Abstract
Object
Hypertension is the main cause of spontaneous intracerebral hemorrhages (ICH), but the effects of hypertension on ICH-induced brain injury have not been well studied. In this study, we examined ICH-induced brain injury in spontaneously hypertensive rats (SHR).
Methods
This two-part study was performed on 12 weeks old male SHR and Wistar Kyoto (WKY) rats. First, rats received an intracaudate injection of 0.3 units collagenase and hematoma sizes were determined at 24 hours. Second, rats were injected with 100-μL autologous whole blood into the right basal ganglia. Brain edema, neuronal death, ferritin expression, microglia activation, and neurological deficits were examined.
Results
Hematoma sizes were the same in SHR and WKY rats 24 hours after collagenase injection. SHR had greater neuronal death and neurological deficits after blood injection. ICH also resulted in higher brain ferritin levels and stronger activation of microglia in SHR. However, perihematomal brain edema was same in the SHR and WKY rats.
Conclusion
Moderate chronic hypertension resulted in more severe ICH-induced neuronal death and neurological deficits, but did not exaggerate hematoma enlargement and perihematomal brain edema in the rat ICH models.
Keywords: brain edema, cerebral hemorrhage, ferritin, hypertension, microglia, neuronal death
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a common and often fatal stroke subtype. Studies have indicated a mortality of more than 40%, and many survivors are left with significant neurological deficits17. Hypertension is a major risk factor for ICH. High blood pressure is found in 90% of patients with ICH and persists for a few days5. In the emergency department, about 27% of ICH patients have a systolic blood pressure (SBP) of above 160 mmHg 23. Transient or sustained high blood pressure has increased risk of continuous bleeding, subsequent rebleeding, or both within the first 24 h after the initial hemorrhage4. Several clinical trials of aggressive blood pressure lowering in acute ICH are ongoing3,24. However, the effects of hypertension on pathological changes in the brain after ICH are still not well understood.
Spontaneously hypertensive rats (SHR), derived from Wistar Kyoto (WKY) rats, have been used extensively to study the effects of chronic hypertension on ischemic stroke10,15. Mortality after ICH is higher in SHR8. In the present study, we investigated ICH-induced brain injury in SHR and WKY rats using both collagenase and blood injection models.
Materials and Methods
Animal Preparation and Blood Pressure Measurement
Male SHR and WKY rats were purchased from Charles River Laboratories at 9 weeks of age and used for experiments at 12 weeks old. The protocols for these animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. All animals were housed under standard 12:12-h light-dark conditions and allowed free access to food and water. Systolic and mean blood pressure measurements (in triplicate) were taken using a noninvasive tail cuff blood pressure system (IITC Life Science Inc., Model 229) once a week before surgery for three weeks. Blood pressure was also measured during surgery and at one day after surgery.
Intracerebral Injection
Animals were anesthetized with pentobarbital (45 mg/kg, i.p.). The right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. The rats were placed in a stereotactic head frame (Kopf Instruments) and a 1-mm cranial burr hole drilled on the right coronal suture 3.5 mm lateral to midline. A 26-gauge needle was inserted stereotaxically into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm lateral, and 5.5 mm ventral to the bregma). This needle was used for injections of autologous blood or collagenase14. Body temperature was maintained at 37.5°C with a feedback-controlled heating pad. Blood pH, PaO2, PCO2, hematocrit, and glucose levels were monitored. After infusion, the needle was removed, the skin was closed and the animals were allowed to recover.
Experimental Groups
There were two parts to this study which used a total of 96 rats.
Part 1. To determine whether there are differences in hematoma volume between SHR and WKY rats after intracaudate injection of collagenase. Rats (n=6 each) received a 1.5-μL injection (at 0.1-μL/min) of saline containing 0.3 U bacterial collagenase (type VIIs, Sigma). The syringe was left in place for 10 minutes after the infusion was completed. Animals were killed at 24 hours for brain hemoglobin content determination.
Part 2. There were three sets of experiments in this part. All rats received an infusion of 100-μL autologous blood into the right basal ganglia at a rate of 10-μL/min. In the first set of experiments, SHR and WKY rats were killed at days 1 and 3 (n=6 each time point) for brain water content measurement. In the second set, rats were killed at days 1, 3 and 14 (n=6 each time point) for immunohistochemistry. Behavioral test were performed at days 1, 7 and 14. In the third set, rats were killed at days 1 and 3 (n=6 each time point) for Western blotting.
Brain Water Content
Animals were reanesthetized with pentobarbital (60 mg/ kg, i.p.) and decapitated at one and three days after ICH33. Brains were removed and a 3-mm thick coronal brain slice cut 4 mm from the frontal pole, and dissected into ipsi-and contralateral cortex, and ipsi- and contralateral basal ganglia. The cerebellum was taken as a control. Brain samples were immediately weighed on an electronic analytical balance to obtain the wet weight, and then dried at 100°C for 24 hours to obtain the dry weight. Percent water content was determined as: ((wet weight- dry weight)/wet weight)*100%.
Hemoglobin Contents
Hematoma size was measured by determining hemoglobin content with a spectrophotometer22. Animals were perfused transcardially with 0.1mol/L phosphate-buffered saline under deep anesthesia until the outflow fluid from the right atrium was colorless. The brain was rapidly removed and dissected into left and right hemisphere. The brain tissue was then homogenized in 0.1mol/L phosphate-buffered saline followed by 30-minute centrifugation (13,000 g). Fifty micro-liter supernatant was mixed with 200-μL reagent (QuantiChrom Hemoglobin Assay Kit; BioAssay Systems). After 15 minutes, optical density was measured at 400 nm with a spectrophotometer (Ultrospec 3; Pharmacia LKB) and hemispheric hemoglobin content expressed as milligrams per hemisphere.
Immunohistochemistry and Fluoro-Jade C Staining
The immunohistochemistry method has been described previously 33. Briefly, rats were anesthetized and perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH7.4). Brains were removed and kept in 4% paraformaldehyde for 6 h, then immersed in 25% sucrose for 3–4 days at 4 °C. After embedding in a mixture of 25% sucrose and OCT (SAKURA Finetek, USA), 18-μm sections were taken on a cryostat. The avidin–biotin complex technique was used for staining with hematoxylin as counter stain. The primary antibodies were mouse anti-rat OX6 (1:400 dilution, AbD Serotec) and rabbit anti-human ferritin (1:400 dilution, Sigma). Normal rabbit or mouse serum and the absence of primary antibody were negative controls.
For Fluoro-Jade C Staining, brain sections were incubated with 0.0001% Fluoro-Jade C (Histo-Chem Inc.) in 0.1% acetic acid in the dark at room temperature for 10 min. Following incubation, sections were rinsed and allowed to dry in the dark. Slides were covered with coverslip and examined with an Olympus BX51 fluorescence microscope.
For cell counting, we used 18-μm thick coronal sections from both 1 mm anterior and 1 mm posterior to the blood injection site. Three high-power images (x40 magnification) were taken adjacent to the hematoma and the positive cells were manually counted.
Western Blot Analysis
Western blot analysis was performed as previously described33. Briefly, brain tissue was immersed in Western sample buffer and sonicated. Protein concentration was determined by Bio-Rad protein assay kit and 50-μg protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham). Membranes were probed with primary antibodies: rabbit anti-ferritin heavy chain polyclonal antibody (1:2000, Cell Signaling Technology) and goat anti-ferritin light chain polyclonal antibody (1:2000, Abnova). The antigen-antibody complexes were visualized with the ECL chemiluminescence system (Amersham) and exposed to Kodak X-OMAT film. The relative densities of bands were analyzed with NIH ImageJ.
Behavioral Tests
Three behavioral tests, including the forelimb placing test, the forelimb use asymmetry test and the corner turn test were used in this study as described before12. All animals were scored by a behavioral tester who was blind to the neurological condition.
Statistical Analysis
Student t test was used. Values are mean±SD. Statistical significance was set at p<0.05.
Results
Blood Pressure
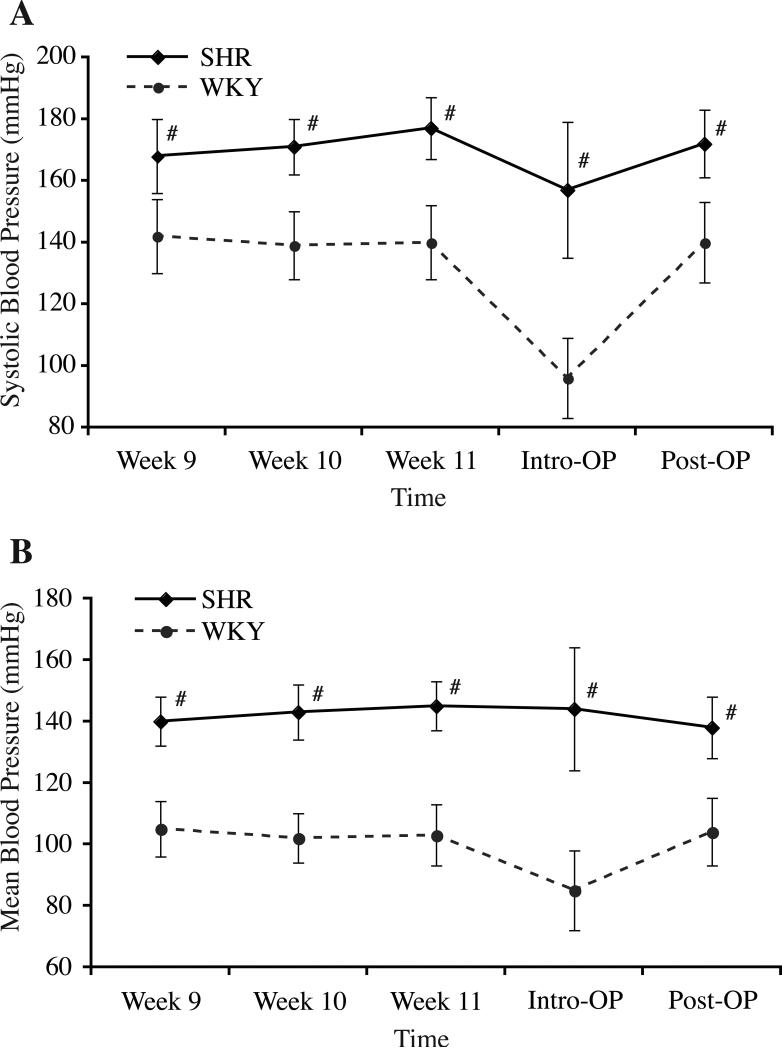
Systolic artery blood pressure (SBP) and mean artery blood pressure (MBP) of the rats were measured once a week from 9 weeks old until the end of study (Figure 1). SBP and MBP were significantly higher in the SHR (e.g., SBP/MBP at 11 weeks, 177±10/145±8 mmHg vs. 140±12/103±10mmHg in WKY rats, p<0.01). Other physiological parameters were also recorded during intracerebral injections and all were in the normal range except that hematocrit was higher in SHR strain (48±4.2% vs. 42.3±3.5 in WKY rats, p<0.01).
Figure 1.
Graphs showing systolic blood pressure (A) and mean blood pressures (B) in age-matched spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto (WKY) rats since 9 weeks of age (Week 9) till the end of this study. Data are expressed as mean±SD. #p<0.01 vs. WKY.
Collagenase Model
Brain hemoglobin content was measured 24 hours after collagenase injection to estimate the hematoma size. We found that hemoglobin content in the ipsilateral hemisphere was the same in SHR and WKY rats (13.6±3.2 mg vs. 13.2±2.1 mg, p=0.817, Figure 2).
Figure 2.
Brain hemoglobin content (a measure of hematoma size) in the ipsilateral hemisphere one day after intracerebral injection of collagenase in SHR and WKY rats. Values are expressed as mean±SD.
Blood Injection Model
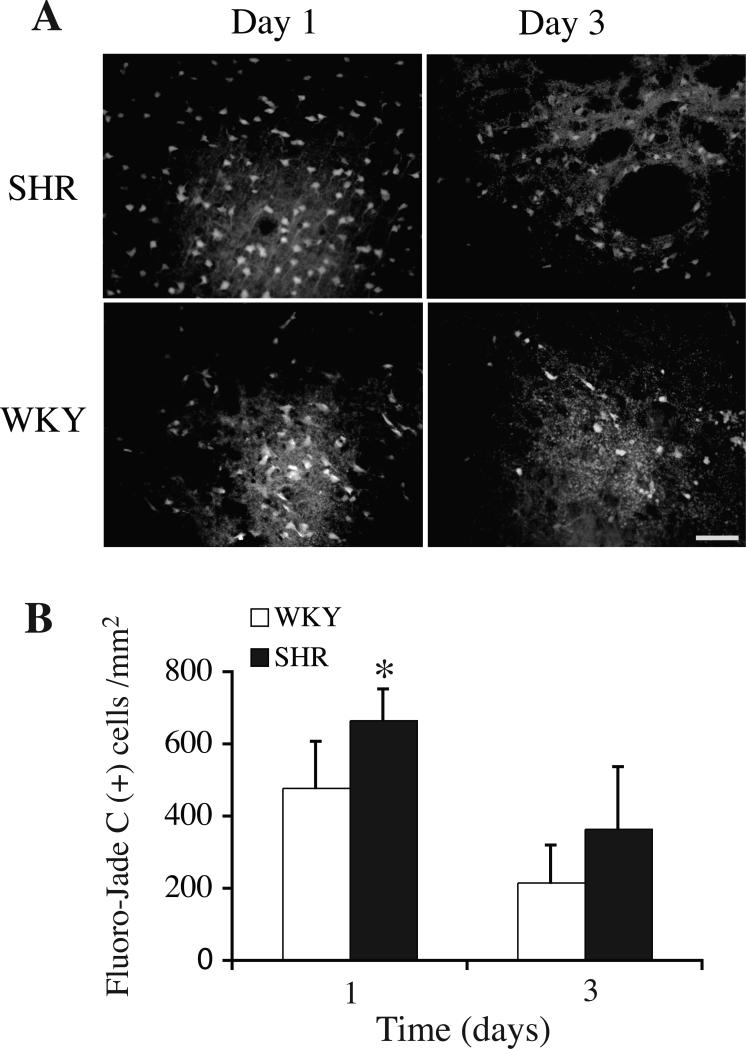
Brain injury after an ICH was examined using the blood injection model. Degenerating neurons were detected by Fluoro-Jade C staining in the perihematomal zone after both 1 and 3 days. ICH caused more neuronal death around the hematoma in SHR strain at the first day (666±86/mm2 versus 478±129/mm2 in WKY rats, p<0.05, Figure 3). There still was a trend for more neuronal death in SHR strain at day 3 (365±172/mm2 versus 216±104/mm2 in WKY rats, p=0.09, Figure 3).
Figure 3.
(A) Fluoro-Jade C staining showing degenerating neurons in the ipsilateral basal ganglia of SHR and WKY rats one and three days after ICH. Bar=50 μm. (B) Bar graph showing numbers of Fluoro–Jade C staining positive cells around the hematoma. Values are expressed as mean±SD. *p<0.05 vs. WKY.
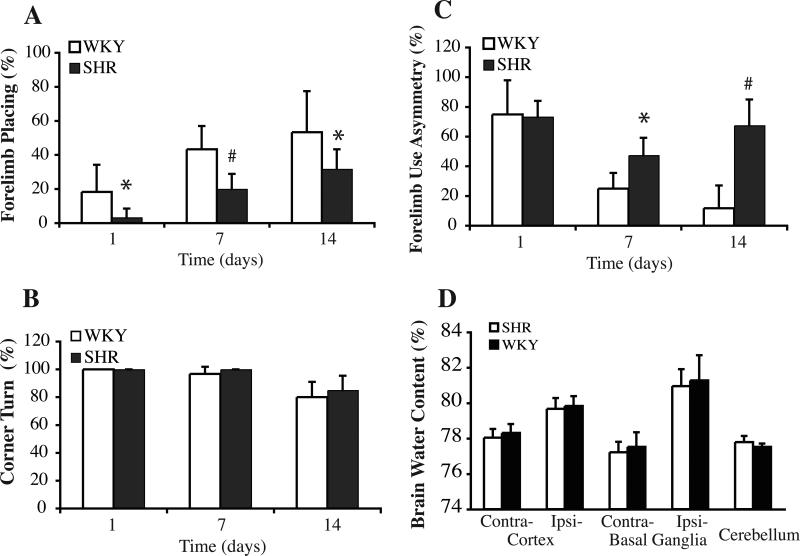
ICH induced neurological deficits in both SHR and WKY rats. Both strains had forelimb placing recovery at day 7 and day 14 after ICH but the WKY rats had better forelimb placing scores than SHR strain at all the time points (Figure 4). Forelimb use asymmetry scores recovered at day 7 and 14 in WKY rats, but there was little recovery in the SHR strain which had more severe deficits at days 7 and 14 (Figure 4). There were no differences between the two groups for corner turn deficits which were at or close to maximal at days 1, 7 and 14 (Figure 4). There was also no difference in brain edema between SHR and WKY rats at day 1 (Figure 4) and day 3 (data not shown).
Figure 4.
Bar graphs showing the results of the forelimb placing (A, normal=100%), corner turn (B, normal=50%), and forelimb use asymmetry (C, normal=0%) tests in SHR and WKY rats after ICH. Values are mean±SD. *p<0.05, #p<0.01 vs. WKY. (D) Brain water content one day after ICH in SHR and WKY rats. Values are expressed as mean±SD. There were no differences in water content in the ipsilateral basal ganglia and cortex between the two strains.
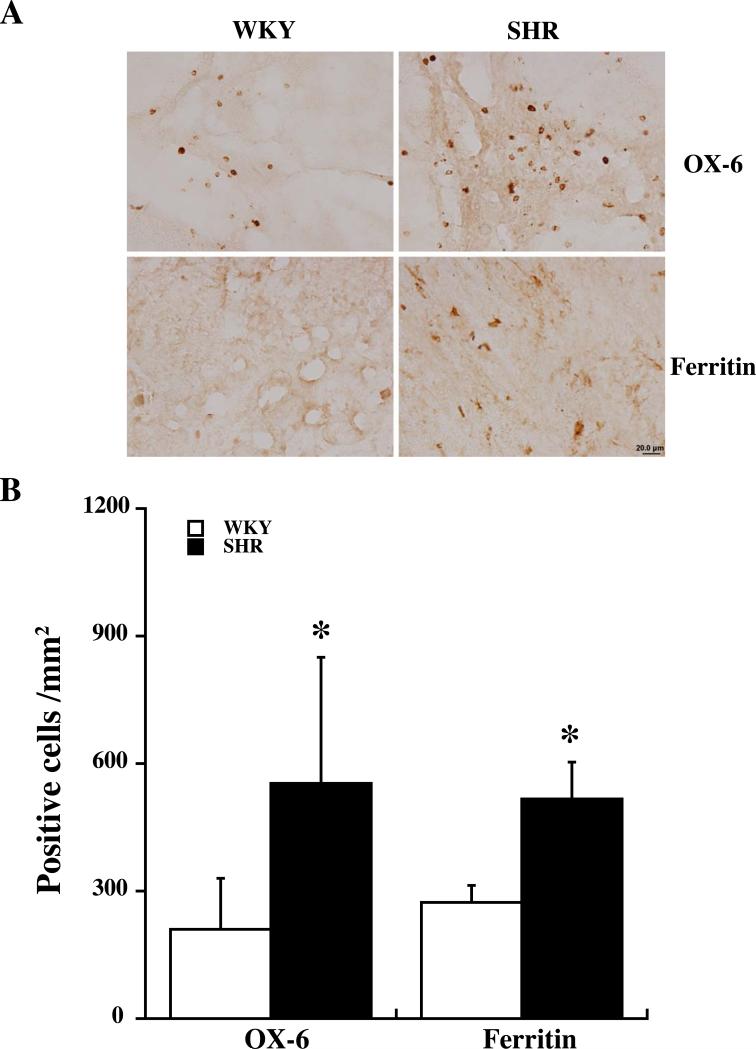
Activated microglia (OX-6 positive) were found around the hematoma in both strains. The SHR strain had significantly more OX-6 positive cells in the perihematomal area at day 1 (p<0.05, Figure 5) and day 3 (523±225 vs. 327±51 positive cells/mm2 in WKY rats, p<0.05).
Figure 5.
OX-6 and ferritin positive cells in the ipsilateral basal ganglia of SHR and WKY rats one day after ICH. (A) OX-6 and ferritin positive cells. Scale bar=20 μm. (B) Cell count. Values are mean±SD. *p<0.05 vs. WKY.
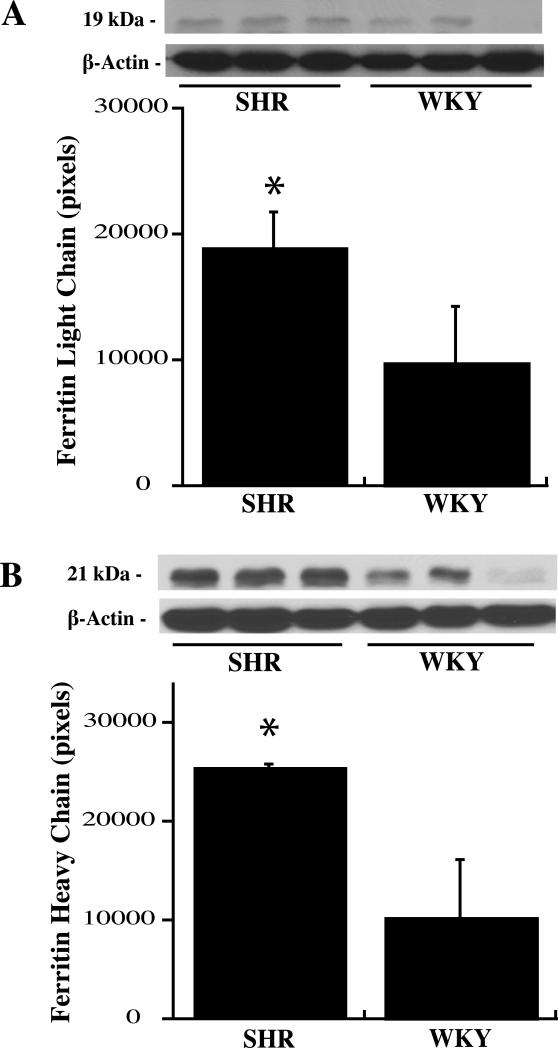
Protein levels of ferritin, a key iron storage protein in the brain, were examined in the ipsilateral basal ganglia of both strains at day 1 and 3 after ICH. Immunohistochemistry showed more ferritin positive cells present around the hematoma in SHR strain at day one (516±89 vs. 272±42 positive cells/mm2 in WKY rats, p<0.05, Figure 5). Very weak ferritin immunoreactivity was present in the contralateral basal ganglia in both strains. Brain ferritin levels were also quantified by Western blots. Both ferritin light and heavy chain levels were higher in SHR compared with WKY rats one day after ICH (Figure 6) but not at day 3 (light chain: 21203±2666 vs. 22224±5623 pixels in WKY, p>0.05; heavy chain: 26081±2439 vs. 24976±5190 pixels in WKY, p>0.05).
Figure 6.
Western blot analysis showing ferritin light chain (A) and ferritin heavy chain (B) levels in the ipsilateral basal ganglia of SHR and WKY rats one day after ICH. Values are mean±SD. *p<0.05 vs. WKY.
Discussion
Our present study demonstrated that intracerebral hematomas caused more severe neuronal death, microglia activation, ferritin upregulation and neurological deficits in SHR compared to WKY rats and these results indicate that chronic hypertension has a role in brain damage following ICH. Interestingly, though, hematoma size was not affected by hypertension in the collagenase model.
ICH resulted in more neuronal death in the brain. It is well known that thrombin and iron are two major factors causing brain injury after ICH11. Hypercoagulable state and severe iron overload may contribute to more severe neuronal death in SHR rats after ICH. However, brain injury after ICH is complicated with many potential factors affecting ICH-induced neuronal death. For example, studies showed there is an intrinsic vulnerability participating in the exacerbation of brain damage after cerebral insults in SHR27,34. In addition, intracerebral injection of AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) provoked greater damage and higher mortality in adult SHR15.
The main factor which affects ICH patient outcome is hemorrhagic volume. Hematoma enlargement occurs in about a third of ICH patients32 which may be related with hypertension. Recent studies showed that early intensive blood pressure-lowering treatment can reduce hematoma growth4. Therefore, we predicted larger hematomas in SHR rats. However, we found the hematoma sizes were the same in SHR and WKY rats. The reasons resulted in same hematoma sizes could be: 1) a moderate increase of systolic blood pressure (<200mmHg) may not enlarge the hematoma; 2) a hypercoagulable state occurs in SHR rats,2 and the blood prothrombin time is much shorter in SHR rats compared with that in WKY rats 19, which may limit hematoma growth. A prothrombotic state has also been reported in human hypertension26. This may have multiple underlying causes 26 and might be an adaption to prevent hemorrhage; 3) the type of blood vessel disruption that occurs in the collagenase model differs from that occurring in spontaneous ICH in man with regards to the mechanism of disruption and the type of vessels that hemorrhages. It should also be noted that while the hypertensive state is well established by 12 weeks in the SHR rat, it is possible that the effects of hypertension on hematoma size may vary with the duration of hypertension. It is known, for example, that there continues to be changes in the vascular wall of the SHR rat with age, even when the blood pressure has ceased to increase 29. Further experiments are needed to examine the effect duration of hypertension on hematoma size in the SHR rat.
Edema formation following ICH elevates intracranial pressure and may result in herniation 25. There are several phases of edema formation after ICH, including a very early phase involving hydrostatic pressure and clot retraction with movement of serum from the clot into the surrounding tissue, and a second phase related to the coagulation cascade and thrombin production and a third phase related to erythrocyte lysis and iron toxicity32. We hypothesized that perihematomal edema would be more severe in SHR because of the hypertension, the hypercoagulable state, and higher hematocrit, but in this study we found that hypertension has no effects on perihematomal edema formation. This result is supported by a recent study indicating blood pressure lowering treatment has no effects on edema formation in ICH patients3. Whether or not edema resolution is enhanced in SHR should be examined. CSF is the primary pathway for edema clearance and the CSF turn-over rate is more than twice greater in SHR than that in WKY 1.
Enhanced activation of microglia was present in the SHR strain after ICH. It is well known that inflammation exacerbates hemorrhagic brain damage. An inflammatory response in the surrounding brain with microglial activation occurs shortly after ICH 6,13and activated microglial cells persist for at least a month 7,13. Inhibition of microglia activation with tuftsin fragment 1-3 and minocycline reduces brain damage after ICH in animals 28,31. Whether enhanced microglia activation contributes to the worse behavioral outcome in SHR needs elucidation.
Ferritin is a major iron storage protein that can be produced in brain. Ferritin has two subunits, heavy and light chains. Ferritin protein synthesis is regulated by both iron mediated and non-iron mediated induction. The greater ferritin upregulation in the SHR strain in this study suggests more severe iron release. We have gathered substantial evidence that iron release from hemoglobin breakdown has a major role in ICH-induced brain injury. There is a build-up in non-heme brain tissue iron and the appearance of iron positive cells around the perihematomal zone as early as the first day 30. Increased brain iron levels cause oxidative stress, neuronal death and neurological deficits following ICH 32. Deferoxamine, an iron chelator, reduces hemorrhagic brain injury in aged rats and piglets 9,20. Recent clinical studies also showed that high ferritin levels in serum are associated with severe perihematomal brain edema and poor outcome in ICH patients16,21.
However, non-iron mediated ferritin upregulation may also occur in the brain after ICH, although hemoglobin degradation products such as iron and heme are strong ferritin inducers. For example, ICH causes a significant increase of tumor necrosis factor-α in the brain and this inflammatory mediator can induce ferritin synthesis18. Therefore, future studies need to determine the precise mechanisms underlying the greater ferritin upregulation in SHR including the greater hematocrit in that strain.
In summary, moderate chronic hypertension (< 200mmHg SBP) did not enlarge the hematoma or exacerbate brain edema after ICH. However, it did result in increased neuronal death and worse function outcome, which may be associated with microglia activation and iron toxicity.
Acknowledgment
This study was supported by grants NS 17760, NS-039866 and NS-057539 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
References
- 1.Al-Sarraf H, Philip L. Effect of hypertension on the integrity of blood brain and blood CSF barriers, cerebral blood flow and CSF secretion in the rat. Brain Res. 2003;975:179–188. doi: 10.1016/s0006-8993(03)02632-5. [DOI] [PubMed] [Google Scholar]
- 2.Amagasa H, Okazaki M, Iwai S, Kumai T, Kobayashi S, Oguchi K. Enhancement of the coagulation system in spontaneously hypertensive and hyperlipidemic rats. J Atheroscler Thromb. 2005;12:191–198. doi: 10.5551/jat.12.191. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41:307–312. doi: 10.1161/STROKEAHA.109.561795. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 6.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Darder JM, Duran-Cabral J. Experimental intracerebral haemorrhage in normotensive and spontaneously hypertensive rats. Acta Neurochirurgica. 1990;107:102–107. doi: 10.1007/BF01405787. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henning EC, Latour LL, Hallenbeck JM, Warach S. Reperfusion-associated hemorrhagic transformation in SHR rats: evidence of symptomatic parenchymal hematoma. Stroke. 2008;39:3405–3410. doi: 10.1161/STROKEAHA.108.520304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua Y, Keep R, Hoff J, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 12.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins A, Maxwell W, Graham D. Experimental intracerebral hematoma in the rat: sequential light microscopic changes. Neuropathol Appl Neurobiol. 1989;15:477–486. doi: 10.1111/j.1365-2990.1989.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 14.Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke. 2002;33:3012–3018. doi: 10.1161/01.str.0000037673.17260.1b. [DOI] [PubMed] [Google Scholar]
- 15.Lecrux C, Nicole O, Chazalviel L, Catone C, Chuquet J, MacKenzie ET, et al. Spontaneously hypertensive rats are highly vulnerable to AMPA-induced brain lesions. Stroke. 2007;38:3007–3015. doi: 10.1161/STROKEAHA.107.491126. [DOI] [PubMed] [Google Scholar]
- 16.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–1170. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- 17.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 18.Miller LL, Miller SC, Torti SV, Tsuji Y, Torti FM. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci USA. 1991;88:4946–4950. doi: 10.1073/pnas.88.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munakata M, Komiyama Y, Masuda M, Kagawa H, Nomura S, Fukuhara S, et al. Whole blood prothrombin time using diluted tissue factor is shortened in spontaneous hypertensive rats. Semin Thromb Hemost. 2000;26:97–100. doi: 10.1055/s-2000-9810. [DOI] [PubMed] [Google Scholar]
- 20.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez de la Ossa N, Sobrino T, Silva Y, Blanco M, Millan M, Gomis M, et al. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke. 2010;41:810–813. doi: 10.1161/STROKEAHA.109.570168. [DOI] [PubMed] [Google Scholar]
- 22.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Hussein HM, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25:32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570–576. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New England Journal of Medicine. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 26.Varughese GI, Lip GYH. Is hypertension a prothrombotic state? Current Hypertension Reports. 2005;7:168–173. doi: 10.1007/s11906-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 27.Veerasingham SJ, Yamazato M, Berecek KH, Wyss JM, Raizada MK. Increased PI3-kinase in presympathetic brain areas of the spontaneously hypertensive rat. Circ Res. 2005;96:277–279. doi: 10.1161/01.RES.0000156275.06641.b2. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Annals of Neurology. 2003;54:655–664. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- 29.Wirth KJ, Linz W, Wiemer G, Scholkens BA. Differences in acetylcholine- and bradykinin-induced vasorelaxation of the mesenteric vascular bed in spontaneously hypertensive rats of different ages. Naunyn-Schmiedeberg's Arch Pharmacol. 1996;354:38–43. doi: 10.1007/BF00168704. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31:183–188. doi: 10.1179/174313209X385680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 33.Xi G, Keep RF, Hua Y, Xiang JM, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Raizada MK. MAP kinase-independent signaling in angiotensin II regulation of neuromodulation in SHR neurons. Hypertension. 1998;32:473–481. doi: 10.1161/01.hyp.32.3.473. [DOI] [PubMed] [Google Scholar]