Abstract
Angiogenesis, the formation of new blood vessels from preexisting vessels, is critical to most physiological processes and many pathological conditions. During zebrafish development, angiogenesis expands the axial vessels into a complex vascular network that is necessary for efficient oxygen delivery. Although the dorsal aorta (DA) and the axial vein (AV) are spatially juxtaposed, the initial angiogenic sprouts from these vessels extend in opposite directions, suggesting that distinct cues may regulate angiogenesis of the axial vessels. In this report, we found that angiogenic sprouts from the DA are dependent on Vegf-A signaling, and do not respond to Bmp signals. In contrast, sprouts from the AV are regulated by Bmp signaling independent of Vegf-A signals, suggesting that Bmp is a vein-specific angiogenic cue during early vascular development. Our results support a paradigm, whereby different signals regulate distinct programs of sprouting angiogenesis from the AV and DA, and suggest that signaling heterogeneity contributes to the complexity of vascular networks.
The DA and AV form a primitive circulatory loop, and subsequent angiogenesis from these vessels is essential to generate the complex vascular networks found in vertebrates. In zebrafish, the initial sprouts from the DA project dorsally to form the intersegmental arteries (ISAs)1 (arrows, Suppl. Fig. S1a), while those from the posterior AV extend ventrally (arrowheads, Suppl. Fig. S1a) to form a honeycomb-like network termed the caudal vein plexus (CVP), which is composed of a dorsal and ventral vein with interconnecting vessels (Suppl. Fig. S1a). Since the neighboring axial vessels extend angiogenic sprouts in opposite directions and form distinct vascular networks, we hypothesized that the DA and AV respond to different angiogenic stimuli.
The Vascular Endothelial Growth Factor-A (Vegf-A) signaling cascade is a critical angiogenic stimulus for many vascular beds2, so we first assessed the role of Vegf-A in regulating sprouting angiogenesis from the axial vessels. Co-injection of morpholinos (MOs) against two Vegf-A receptors in zebrafish, kdrl and kdr3, caused severe vascular defects. The DA and the CV incompletely segregate4 (Fig 1a), endothelial cell apoptosis was significantly increased5 (Suppl. Fig. S1b), and ISA sprouts were blocked5 (Fig. 1a). While the percentage of segments (the area defined by two adjacent somite boundaries) containing an ISA was drastically reduced, the percentage containing a CVP was largely unaffected in kdrl/kdr morphants (Fig. 1b) (see Materials and Methods for quantification specifics). The venous sprouts still formed a primitive plexus in kdrl/kdr morphants, and only displayed marginal defects in branching (Fig. 1 and Suppl. Fig. S1c). This vascular network was unstable and ultimately regressed, as previously reported4. While our data corroborate the role of Vegf-A signaling in regulating ISA formation and endothelial cell stability3, they suggest that another angiogenic stimulus regulates sprouting from the AV.
Figure 1. The AV forms angiogenic sprouts despite loss of Vegf receptor activity, and expresses Bmp pathway components.
(a) Epiflourescent images of 34hpf Tg(kdrl:GFP) control and kdrl/kdr MO injected embryos; insets show higher magnification of the CVP region. Asterisks denote the lack of intersegmental arteries in kdrl/kdr MO injected embryos. Scale bar, 250µm. (b) The percentage of segments that contain an ISA (red bars) or a CVP (blue bars) was quantified in control (n=9) and kdrl/kdr (n=10) MO injected embryos. kdrl/kdr MOs completely blocked the formation of arteries but not veins. Error bars represent mean ± SEM. ***P<0.001 versus control, Student’s t test. (c) Expression pattern of bmp2b, bmpr2a, and bmpr2b in the developing CVP region (black arrowheads) at 32hpf, as detected by in situ hybridization. Cross sections from different 32hpf embryos were taken at the area marked by dashed line. Abbreviations: DA, DA; VV, ventral vein; DV, dorsal vein.
To identify the angiogenic signal required for sprouting from the AV, we analyzed the expression of components from several signaling pathways (data not shown), and found that Bone Morphogenetic Protein (Bmp) pathway components were selectively expressed in the developing CVP. Whole mount in situ hybridization indicated that the bmp2b ligand was highly expressed within the CVP and surrounding tissue during plexus formation (26–32 hours post-fertilization (hpf)), and expression subsided as the CVP stabilized at 38hpf (Fig. 1c and Suppl. Fig. S1d). In addition, two Bmp type II receptors, bmpr2a and bmpr2b, were strongly expressed in the endothelial cells of the CVP at 26, 32, and 38hpf consistent with previous studies6 (Fig. 1c and Suppl. Fig. S1d).
Bmp can function as a context-dependent pro-angiogenic cue7. Upon ligand binding, Bmp type II receptors phosphorylate Bmp Type I receptors, which in turn activate Smad and/or MAP kinase signaling8. To test whether Bmp signaling regulates sprouting of the AV, we manipulated expression of Bmp pathway components in the developing zebrafish using a heat shock promoter (hsp70l)9. To determine the expression profile of hsp70l, we heat-shocked Tg(hsp70l:GFP) embryos at 25hpf and found that GFP was expressed in most tissues and cell types (Suppl. Fig. S2a). We next analyzed the effects of decreased Bmp activity on sprouting from the AV by over-expressing noggin3, an endogenous inhibitor of Bmp signaling10. Control embryos heat-shocked at the onset of plexus formation (25hpf) showed no apparent vascular abnormalities (Fig. 2a and Suppl. Movie S1). In contrast, heat-shocked Tg(hsp70l:noggin3) embryos displayed CVP with aberrant sprouts that failed to make proper connections with neighboring sprouts, but showed no ISA defects (arrows, Fig. 2a, Suppl. Fig. S3a, and Suppl. Movie S2). Similar results were also observed in Tg(hsp70l:dnbmprI-GFP) embryos that expressed a dominant negative Bmp receptor type I GFP fusion (DNBmprI-GFP) when heat-shocked (Suppl. Fig. S2b). Since CVP patterning was perturbed while ISA patterning was largely unaffected, these results suggest that decreased Bmp signaling selectively affects vessel patterning from the AV.
Figure 2. Bmp signaling is necessary and sufficient for sprouting from the AV.
(a) Blood vessels in wild-type, Tg(hsp70:noggin3), and Tg(hsp70:bmp2b) embryos in the Tg(kdrl:GFP) transgenic background. The entire vascular network of 42hpf embryos was analyzed using epiflourescent images; dashed boxes represent the trunk and tail areas analyzed below. Z-stacks from the trunk and tail regions were used to make 3-D color projections, where red represents the most proximal (closest to viewer) and blue represents the most distal (farthest from viewer) blood vessels (epiflourescent images and 3-D color projections were taken from different embryos). Scale bar, 50µm. (b)Time lapse imaging of Tg(fli1:nGFP);Tg(kdrl:ras-mCherry) embryos starting at 32hpf. Arrows in panel a and b show sprouts from the AV that fail to make connections in Tg(hsp70:noggin3) embryos. Arrowheads in panel a and b point to ectopic sprouts that branch from the AV in Tg(hsp70:bmp2b) embryos. Scale bar, 20µm. Abbreviations: DA, DA; VV, ventral vein; DV, dorsal vein; NC, notocord; NT, neural tube; ISA, intersegmental artery.
We next asked whether increased Bmp signaling could induce angiogenesis. bmp2b expression was increased in heat-shock treated Tg(hsp70l:bmp2b) embryos at the onset of CVP formation. bmp2b over-expression induced ectopic sprouts along the AV, with the most robust ectopic sprouting occurring in the CVP (arrowheads, Fig. 2a, Suppl. Fig. S3b, and Suppl. Movie S3). Bmp-induced ectopic sprouts extended from the AV and migrated between the epithelial surface and the somite boundary, forming an additional plexus in a region that is avascular in wild-type (WT) embryos (arrowheads, Suppl. Fig. S4a). The Bmp-induced plexus highly expressed a venous marker, dab2, indicating that it has venous identity (Fig. S4b). Although Bmp over-expression induced robust sprouting from the AV, ectopic sprouts were never observed from the DA (Fig 2a). To further delineate the specificity of Bmp signaling, WT and Tg(hsp70l:bmp2b) embryos were heat-shocked at 2.5dpf, when ventral sprouts from the AV form the subintestinal vein plexus (SIVP) (arrows, Suppl. Fig. S4c). The SIVP in bmp2b over-expressing embryos was shifted dorsally (arrows, Suppl. Fig. S4c) and contained ectopic vessels (arrowhead, Suppl. Fig. S4c), suggesting that the SIVP is also responsive to Bmp signaling. These data indicate that sprouting angiogenesis from the AV during early development is uniquely dependent on Bmp signaling.
To assess the cellular effects of Bmp signaling on venous endothelial cell behavior, we performed time-lapse imaging. WT embryos formed a honeycomb-like plexus by 32hpf, and this plexus began to retract filopodia and stabilize by 35hpf (Fig. 2b and Suppl. Movie S1). However, Tg(hsp70l:noggin3) embryos contained atypical angiogenic sprouts that failed to make connections and never formed a proper plexus (Fig. 2b and Suppl. Movie. S2). In contrast, Tg(hsp70l:bmp2b) embryos contained ectopic endothelial sprouts. These ectopic sprouts branched and sprouted from the dorsal vein of the CVP as early as 6.5 hours after heat-shock treatment (32hpf) and rapidly migrated dorsally (Fig. 2b and Suppl. Movie S3).
To better characterize the noggin3 and bmp2b over-expression phenotypes, we counted venous endothelial cell nuclei and performed branch point analyses in WT, noggin3, and bmp2b over-expressing embryos. While the number of venous endothelial cells in the CVP remained relatively unchanged in noggin3 over-expressing embryos, we observed a slight but significant increase in endothelial cell numbers in bmp2b over-expressing embryos (Supp Fig S3c). Venous branch points, however, were significantly altered in both noggin3 and bmp2b over-expressing embryos (Suppl. Fig. S3d). We found that the number of branch points was decreased more than 7 fold by noggin3 over-expression, and increased approximately 2.5 fold by bmp2b over-expression (Suppl. Fig. S3d). In addition, bmp2b over-expression caused a significant increase in the number of filopodia (Suppl. Fig. S4d–e), and randomized their direction of extension (Suppl. Fig. S4f). Taken together, our data indicate that Bmp is a pro-angiogenic cue that regulates angiogenesis in the AV.
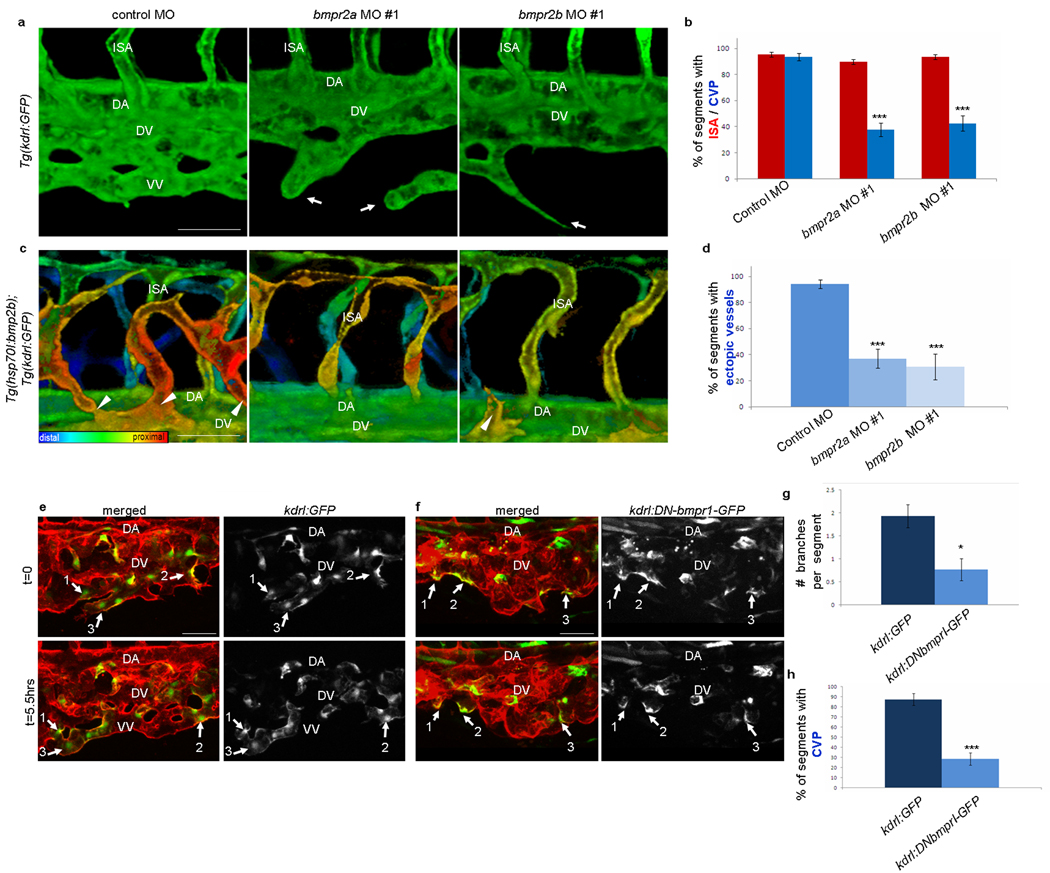
To investigate whether the Bmp type II receptors expressed in the developing CVP regulate Bmp-mediated angiogenesis, we analyzed bmpr2a or bmpr2b morphants (Fig. 3a–d and Suppl. Fig. S5). While the number of arterial sprouts did not differ significantly from control embryos, sprouts from the AV were significantly reduced in bmpr2a and bmpr2b morphants (Fig. 3a–b and Suppl. Fig. S5c). Moreover, knock-down of bmpr2a or bmpr2b in bmp2b over-expressing embryos inhibited formation of ectopic sprouts (Fig. 3c–d and Suppl. Fig. S5d). Therefore, Bmpr2a and Bmpr2b regulate Bmp-mediated angiogenesis from the AV.
Figure 3. Angiogenesis from the AV requires bmpr2a and bmpr2b and involves endothelial cell autonomous activation of Bmp signaling.
(a) Confocal monochrome projections of Tg(kdrl:GFP) embryos injected with a standard control, bmpr2a, or bmpr2b MO. The sprouts from the AV are disrupted with bmpr2a and bmpr2b MO (arrows). (b) The percentage of segments that contain an ISA (red bars) or a CVP (blue bars) was quantified. Total of eight embryos were used for the quantification in each case. bmpr2a or bmpr2b MOs blocked the formation of veins but not arteries. (c) Confocal color depth-code projections of Tg(hsp70l:bmp2b);Tg(kdrl:GFP) heat-shocked embryos injected with a standard control, bmpr2a, or bmpr2b MO. The ectopic sprouts (arrowheads) are reduced in both bmpr2a and bmpr2b morphants. (d) The percentage of segments that contain an ectopic sprout was quantified in control (n=37), bmpr2a #1 (n=27), and bmpr2b #1 (n=15) MO injected embryos. The number of Bmp-induced ectopic sprouts was significantly reduced in both bmpr2a and bmpr2b morphants. (e–f) Time-lapse confocal images of Tg(kdrl:GFP) (e) and Tg(kdrl:DNBmpr1-GFP) (f) mosaic embryos in a Tg(kdrl:ras-mCherry) background. Numbered arrows indicate mosaic endothelial cells. (g) The number of branch points and (h) the percent of segments containing a CVP were quantified in mosaic segment containing GFP or DNBmprI-GFP cells. Total of 29 segments in 7 embryos for Tg(kdrl:GFP) and 34 segments in 11 embryos for Tg(kdrl:DNBmpr1-GFP) were used for quantification (See Methods for detailed quantification method). DNBmprI-GFP-expressing endothelial cells contain fewer branches (g) and fail to form proper CVP connections (h). Scale bar, 50µm. Error bars represent mean ± SEM. **P<0.01 and ***P<0.001 versus control, Student’s t test. Abbreviations: DA, DA; ISA, intersegmental artery; VV, ventral vein; DV, dorsal vein.
Considering the expression and function of bmpr2a and bmpr2b in CVP formation, it is likely that Bmp activation is required in endothelial cells. To investigate this hypothesis, we generated mosaic embryos by injecting either kdrl:GFP or kdrl:DNBmprI-GFP in the Tg(kdrl:mCherry) background. The resulting embryos contained patches of endothelial cells that strongly expressed GFP or DNBmprI-GFP. The GFP-expressing control cells extended venous sprouts, which made connections and formed a honeycomb-like plexus (Fig. 3e and Suppl. Movie S4). In contrast, the DNBmprI-GFP-expressing cells were unable to extend sprouts from the AV, and they failed to connect with neighboring endothelial cells to form a honeycomb-like plexus (Fig. 3f and Suppl. Movie S5).
The segments that contained DNBmprI-GFP-expressing cells had fewer branch points than GFP expressing control cells (Fig. 3g), indicating that Bmp signaling within endothelial cells is important during branching morphogenesis. In addition, the frequency with which the branches connected to form a plexus was significantly reduced in DNBmprI-GFP-expressing cells, suggesting that Bmp signaling within endothelial cells is critical for the formation of endothelial networks (Fig. 3h). Taken together, our results indicate that Bmp-mediated angiogenesis requires Bmp activation in endothelial cells.
Bmp signaling activates the Smad signaling cascade and/or alternative MAP kinase signaling cascades such as Erk and p3811,12. To delineate the downstream factors critical for Bmp-mediated angiogenesis, we first analyzed the activity/phosphorylation status of Smad1/5/8 (R-Smads) and Erk. Activated R-Smads and Erk were present within the ectopic sprouts from the AV (Suppl. Fig. S6a–b). To assess the function of R-Smad and Erk signaling in Bmp-mediated angiogenesis, we blocked the activity of R-Smad or Erk by treating embryos with small chemical inhibitors. To inhibit the R-Smad signaling cascade, we used DMH1, which inhibits Alk2/3 and selectively abrogates activation of R-Smads without affecting MAP kinase activity13. In addition, we inhibited the p38 pathway with SB203580, and the Erk pathway with either U0126 (data not shown) or SL327. While both arterial and venous angiogenesis was unaffected by treatment with DMSO or the p38 inhibitor, inhibition of R-Smad activation selectively blocked formation of the CVP without affecting ISAs, and inhibition of Erk activity blocked formation of both the CVP and ISAs (Fig. 4a–b). Moreover, inhibiting R-Smad or Erk activation in Bmp over-expressing embryos efficiently inhibited the percentage of segments with ectopic vessels, while p38 inhibition had no effect on the percent of segments with ectopic vessels (Fig. 4c–d). Interestingly, the Erk inhibitor also drastically attenuated the length and progression of the ectopic sprouts (Fig. 4e). Collectively, these results suggest that R-Smad activation selectively regulates venous sprouting angiogenesis, and Erk (but not p38) activation is involved in the progression of Bmp-mediated venous sprouts as well as arterial sprouts.
Figure 4. Activation of R-Smad and Erk mediates Bmp-induced angiogenesis.
(a) Epiflourescent micrographs of Tg(kdrl:GFP) embryos at 38hpf were taken after treatment with DMSO, DMH1 (R-Smad inhibitor), SB203580 (p38 inhibitor), and SL327 (Erk inhibitor). Arrows point to defects in the formation of venous vessels in DMH1- or SL327-treated embryos. (b) The percentage of segments that contain an ISA (red bars) or a CVP (blue bars) was quantified in DMSO (n=14), DMH1 (n=13), SB203580 (n=8), or SL327 (n=10) treated embryos. (c) Confocal depth-code color projections of Tg(hsp70l:bmp2b);Tg(kdrl:GFP) embryos at 46hpf were taken after treatment with small molecule inhibitors. Addition of DMH1 or SL327 to bmp2b over-expressing embryos inhibited Bmp-induced ectopic sprouts. Arrowheads point to ectopic sprouts from the AV. (d) The percentage of segments that contain an ectopic vessel was quantified in DMSO (n=11), DMH1 (n=13), SB203580 (n=4), or SL327 (n=6) treated embryos. (e) The average ectopic vessel length was quantified in DMSO (n=15), DMH1 (n=14), SB203580 (n=16), or SL327 (n= 22) treated embryos. Inhibition of either R-Smad or Erk activation significantly reduced the formation ectopic vessels and the average length of ectopic vessels. Error bars represent mean ± SEM. **P<0.01 and ***P<0.001 versus control, Student’s t test.
Since activation of the Bmp signaling cascade transcriptionally regulates multiple genes and pathways, we analyzed the transcriptional levels of important regulators of angiogenesis using quantitative RT-PCR. Transcription levels of Tg(hsp70l:bmp2b) embryos were compared to WT at 2 and 5 hours post heat-shock induction of bmp2b. id2a, a downstream transcriptional target of Bmp signaling, was used as a positive control. vegfa, vegfc (also a major stimulus of lymphangiogenesis14), vegfr3/flt4 (a venous marker and receptor for Vegf-C), and dll4 (an arterial marker and tip cell marker15,16) were also tested. Bmp over-expression upregulated id2a by over 3-fold at 2 hours post heat-shock, while vegfa, vegfc, flt4, and dll4 were either marginally affected or not affected at all (Suppl. Fig. S7). In addition vegfa, vegfc, flt4, and dll4 transcript levels were unaffected 5 hours post heat-shock induction of bmp2b (Suppl. Fig. S7).
To test the physiological relevance of the moderate increase in vegfa transcription at 2 hours post heat-shock, Bmp over-expression was induced in embryos lacking Vegf receptors. Co-injection of the kdrl/kdr MOs resulted in a single AV at 2dpf (Fig. 5a). Despite the severely disrupted vascular network, Bmp-induced ectopic blood vessels were unaffected in kdrl/kdr morphants, demonstrating that Bmp is capable of inducing angiogenesis when Vegf receptors are inhibited (Fig. 5a–b). We next analyzed the effects of Bmp and Vegf-A small molecule inhibitors during sprouting angiogenesis of the axial vessels12. Addition of dorsomorphin, a chemical inhibitor of both the Bmp and Vegf-A signaling pathways, effectively inhibited vessels from the DA and AV and blocked Bmp-induced ectopic vessels (Suppl. Fig. S8). DMH4, an inhibitor of Vegf-A signaling, preferentially blocked vessels from the DA and had no effect on Bmp-induced ectopic vessels, while DMH1, an inhibitor of Bmp signaling, selectively inhibited vessels from the AV and disrupted Bmp-induced ectopic vessels (Suppl. Fig. S8). Taken together, these findings demonstrate that Bmp is the major stimulus for sprouting angiogenesis from the AV, and that Vegf-A is the major stimulus for sprouting from the DA. Secondly, these results suggest that Bmp mediates angiogenesis independent of a significant contribution from Vegf-A signaling.
Figure 5. Bmp signaling regulates AV angiogenesis independent of Vegf receptor activity.
(a) Control and kdrl/kdr MOs were injected into Tg(hsp70:bmp2b); Tg(kdrl:GFP) heat-shocked embryos and shown as 3D color projections. The number of Bmp-induced ectopic sprouts (arrowheads) was not affected by the loss of Kdrl/Kdr activity. Scale bar, 50µm. (b) The percentage of segments that contain ectopic vessels was quantified (n=3 for control, and 6 for kdrl/kdr MO). There was no statistically significant difference between control and kdrl/kdr MOs injected embryos. Error bars represent mean ± SEM. (c) 3-D color projections were taken from the trunk and tail region of 42hpf heat-shocked embryos. Over-expression of bmp2b induced ectopic sprouts in venous endothelial cells (arrowheads), while over-expression of vegfa stimulated ectopic sprouts in arterial endothelial cells in the trunk (arrows). (d) In this model, Bmp signaling is the dominant regulator of AV angiogenesis, while Vegf-A is the main regulator of angiogenesis from the DA. (e) In venous endothelial cells, Bmp2b ligand binds to a Bmpr2a and/or Bmpr2b and Alk2/Alk3 hetero-tetrameric complex, which phosphorylates R-Smad and Erk to promote angiogenesis, while arterial cells utilize the classical Vegf-A signaling cascade to induce angiogenesis. Scale bar, 50µm. Abbreviations: DA, DA; VV, ventral vein; DV, dorsal vein; ISA, intersegmental artery.
To compare the angiogenic effects of Bmp and Vegf-A, we induced over-expression of bmp2b or vegfa121 by heat-shock treatment of Tg(hsp70l:bmp2b) or Tg(hsp70l:vegfa121) transgenic lines, respectively. As expected, bmp2b over-expression induced robust ectopic sprouts along the AV, but not from the DA (Fig. 5c). In contrast, vegfa121 over-expression did not induce ectopic sprouts from the AV, but increased sprouting along the DA was observed (Fig. 5c). The distinct angiogenic responses between bmp2b over-expressing embryos and vegfa121 over-expressing embryos demonstrate that Bmp is a distinct and potent pro-angiogenic factor.
Taken together, our findings support a paradigm whereby Bmp signaling mediates venous angiogenesis, while Vegf-A signaling directs arterial angiogenesis. In our model, this differential response to angiogenic stimuli permits neighboring venous and arterial vessels to extend distinct angiogenic sprouts and form non-overlapping vascular networks (Fig. 5d). The venous sensitivity observed during Bmp-mediated angiogenesis may be provided by the notochord, which lies above the DA and expresses Bmp antagonists that inhibit blood vessel growth17,18. Collectively our results suggest a model of Bmp mediated angiogenesis in which Bmp2b binds Bmpr2a/b and Alk2/Alk3 hetero-tetrameric receptor complexes in venous endothelial cells and activates R-Smad and Erk, which elicits angiogenic responses that include sprout migration and fusion (Fig. 5e).
The zebrafish embryo contains a relatively simple and streamlined vascular system, and this simplicity allows for elucidation of binary choices that likely underlie vascular development in more complex organisms. It will be important to determine if there is a similar role for Bmp signaling during mammalian development and tumor angiogenesis. Although published work in mammalian systems does not identify a selective requirement for Bmp signaling in venous angiogenesis, mammalian vascular systems are more complex, and the requirement for BMP signaling in early development makes specific interrogation of later requirements difficult19–22. In addition, several types of carcinomas express high levels of BMP growth factors23–25, and anti-angiogenesis cancer drugs that singularly antagonize VEGF-A activity are only partially effective26. Therefore, future studies that target both Bmp and Vegf-A signaling may be more successful at manipulating blood vessel growth.
Methods
Zebrafish husbandry
Zebrafish (Danio rerio) embryos were raised as previously described27. The following transgenic lines were used: Tg(fli1:nEGFP)y7 28, Tg(kdrl:GFP)s843 29, Tg(kdrl:ras-mCherry)s896 30, Tg(hsp70l:bmp2b)fr13 31, Tg(hsp70l:noggin)fr13 31, Tg(hsp70l:dnbmprI-GFP)w30 32, and Tg(hsp70l:vegfaa121;cmlc2:EGFP)nc2 (this study).
In situ hybridizations and immunohistochemistry
Whole mount in situ hybridization was performed as previously described33,34 to probes for bmp2b, bmpr2a, bmpr2b, and dab2 were synthesized as previously described6, and documented with a Leica MF16 microscope. For transverse sections, embryos were mounted in 4% agarose, embedded in paraffin, and sectioned into 8, 7, and 5µm slices respectively. Fast red staining was used to visualize tissue morphology.
Immunohistochemistry was performed as previously described29. Following antibodies were used: anti-Caspase3, cleaved (Cat#:PC679, Calbiochem), β-tubulin (Cat#:61053, BD Transduction Laboratories) at 1:200, and Alexa Fluor secondary antibodies (Invitrogen) at 1:400. To sagittally mount embryos, the head and yolk were removed and the trunk was covered in 1% low melt agarose and sealed with a cover slip. For transverse sections, embryos were mounted in 4% agarose and sectioned on a Leica VT 1000s vibratome.
Heat-shock treatment
Tg(hsp70l:noggin), Tg(hsp70l:bmp2b), Tg(hsp70l:vegfaa121;cmlc2:GFP), and Tg(hsp70l:dnbmprI-GFP) embryos were heat-shocked 25–26hpf for 30minutes at 42°C. Tg(hsp70l:noggin) and Tg(hsp70l:bmp2b) embryos were genotyped by PCR, and Tg(hsp70l:vegfaa121;cmlc2:GFP) and Tg(hsp70l:dnbmprI-GFP) embryos were identified by the expression of GFP.
Quantification
To quantify and compare arterial and venous angiogenesis in Fig. 1b, 3b, 3h, 4b, 5b, Suppl. Fig. S6a, S14a–b, and S18b, we calculated the percentage of segments that form an angiogenic vessel from the DA (ISA) or from the AV (CVP) between 36–40hpf. Each segment is defined as the area on the A-P axis between two adjacent somite boundaries. The first 12 segments starting at the end of the yolk extension (roughly corresponding to the 14th to 26th somite) were analyzed. To quantify arterial angiogenesis (red bars), each segment that contained an ISA (at the anterior somite boundary) that reached the DLAV was given a value of 1, while each segments that lacked an ISA was given a value of 0. Similarly, to quantify venous angiogenesis (blue bars), each segment that contained a CVP with a fused ventral vein (therefore, completed the CV remodeling) was given a value of 1, and segments that lacked a fused ventral vein in the CVP were given a value of 0. These values were then used to calculate the percentage of segments with either ISA (red bars) or CVP (blue bars).
To quantify ectopic vessels in bmp2b over-expressing embryos in Fig. 3d, 4d, 5b, Suppl. Fig. S6b, S14b, and S18c embryos were examined between 44–50hpf. Since the ectopic sprouts and pairs of ISAs formed on in both the left and right side of embryos, only the ISAs and sprouts closest to the objective were analyzed.
To quantify embryos with somatic mosaicism in Fig. 3g–h, embryos were presorted for GFP expression in endothelial cells between 44–50hpf. Only the mosaic segments (area between two adjacent somite boundaries) which contained patches of kdrl:GFP or kdrl:DNBmprI-GFP expressing endothelial cells were quantified. The number of endothelial branches per segment was counted, and an average was calculated (Fig. 3g). To calculate the percentage of mosaic segments that form a CVP (Fig. 3h), each segment that contained a CVP with a fused ventral vein was given a value of 1, and segments that lacked a fused ventral vein in the CVP were given a value of 0. These values were then used to calculate the percentage of segments with a CVP.
In all cases, embryos with gross morphological defects were presorted and excluded from analysis prior to quantification.
Morpholino injections and small molecule treatment
Microinjections of MOs were performed as previously described35. Briefly, embryos were injected at the single cell stage with 4–12ng of control MO (Gene Tools), 12ng of bmpr2a splicing MO #1, 12ng of bmpr2a splicing MO #2, 8ng of bmpr2b splicing MO #1, 12ng of bmpr2b splicing MO #2 and a combination of 2ng of kdrl, and 2ng of kdr MO (Gene Tools). Embryos were co-injected with 2ng of p53 MO (Gene Tools) and embryos with gross morphological defects were presorted and excluded from quantification. The sequences for the MOs used in this study are: bmpr2a #1: 5′-AGAGAAACGTATTTGCATACCTTGC-3′; bmpr2a #2: 5’-TCATTACGGAAACATACCTCTTAGC-3’;bmpr2b #1: 5′AGTTGATTCTGACCTTGTTTGACCA-3′; bmpr2b #2: 5’-CGGCTTCATCTTGTTCTGACCTCAC-3’; kdrl: 5′-CACAAAAAGCGCACACTTACCATGT-3′5; and kdr: 5′-GTTTTCTTGATCTCACCTGAACCCT -3′5.
Embryos were treated with chemical inhibitors at 26hpf. The final concentration of small molecule inhibitors was 60μM of SL327, 200µM of SB203580, 40µM of dorsomorphin, 10µM of DMHI, and 5µM of DMHI in 2% DMSO.
Live Imaging and 3-D Image Processing
Embryos were dechorionated, and embedded in 1% agarose (containing egg water with tricaine) in the center of a glass bottom petri dish (MatTek). Once agarose solidified, egg water with tricaine was added. Embryos were imaged using a Zeiss 510 Meta confocal microscope.
Zeiss LSM software was used to generate monochrome projections and 3-D color projections from confocal Z-stacks. The color bar on the 3-D color projections represents the z-axis location of objects with red representing the most proximal (closest to viewer) and blue representing the most distal blood vessels (farthest from viewer).
Real-Time PCR
Quantitative RT-PCR for zebrafish id2a, vegfa, vegfc, dll4, and flt4 was performed using the TaqMan gene expression assay (Applied Biosystems). Wild-type and Tg(hsp70l:bmp2b)+/− fish were incrossed and heat-shocked as previously described. Total RNA was extracted from ~50 embryos 2 hours post heat-shock and 5 hours post heat-shock. gapdh was used as an endogenous control to normalize expression levels. The expression of id2a, vegfa, vegfc, dll4, and flt4 were displayed as a ratio of bmp2b-induced to wild-type.
Generating Transgenic Constructs
The vegfa gene was amplified from cDNA of 32hpf embryos. The PCR product was ligated into the pCR8 vector (Invitrogen). The vegfa gene was sequenced and found to be the vegfaa121 splicing isoform. The gateway tol2 kit36 was used to create the hsp70l:vegfaa121 construct, which was injected with transposase RNA into 1-cell embryos to create stable Tg(hsp70l:vegfaa121;cmlc2:EGFP) transgenic lines.
The dominant negative form of Bmp receptor type I (DNbmprI-GFP) gene was amplified from the cDNA of Tg(hsp70l:DNbmprI-GFP) embryos32, and ligated into the pCR8 vector (Invitrogen). The gateway tol2 kit was used to generate the kdrl:DNbmprI-GFP construct. The resulting construct was injected with transposase RNA into 1-cell embryos, which generated patches of endothelial cells that over-express the DNbmprI-GFP fusion protein.
Supplementary Material
Acknowledgments
The authors thank Ed Flynn for excellent fish care; the members of the Jin and Bautch labs for fruitful discussions; the UNC Histology Facility; Michael Hooker Microscopy Facility; William Comb and Maria Aleman for technical assistance; and Drs. Matthias Hammerschmidt and Neil Chi for providing transgenic lines, and Chi-Bin Chien for the kdrl 5’ entry gateway vector. In addition, the authors thank Drs. Frank Conlon, Mark Majesky, Cam Patterson, and John Rawls for invaluable discussion and critical reading of the manuscript. This study was supported by grants from the NIH to S.-W. J. (HL090960) and to V. L. B. (HL43174 and HL86564), and the UNC Integrative Vascular Biology Training Grant (T32HL69768) and an American Heart Association Pre-doctoral Fellowship to D. M. W.
Footnotes
Author Contributions
D.M.W., V.L.B., and S.-W.J. designed the experiments, D.M.W. performed the experiments, J.-D.K. helped with in situ hybridization, J.H. and C.C.H. provided key reagents, D.M.W., V.L.B., and S.-W.J. wrote the manuscript.
References
- 1.Isogai S, Lawson N, Torrealday S, Horiguchi M, Weinstein B. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Covassin L, Villefranc J, Kacergis M, Weinstein B, Lawson N. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. U S A. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbert S, et al. Arterial-Venous Segregation by Selective Cell Sprouting: An Alternative Mode of Blood Vessel Formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro R, et al. Two novel type II receptors mediate BMP signalling and are required to establish left-right asymmetry in zebrafish. Dev. Biol. 2008;315:55–71. doi: 10.1016/j.ydbio.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- 8.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Halloran MC, et al. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- 10.Fürthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 11.Derynck R, Zhang Y. Smad-dependent and Smad-independent pathways in TGF-B family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 12.Pi X, et al. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem. Biol. 2010;19:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeltsch M, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 15.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 16.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 17.Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev. Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- 18.Bressan M, Davis P, Timmer J, Herzlinger D, Mikawa T. Notochord-derived BMP antagonists inhibit endothelial cell generation and network formation. Dev. Biol. 2009;326:101–111. doi: 10.1016/j.ydbio.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Z, et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 20.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev. Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 21.Beppu H, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol. 2000;221:49–58. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 22.Hong KH, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleeff J, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–1216. doi: 10.1016/s0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, et al. Overexpression of BMP-2/4, -5 and BMPR-IA associated with malignancy of oral epithelium. Oral Oncol. 2001;37:225–233. doi: 10.1016/s1368-8375(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 25.Langenfeld EM, et al. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24:1445–1454. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield M. The zebrafish book, A Guide for the laboratory use of zebrafish (Danio rerio) 4th Ed. Eugene: University of Oregon Press; 2000. [Google Scholar]
- 28.Roman BL, et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- 29.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 30.Chi NC, et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev. Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- 33.Thisse B, et al. Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission ( http://zfinorg) 2001 [Google Scholar]
- 34.Thisse C, Thisse B. Zebrafish Science Monitor. Vol. 5. Eugene: University of Oregon Press; 1998. High resolution whole-mount in situ hybridization. [Google Scholar]
- 35.Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 36.Kwan, et al. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.