Abstract
Introduction
Chimeric antigen receptors (CARs) usually combine the antigen binding site of a monoclonal antibody with the signal activating machinery of a T cell, freeing antigen recognition from major histocompatibility complex restriction and thus breaking one of the barriers to more widespread application of cellular therapy. Similar to treatment strategies employing monoclonal antibodies, T cells expressing CARs are highly targeted, but additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion and long term persistence. Furthermore, gene transfer allows the introduction of countermeasures to tumor immune evasion and of safety mechanisms.
Areas covered
The authors review the basic structure of so-called first and later generation CARs and their potential advantages over other immune therapy systems. It is described how these molecules can be grafted into immune cells (including retroviral and non-retroviral transduction methods) and strategies to improve the in vivo persistence and function of immune cells expressing CARs are discussed. Examples of tumor associated antigens that have been targeted in preclinical models are presented and clinical experience with these modified cells is summarized. Finally, a discussion on safety issues surrounding CAR gene transfer into T cells and potential solutions to them, are presented.
Expert opinion
Because of recent advances in immunology, genetics and cell processing, CAR-modified T cells will likely play an increasing role in the cellular therapy of cancer, chronic infections and autoimmune disorders.
Keywords: Chimeric antigen receptor, T-body, Gene therapy, Cellular therapy, Adoptive T cell therapy
1. Introduction
Allogeneic hematopoetic stem cell transplantation is probably the first example of a cellular therapy exploiting an antitumor immune response, although this effect remained largely unappreciated until follow-up studies demonstrated decreased relapse rates of allogeneic versus syngeneic transplants.1 The recognition of a graft-versus-tumor effect led to the development of strategies using donor lymphocyte infusion (DLI) after transplantation, which were successful against relapsed chronic myelogenous leukemia2 and Epstein-Barr Virus (EBV)-associated post-transplant lymphoproliferative disorder (PTLD).3 The high incidence of severe graft-versus-host-disease (GVHD) after DLI sparked the development of approaches to minimize that risk by ex vivo selecting and expanding virus-specific T cells, which proved very effective.4 For example, in vitro expanded, donor-derived EBV-specific cytotoxic T lymphocytes (CTLs) have been safely administered, or adoptively transferred, to numerous patients and shown to proliferate in vivo, persist for more than a decade and localize to sites of EBV-associated tumors. No disease has been detected in more than one hundred patients at high risk of developing EBV-PTLD who received CTL prophylaxis (versus 12% of controls), and complete and sustained resolution of these tumors has been observed in 11 out of 13 patients with resistant lymphoma.5
In contrast to tumor-associated viral antigens, which have been successfully targeted by in vitro selected T cells, most other tumor-associated antigens (TAAs) have proved more challenging, a fact no doubt related to most known TAAs being endogenous and thus more likely to evoke tolerance.6, 7 One approach to obtain consistent manufacture of T cells reactive against weakly immunogenic TAAs has been the expression of transgenic T cell receptors (TCR) in lymphocytes. While some efforts have focused on expressing rare, naturally occurring, self- or allo-reactive, tumor-specific TCRs in T cells,8 many groups have developed instead artificial receptors that are engineered to bind specifically to TAAs. These receptors couple a major histocompatibility complex (MHC)-unrestricted interaction between a TAA and its recognizing molecule to the activating signal machinery of T cells and, because they combine portions of different molecules, they are usually referred to as chimeric antigen receptors (CARs). Here, we will review the basic structure of CARs and their potential advantages over other systems. We will describe at length how these molecules can be grafted into immune cells, discuss issues related to improving their persistence and function in vivo, and give examples of antigens that have been targeted. We will finish with a discussion on safety issues surrounding CAR gene transfer into T cells and potential solutions for these problems.
2. CARs
2.1. Basic structure
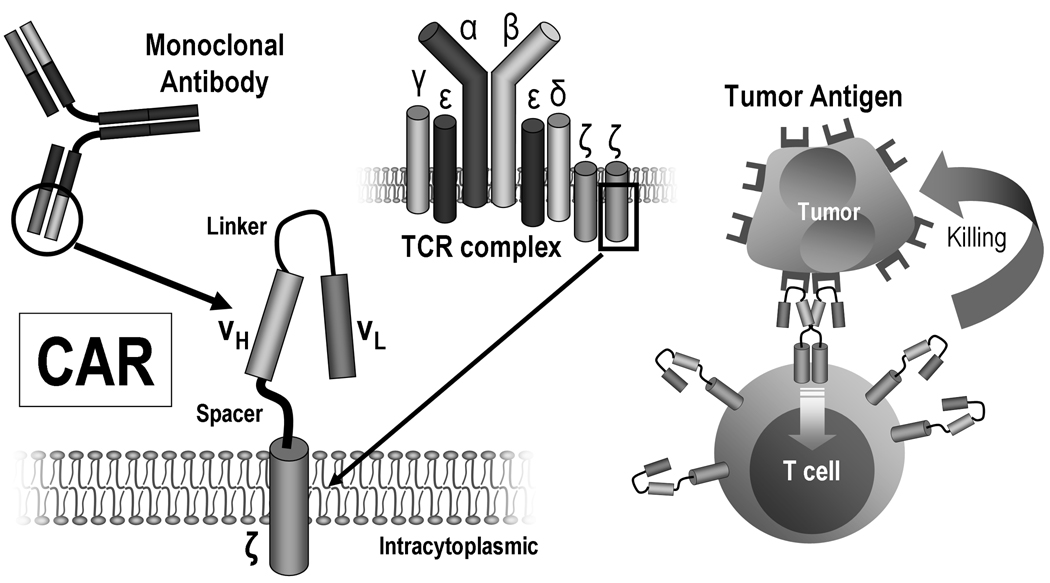
A CAR combines the binding site of a molecule that attaches strongly to the antigen being targeted (i.e., a “binding portion”) with the cytoplasmic domains of conventional immune receptors responsible for initiating signal transduction that leads to lymphocyte activation (the “signaling portion”).9–12 Most commonly, the binding portion used is derived from the structure of the Fab (antigen binding) fragment of a monoclonal antibody (mAb) that has high affinity for the antigen being targeted (Fig. 1). Because the Fab is the product of two genes, the corresponding sequences are usually combined via a short linker fragment that allows the heavy-chain to fold over the light-chain derived peptides into their native configuration, creating a single-chain fragment variable (scFv) region.13, 14 As many of the original CARs systems attached an antibody fragment to a T cell, they were also called “T-bodies”.15 Other possible antigen binding moieties include signaling portions of hormone or cytokine molecules,16, 17 the extracellular domains of membrane receptors18 and peptides derived from screening of libraries (e.g. phage display).19, 20
Figure 1. The basic structure of a monoclonal antibody (mAb)-derived chimeric antigen receptor (CAR).
The most common CARs combine the extracellular antigen recognition site of a mAb and the intracellular domains of a T cell receptor complex (TCR) molecule, such as the ζ-chain. Clustering of CARs induced by antigen binding is thought to be responsible for initiating signal transduction that leads to T-cell activation and killing of the cells expressing the target antigen.
Since some degree of flexibility between the binding and the signaling portions of the CAR may be desirable and because projection of the antigen recognition domains away from cell surface may be required for better binding to the antigen, a hinge region bridging the binding and signaling portions is generally included in the construct. Examples include the CH2CH3 portion of an immunoglobulin molecule such as IgG1. The importance of the hinge region is illustrated by studies which demonstrated that, for the same targeting construct, optimal T cell activation depends on the relative length of this spacer region and the distance of the epitope from the target cell membrane.21–24 For instance, juxtamembrane epitopes require in general longer spacer regions than those farther away from the membrane.
The signaling portion of CARs contains usually the intracellular domains of the zeta (ζ) chain of the TCR/CD3 complex25 or, less commonly, of the gamma (γ) chain of the immunoglobulin receptor FcεRI26, 27 or the CD3-epsilon (ε) chain,28 with the transmembrane region being derived from the same molecules or other type I transmembrane proteins such as CD4, CD8 or CD28.
2.2. Advantages and disadvantages of using CARs
Similar to treatment strategies employing monoclonal antibodies, T cells expressing transgenic native TCRs or CARs are highly targeted, but additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion and long term persistence. Furthermore, gene transfer allows the introduction of countermeasures to tumor immune evasion and of safety mechanisms. Compared to transgenic native TCRs, though, chimeric antigen receptors have human leukocyte antigen (HLA)-unrestricted activity and can target non-protein antigens (Table I). This is important as many tumors adopt immune evasion strategies affecting MHC processing and presentation,29–32 and several TAA are carbohydrates or glycolipids. Additionally, because CARs are single molecules that do not, in principle, interact with either of the native TCR chains, they are not subject to mispairing with complementary chains, a problem that can occur when four (two native and two transgenic) TCR chains are expressed in the same lymphocyte: unless the two native TCR chains are selectively ablated, pairing of a native chain with a transgenic chain can lead to decreased antigen recognition through the transgenic receptor and, even worse, off-target effects if an unwanted specificity happens to be created. This has been recently documented in vitro33 and in a murine model,34 in which mixed TCRs (formed from pairing of one endogenous and one exogenous chain) were shown to drive autoreactivity. In the latter study, a syndrome resembling transfusion-associated GVHD was observed, the manifestations of which could be ameliorated by the use of systems that reduced pairing of endogenous and exogenous TCR chains. After publication of these studies, however, it was noted that no such complications have been observed after infusion of transgenic TCR-modified T cells in more than 100 human subjects.35
Table I.
Examples of tumor associated antigens that have been targeted with CARs.
| Tumors/Antigens | Description | Trials |
|---|---|---|
| Gastrointestinal | ||
| EGP2/EpCam27, 111 | Epithelial glycoprotein 2/Epithelial cell adhesion molecule | No |
| EGP40112 | Epithelial glycoprotein 40 | No |
| TAG72/CA72-419, 113 | Tumor associated glycoprotein 72/Cancer antigen 72-4 | No |
| Glioblastoma | ||
| IL13Rα216 | Interleukin 13 receptor alpha-2 subunit | Yes* |
| Kidney | ||
| G250/MN/CA IX114 | Carbonic anhydrase IX | Yes100 |
| Lymphoid malignancies | ||
| CD1973, 75, 115–117 | – | Yes38, 82, 102, 106 |
| CD2041, 47, 52, 118 | Membrane-spanning 4-domains subfamily A member 1 | Yes38, 41, 118 |
| CD2223 | Sialic acid-binding Ig-like lectin 2 | No |
| CD3076, 119 | Tumor necrosis factor receptor superfamily member 8 | Yes* |
| κ107 | Kappa light chain | Yes* |
| Melanoma | ||
| GD3120 | GD3-Ganglioside | No |
| HLA-A1+MAGE-1121, 122 | Human leukocyte antigen A1 + Melanoma antigen 1 | No |
| Neuroblastoma | ||
| CD171123 | L1 cell adhesion molecule | Yes39 |
| GD2124 | GD2-Ganglioside | Yes40 |
| NCAM36 | Neural cell adhesion molecule | No |
| Ovary (mainly) | ||
| FBP/αFR26, 74 | Folate binding protein/alpha folate receptor | Yes101 |
| Le(Y)125, 126 | Lewis-Y antigen | No |
| MUC124 | Mucin 1 | No |
| Prostate | ||
| PSCA127 | Prostate stem cell antigen | No |
| PSMA45, 128 | Prostate-specific membrane antigen | Yes* |
| Rhabdomyosarcoma | ||
| FAR129 | Fetal acetylcholine receptor | No |
| Several solid tumors | ||
| CEA28, 130–132 | Carcinoembryonic antigen | Yes* |
| ERBB2/HER248, 133–137 | Avian erythroblastic leukemia viral oncogene homolog 2/Human epidermal growth factor receptor 2 | Yes53 |
| ERBB3+ERBB417, 138 | Avian erythroblastic leukemia viral oncogene homolog 3 + 4 | No |
| Mesothelin49 | – | No |
| Various tumors | ||
| CD44v6139 | Hyaluronate receptor variant 6 | No |
| VEGFR2/FLK1/KDR140–142 | Vascular endothelial growth factor receptor 2/Fetal liver kinase 1/Kinase domain insert receptor | Yes* |
Clinical trials listed on http://www.clinicaltrials.gov, but whose results have not been published.
Nonetheless, a limitation of CARs not relying on antigen processing is that only surface antigens can be recognized by them. Also, the presence of soluble antigen shed by tumors could compete with binding to and killing of the malignant cells,15 although at least in the settings investigated so far this seems not to be a major issue. In vitro studies have shown that anti-CEA CARs are not inhibited by soluble CEA, even at high concentrations.28, 36, 37
2.3. First and later generation CARs
The initially published CARs were designed with a single signaling domain.9–12 Several studies employing T cells modified with these so-called first generation CARs established the feasibility of the approach, but showed very limited clinical benefit.38–41 This has been primarily thought to be due to ineffective or incomplete activation of these cells, leading to a very limited persistence, compared for instance with that of EBV-specific CTLs, which have been detected in circulation up to 15 years after infusion.5
To exert its function, a T cell requires binding through its TCR to its cognate (native) antigen presented by an HLA molecule (resulting in the so-called “signal 1”). So as to become fully activated, however, a naïve T cell requires additional stimulatory events prompted by neighboring cells. Otherwise, the end result of stimulation through the TCR is T cell apoptosis or anergy. Many of these additionally required pathways have been described, including activating ligands displayed on the surface of the cells presenting the antigen, which bind costimulatory molecules in T cells (leading to the generation of a “signal 2”), and stimulatory cytokines secreted by the same or other nearby cells (sometimes referred to as “signal 3”).42 Examples of these ligands include CD80 and CD86, normally present in activated antigen presenting cells, which bind the costimulatory CD28 receptor, expressed by T cells.
As tumor cells often lack expression of the costimulatory ligands involved in physiologic activation of T cells,6 this has been assumed to be the basis for the modest activation, expansion and persistence of T cells expressing first generation CARs. Additionally, the prolonged expansion period of T cells in vitro may also be associated with downregulation of the receptors for those costimulatory ligands, further compounding the problem.
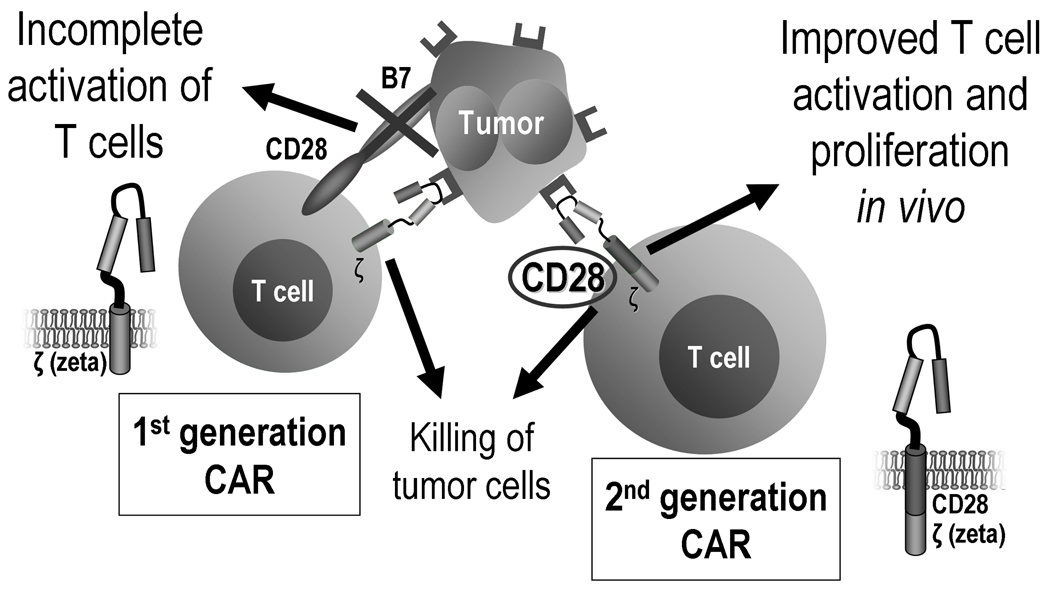
So as to provide T cells with additional activating signals, more recently developed second generation CARs have been engineered to include another stimulatory domain, usually derived from the intracytoplasmatic portion of costimulatory molecules, such as CD28 (Fig. 2), CD134/OX40, CD137/4-1BB, Lck, ICOS and DAP10.43–51 As these costimulatory domains are incorporated in the CAR, its activation by engagement with the respective antigen delivers both signal 1 and signal 2 to T cells, bypassing the need for costimulatory ligands and preventing potential anergy or apoptosis resulting from a solitary signal 1. Many in vitro and preclinical studies comparing first and second generation CARs demonstrate improved function of T cells bearing the latter.43–51 Third generation CARs incorporating 3 or more stimulatory domains have also been described,24, 48, 52 but it is unclear whether the strong costimulation potentially obtained will always be advantageous.53
Figure 2. First versus second generation CARs.
First generation CARs include a single stimulatory domain. Because most tumors do not express costimulatory molecules (“signal 2”), T cells are incompletely activated even when the CAR is engaged by the target antigen. Second generation CARs contain one additional costimulatory endodomain, which is thought to improve T cell activation and proliferation, and thus promote better killing of the target tumor cells.
3. Grafting CARs into immune cells
3.1. Gene transfer methods
Although it is possible to transiently express CARs in T cells by transfecting them with naked DNA plasmids or mRNA (for instance, to quickly and reversibly test them for toxicity), in most instances the goal is to obtain constitutive expression of the CAR. As such, techniques that achieve permanent genetic modification of T cells are usually employed.54 The most frequently used strategy uses gammaretroviruses or lentiviruses (both members of the retrovirus family) that are engineered to encode the full length CAR molecule. Upon infection of the target cells, the viral genomic RNA gets retrotranscribed by the virus reverse transcriptase into DNA, which in turn gets randomly inserted into the host cell DNA through the action of a viral integrase, thus becoming part of that cell’s genome.55 Because they lack key genes (including gag, pol and env) the retroviruses used are replication-defective, meaning that they are unable to complete their life cycle by proliferating and infecting other cells.56 The probability of generating replication competent retroviruses (RCR) during their mass production or spontaneously after target cell infection is very low, but ruling out these events are part of the quality assurance and quality control processes that are required during manufacture of the CAR-expressing T cells employing these manufacturing methods.57
In contrast to lentiviruses, which are capable of infecting resting cells, gammaretroviruses require that their target cells are dividing for successful integration.58 Because peripheral blood lymphocytes, the usual starting population, are not actively cycling, they need to be activated in vitro before they can be successfully transduced by gammaretroviruses (in fact, even lentiviruses integrate better into dividing than resting cells).
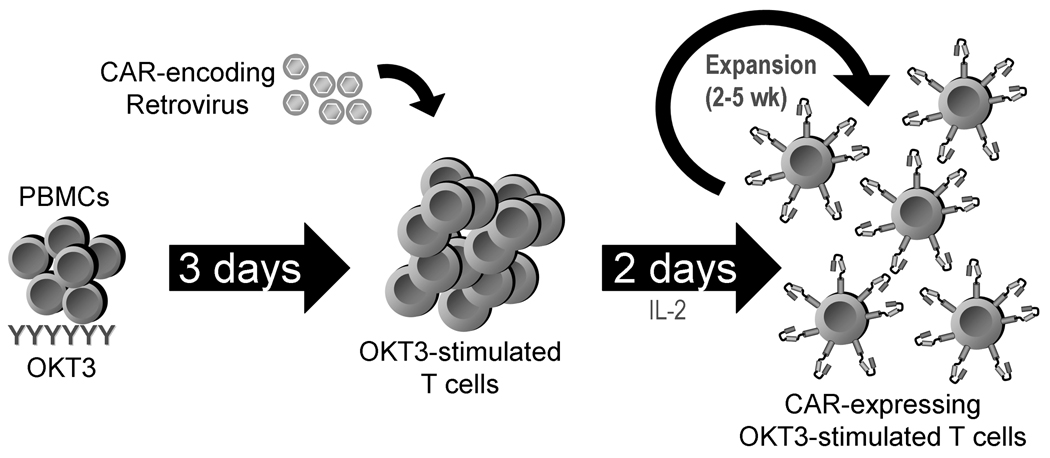
Two methods can be employed to obtain a population of activated T cells. Most commonly, polyclonal stimulation of T cells is obtained by using an activating monoclonal antibody anti-CD3 (OKT3). The “lymphoblasts” (OKT3-blasts) thus obtained are easily transducible by retroviruses (Fig. 3). Concomitant stimulation of native CD28 receptors by another agonistic monoclonal antibody facilitates T cell activation further. After stimulation and transduction, which takes approximately 48 hours, the T cells are kept in culture for 2 to 3 weeks in IL-2 containing medium until sufficient numbers for clinical application are reached.59 Alternatively, T cells with particular specificities can be selectively expanded in the presence of antigen presenting cells (APCs), such as dendritic cells or lymphoblastoid cell lines (LCL).60 As antigen recognition and interaction with APCs drives activation of T cells, these antigen-specific T cells can then be transduced with a retrovirus encoding a CAR, generating bispecific cell lines, which may have some advantages over OKT3-activated lines (discussed below).
Figure 3. Retroviral transduction of T cells and expansion of CAR-expressing T cells.
T cells are activated from the patient’s peripheral blood mononuclear cells (PBMC) by stimulation with anti-CD3 antibody (OKT3) and these activated T cells are expanded with interleukin 2 (IL-2) and transduced with a replication incompetent retrovirus encoding the CAR. Further expansion in IL-2-containing medium is done until sufficient numbers for clinical application are reached.
Because retroviral production under good manufacturing practices (GMP) is expensive, some groups have adopted non-viral methods for permanent transduction, specifically transposon-based systems, including Sleeping Beauty61 and PiggyBac.62 These usually require double transfection with one plasmid containing the expression cassette for the desired CAR and another encoding a transposase. Once expressed in the target cells, the transposase catalyzes the integration of the CAR gene into specific, though pretty much randomly distributed, sites throughout the genome. The reaction is essentially irreversible and thus leads to stable integration of the gene of interest.
Selection of a particular transduction method depends on its potential advantages and disadvantages and also on each center’s experience and logistics. Retroviral transduction is very efficient, minimizing the time to achieve clinically useful cell numbers. However, as noted above, it is an expensive method, there is a potential concern of generating replication competent retrovirus (although this has never been observed in any trial so far), and viral genes may be immunogenic, curtailing survival of the transduced cells. Earlier non-viral methods relied on electroporation of cells with a naked DNA plasmid encoding the CAR and its illegitimate recombination for stable genomic integration.63 The efficacy of this procedure is low and requires long cell culture periods to allow selection of T cells with stable integrants, during which cells may become terminally differentiated or senescent. The newer non-viral methods described above are more effective and may circumvent some of these issues, but they have not been directly compared to retroviral transduction.
Depending on the final application and the overall efficacy of the transduction method, a selectable marker (such a surface antigen or an antibiotic resistance gene) can be used to facilitate purification of the transduced cells. This is especially important when the transduction is done using methods that have lower success rates.63 On the other hand, because some selectable markers may be immunogenic and compromise survival of the transduced cells in vivo, alternative selection methods have been proposed. One such approach engineers T cells to express a cytokine receptor, such as that for IL-4, in cis with the CAR.64 After T-cell transduction, if the resulting cellular product is grown in the presence of that cytokine, those cells successfully expressing the transgenic cassette will expand preferentially over the untransduced cells. In addition, administration of that cytokine after adoptive transfer of the modified T cells may selectively support their growth in vivo, although their behavior in this setting is currently unknown.
3.2. Populations transduced
Most studies have focused on the genetic modification of the most abundant of the lymphocytes subsets, i.e. αβ-TCR+ T cells, in particular, CD8+ cells, as these are thought to be the immediate effectors of tumor cell destruction after CAR engagement. Unless PBMCs are fractionated prior to gene transduction, however, CD4+ cells are also activated and included in the CAR-transduced population. This is usually not a problem, as no additional toxicity has been described for CD4+ CAR+ T-cells and because these cells may provide help for CD8+ CAR+ T cells in vivo or have themselves significant cytotoxic activity.44, 65 Indeed, several publications have highlighted the synergistic effects of CAR-bearing CD8+ and CD4+ T cells in murine models, including HER266 and CD19-specific67 CARs.
As the gene transfer procedures are not specific for T cells, other populations of immune cells, which may offer specific advantages, can also be transduced using the same methods. For instance, natural killer (NK) cells have intrinsic lytic potential and some CARs have been engineered to contain predominantly NK-activating endodomains. This, combined with new NK cell expansion systems, is a possible alternative to T cells.68 Moreover, another population with endogenous killing ability, γδ-TCR+ T cells, can be easily expanded in vitro with bisphosphonates and is amenable to transduction with a CAR.69 One advantage of this system is that bisphosphonates are drugs that have been approved for other medical uses and are routinely used in the treatment of patients with cancer.70 Whether any of these populations is more effective at tumor killing is unclear at this point.
Finally, while most often the aim of CAR-modified T-cell therapy is to trigger immune responses against endogenous TAA, there is also interest in using this approach to dampen immune responses to endogenous antigens by transducing populations of T cells whose primary function is to inhibit immune responses, such as regulatory T cells. The rationale in this setting is that engagement of a CAR expressed by a regulatory T cell will trigger their immunosuppressive machinery and thus limit or prevent immune responses against tissues expressing the targeted antigen.71 If successful, this approach could be used in the management of autoimmune disorders.
4. Expansion and persistence in vivo of CAR-expressing cells
Although many CAR constructs have been shown to be effective in vitro and in animal models at mediating killing of their target cells, few clinical studies have shown consistent anti-tumor effects. This limitation is thought to be in part, at least for trials employing first generation CARs, due to the fact that tumor cell engagement of the CAR alone fails to sustain sufficient T-cell growth and activation for tumor eradication. T cells require multiple additional, or costimulatory, signals to produce optimum activation, proliferation and survival following antigen receptor engagement.72 As discussed above, incorporating one or more endodomains from the required costimulatory molecules into the CAR itself can enhance T cell performance following chimeric receptor engagement. However, even T cells expressing second generation CARs have been observed to have limited persistence in vivo after infusion in patients, highlighting the need to find alternative methods of optimizing their expansion after adoptive transfer.
4.1. Bispecific cells
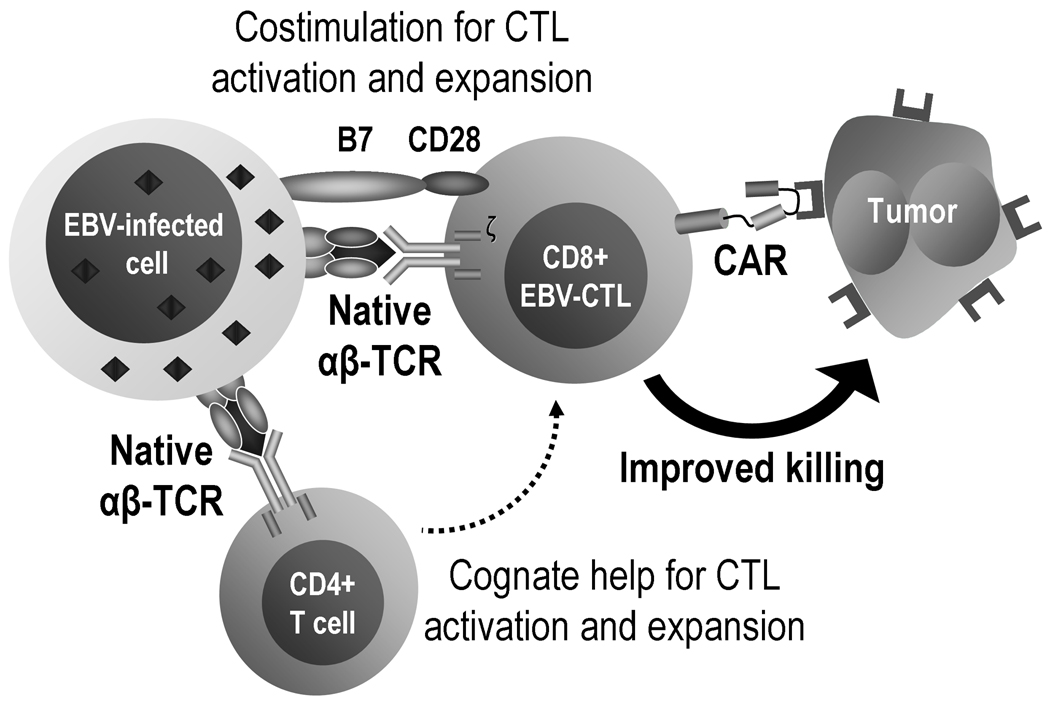
Under normal conditions, costimulation occurs in a temporo-spatially coordinated manner,72 which may not be fully replicated within the T cells when the CAR simultaneously generates costimulatory signals after antigen engagement, as is the case with later generation CARs. An alternative to achieve a more physiological solution to this problem has been to graft the CAR into virus specific CTLs40, 73–76 instead of a non-specifically, polyclonally activated T cell population. CTLs with native receptor (αβ-TCR) specificity directed to persistent human viruses, such as Epstein-Barr virus (EBV) or cytomegalovirus (CMV), should receive physiological costimulation in vivo during their encounters with persistent viral antigens on professional APCs.77 As such CTLs divide and persist in vivo for many years following infusion,4, 5 if they are modified to coexpress a CAR directed at a TAA, they too should obtain physiologic costimulatory signals following engagement of their native receptors, which should in turn enhance their survival and their CAR-mediated anti-tumor activity (Fig. 4). This hypothesis has obtained preliminary support in patients receiving first generation CTLs expressing a CAR directed to the GD2 antigen on neuroblastoma (summarized in section 6.1).40 In any case, the levels of expansion observed appear lower than those expected for untransduced virus-specific T cells, which have been seen to persist for several years.5 Potential reasons for this finding include adverse effects of the retroviral transduction on the physiology of the T cells and the lack of lymphodepletion prior to adoptive transfer of these cells in this particular trial.
Figure 4. Epstein-Barr Virus (EBV) and tumor bispecific cytotoxic T lymphocytes (CTLs).
Unlike most tumor cells (including malignant lymphocytes), EBV-transformed cells not only express specific antigens but also high levels of many different costimulatory molecules, including CD40L, CD80 and CD86. Because they express both class I and class II human leukocyte antigen (HLA) molecules, they can present viral epitopes that will stimulate both CD8 and CD4 virus-specific cells, favoring the cognate interactions between T cell subsets that are critical for optimal and sustained immune responses. CTLs with native receptor (αβ-TCR) specificity directed to EBV receive physiological costimulation in vivo during their encounters with persistent viral antigens on professional antigen presenting cells. If these EBV-specific CTLs are modified to co-express a CAR directed to a tumor associated antigen, they will obtain physiologic costimulatory signals following engagement of their native receptors, which in turn will enhance their survival and their CAR-mediated anti-tumor activity.
Whether the physiological co-stimulation received by expressing a first generation CAR directed against a tumor-associated antigen in virus-specific CTLs will be of greater functional benefit in vivo than the incorporation of other costimulatory endodomains (such as CD28) into the same CAR and expressing it in OKT3-blasts is the object of ongoing clinical trials, including a comparison between EBV-specific CTLs carrying a first generation CD19-specific CAR and OKT3-activated T cells bearing a second generation CAR (differing only by the inclusion of a CD28 endodomain) to treat subjects with advanced B-cell malignancies (ClinicalTrials.gov Identifier NCT00709033).
4.2. Memory populations
The result of a physiologic immune response to a foreign antigen is a heterogenous population of cells that play different roles. In particular, a fraction of the cells involved becomes long-lived and is responsible for establishing immunologic memory against the antigen that triggered that response.78 The mechanisms involved in segregating this primarily memory population, akin to a stem cell compartment, from the rest of the immune effectors are incompletely understood, but it is unlikely that polyclonal activation of circulating T cells via the CD3 and CD28 receptors would be able to reproduce the hierarchy of immune cells that is generated during native immune responses. As a consequence, it is possible that most cells that are transduced ex vivo with CARs have mainly effector or effector memory properties but lack memory potential. This offers an alternative explanation for the limited persistence of CAR T cells observed in vivo so far: although, as proven by a plethora of in vitro experiments, these cells are effective at killing targets, they may not able to expand and persist as a memory population. In addition, this could also account for why bispecific cells may have improved persistence in vivo: in a way, expanding and selecting cells in the presence of APCs (the first step in the generation of bispecific cells) likely enlists more physiologic pathways that those achieved by TCR activation outside of an immune synapse.
While we know little about how immune memory is generated, it is commonly accepted that different subsets of circulating T cells, identifiable by distinct surface antigen expression, represent separate compartments of this immune memory hierarchy. For instance, antigen-experienced T cells (i.e. memory cells) can be further divided into central memory and effector memory cells, which differ in functional and homing properties.79 Recently, it has been demonstrated, in a primate model, that adoptively transferred T cells derived from central memory cells sorted from an otherwise homogenous population of T cells are able to persist and give rise to effector cells in vivo longer than a comparable T cell population generated from effector memory T cells.80
Understanding the factors involved in generating and maintaining a memory population, with increased intrinsic replicative potential and self-renewal ability, would thus have tremendous implications for our ability to establish long lasting CAR-modified cell lines. On the other hand, this information would also give us full control to generate a population with a limited lifespan, if a transient effect is being deliberately sought.
4.3. Avoiding immune response against transduced cells
One of the potential problems of using an artificial CAR, especially, as it is often the case, when the new molecule contains portions derived from another species, is that the recipient’s immune system may react against the new protein and eliminate the CAR-bearing cells. Humanized CARs, in which the xenotypic sequences of the variable regions are replaced by their human homologues, have been designed and proposed as a measure to limit immune mediated destruction of the modified cells.28, 48 Evidence for immune recognition of CARs has been recently provided,38, 81 with studies showing generation of cellular and humoral responses against retroviral vector, selection gene (neomycin phosphotransferase) and CAR epitopes, underscoring the need to attenuate the immunogenicity of both transgenes and vectors in this setting.
While immune destruction of CAR-bearing T cells could provide another explanation for the limited persistence of T cells, many patients treated with CAR-T cells have received significantly immunosuppressive therapy quite close to receiving those cells (especially true in the management of hematological malignancies) and thus they may be less likely to mount an immune response against the infused cells. Supporting the idea that immune rejection may not be important in all settings is the fact that re-expansion of CAR-T cells has been observed after a second infusion of the same cells, six or more weeks after initial administration.82 Although these were patients with multiply relapsed lymphomas and extensive chemotherapy exposure, none received lymphodepleting chemotherapy before adoptive T cell therapy. If a primary immune response had resulted from the initial exposure to the CAR-T cells, a secondary response should have quickly eliminated the reinfused cells.
4.4. Improving factors extrinsic to T cells
Besides focusing on improving the intrinsic properties of modified T cells used for cancer therapy, there are a few approaches that that may be helpful at prolonging their survival and that do not necessarily depend on gene transfer. First, it is now well established that lymphocytic expansion is subjected to homeostatic mechanisms that control the total numbers of T cells.83 Therefore, it has become apparent that a certain degree of lymphopenia will facilitate expansion of adoptively transferred T cells. Whereas this will always be the case after hematopoietic stem cell transplantation (given the nature of the conditioning regimens, which are myeloablative and lymphodepleting), cancer patients who have been off chemotherapy for long periods of time may have a full lymphocytic compartment. In this case, induction of lymphodepletion prior to infusion of CAR-T cells may facilitate their expansion after infusion. Presumably, this occurs because lymphopenia creates space for the oncoming adoptively transferred cells and induces their homeostatic expansion. This effect is mediated, on one hand, through chemotherapeutic ablation of endogenous regulatory T cells, which normally secrete inhibitory cytokines, such as TGF-β and interleukin (IL)-10, that limit effector T cell expansion.84 On the other hand, T-cell growth homeostatic cytokines, such as IL-7 and IL-15, which ordinarily exist in limiting amounts, may become readily available due to less competition and increased production by lymphopoetic stromal cells.85 The approach has been previously used and encouraging results have been seen,86–88 although it is difficult to tease out the antitumor effects mediated by the T cells from those of the lymphodepleting drug (which is usually a chemotherapeutic agent that may have intrinsic antitumor activity).
Furthermore, as previously described, provision of T-cell growth factors, such as IL-2,41 IL-789 or IL-15,90 may improve survival of CAR-T cells, especially if these cells are modified to improve the responsiveness to these cytokines.91 Finally, the levels of expression of some TAA may be amenable to modulation using drugs that exert epigenetic effects, such as hypomethylating agents and histone deacetylase (HDAC) inhibitors. Increasing the density of surface antigens may facilitate engagement of CARs and, secondarily, activation and proliferation of the cells bearing those receptors. For instance, in a murine model of melanoma, administration of an HDAC inhibitor was shown to increase MHC and TAA expression by tumor cells, presumably by upregulating their gene expression.92 This, together with beneficial effects on the adoptively transferred cells (mediated by a decrease in competing endogenous lymphocytes also brought about by the drug), led to enhanced tumor immunotherapy.
5. Effector mechanisms
5.1. Killing
Apart from a suggestion that CARs containing a ζ transmembrane domain can form a complex with the endogenous TCR,93 which in turn may drive optimal T cell activation, little is known about the details of the initial activation of CARs. The common assumption is that dimerization or multimerization of the receptors resulting from binding target antigen epitopes brings together their cytoplasmatic signaling domains, which then become targets for intracellular kinases, such as Lck.72 Phosphorylation of the immunoreceptor tyrosine-based activation motives (ITAM) contained in the signaling portion of the CARs then recruit adapter molecules, such as ZAP-70, which in turn stimulate downstream pathways that lead to activation of the transduced cells. Depending on the exact nature of the transduced cell, this leads to release of cytotoxic molecules (such as perforin or granzymes), expression of proapoptotic ligands (such as Fas ligand – FasL, and tumor necrosis factor-related apotosis inducing ligand – TRAIL) or secretion of proinflammatory cytokines (such as IL-2, IFN-γ and TNF-α). As a consequence, tumor cells can be eliminated directly by the CAR-T cells or other immune cells can be recruited to the tumor microenvironment, which usually is hostile to effector T cells because of the presence of immunosuppressive cells (such as regulatory T cells, stromal cells and myeloid derived stromal cells) and inhibitory cytokines secreted by them (such as TGF-β and IL-10).6 As discussed above, incorporation of additional signaling domains may be able to bypass some of the stimulatory events or amplify these effects.
5.2. Improving function
A few additional strategies may be important for optimizing the effector function of CAR-modified T cells. One aspect that has received some attention is that of modulating the affinity of the CAR for its corresponding antigen. Although it would seem beneficial to ensure increased affinity, this may not be necessarily the case.15 On one hand, the binding affinity of TCRs is usually lower than that of antibodies and so having high affinity may not be needed to trigger activation. On the other hand, having lower affinity for the antigen may target preferentially tissues that have higher density of tumor associated antigens (sparing normal tissues), may facilitate disengagement of the CAR and allow the recycling of the T cells, and may decrease inhibition of these cells by soluble antigen. It has been shown, for instance, that the activation threshold for an HER2-specific CAR is inversely correlated with the affinity of the CAR binding domain, such that T cells expressing a lower affinity CAR are activated exclusively by cells with high amounts of HER2, in contrast to cells with a high affinity CAR, which are activated against cells displaying a wide range of HER2 levels. However, the maximum level of cellular activation achieved is comparable in either setting and independent of the binding affinity.94 Therefore, higher affinity CARs do not necessarily induce a more potent activation of T cells than low affinity counterparts and may in fact be associated with less discrimination between target cells with high or low target antigen expression levels. Fine tuning the affinity of the CAR may thus improve the efficacy of CAR-bearing cells.
Moreover, successful effector function requires the CAR-expressing T cell to be able to travel and localize to the targeted tumor sites. This homing process depends on the activation of receptors in the T cells by chemokines released by the tumor. For instance, forcing expression of chemokine receptors, such as CCR4, which may not be adequately upregulated during in vitro activation, may thus improve migration of the CAR-expressing cells.95
Finally, several tumors are intrinsically immunosuppressive because they secrete inhibitory cytokines or because they actively recruit inhibitory cells through, for example, production of CCL2, a chemokine that promotes trafficking of regulatory T cells to tumors.96 To protect CAR-expressing cells from these effects, they can be engineered to express dominant negative receptors for inhibitory cytokines, to display a higher density of receptors for activating cytokines or to secrete stimulating cytokines in an autocrine fashion.
For instance, EBV-positive Hodgkin and non-Hodgkin lymphoma cells secrete large amounts of TGF-β, with suppressive activity over effector T cells that could potentially recognize and destroy these tumors. The expression of a dominant negative TGF-β receptor (TGFβR) on EBV-specific CTLs inactivates the native TGFβR and blocks the inhibitory activity of TGF-β over those cells. TGF-β-resistant CTLs have a functional advantage over unmodified CTLs in the presence of TGF-β-secreting EBV-positive lymphomas and have enhanced antitumor activity.97 Furthermore, it is known that expression of the IL-7 receptor alpha chain (IL-7Rα) is lost during activation of CTLs, precluding their response to this homeostatic cytokine. By genetically modifying CTLs to re-express IL-7Rα, response of these cells to IL-7 can be restored, without apparent modification of their antigen specificity or dependency.91 Finally, transgenic expression of IL-2 and IL-15 in EBV-specific CTLs has been seen to increase the expansion of these CTLs (both in vitro and in vivo in a SCID mouse model) and to enhance their antitumor activity, with their proliferation remaining strictly antigen dependent. Because, for example, systemic administration of IL-2 is associated with toxicity and can cause expansion of unwanted cells, including regulatory T cells, that strategy may overcome these problems.98
6. Targeted antigens
The great majority of the work with CARs has been done in vitro or in animal models. At this point, however, a large number of clinical trials building on this large body of preclinical data is being conducted and their results are beginning to be published. Table I summarizes many of the TAAs that have been targeted so far and provides references detailing the preclinical and, when available, clinical studies. While most studies are geared toward treating malignancies, a few studies have addressed chronic viral infections, such as HIV,18 and autoimmune disorders.99 Because CAR-expressing cells infused with therapeutic intent get quickly diluted in circulation, their direct detection in peripheral blood is usually challenging. To improve sensitivity, quantitative real time polymerase chain reaction (PCR) techniques for amplification of the transgene are commonly used to document their presence and quantify their expansion.
6.1. Summary of clinical experience in cancer patients
As in most phase I trials, the clinical studies published (Table II) have mostly included patients whose disease is unresponsive to standard therapies, with many of them having received multiple chemotherapy regimens before being given adoptive T-cell therapy. In one of the first published clinical studies in cancer, 3 patients were treated with retrovirally transduced T cells bearing a CAR specific for carbonic anhydrase IX, a membrane antigen expressed by renal cell carcinoma cells, and low-dose IL-2. CAR-bearing T cells were transiently detected up to 7 weeks after infusion and no tumor responses were observed. All patients developed low levels of anti-idiotype antibody and evidence of liver toxicity, the latter thought to be caused by off-target effects related to the expression of CA IX in biliary ducts.100 These data have been recently updated (Table II).81
Table II.
Clinical trials using CAR-modified T cells with published results
| Antigen | Targeted disease |
Construct | CAR gene transfer |
Selection | T cell activation | Auxiliary therapy | Cell dose range (×106) |
N | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| CA IX81, 114 | Renal cell carcinoma | scFv + CD4TM + FcεRIγ (1st generation) |
Retroviral | – | Non-specific Non-specific Non-specific |
IL-2 IL-2 IL-2 + anti-CA IX mAb |
20–2000/pt 100/pt 100/pt |
3 5 3 |
NR NR NR |
| CD17139 | Neuroblastoma | scFv + CH2CH3 + CD4TM + CD3ζ (1st generation) |
Electroporation | Hygromycin | Nonspecific | – | 100–1000/m2 | 6 | NR |
| CD1982 | B-cell NHL and CLL | scFv + CH2CH3 ± CD28 + CD3ζ (1st and 2nd generation) |
Retroviral | – | Non-specific | – | 40–400/m2 | 6 | 2 SD, 4 NR |
| CD1938 | B-cell NHL | scFv + CH2CH3 + CD4TM + CD3ζ (1st generation) |
Electroporation | Hygromycin | Non-specific | FLU* and IL-2 | 100–2000/ m2 | 2 | NR |
| CD19102 | B-cell NHL | scFv + CD28 + CD3ζ (1st generation) |
Retroviral | – | Non-specific | Lymphodepletion (CTX/FLU) and IL-2 | 40/pt | 1 | PR |
| CD19106 | B-cell CLL | scFv + CD28 + CD3ζ (2nd generation) |
Retroviral | – | Non-specific | Lymphodepletion (CTX) | 10–30/kg | 1 | Death |
| CD2038 | B-cell NHL | scFv + CH2CH3 + CD4TM + CD3ζ (1st generation) |
Electroporation | Hygromycin | Non-specific | ASCT (infusion at day 28) | 100–2000/ m2 | 2 | cCR |
| CD2041 | B-cell NHL | scFv + CH2CH3 + CD4TM + CD3ζ (1st generation) |
Electroporation | Neomycin | Non-specific | – or IL-2 | 100–3300/m2 | 7 | 2 cCR, 1 PR, 4 SD |
| αFR101 | Ovarian carcinoma | scFv + FcεRIγ (1st generation) |
Retroviral | G418 | Non-specific Allogeneic PBMC |
IL-2 Allogeneic cell immunization |
3000–50,000/pt | 8 6 |
NR NR |
| GD240 | Neuroblastoma | scFc + CH2CH3 + CD3ζ (1st generation) |
Retroviral | – | Non-specific and EBV-LCL | – | 40–400/m2 | 11 | 2 CR, 3 cCR, 2 tn, 2 SD, 2 NR |
| HER253 | Colorectal cancer | scFv + CD8TM + CD28 + CD137 + CD3ζ (3rd generation) | Retroviral | Non-specific | Lymphodepletion (CTX/FLU) | 10,000/pt | 1 | Death |
Used after first cell infusion.
Abbreviations: TM: transmembrane segment, –: none, PBMC: peripheral blood mononuclear cells, EBV-LCL: Epstein-Barr virus lymphoblastoid cell line, mAb: monoclonal antibody, CTX: cyclophosphamide, FLU: fludarabine, ASCT: autologous stem cell transplantation after high dose chemotherapy, pt: patient, NR: no response, SD: stable disease, PR: partial response, cCR: continued complete response (i.e. patient had no evidence of disease before and after infusion), CR: complete response; tn: tumor necrosis (without reaching criteria for PR).
A study employing a CAR directed at the alpha-folate receptor (αFR) expressed in ovarian carcinoma demonstrated by PCR analysis that gene-modified T cells were present in the circulation in large numbers for the first 2 days after transfer, but that they quickly declined to almost undetectable 1 month later in most of the 14 patients treated. T cells were transduced via a retrovirus and activated with OKT3 or with allogeneic cells. An inhibitory factor, most likely an antibody, developed in half of 6 patients tested over the period of treatment, which significantly reduced the ability of gene-modified T cells to respond against tumor cells.101
T cells modified with a CAR specific for CD171, a cell adhesion molecule that is overexpressed in metastatic neuroblastoma and that may be involved in progression of the disease, were used for treatment of neuroblastoma patients in another CAR clinical trial. These cells were subjected to polyclonal activation, electroporation with a plasmid encoding the CAR and a marker, and hygromycin selection. No overt toxicities to tissues known to express CD171, such as the central nervous system, were observed. The persistence of T cells by PCR was shorter than 1 week in most patients with bulky disease, but up to 6 weeks in a patient with smaller disease burden.39 In another clinical trial in neuroblastoma, 11 patients were treated with identical numbers of polyclonal OKT3-activated T cells and EBV-specific LCL-stimulated T cells, with both cell populations expressing a GD2-ganglioside-specific CAR. No toxicities were observed and T-cell infusion was associated with tumor regression or necrosis in half of the subjects tested. The modified EBV-specific T cells demonstrated longer survival, of up to 6 weeks.40
Several trials addressing treatment of lymphoid malignancies have been reported recently. In one of the studies, 7 patients with follicular or mantle cell lymphomas received CD20-specific CAR-modified T-cell infusions, with minimal toxicities. T cells were subjected to polyclonal activation, plasmid electroporation and neomycin selection. The modified T cells persisted in vivo up to 9 weeks in patients who also received low-dose subcutaneous IL-2 injections. Two patients had continued complete response, one achieved partial response, and 4 had stable disease.41 In another study, 2 patients with recurrent diffuse large B cell lymphoma were treated with cloned CD8+ T cells expressing a CD20-specific CAR (and neomycin resistance) after autologous hematopoietic stem cell transplantation, and 2 patients with refractory follicular lymphoma were treated with polyclonally activated T cells bearing a CD19-specific CAR (and hygromycin resistance) and low-dose IL-2. Neither clinical responses nor overt toxicities were observed. Detection of transferred T cells by PCR was shorter than 7 days. Cellular antitransgene immune rejection responses were noted in 2 patients.38 Another recent report describes a patient with advanced follicular lymphoma treated with a preparative chemotherapy regimen followed by autologous T cells retrovirally modified to express a CD19-specific CAR. The patient's tumor underwent significant partial regression and B cells were absent from circulation for at least 39 weeks after T-cell infusion, despite recovery of other blood cell counts. The CD19-CAR transgene was detected in the peripheral blood up to 27 weeks after infusion.102
Finally, results of a phase I trial in which subjects with refractory/relapsed B cell lymphomas were simultaneously infused with two autologous T cell products, both expressing a CD19-specific CAR but with one CAR encoding both CD28 and ζ endodomains while other including only the ζ endodomain, are now available. This strategy allowed direct measurement of the consequences of adding a CD28 co-stimulatory endodomain to CAR-redirected T cells in the same subject and established that T cells bearing a CAR that contains the CD28 endodomain have enhanced in vivo proliferation and survival compared to T cells expressing a CAR lacking CD28. Clinical responses were limited, with two patients having transient stable disease and four showing progression of disease.82
7. Safety concerns
7.1. Insertional mutagenesis
One of the concerns with any technique that causes permanent genetic changes is that of insertional mutagenesis, especially when there is little to no control over the sites of integration. Although these fears have materialized in studies involving genetic modification of hematopoietic stem cells, in which some children with severe combined immunodeficiency (SCID) who were treated with common gamma-chain receptor genes developed leukemia,103 this has not been seen in any of the preclinical and clinical studies reported so far. That CAR-based gene transfer targets differentiated T cells, which have a lower risk of malignant transformation, instead of hematopoietic stem cells, and that it delivers transgenes whose stimulation is antigen driven, so that positive selection would occur only in the presence of the target antigen, and whose signal transduction augments committed cytotoxic effector cell function, could explain why this has not been a problem so far. Furthermore, at least in a model in which murine T cells were retrovirally transduced and adoptively transferred into congenic mice, there was no excess tumor incidence compared to controls and none of the tumors observed were of donor T-cell origin.104
7.2. Off-target effects
A more significant concern has been potential off-target effects of the CAR-modified T cells. Most antigens targeted by CARs are not tumor “specific”, but simply tumor “associated”. This means that even though tumors may display higher densities of these antigens, they are shared with normal tissues in the body. While some of those tissues may be shielded from immune attack by physiological barriers, such as testes, many are not. In some instances, bystander attack of normal tissues may be acceptable, as would be the case with the destruction of normal B cells by CARs targeting CD19 or CD20, provided that the malignancy being treated is controlled by the modified T cells. In other cases, however, the implications are more serious. For instance, as described above, CA IX is expressed both in renal cell carcinoma and the biliary tree, which may explain the observation that patients treated with T cells expressing a CAR targeting that antigen developed significant liver toxicity.100 Furthermore, a recently reported fatal adverse event in a trial employing a HER2-specific CAR for treatment of metastatic colorectal cancer, in which a patient developed acute respiratory failure, is thought to be related to low level expression of HER2 in the pulmonary parenchyma or vasculature.53
Identical problems have been observed with transgenic native TCRs. In a trial of melanoma patients with T cells expressing TCRs against MART-1 or gp100 (antigens expressed by melanoma and pigmented epithelia), several patients experienced transient skin rash, uveitis or hearing loss, thought to be due to cytotoxic effects against normal melanocytes.8 In another protocol using CEA-specific (an antigen expressed by colorectal cancer but also, in lower levels, by normal colonic mucosa) transgenic TCRs, all three patients treated developed dose limiting transient inflammatory colitis.105
7.3. Systemic inflammatory reaction, cytokine storm and tumor lysis syndrome
As with any therapy achieving significant tumor cell destruction, adoptive CAR-T cell therapy can in theory lead to release into circulation of nucleic acid catabolites (including urates and phosphates) and intracellular ions (mainly potassium), a condition known as tumor lysis syndrome. In parallel, massive cell activation resulting from engagement of CARs by tumor antigens could result in release of significant amounts of proinflammatory cytokines into circulation, such as IFN-γ and TNF-α, a situation that has been referred to as cytokine storm. These syndromes are life threatening and, therefore, close monitoring of patients receiving these cells is required.
Of note, a patient with bulky chronic lymphocytic leukemia, treated with cyclophosphamide followed 2 days after by autologous T cells retrovirally transduced with a second generation CD19-specific CAR, developed a fatal syndrome of hypotension, dyspnea, renal failure and fever (Table II). An autopsy failed to reveal an obvious cause of death and sepsis was thought to be the most likely precipitating factor, but the possibility that a cyclophosphamide-induced “cytokine storm” that may have enhanced the in vivo activation of modified T cells was also considered, as elevated cytokine levels were seen before the T-cell infusion.106
7.4. Safety mechanisms
In some circumstances, it may be possible to identify alternative, more restricted tumor antigens. An example of this is targeting the kappa107 or lambda light chains, a strategy which would spare B cells expressing the alternative light chain. Most often, however, the introduction of a safety mechanism allowing quick, on-demand elimination of gene modified T cells should any untoward effects occur is desirable. Several inducible suicide systems, which could be encoded in cis with the CAR gene, have been described.108 Strategies involving nucleoside analogues, such as those combining Herpes Simplex Virus thymidine kinase (HSV-tk) with ganciclovir (GCV) and bacterial or yeast cytosine deaminase (CD) with 5-fluoro-cytosine (5-FC) have been used successfully in some cases, especially as a way to eliminate alloreactive T cells infused as part of a hematopoietic stem cell transplant.109 A drawback of these systems is that they are cell-cycle dependent and unlikely to be effective in non dividing cells. Additionally, the prodrugs required for suicide may themselves have therapeutic uses that are therefore excluded (e.g. GCV), or may be toxic (e.g. 5-FC). Also, nonhuman enzymatic systems, such as HSV-tk and CD, carry a high risk of destructive immune responses against transduced cells.
An inducible caspase-9 (iCasp9) system, which addresses some of the problems of existing approaches, has also been developed. This engineered protein can be activated using a specific chemical inducer of dimerization (CID), a functionally identical analogue of which (AP1903) has been safely tested in a phase I study in humans. The iCasp9 suicide gene is of human origin, and thus should be less likely to induce unwanted immune responses. Transduction of lymphocytes by this gene construct is stable and the cells are quickly and specifically eliminated less than 24 hours after exposure to CID.110 It has recently been shown that this approach can also be extended to other cell types, including post-mitotic tissues.108 All this suggests that an inducible suicide system could be used for increased safety of CAR-modified T cells.
8. Conclusion
CARs endow T cells with MHC-unrestricted ability to recognize cellular surface antigens, freeing antigen recognition from HLA restriction and thus breaking one of the barriers to more widespread application of cellular therapy. Similar to treatment strategies employing monoclonal antibodies, T cells expressing CARs are highly targeted, but additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion and long-term persistence. Furthermore, gene transfer allows the introduction of countermeasures to tumor immune evasion and of safety mechanisms. Although CAR-modified T cells have been used in several clinical trials, the benefits observed so far have been modest. Incorporation of more potent costimulatory domains into the CAR-molecule, better gene transfer methods, transduction of specific memory populations, infusion of the modified T cells into a lymphodepleted host and provision of T-cell-specific cytokines are expected to improve these results, by enhancing the activation of the engineered T cells and prolonging their persistence in vivo. As we learn more about the mechanisms responsible for immune activation and generation of memory, CAR-modified T cells will likely play an increasing role in cellular therapy of cancer, chronic infections and autoimmune disorders.
9. Expert opinion
The long and winding road of immunotherapy has recently led us to some successes. CAR-based technology is a major step forward towards off-the-shelf cellular therapy. Although still requiring patient-specific (autologous or HLA-matched allogeneic) lymphocytes, these cells can be modified to express an identical antigen receptor regardless of the HLA context, meaning that each CAR is truly universal.
Improved gene transfer methods, better understanding of the cellular processes involved in T cell activation and memory, and superior design of CARs to contain optimal activation domains have allowed this strategy to be successfully brought to the clinic. Countermeasures to tumor evasion mechanisms, such as rendering CAR-modified T cells resistant to inhibitory cytokines, and enhancement of tumor trafficking, such as the coexpression of effective homing receptors, will broaden the range of tumors that can be treated using this approach. A tremendous body of knowledge will be available to us in the coming years as many trials complete accrual.
Beyond these scientific developments, recent technical improvements in cell processing, including automation and better culture systems, will make production of the modified cells simpler and faster, allowing the use of these therapies outside the limited number of centers where they can currently be administered. In parallel, these technical advances will drive down the costs of production, which together with the low toxicity of this approach will translate into a vastly favorable cost/benefit ratio when compared to conventional or targeted chemotherapy drugs.
While it is hard to imagine that, in the near future, cellular immunotherapy alone will be able to cure established cancers (the ultimate goal of the field), there is increasing expectation that — like monoclonal antibodies — it will come to play an increasing role in a multidisciplinary approach to these diseases. The last decades have shown us that, with few exceptions, no single drug or therapeutic modality is able to cure advanced neoplasms. Ultimate control and eradication of cancer will likely require a combination approach that includes immunotherapy. The numerous ongoing clinical trials will allow us to gather information regarding the best approaches to achieve this goal.
Acknowledgments
This work is supported in part by R01 CA142636 NIH-NCI, P50 CA126752 NIH-NCI and PR093892 Department of Defense.
List of abbreviations
- 5-FC
5-Fluoro-Cytosine
- APC
Antigen Presenting Cell
- CA IX
Carbonic Anhydrase 9
- CAR
Chimeric Antigen Receptor
- CD
Cytosine Deaminase
- CID
Chemical Inducer of Dimerization
- CTL
Cytotoxic T Lymphocyte
- DLI
Donor Lymphocyte Infusion
- EBV
Epstein-Barr Virus
- Fab
Fragment, antigen binding
- GCV
Ganciclovir
- GVHD
Graft-Versus-Host-Disease
- HIV
Human Immunodeficiency Virus
- HLA
Human Leukocyte Antigen
- HSV-tk
Herpes Simplex Virus thymidine kinase
- iCasp9
inducible Caspase-9
- IFN
InterFeroN
- IL
InterLeukin
- ITAM
Immunoreceptor Tyrosine-based Activation Motives
- LCL
Lymphoblastoid Cell Line
- mAb
monoclonal Antibody
- MHC
Major Histocompatibility Complex
- NK
Natural Killer
- PBMC
Peripheral Blood Mononuclear Cells
- PCR
Polymerase Chain Reaction
- PTLD
Post-Transplant Lymphoproliferative Disorder
- RCR
Replication Competent Retrovirus
- scFv
single-chain Fragment variable
- SCID
Severe Combined ImmunoDeficiency
- TAA
Tumor-Associated Antigen
- TGF
Transforming Growth Factor
- TNF
Tumor Necrosis Factor
- ZAP-70
Zeta-chain-Associated Protein kinase 70
- αFR
Alpha Folate Receptor
Footnotes
Declaration of interest
CA Ramos is a recipient of an American Society of Clinical Oncology Career Development Award.
References
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 1;75(3):555–562. [PubMed] [Google Scholar]
- 2.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990 Dec 15;76(12):2462–2465. [PubMed] [Google Scholar]
- 3.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994 Apr 28;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 4.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995 Jan 7;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 5.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010 Feb 4;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009 Jul 16;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becker ML, Near R, Mudgett-Hunter M, Margolies MN, Kubo RT, Kaye J, et al. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989 Sep 8;58(5):911–921. doi: 10.1016/0092-8674(89)90943-4. One of four original reports describing the feasibility of fusing the Fab portion of an antibody to the signaling domains of T cell receptors and demonstrating that such molecules are functional.
- 10. Goverman J, Gomez SM, Segesman KD, Hunkapiller T, Laug WE, Hood L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell. 1990 Mar 23;60(6):929–939. doi: 10.1016/0092-8674(90)90341-b. One of four original reports describing the feasibility of fusing the Fab portion of an antibody to the signaling domains of T cell receptors and demonstrating that such molecules are functional.
- 11. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. One of four original reports describing the feasibility of fusing the Fab portion of an antibody to the signaling domains of T cell receptors and demonstrating that such molecules are functional.
- 12. Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987 Dec 31;149(3):960–968. doi: 10.1016/0006-291x(87)90502-x. One of four original reports describing the feasibility of fusing the Fab portion of an antibody to the signaling domains of T cell receptors and demonstrating that such molecules are functional.
- 13.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotny J, Margolies MN, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, et al. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 15.Eshhar Z. Tumor-specific T-bodies: towards clinical application. Cancer Immunol Immunother. 1997 Nov–Dec;45(3–4):131–136. doi: 10.1007/s002620050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004 Dec 15;64(24):9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 17.Muniappan A, Banapour B, Lebkowski J, Talib S. Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 2000 Jan;7(1):128–134. doi: 10.1038/sj.cgt.7700100. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000 Aug 1;96(3):785–793. [PubMed] [Google Scholar]
- 19.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Kruis W, et al. T cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology. 1997 Oct;113(4):1163–1170. doi: 10.1053/gast.1997.v113.pm9322511. [DOI] [PubMed] [Google Scholar]
- 20.Pameijer CR, Navanjo A, Meechoovet B, Wagner JR, Aguilar B, Wright CL, et al. Conversion of a tumor-binding peptide identified by phage display to a functional chimeric T cell antigen receptor. Cancer Gene Ther. 2007 Jan;14(1):91–97. doi: 10.1038/sj.cgt.7700993. [DOI] [PubMed] [Google Scholar]
- 21.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005 May–Jun;28(3):203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 22.Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007 Apr 1;178(7):4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 23.James SE, Greenberg PD, Jensen MC, Lin Y, Wang J, Till BG, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008 May 15;180(10):7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008 Apr 1;180(7):4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 25.Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993 Jul 1;178(1):361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren-Heidenreich L, Mordini R, Hayman GT, Siebenlist R, LeFever A. Comparison of the TCR zeta-chain with the FcR gamma-chain in chimeric TCR constructs for T cell activation and apoptosis. Cancer Immunol Immunother. 2002 Oct;51(8):417–423. doi: 10.1007/s00262-002-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, et al. Bypassing immunization: optimized design of "designer T cells" against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999 Dec;5(12):3928–3941. [PubMed] [Google Scholar]
- 29.Jakobsen MK, Restifo NP, Cohen PA, Marincola FM, Cheshire LB, Linehan WM, et al. Defective major histocompatibility complex class I expression in a sarcomatoid renal cell carcinoma cell line. J Immunother Emphasis Tumor Immunol. 1995 May;17(4):222–228. doi: 10.1097/00002371-199505000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lou Y, Basha G, Seipp RP, Cai B, Chen SS, Moise AR, et al. Combining the antigen processing components TAP and Tapasin elicits enhanced tumor-free survival. Clin Cancer Res. 2008 Mar 1;14(5):1494–1501. doi: 10.1158/1078-0432.CCR-07-1066. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007 Mar 1;67(5):1887–1892. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- 32.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009 Jul 30;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 33.van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010 May;16(5):565–570. doi: 10.1038/nm.2128. 1p following 70. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg SA. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol Ther. 2010 Oct;18(10):1744–1745. doi: 10.1038/mt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilham DE, O'Neil A, Hughes C, Guest RD, Kirillova N, Lehane M, et al. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002 Mar–Apr;25(2):139–151. doi: 10.1097/00002371-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Hombach A, Koch D, Sircar R, Heuser C, Diehl V, Kruis W, et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 1999 Feb;6(2):300–304. doi: 10.1038/sj.gt.3300813. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010 Sep;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007 Apr;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 40. Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–1270. doi: 10.1038/nm.1882. A report demonstrating that in vitro expanded virus-specific T cells can be modified to express functional CARs (bispecific cells) and that these cells persist longer in vivo than non-specifically activated T cells. Infusion of these cells is associated with clinical responses.
- 41.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008 Sep 15;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T-cell priming by type-1and type-2 polarized dendritic cells: the concept of a third signal. Immunology Today. 1999;20(12):561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 43. Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998 Sep 15;161(6):2791–2797. The first report on the use of CD28 as an effective costimulatory domain for CARs.
- 44.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001 Dec 1;167(11):6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 45.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002 Jan;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 46. Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004 Apr;18(4):676–684. doi: 10.1038/sj.leu.2403302. The first report on the use of CD137 (4-1BB) as an effective costimulatory domain for CARs.
- 47.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007 Aug;18(8):712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009 Nov 1;183(9):5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009 Aug;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yvon E, Del Vecchio M, Savoldo B, Hoyos V, Dutour A, Anichini A, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009 Sep 15;15(18):5852–5860. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004 Apr;9(4):577–586. doi: 10.1016/j.ymthe.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 53. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010 Apr;18(4):843–851. doi: 10.1038/mt.2010.24. A report of a fatal event occurred in a clinical trial after infusion of T cells carrying a third generation CAR (including ζ, CD28 and CD137) targeting ERBB2/HER2 in metastatic colorectal cancer, thought to be related to the large number of administered cells localizing to the lung immediately following infusion and being triggered to release cytokines by the recognition of low levels of ERBB2 on lung epithelial or endothelial cells.
- 54.Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990 Aug 30;323(9):570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 55.Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- 56.Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 57.Nordling D, Kaiser A, Reeves L. Release testing of retroviral vectors and gene-modified cells. Methods Mol Biol. 2009;506:265–279. doi: 10.1007/978-1-59745-409-4_18. [DOI] [PubMed] [Google Scholar]
- 58.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 59.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003 Apr 1;275(1–2):251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 60.Smith CA, Ng CY, Heslop HE, Holladay MS, Richardson S, Turner EV, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995 Apr;4(2):73–79. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 61.Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, et al. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003 Jul;8(1):108–117. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 62.Wilson MH, Coates CJ, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007 Jan;15(1):139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 63.Jensen MC, Clarke P, Tan G, Wright C, Chung-Chang W, Clark TN, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000 Jan;1(1):49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 64.Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010 Aug 13;285(33):25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hombach A, Kohler H, Rappl G, Abken H. Human CD4+ T cells lyse target cells via granzyme/perforin upon circumvention of MHC class II restriction by an antibody-like immunoreceptor. J Immunol. 2006 Oct 15;177(8):5668–5675. doi: 10.4049/jimmunol.177.8.5668. [DOI] [PubMed] [Google Scholar]
- 66.Moeller M, Haynes NM, Kershaw MH, Jackson JT, Teng MW, Street SE, et al. Adoptive transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. Blood. 2005 Nov 1;106(9):2995–3003. doi: 10.1182/blood-2004-12-4906. [DOI] [PubMed] [Google Scholar]
- 67.Cheadle EJ, Hawkins RE, Batha H, Rothwell DG, Ashton G, Gilham DE. Eradication of established B-cell lymphoma by CD19-specific murine T cells is dependent on host lymphopenic environment and can be mediated by CD4+ and CD8+ T cells. J Immunother. 2009 Apr;32(3):207–218. doi: 10.1097/CJI.0b013e318194a921. [DOI] [PubMed] [Google Scholar]
- 68. Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005 Jul 1;106(1):376–383. doi: 10.1182/blood-2004-12-4797. One of the first reports on redirecting NK cells to recognize a tumor associated antigen via a CAR.
- 69. Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. 2004 Aug;126(4):583–592. doi: 10.1111/j.1365-2141.2004.05077.x. One of the first reports on redirecting γδ T cells to recognize a tumor associated antigen via a CAR.
- 70.Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10(8):842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 71.Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008 Jun;134(7):2014–2024. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 72.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper LJ, Al-Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005 Feb 15;105(4):1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 74.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002 Dec;20(12):1221–1227. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- 75.Roessig C, Scherer SP, Baer A, Vormoor J, Rooney CM, Brenner MK, et al. Targeting CD19 with genetically modified EBV-specific human T lymphocytes. Ann Hematol. 2002;81 Suppl 2:S42–S43. [PubMed] [Google Scholar]
- 76.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007 Oct 1;110(7):2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009 Nov 5;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lefrancois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010 May;235(1):206–218. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008 Jan;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011 Jan 6;117(1):72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 82.Savoldo B, Ramos CA, Liu E, Mims M, Keating M, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011 doi: 10.1172/JCI46110. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007 Oct;19(5):318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antony PA, Piccirillo CA, Akpinarli Al, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T Cell Immunity Against a Tumor/Self-Antigen Is Augmented by CD4+ T Helper Cells and Hindered by Naturally Occurring T Regulatory Cells. The Journal of Immunology. 2005 March 1;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005 Oct 3;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008 Nov 10;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4(3):e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010 Jan;33(1):1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006 May–Jun;29(3):313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pouw N, Treffers-Westerlaken E, Kraan J, Wittink F, ten Hagen T, Verweij J, et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol Immunother. 2010 Jun;59(6):921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009 May;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009 Nov 15;69(22):8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010 Jun 15;184(12):6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 94.Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004 Dec 15;173(12):7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 95. Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009 Jun 18;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. The first report on the coexpression of a homing receptor and a CAR to improve the functionality of adoptively transferred CAR-modified T cells.
- 96.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 97.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008 Jun;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007 Oct 15;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hombach AA, Kofler D, Rappl G, Abken H. Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther. 2009 Sep;16(9):1088–1096. doi: 10.1038/gt.2009.75. [DOI] [PubMed] [Google Scholar]
- 100.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006 May 1;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 101.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006 Oct 15;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010 Nov 18;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008 Sep;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westwood JA, Murray WK, Trivett M, Shin A, Neeson P, MacGregor DP, et al. Absence of retroviral vector-mediated transformation of gene-modified T cells after long-term engraftment in mice. Gene Ther. 2008 Jul;15(14):1056–1066. doi: 10.1038/gt.2008.47. [DOI] [PubMed] [Google Scholar]
- 105.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T Cells Targeting Carcinoembryonic Antigen Can Mediate Regression of Metastatic Colorectal Cancer but Induce Severe Transient Colitis. Mol Ther. 2010 Dec 14; doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010 Apr;18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006 Dec 1;108(12):3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010 Jun;28(6):1107–1115. doi: 10.1002/stem.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009 May;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 110.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007 Aug;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meier R, Golovko D, Tavri S, Henning TD, Knopp C, Piontek G, et al. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn Reson Med. 2010 Oct 6; doi: 10.1002/mrm.22652. [DOI] [PubMed] [Google Scholar]
- 112.Daly T, Royal RE, Kershaw MH, Treisman J, Wang G, Li W, et al. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 2000 Feb;7(2):284–291. doi: 10.1038/sj.cgt.7700121. [DOI] [PubMed] [Google Scholar]
- 113.McGuinness RP, Ge Y, Patel SD, Kashmiri SV, Lee HS, Hand PH, et al. Anti-tumor activity of human T cells expressing the CC49-zeta chimeric immune receptor. Hum Gene Ther. 1999 Jan 20;10(2):165–173. doi: 10.1089/10430349950018968. [DOI] [PubMed] [Google Scholar]
- 114.Weijtens ME, Willemsen RA, van Krimpen BA, Bolhuis RL. Chimeric scFv/gamma receptor-mediated T-cell lysis of tumor cells is coregulated by adhesion and accessory molecules. Int J Cancer. 1998 Jul 17;77(2):181–187. doi: 10.1002/(sici)1097-0215(19980717)77:2<181::aid-ijc2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 115.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 116.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003 Feb 15;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 117.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006 Oct;20(10):1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 118.Jensen M, Tan G, Forman S, Wu AM, Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant. 1998;4(2):75–83. doi: 10.1053/bbmt.1998.v4.pm9763110. [DOI] [PubMed] [Google Scholar]
- 119.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res. 1998 Mar 15;58(6):1116–1119. [PubMed] [Google Scholar]
- 120.Yun CO, Nolan KF, Beecham EJ, Reisfeld RA, Junghans RP. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia. 2000 Sep–Oct;2(5):449–459. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Willemsen RA, Debets R, Chames P, Bolhuis RL. Genetic engineering of T cell specificity for immunotherapy of cancer. Hum Immunol. 2003 Jan;64(1):56–68. doi: 10.1016/s0198-8859(02)00730-9. [DOI] [PubMed] [Google Scholar]
- 122.Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005 Jun 15;174(12):7853–7858. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez S, Naranjo A, Serrano LM, Chang WC, Wright CL, Jensen MC. Genetic engineering of cytolytic T lymphocytes for adoptive T-cell therapy of neuroblastoma. J Gene Med. 2004 Jun;6(6):704–711. doi: 10.1002/jgm.489. [DOI] [PubMed] [Google Scholar]
- 124.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001 Oct 15;94(2):228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 125.Mezzanzanica D, Canevari S, Mazzoni A, Figini M, Colnaghi MI, Waks T, et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 1998 Nov–Dec;5(6):401–407. [PubMed] [Google Scholar]
- 126.Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):19051–19056. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morgenroth A, Cartellieri M, Schmitz M, Gunes S, Weigle B, Bachmann M, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007 Jul 1;67(10):1121–1131. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 128.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999 Jun;1(2):123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gattenlohner S, Marx A, Markfort B, Pscherer S, Landmeier S, Juergens H, et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006 Jan 1;66(1):24–28. doi: 10.1158/0008-5472.CAN-05-0542. [DOI] [PubMed] [Google Scholar]
- 130.Darcy PK, Kershaw MH, Trapani JA, Smyth MJ. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur J Immunol. 1998 May;28(5):1663–1672. doi: 10.1002/(SICI)1521-4141(199805)28:05<1663::AID-IMMU1663>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 131.Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 2002 Nov 15;169(10):5780–5786. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- 132.Sheen AJ, Sherlock DJ, Irlam J, Hawkins RE, Gilham DE. T lymphocytes isolated from patients with advanced colorectal cancer are suitable for gene immunotherapy approaches. Br J Cancer. 2003 Apr 7;88(7):1119–1127. doi: 10.1038/sj.bjc.6600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chmielewski M, Hombach AA, Abken H. CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene Ther. 2010 Oct 14; doi: 10.1038/gt.2010.127. [DOI] [PubMed] [Google Scholar]
- 134.Pinthus JH, Waks T, Kaufman-Francis K, Schindler DG, Harmelin A, Kanety H, et al. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. Cancer Res. 2003 May 15;63(10):2470–2476. [PubMed] [Google Scholar]
- 135.Pinthus JH, Waks T, Malina V, Kaufman-Francis K, Harmelin A, Aizenberg I, et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J Clin Invest. 2004 Dec;114(12):1774–1781. doi: 10.1172/JCI22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993 Dec 1;151(11):6577–6582. [PubMed] [Google Scholar]
- 137.Teng MW, Kershaw MH, Moeller M, Smyth MJ, Darcy PK. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 2004 Jul;15(7):699–708. doi: 10.1089/1043034041361235. [DOI] [PubMed] [Google Scholar]
- 138.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996 Jun;2(6):1001–1008. [PubMed] [Google Scholar]
- 139.Hekele A, Dall P, Moritz D, Wels W, Groner B, Herrlich P, et al. Growth retardation of tumors by adoptive transfer of cytotoxic T lymphocytes reprogrammed by CD44v6-specific scFv:zeta-chimera. Int J Cancer. 1996 Oct 9;68(2):232–238. doi: 10.1002/(SICI)1097-0215(19961009)68:2<232::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 140.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010 Nov 1;120(11):3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kershaw MH, Westwood JA, Zhu Z, Witte L, Libutti SK, Hwu P. Generation of gene-modified T cells reactive against the angiogenic kinase insert domain-containing receptor (KDR) found on tumor vasculature. Hum Gene Ther. 2000 Dec 10;11(18):2445–2452. doi: 10.1089/10430340050207939. [DOI] [PubMed] [Google Scholar]
- 142.Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7009–7014. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]