was largely without effect, expression of BRAFV600E in thyrocytes of adult mice initiated the process of thyroid carcinogenesis. Moreover, unlike the lung epithelium or melanocytes where expression of BRAFV600E elicits benign tumors, expression of BRAFV600E in the thyroid epithelium resulted in malignancy with 100% penetrance but without requiring investigator-initiated silencing of tumor suppressor genes.
Thyrocyte specific BRAFV600E expression leads to hypothyroidism
Given the rapid and dramatic increase in thyroid size combined with the observed loss of colloid, we assessed effects of oncogenic BRAFV600E on thyroid endocrine function. One month old Thyro::CreERT2; BRafCA mice were treated with tamoxifen and were euthanized 1 or 3 months days later with serum samples collected for analysis of the concentration of Thyroid Stimulating Hormone (TSH) and the most abundant thyroid hormone 3,5,3′,5′-tetraiodothyronine (Thyroxine or T4) by radioimmune assay. Both 1 and 3 months following expression of BRAFV600E, we detected a profound decrease in serum T4 levels to the lower limit of detection of the assay (Fig. 1C), indicating a deficit in the synthesis and/or secretion of thyroid hormones. Concomitant with decreased T4, we also detected a 100-fold increase in serum TSH at the same time points (Fig. 1D). The robust elevation of TSH is an expected homeostatic response of the pituitary to the striking decrease in serum T4 and is a manifestation of the profound hypothyroidism associated with widespread BRAFV600E expression in thyrocytes. Consistent with the absence of an overt thyroid phenotype in Thyro::CreERT2; KRasLSL mice, we detected no perturbation in serum T4 or TSH levels 1 month after tamoxifen administration of these mice (Figs. 1C & 1D).
BRAFV600E-induced PTC in the mouse expresses markers of human PTC
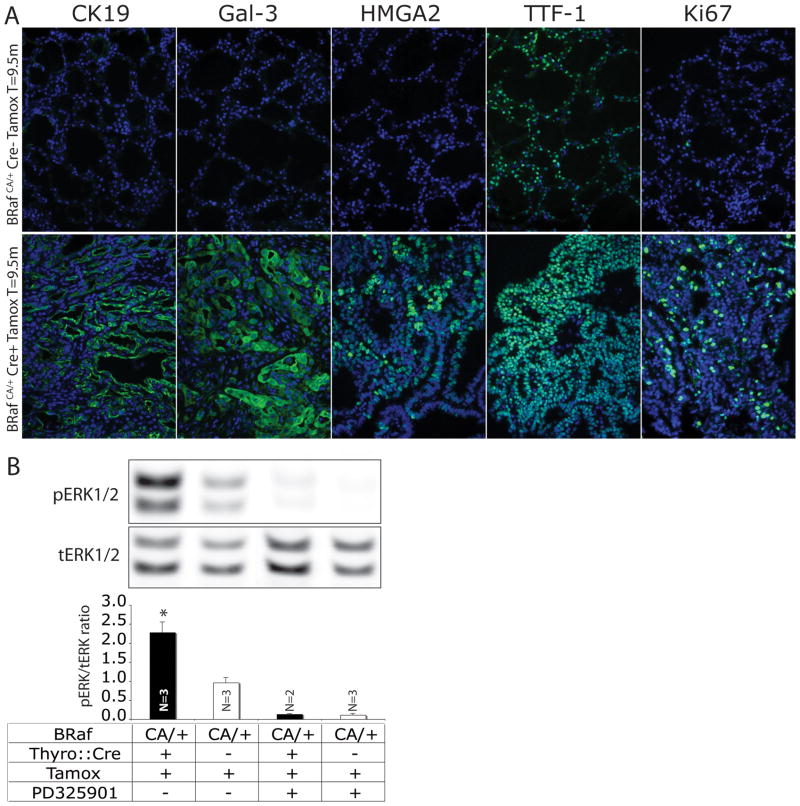
In addition to characteristic cytological features, human PTCs express a number of marker proteins including Galectin-3 (Gal-3) and Cytokeratin-19 (CK19), which are used in the clinical setting to identify lesions, aid diagnosis and stage disease (11). In addition, expression of high mobility group AT-hook 2 (HMGA2) is useful for distinguishing benign thyroid lesions from malignant ones (12). Many thyroid cancers display expression of Thyroid Transcription Factor-1 (TTF-1/NKX2.1), which is a master regulator of thyroid (13) and lung development (14). To determine if BRAFV600E-induced mouse PTC express these markers, tissue sections from control and PTC bearing Thyro::CreERT2; BRafCA mice (~9 months post-tamoxifen) were stained with antisera against CK19, Gal-3, HMGA2, TTF-1 and Ki67, the last being a general marker of cell proliferation (Fig. 3A as indicated). Whereas normal thyroid (Cre-) was largely negative for CK19, Gal-3 and HMGA2 expression (Figs. 2A & 2C), these proteins were readily detected in BRAFV600E-induced PTC (Cre+). As expected, TTF-1 expression was readily detected both in normal thyrocytes and in PTC (Fig. 3A), which further serves to confirm thyrocytes as the cells of origin of papillary tumors in the Thyro::CreERT2; BRafCA mice. Finally, as expected, normal thyrocytes displayed low Ki67 expression, consistent with the fact that these cells proliferate slowly. By contrast, BRAFV600E-induced PTC displayed readily detectable Ki67 in a high percentage of cells indicating sustained cell proliferation. Similar results were observed by measuring DNA synthesis following injection of mice with BrdU (data not shown).
Figure 3. Biochemical characterization of BRAFV600E-induced thyroid phenotype.
A: Immunofluorescence analysis of histological sections of thyroid from control BRafCA/+ (upper panels) and Thyro::CreERT2; BRafCA/+ (lower panels) 12 months after tamoxifen administration. DAPI in blue and CK19, Galectin-3, HGMA2, TTF-1 and Ki67 in green as indicated. Magnification is 200x.
B: Representative immunoblots of phosho- (p) and total (t)-ERK1/2 in extracts of thyroids from control or PD325901 treated BRafCA/+ or Thyro::CreERT2; BRafCA/+ as indicated below. Specimens were isolated from mice 2 weeks after tamoxifen administration. Immunoblots were imaged and quantified using a Li-Cor Odyssey imaging system. The comparison of tamoxifen treated Thyro::CreERT2; BRafCA/+ mice (lane 1) to control (lane 2) has P ≤ 0.05. N=number of samples in each group.
To determine if PTC developing in tamoxifen-treated Thyro::CreERT2; BRafCA mice displayed evidence of ERK1/2 MAP kinase pathway activation, protein extracts were prepared 2 weeks following tamoxifen administration to BRafCA mice that either did or did not carry the Thyro::CreERT2 transgene (Fig. 3B). Western blots were probed with antisera against either phospho- (pERK1/2) or total ERK1/2 (tERK1/2). In parallel, mice were treated with PD325901, a specific and selective MEK1/2 inhibitor prior to euthanasia (Fig. 3B). As expected, tamoxifen-induced (+Tamox), thyrocyte-specific expression (Cre+) of BRAFV600E led to an elevation of pERK1/2 compared to similarly treated controls (Cre-, +Tamox, Fig. 3B). Moreover, both BRAFV600E-mediated elevation and baseline pERK1/2 was strongly inhibited by PD325901. Similar results were obtained in a second cohort of mice analyzed 4 weeks after tamoxifen administration. These data indicate that thyroid specific expression of BRAFV600E leads to elevated ERK1/2 MAP kinase signaling.
BRAFV600E-induced elevation of serum TSH is not required for thyroid tumorigenesis
Previous research indicated that BRAFV600E expression in the lung epithelium or in melanocytes leads to a clearly defined state of benign tumorigenesis in which initiated benign tumor cells are restrained in their progression to malignancy unless tumor suppressor genes such as Trp53, Cdkn2a or Pten are deliberately silenced (6, 7). By contrast, BRAFV600E expression in thyrocytes appears to promote malignant progression to PTC without deliberate manipulation of tumor suppressors. One possible reason for this may be due to the highly elevated serum TSH which, acting through the TSH receptor and its downstream signaling pathways in BRAFV600E expressing thyrocytes, may prevent the senescent phenotype associated with BRAFV600E-induced benign tumor cells (6, 7).
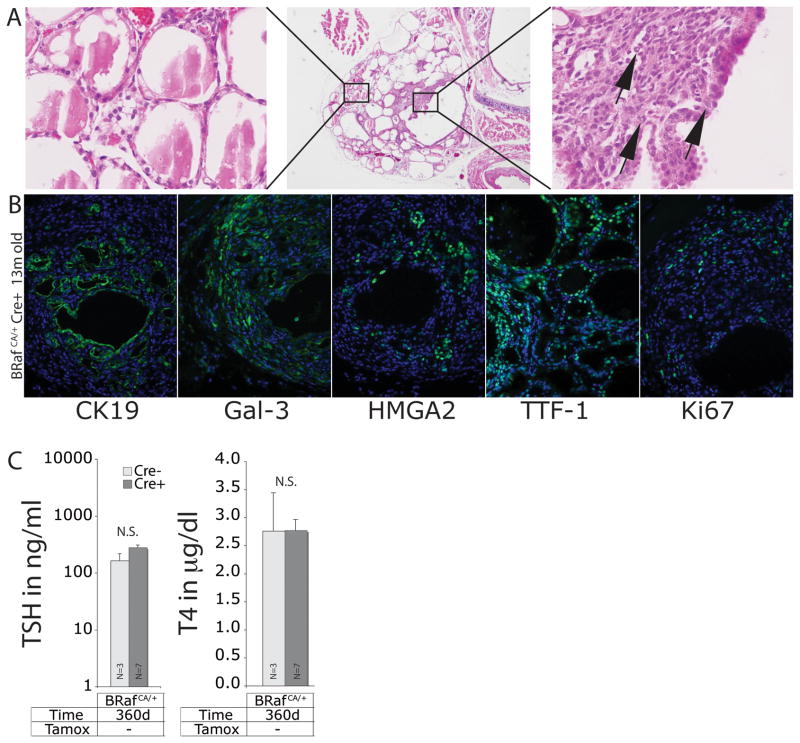
To test this, we took advantage of the fact that the Thyro::CreERT2 transgene displays 4-hydroxytamoxifen independent activity in untreated mice. Consequently, these mice were predicted to have stochastic activation of BRAFV600E expression in thyrocytes without the wholesale dysregulation of thyroid function that occurs in tamoxifen treated Thyro::CreERT2; BRafCA mice. Consequently, we aged a cohort of 9 Thyro::CreERT2; BRafCA mice to 12 months and assessed them for evidence of thyroid tumorigenesis (Figs. 4A & 4B). In addition, serum samples were collected for analysis of TSH and T4 levels (Fig. 4C). At euthanasia, the thyroid of all nine animals presented regions of papillary thyroid cancer with adjacent histologically normal thyroid architecture (Fig. 4A). Such thyroid lesions were genotype dependent in that they were only detected in compound Thyro::CreER; BRafCA mice. Consistent with a diagnosis of PTC, all of the tumor lesions stained positive for Cytokeratin 19, Galectin-3, HMGA2, TTF-1 and Ki67 (Fig. 4B, as indicated). Importantly, analysis of serum revealed no statistically significant elevation of TSH or decrease in T4 compared to controls (Fig. 4C). Although these results do not rule out a possible role for normal levels of serum TSH in promoting thyroid tumorigenesis, they indicate that the logarithmic elevation of TSH observed in tamoxifen treated Thyro::CreER; BRafCA mice is not required for PTC development.
Figure 4. Thyro::CreERT2; BRafCA/+ mice develop PTC without tamoxifen administration.
A. H&E staining of histological sections of thyroid from a representative 13 month old Thyro::CreERT2; BRafCA/+ mouse that was not administered tamoxifen at low (40x, middle panel) and high power magnification (400x, left and right panels). Cells displaying abnormal nuclear cytology are indicated with arrows (right panel).
B. Immunofluorescence analysis of histological sections of thyroid from Thyro::CreERT2; BRafCA/+ without tamoxifen injection. DAPI in blue and CK19, Galectin-3, HMGA2, TTF-1, and Ki67 in green as indicated. Magnification is 200x.
C. Analysis of serum concentration of TSH and T4 from ~1 year old Thyro::CreERT2; BRafCA/+ mice that were not administered tamoxifen. N=number of mice in each group.
Pharmacological blockade of MEK inhibits BRAFV600E-induced thyroid tumorigenesis
Genetically engineered mouse models of human cancer provide useful pre-clinical platforms for testing novel anti-cancer therapies (15). However, for such models to be relevant and useful, they must recapitulate key features of the genetics and histopathology of the cognate human disease. We have previously demonstrated that pharmacological inhibition of MEK1/2 using PD325901 has potent anti-tumor activity in mouse models of BRAFV600E-induced lung tumorigenesis and melanoma (6, 7). Given the frequency of BRAF mutation in human PTC, we sought to test the same approach in this new mouse model of BRAFV600E-induced PTC.
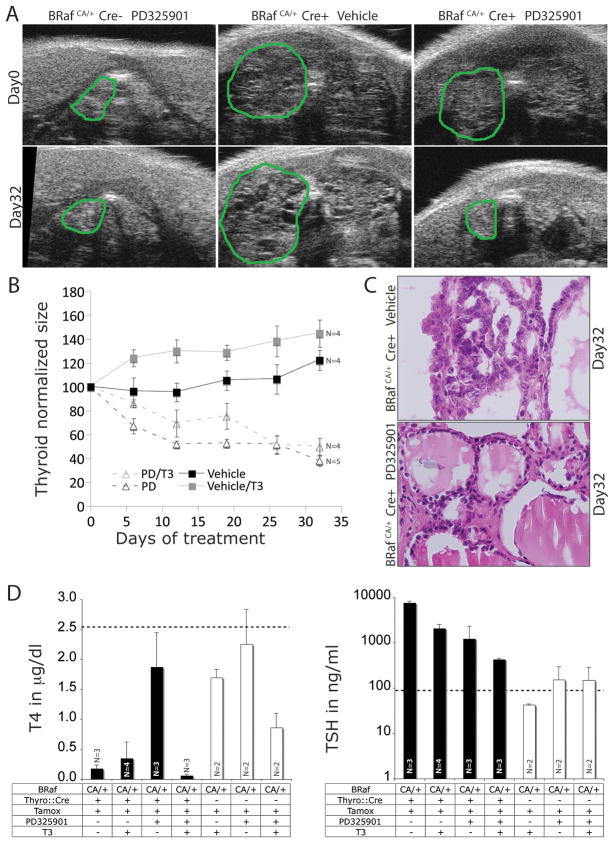
To do so, we administered tamoxifen to a cohort of 17 adult Thyro::CreERT2; BRafCA mice and aged them for a further 5 months at which time, and as expected, all of the mice were demonstrated to have an enlarged thyroid using ultrasound imaging. Mice were randomly assigned into four treatment groups: Group 1 (4 mice) received vehicle control; Group 2 (5 mice) were administered with PD0325901 by oral gavage; Group 3 (4 mice) were treated with synthetic thyroid hormone (T3) in drinking water in an attempt to normalize TSH levels and; Group 4 (4 mice) were treated with a combination of PD325901 and T3. Treatments were administered for 1 month with effects on thyroid size monitored weekly (Fig. 5A) by ultrasound imaging and enumerated by pixel counting as described in the Materials and Methods (Fig. 5B). At the end of the treatment period mice were euthanized and their thyroid subject to histological analysis for the presence of PTC (Figs 5C). Serum TSH and T4 were analyzed as previously described (Fig. 5D).
Figure 5. MEK1/2 inhibition (PD0325901) elicits regression of BRAFV600E-induced PTC.
A. Ultrasonogram images from cranio-caudal scanning of mice where the green line outlines the left lobe of the thyroid. Representative images of control BRafCA/+ mice prior to (Day 0, left) and following PD325901 administration (Day 32, left), Thyro::CreERT2; BRafCA/+ mice prior to (Day 0; middle) and following vehicle administration (Day 32, middle), and Thyro::CreERT2; BRafCA/+ mice prior to (Day 0, right) and following PD325901 administration (Day 32, right).
B. Quantification of normalized thyroid sizes calculated from ultrasonograms during the 32 days of PD325901 or T3 administration. N=number of mice in each group.
C. H&E staining of histological sections of thyroid from Thyro::CreERT2; BRafCA/+ mice 32 days after vehicle (upper) or PD325901 administration (lower). Magnification is 200x.
D. Analysis of serum concentrations of TSH and T4 in tamoxifen treated control BRafCA or Thyro::CreER; BRafCA mice subjected to either PD325901 or T3 as single agents or in combination as indicated. N=number of mice in each group.
Both vehicle and T3 treated mice displayed a progressive 15–20% or 20–30% increase respectively in thyroid size over the 32 days observation period (Figs. 5A & 5B). Mice treated with T3 displayed a greater increase in thyroid size compared to vehicle, but the difference was not statistically significant. By contrast, mice treated with PD325901, either alone or in combination with T3, displayed a progressive decrease in thyroid size that reached ~60% of the starting size by the end of the treatment period. The addition of T3 did not influence the reduction in thyroid size elicited by PD325901. Results inferred by ultrasonography were confirmed by post-mortem analysis of thyroid size (data not shown). It should be noted that although the thyroid of PD325901 treated mice displayed a 60% decrease in relation to the starting size, they did not return to that of normal animals. Indeed, at the end of the treatment period, the thyroid of these animals remained approximately twice that of normal mice. Nevertheless, compared to vehicle or T3 treated mice, MEK1/2 inhibition had a dramatic inhibitory effect on the ability of BRAFV600E to promote increased thyroid size.
Histological analysis of the variously treated animals demonstrated that vehicle or T3 treated animals presented with PTC with characteristic cytological features and altered follicular architecture (Fig. 5C) as described above (Fig. 1). By contrast, and consistent with the inhibitory effects on thyroid size, PD325901 treated mice displayed a more normal appearing follicular architecture with an increased prevalence of normal follicles lined by cuboidal thyrocytes with no evidence of nuclear atypia (Fig. 5C). Moreover, the follicles of PD325901 treated mice contained readily detectable eosinophilic colloid. Although aberrant cells were not entirely eradicated from the thyroid of PD325901 treated mice, the abundance of abnormal PTC cells was greatly diminished compared to vehicle treated mice.
Consistent with effects of PD325901 on thyroid size and architecture, treatment with PD325901 led to a normalization of serum T4 levels and a 6-fold decrease in serum TSH levels (Fig. 5D) showing at least partial restoration of thyroid endocrine function. The effect of PD325901 on serum T4 and TSH was specific to mice with thyroid specific BRAFV600E expression (black bars) since similar treatment of control BRafCA mice lacking the Thyro::CreERT2 transgene had no significant effect on either serum T4 or TSH levels (white bars). Single agent administration of T3 led to a decrease in serum TSH indicating that hormonal supplementation was at least partially effective. Furthermore, mice administered with T3 plus PD325901 displayed very low serum T4 levels due to inhibitory effects of exogenous T3 on T4 production. A co-operative effect of PD325901 plus T3 is evidenced since co-administration led to an even more striking decrease in serum TSH than either agent alone. T3 administration showed that hormonal supplementation alone did not provide any therapeutic benefit unless co-administered with PD325901. Overall, these results demonstrate that the effects of BRAFV600E on thyroid function and tumorigenesis are highly reliant on MEK→ERK signaling.
DISCUSSION
Dysregulated RTK signaling, mediated by mutational activation of RET/PTC, K- or NRAS or BRAF, appears to be a feature common to the majority of thyroid cancers (16, 17). Consequently, pharmacological targeting of RTK-activated signaling pathways may be useful in the treatment of this disease. Here we describe a new mouse model of BRAFV600E-induced papillary thyroid tumorigenesis that recapitulates key features of the human disease. Despite the profound hypothyroidism and tumorigenesis induced by widespread expression of BRAFV600E in thyrocytes, the mice displayed no overt signs of illness and none required euthanasia due to PTC. This stands in contrast to mice with constitutive, embryonic-onset expression of BRAFV600E (18). This may reflect the timing of BRAFV600E expression (embryonic vs. adult) in the two models and the fact that no obvious progression to more aggressive anaplastic thyroid cancer or metastasis was detected in our model. However, in both models, BRAFV600E is likely expressed in the majority of thyrocytes resulting in profound hypothyroidism and a field cancerization that is not common in human PTC. To that end, untreated Thyro::CreER; BRafCA mice that display stochastic recombination of the BRafCA allele in thyrocytes to express BRAFV600E may be a more accurate model of human PTC since, under these circumstances, there is no goiter or hypothyroidism associated with PTC initiation or progression.
In previous studies we have documented that expression of BRAFV600E in the lung epithelium or in melanocytes leads to a distinct phase of benign tumorigenesis that fails to progress to malignancy unless tumor suppressor genes are deliberately silenced (6, 7). Furthermore, it has been proposed that BRAFV600E-induced benign tumorigenesis reflects the engagement of senescence as a cancer suppression mechanism (19, 20). However in the thyroid, BRAFV600E expression (whether induced by tamoxifen or not) invariably leads to development of PTC displaying characteristic cytological features and protein marker expression of the cognate human disease. It remains unclear why thyrocytes expressing BRAFV600E do not undergo senescence as a cancer suppression mechanism. It is possible that the 10–100 fold elevation of serum TSH might provide co-stimulatory signals through the heterotrimeric G-protein coupled TSH receptor that might prevent engagement of senescence mechanisms. Indeed, TSH receptor couples to cAMP production through activation of Gαs.GTP and to PI3’-kinase and/or PLCβ through βγ subunits. However, progression to PTC was detected in non-tamoxifen treated Thyro::CreERT2; BRafCA mice in which no alterations in serum TSH or T4 were detected. Moreover, administration of exogenous T3 to tamoxifen treated Thyro::CreERT2; BRafCA mice did not diminish the BRAFV600E-induced increase in thyroid size and development of PTC. Although these results tend to rule out a role for elevated TSH in promoting PTC progression, they do not rule out a role for normal levels of serum TSH displayed by these mice. Consistent with this, while this manuscript was under review, Franco et al., described a similar mouse model in which embryonic-onset expression of BRAFV600E led to PTC (21). In that model, concomitant silencing of either TSH receptor or Gsα expression delayed, but did not abrogate, BRAFV600E- induced PTC. In addition, exogenous thyroid hormone had no effect on PTC maintenance. Notwithstanding differences in timing of the initiating genetic event, TSH receptor signaling plays a role in PTC initiation but not maintenance.
To our surprise, thyrocyte specific expression of KRASG12D had no obvious effect on thyroid architecture and did not predispose to thyroid tumorigenesis. The failure of KRASG12D to elicit thyroid tumors might reflect a lack of KRAS expression in mouse thyrocytes. However, it may also reflect an inability of KRASG12D to functionally engage downstream signaling effectors. Our results confirm those of others who demonstrated that KRASG12D expression in the developing thyroid was largely without effect (10). However, in this model, KRASG12D cooperated with PTEN silencing to induce thyroid cancer indicating that KRAS is expressed in mouse thyrocytes.
Considerable interest and excitement is focused on the effectiveness of pharmacological agents that target BRAF→MEK→ERK signaling in the treatment of cancers expressing mutationally activated BRAF. Indeed PLX-4032, a BRAF inhibitor, has demonstrated dramatic anti-tumor effects against metastatic melanoma and thyroid cancers expressing BRAFV600E (5). Our data suggest that BRAFV600E-induced PTC critically relies on MEK1/2 signaling since pharmacological blockade of these enzymes had striking effects on thyroid size and function and clear anti-tumor activity. However, a more effective test of such agents might be in the context of BRAFV600E-induced thyroid cancers with concomitant silencing of relevant tumor suppressor genes such as Trp53, Cdkn2a or Pten (6, 7). Indeed, preliminary evidence suggests that BRAFV600E can cooperate with dominant-negative TP53R270H for thyroid cancer progression (Date not shown). Finally, primary treatment for thyroid cancer often involves systemic radioiodide therapy. However, the effectiveness of such therapy is reported be limited by the ability of BRAFV600E to inhibit sodium-iodide symporter (NIS) expression. Hence, agents that target BRAFV600E signaling might promote NIS re-expression thereby sensitizing thyroid tumor cells to radioiodide therapy.
In conclusion, we describe here a new mouse model of adult onset thyroid cancer that displays key features of the human disease, which will complement studies on human thyrocytes and thyroid cancer lines (22). In addition, we demonstrate the utility of this model system to test the anti-tumor effects of pharmacological inhibitors of BRAF→MEK→ERK signaling. It will be interesting to test the effects of deliberate tumor suppressor gene silencing on the propensity of BRAFV600E-induced PTC to progress to more aggressive disease and on the response of thyroid cancer cells to pathway targeted therapy.
Acknowledgments
We acknowledge all members of the McMahon lab for support and helpful discussions. In particular we thank Takashi Hirano for technical support, Christy Trejo for assistance with tumor analysis and Victoria Marsh for advice on the manuscript. We acknowledge support for mouse husbandry from Allen Villanueva and support from the UCSF Mouse Pathology core in tissue preparation. We thank Dr. Shioko Kimura (National Institutes of Health) for provision of TPO::Cre transgenic mice, Drs. Raymond Grogan and Orlo Clark (UCSF Dept. of Surgery) for interesting and informative discussions on the pathology of human thyroid cancer, Drs. James Fagin and Ronald Ghossein (Sloan Kettering Institute) for advice on thyroid histology and for communicating unpublished results and Dr. Byron Hann (UCSF Pre-clinical Therapeutics core) for instruction and support for Vevo770 ultrasound imaging and drug administration. Finally, we thank Dr. A. Parlow (National Hormone and Peptide Program) for timely support for serum TSH and T4 measurements. This research was supported by a grant from the National Cancer Institute (CA131261). RPC was supported by a fellowship from the Swiss National Science Fund.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–84. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusakabe T, Kawaguchi A, Kawaguchi R, Feigenbaum L, Kimura S. Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis. 2004;39:212–6. doi: 10.1002/gene.20043. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–94. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazaitou-Panayiotou K, Mygdakos N, Boglou K, Kiziridou A, Chrisoulidou A, Destouni C. The Immunocytochemistry Is a Valuable Tool in the Diagnosis of Papillary Thyroid Cancer in FNA’s Using Liquid-Based Cytology. J Oncol. 2010;2010:963926. doi: 10.1155/2010/963926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belge G, Meyer A, Klemke M, et al. Upregulation of HMGA2 in thyroid carcinomas: a novel molecular marker to distinguish between benign and malignant follicular neoplasias. Genes Chromosomes Cancer. 2008;47:56–63. doi: 10.1002/gcc.20505. [DOI] [PubMed] [Google Scholar]
- 13.Civitareale D, Lonigro R, Sinclair AJ, Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989;8:2537–42. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray MK, Chen CY, Schwartz RJ, DeMayo FJ. Transcriptional regulation of a mouse Clara cell-specific protein (mCC10) gene by the NKx transcription factor family members thyroid transciption factor 1 and cardiac muscle-specific homeobox protein (CSX) Mol Cell Biol. 1996;16:2056–64. doi: 10.1128/mcb.16.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–87. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 16.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:955–69. doi: 10.1016/j.beem.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauf JA, Ma X, Smith EP, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–45. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 19.Haigis KM, Wistuba, Kurie JM. Lung premalignancy induced by mutant B-Raf, what is thy fate? To senesce or not to senesce, that is the question. Genes Dev. 2007;21:361–6. doi: 10.1101/gad.1532107. [DOI] [PubMed] [Google Scholar]
- 20.Merlino G. Building the perfect beast: complex mouse models teach surprisingly simple melanoma lessons. Pigment Cell Melanoma Res. 2009;22:246–7. doi: 10.1111/j.1755-148X.2009.00574.x. [DOI] [PubMed] [Google Scholar]
- 21.Franco AT, Malaguarnera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci U S A. 108:1615–20. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonelli A, Bocci G, La Motta C, et al. Novel pyrazolopyrimidine derivatives as tyrosine kinase inhibitors with antitumoral activity in vitro and in vivo in papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:288–96. doi: 10.1210/jc.2010-1905. [DOI] [PubMed] [Google Scholar]