Abstract
How individual cells respond to mechanical forces is of considerable interest to biologists as force affects many aspects of cell behavior1. Application of force on integrins triggers cytoskeletal rearrangements and growth of the associated adhesion complex, resulting in increased cellular stiffness2,3, also known as reinforcement4. While RhoA has been shown to play a role during reinforcement3, the molecular mechanisms that regulate its activity are unknown. By combining biochemical and biophysical approaches, we identified two guanine nucleotide exchange factors (GEFs), LARG and GEF-H1, as key molecules that regulate the cellular adaptation to force. We show that stimulation of integrins with tensional force triggers activation of these two GEFs and their recruitment to adhesion complexes. Surprisingly, activation of LARG and GEF-H1 involves distinct signaling pathways. Our results reveal that LARG is activated by the Src family tyrosine kinase Fyn, whereas GEF-H1 catalytic activity is enhanced by ERK downstream of a signaling cascade that includes FAK and Ras.
To analyze the effect of force on RhoA activity we applied a constant force for different amounts of time on fibronectin (FN)-coated beads using a permanent magnet. Consistent with what has been found previously5,6, we observed that tensional forces increased RhoA activity (figure 1a-b). Pre-incubation with a function blocking anti-β1 antibody (P4C10) prevented RhoA activation in response to force (figure 1a), suggesting that β1 integrins are the major extra cellular matrix (ECM) receptors involved. When cells were incubated with beads coated with RGD peptides and then subjected to tensile force, similar activation of RhoA was observed (supplementary figure 1a). Likewise RhoA was activated by pulling on beads coated with an activating anti-β1 antibody (TS2/16) (supplementary figure 1b), whereas no change in RhoA activity was detected when beads were coated with a non-activating anti-β1 (supplementary figure 1c), indicating that integrin engagement is necessary for RhoA activation in response to force.
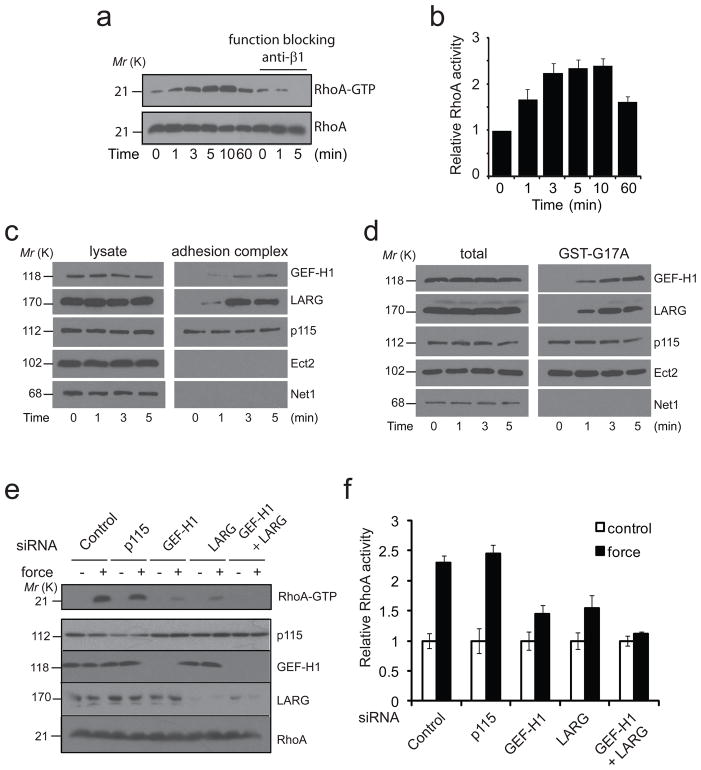
Figure 1. LARG and GEF-H1 activate RhoA in response to force.
a, b, REF52 cells were incubated without or with the function blocking anti-β1antibody (P4C10) for 30 min and then with FN-coated magnetic beads. A permanent magnet was used to generate tensional force for different amounts of time. Active RhoA (RhoA-GTP) was isolated with GST-RBD and analyzed by western blot (a). Corresponding densitometric analysis of RhoA-GTP normalized to RhoA levels and expressed as relative to the control in the absence of stimulation by force (error bars represent s.e.m., n=5) (b). c, REF52 cells were incubated 30 min with FN-coated beads and stimulated with tensional force using a permanent magnet for different amounts of time before cell lysis. After magnetic separation of the adhesion complex fraction, the lysate and the adhesion complex fraction were analyzed by western blot. All results are representative of at least three independent experiments. d, REF52 cells were incubated 30 min with FN-coated beads and stimulated with tensional force using a permanent magnet for different amounts of time before cell lysis. Active GEFs were sedimented with GST-RhoA(G17A) and analyzed by western blot. All results are representative of at least three independent experiments. e, f, REF52 cells were transfected 48 h with control siRNA or siRNA targeting p115, GEF-H1, LARG or both GEF-H1 and LARG, and incubated 30 min with FN-coated beads. After stimulation with tensional force for 5 min cells were lysed and active RhoA (RhoA-GTP) was isolated with GST-RBD and analyzed by western blot (e). f, corresponding densitometric analysis. RhoA-GTP is normalized to RhoA levels and expressed relative to the control (error bars represent s.e.m., n=4). Uncropped images of blots are shown in Supplementary Fig. S5.
GEFs increase the activity of RhoA by promoting the exchange of GDP for GTP7. Considering that application of force on integrin-based adhesions activates RhoA, we hypothesized that GEFs specific for RhoA may be recruited to the adhesion complex (AC). To test this hypothesis, we isolated ACs by separating the FN-coated beads from the lysates of cells stimulated with constant force for different amounts of time. As expected we found vinculin and Focal Adhesion Kinase (FAK) but not tubulin in the fraction (supplementary figure 1d). Similar to previous studies8,9, we found that force induced recruitment of vinculin to the adhesion complex (supplementary figure 1d). When we looked for the presence of RhoA GEFs, we found that p115, GEF-H1 and LARG were present in the AC (figure 1c). Interestingly, application of force induced the recruitment of LARG and GEF-H1 to the AC, whereas p115 localization at the adhesion complex was unaffected by tension. To ensure that the detection of p115, LARG and GEF-H1 in the AC was not due to nonspecific association with the beads, we performed the same experiment with beads coated with an anti-transferrin receptor (TfR). As expected, we were able to detect TfR but not p115, LARG nor GEF-H1 in the bead-associated complex (supplementary figure 1e). LARG and p115 have already been described being activated by adhesion and co-localizing with adhesion proteins10. However, finding the microtubule associated GEF, GEF-H1 present in integrin-based ACs was unexpected. We next wanted to know if the activity of these GEFs was affected by mechanical force. To look for activation of GEFs, we performed affinity pulldown assays with a nucleotide-free RhoA mutant, RhoAG17A, as described earlier11. This revealed that force applied to FN-coated beads increased LARG and GEF-H1 activities, but had no effect on the activities of several other RhoA GEFs such as Ect2, p115 or Net1 (figure 1d).
To determine if these GEFs are responsible for RhoA activation in response to force, we depleted their expression using short interfering RNA (siRNA). Depletion of LARG or GEF-H1 significantly decreased RhoA activation in response to force, whereas knockdown of p115 did not affect the force-induced RhoA activation (figure 1e-f). Double knockdown of LARG and GEF-H1 totally abrogated RhoA activation. Two independent siRNA duplexes targeting GEF-H1 and LARG generated similar results (data not shown). Integrin-mediated signaling to RhoA is required for rearrangements of the actin cytoskeleton during adhesion. Early adhesion is associated with transient RhoA inhibition and Rac activation allowing actin protrusion whereas mature adhesions are associated with the development of RhoA-mediated tension12. Previous studies have shown that the transient depression in RhoA activity following integrin engagement involves p190RhoGAP13, while subsequent activation of RhoA involves p115 RhoGEF, LARG and p190 RhoGEF10,14. We show here that application of force on integrins stimulates the RhoA pathway through an overlapping set of regulators.
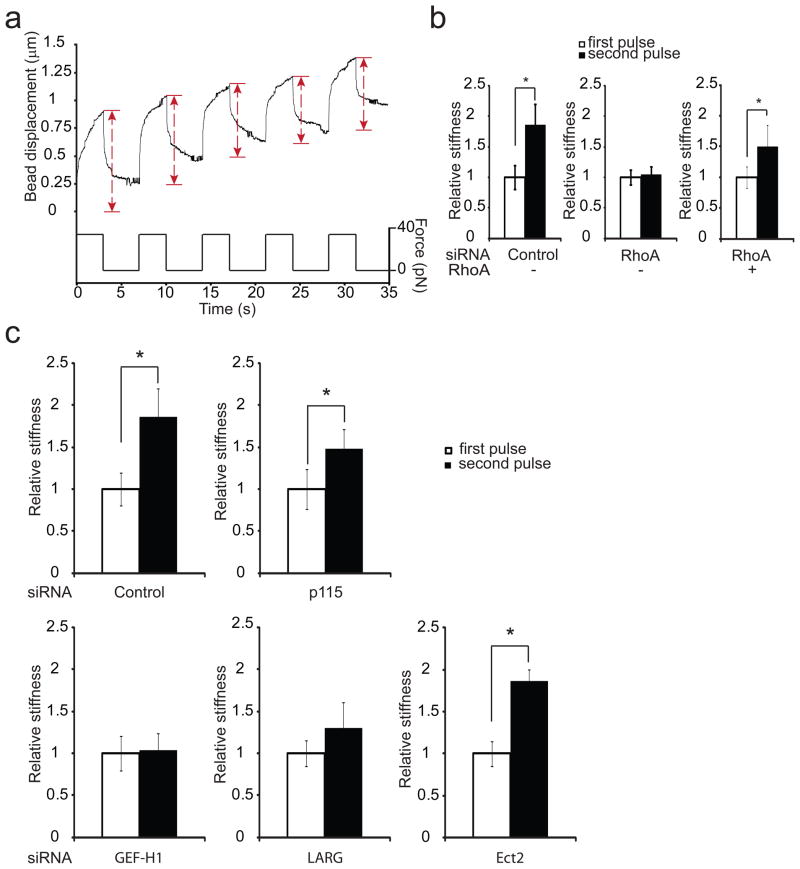
We next wanted to determine the role of these GEFs during reinforcement. To study how cells change their mechanical properties in response to mechanical stresses, we used magnetic tweezers to apply controlled force on magnetic beads coated with FN. The local viscoelastic properties of the cells were determined by measuring bead displacements due to a known force induced by a magnetic field15. Stimulation with successive pulses of constant force triggered a local change in cellular stiffness resulting in decreased bead displacement (figure 2a). To quantify this local increase in stiffness, the spring constant was calculated for each pulse by fitting the bead displacement and force magnitude to a modified Kelvin-Voigt model16,17 (supplementary figure 2). “Relative cellular stiffness” was calculated by normalizing the spring constant for pulses 2, 3, 4 and 5 to that observed during the first pulse. The change in cellular stiffness was already significant between the first and the second pulse (supplementary figure 3a) demonstrating that cellular adaptation to force on integrins is a rapid phenomenon as previous studies have reported3,4. Using pharmacological inhibitors, it has been shown that RhoA is involved in reinforcement3. To examine the role of RhoA during cellular stiffening in our system, we depleted RhoA expression by using siRNA. On depletion of RhoA expression the cells displayed decreased rigidity (supplementary figure 3b-c). Interestingly, the change in cellular stiffness after application of pulses of force was no longer detected in the RhoA knockdown cells (figure 2b). Expression of a siRNA-resistant mutant of RhoA in the knockdown cells restored the cellular stiffening in response to force (figure 2b). Similar results were obtained when we treated the cells with the RhoA inhibitor C3 transferase (supplementary figure 3d), indicating that RhoA activity is necessary for the cellular adaptation to force. To explore the role of the GEFs during the stiffening response, we depleted their expression using siRNA and monitored the change in cellular stiffness during pulses of force application. We found that knockdown of either p115, LARG, GEF-H1 or Ect2 decreased the basal rigidity of the cells (supplementary figure 3e). Cells depleted of LARG and GEF-H1 suppressed the stiffening response following force application, whereas cells depleted of Ect2 or p115 were still able to significantly increase their stiffness in response to force (figure 2c). These results indicate that both LARG and GEF-H1 are necessary for cells to adjust their mechanical properties in response to force applied to integrins. We cannot rule out a potential role for p115 in this response, since the knockdown was never as efficient (figure 1e and supplemental figure 3f) as for LARG and GEF-H1 and because p115 knockdown did decrease the stiffening response (figure 2c).
Figure 2. LARG and GEF-H1 mediate cellular stiffening in response to force applied on integrins.
a, Typical displacement of a FN-coated bead bound to a REF52 fibroblast during force pulse application. b, change in stiffness during 2 force pulses applied to FN-coated beads bound to REF52 cells transfected 48 h with control siRNA or RhoA siRNA or RhoA siRNA and a siRNA-resistant mutant of RhoA (myc-RhoA) (error bars represent s.e.m., n= 20; * p<0.01). c, Change in stiffness during 2 force pulses applied to FN-coated beads bound to REF52 cells transfected 48 h with control siRNA or siRNA targeting p115, GEF-H1, LARG, Ect2 (error bars represent s.e.m., n=20,* p<0.05).
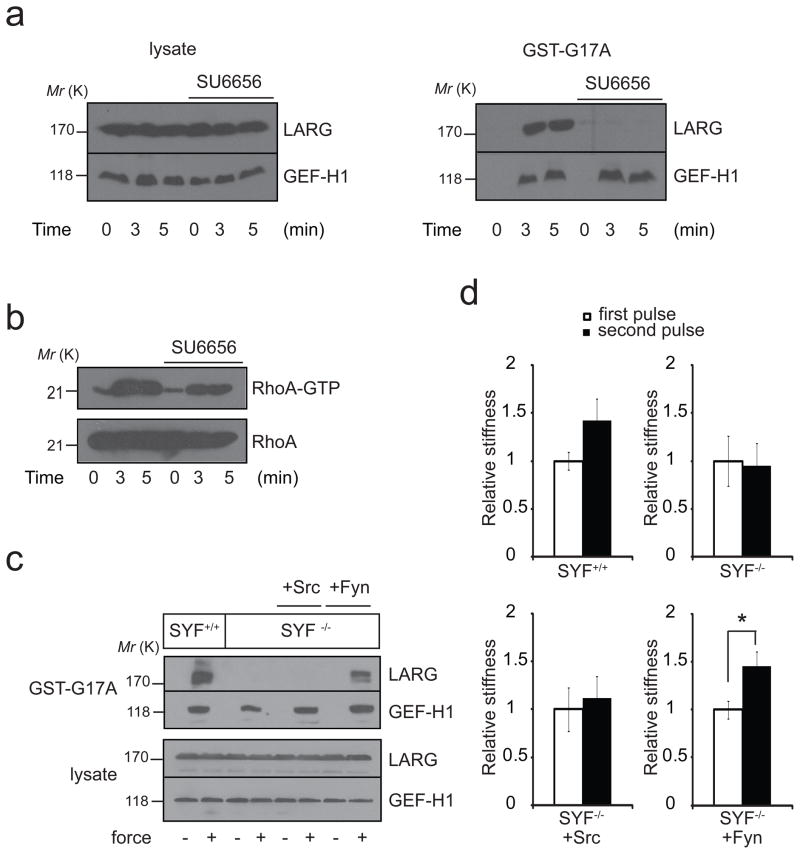
Src family kinases (SFKs) have been shown to be activated in response to force18 and to contribute to cellular stiffening in response to force3. To test if SFKs are involved in LARG and GEF-H1 activation by force, we used the SFK inhibitor SU6656. Pharmacological inhibition of SFKs completely prevented LARG activation in response to force (figure 3a), but had no effect on GEF-H1 activation, suggesting that GEF-H1 and LARG are activated through two independent mechanisms. Consistent with this, inhibition of SFKs by SU6656 partially prevented RhoA activation in response to force (figure 3b). To identify which SFK member is responsible for LARG activation by force, we used the SYF cells (deficient in Src-Yes-Fyn tyrosine kinases). Applying force on FN-coated beads adhering to SYF−/− cells did not increase LARG activity (figure 3c), whereas it stimulated GEF-H1 activity. Surprisingly, expression of Src in the SYF−/− cells did not rescue activation of LARG (figure 3c). However, re-expression of Fyn in SYF−/− cells did restore LARG activation in response to force. Consistent with this observation, analysis of the mechanical properties of SYF cells revealed that only SYF−/− cells re-expressing Fyn but not Src showed a significant increase in stiffness following application of tension on FN-coated beads (figure 3d). We examined whether differences in activity between Fyn and Src in the SYF cells could explain these results, but found that both Src and Fyn are activated by force (supplementary figure 4a). It has been reported that LARG can be activated by FAK phosphorylation on tyrosine19. Interestingly, we observed that LARG phosphorylation on tyrosine was increased in response to force (supplementary figure 4b) and SFK inhibition prevented this increase in phosphorylation. However, we found that FAK inhibition did not affect LARG activation by force (figure 4c), suggesting that Fyn activates LARG in a FAK-independent manner. Fyn has been shown to colocalize at ACs and to play a role in ECM rigidity sensing20. Cells on rigid substrates displayed more stress fibers21 and applied more tension on the ECM through their FAs22. This suggests that the Fyn-LARG pathway can be stimulated by both cell-generated tension as well as by externally applied force, in both cases contributing to increased cellular stiffness.
Figure 3. Fyn mediates LARG activation in response to force.
a, REF52 cells untreated or treated with SU6656 (2.5 μM for 30 min) were incubated with FN-coated beads and stimulated with tensional forces for different amounts of time. Active LARG and GEF-H1 were sedimented with GST-RhoA(G17A) and analyzed by western blot. b, REF52 cells untreated or treated with SU6656 (2.5 μM for 30 min) were incubated with FN-coated beads. After stimulation with tensional force for different amounts of time, cells were lysed and active RhoA (RhoA-GTP) was isolated with GST-RBD and analyzed by western blot. c, SYF−/− cells and SYF cells re-expressing Src, Yes and Fyn (SYF+/+) or re-expressing Src or Fyn were incubated with FN-coated beads and stimulated with tensional forces for 3 min. Active LARG and GEF-H1 were pulled down with GST-RhoA(G17A) and analyzed by western blot. d, Change in stiffness during 2 force pulses applied to FN-coated beads bound to SYF−/− cells and SYF cells re-expressing Src, Yes and Fyn (SYF+/+) or re-expressing either Src or Fyn (error bars represent s.e.m., *p=0.01; n=20). Uncropped images of blots are shown in Supplementary Fig. S5.
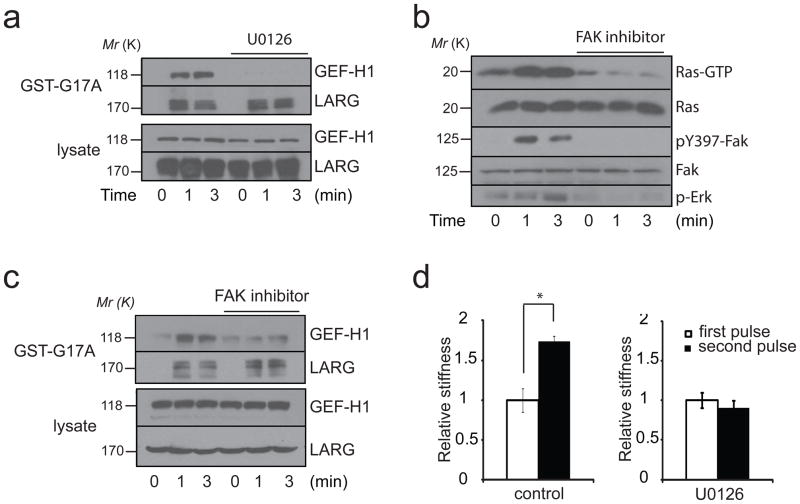
Figure 4. ERK activates GEF-H1 in response to force.
a, REF52 cells untreated or treated with U0126 (5 μM for 30 min) were incubated with FN-coated beads and stimulated with tensional forces for different amounts of time. Active LARG and GEF-H1 were sedimented with GST-RhoA(G17A) and analyzed by western blot. b, REF52 cells untreated or treated with the FAK inhibitor 14 (5 μM for 30 min) were incubated with FN-coated beads and stimulated with tensional forces for different amounts of time. Active Ras (Ras-GTP) was sedimented with Raf1-GST. Phosphorylated FAK (Tyr397), phosphorylated ERK (Thr202-Tyr204), total FAK were analyzed by western blot. c, REF52 cells untreated or treated with the FAK inhibitor 14 (5 μM for 30 min) were incubated with FN-coated beads and stimulated with tensional forces for different amounts of time. Active LARG and GEF-H1 were sedimented with GST-RhoA(G17A) and analyzed by western blot. d, change in stiffness during 2 force pulses applied to FN-coated beads bound to REF52 cells treated with or without U0126 (5 μM for 30 min) (error bars represent s.e.m., *p<0.01; n=20). Uncropped images of blots are shown in Supplementary Fig. S5.
GEF-H1 has been shown to be regulated by microtubule binding23, coupling microtubule depolymerization with RhoA activation in multiple cellular processes such as endothelial barrier permeability, migration and dendritic spine morphology24. To test if GEF-H1 activation could result from microtubule depolymerization we pretreated cells with taxol and analyzed GEF-H1 activity using the nucleotide free RhoA pulldown assay after application of force. We found that taxol did not affect GEF-H1 activation by force (supplementary figure 4d). This result suggests that GEF-H1 is activated independently of microtubule dissociation and is consistent with previous work that showed that treatment with taxol does not affect RhoA-dependent stress fiber formation in response to stretch6. Recent work has shown that the mitogen-activated protein kinase (MAPK) ERK can phosphorylate and activate GEF-H125,26. To test if ERK is necessary for GEF-H1 activation in response to force we used the MEK inhibitor U0126. MEK inhibition prevented GEF-H1 activation by tensional force (figure 4a) but had no effect on LARG activation, confirming that two distinct pathways turn on these two GEFs. ERK has been shown to phosphorylate GEF-H1 on threonine25. Consistent with this, we found that GEF-H1 was phosphorylated on threonine in response to force and MEK inhibition prevented GEF-H1 phosphorylation (supplementary figure 4e).
We next tested if force on integrins activates ERK and its canonical upstream regulator Ras. We observed that ERK and Ras are rapidly activated in response to tensional forces (figure 4b). It has been shown that integrin-mediated cell adhesion causes activation of the Ras-MAPK pathway, but this activation has been reported to be both dependent27 and independent of FAK28. We found that FAK inhibition completely abolished ERK and Ras activation by force (figure 4b). When we looked at GEF-H1 and LARG activation in response to force we found, as expected, that FAK inhibition prevented GEF-H1 activation and had no effect on LARG activity. This result demonstrates that force on integrins activates GEF-H1 through a signaling cascade that includes FAK, Ras and ERK. It has been described that complete activation of FAK during integrin-mediated adhesion requires phosphorylation on Tyr576-577 by Src29. Surprisingly we found that inhibition of SFKs did not affect GEF-H1 activation by force (figure 3a). Moreover, we observed that SFK inhibition did not prevent Ras and FAK activation in response to force (supplementary figure 4f), suggesting that FAK activation by force does not require Src. Analysis of the mechanical properties of cells pretreated with the U0126 revealed that MEK inhibition prevented the significant increase in stiffness following application of tension on FN-coated beads (figure 4d).
To measure the role of the GEFs in the stiffening response, we have used short pulses of force applied to integrins, whereas to measure their contribution to RhoA activation it was necessary to use longer sustained forces. GEF-H1 and LARG, are involved in both the stiffening and the sustained RhoA activation but there is an interesting difference between these two readouts. With the RhoA measurements, inhibiting one of the GEFs decreased the response but did not abolish it (Fig. 1e and f). Similarly, if we blocked the respective upstream signaling pathways, we decreased the level of RhoA activation but only to an intermediate level (Figure 3b – supplementary figure 4g). However, when we examined the stiffening response, inhibiting either pathway blocked the stiffening response (Figure 2c, 3d, 4d). We suspect that the difference reflects that for stiffening to occur in the short time frame following single pulses, the level of RhoA activation beneath a bead has to reach a certain threshold and that this requires both signaling pathways and both GEFs to be activated.
The MAPKs are known to control gene expression, differentiation and growth in response to growth factors30. Here we report for the first time that the Ras-MAPK pathway is activated in response to force on integrins and contributes to reinforcement by activating GEF-H1. Recent work has shown that FAK and ERK are activated when cells are grown on rigid substrates31,32 and contribute to the malignant phenotype observed in breast cancer cells. Our results demonstrate that GEF-H1 acts downstream of the Ras/MAPK pathway to increase cellular rigidity, suggesting that GEF-H1 participates in the control of cellular stiffness in response to substrate rigidity and potentially plays a central role during solid cancer development. Knockdown of GEF-H1 has been reported to not alter the generation of focal adhesions (FAs) 14,33 but to modify their growth. Externally applied forces34 as well as cell-generated tension9,35 are known to play a critical role during FA growth. This suggests that force experienced by FAs, whether externally applied or generated by the actomyosin contractility, activates GEF-H1 that in turn may regulate FA maturation. This potential role in linking force to FA maturation could explain why depletion of GEF-H1 and MEK inhibition affect migration as previously reported33,36.
The external mechanical and stress environment of the cell impacts cell differentiation and gene expression37. The mechanical stiffness of the cell will determine its own strain distribution and hence the specific manner and degree of its mechanically activated signaling. Cytoskeletal stiffening in response to force presumably represents an adaptation that allows a cell to modulate its own mechanically active biochemical network within a mechanical feedback loop. Our identification here of two Rho GEFs that become activated downstream from force applied to integrins increases our understanding of these adaptive pathways. It will be interesting in the future to investigate the universality of these pathways and to determine whether the same or different GEFs are also activated when force is transduced by other cell surface receptors.
Methods
Cell lines and reagents
REF52, SYF MEFs and MRC5 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (Sigma) and antibiotic-antimycotic solution (Sigma). Taxol and SU6656 and U0126 were purchased from Calbiochem. FAK inhibitor 14 was purchased from Tocris. Cell-permeable C3 transferase was from Cytoskeleton.
Antibodies
The anti-RhoA antibody (26C4, 1/300)), anti-Lsc (M-19, 1/500), anti-vinculin (7F9, 1/1000), anti-transferrin receptor (3B8 2A1, 1/300) and anti-Ect2 (C-20, 1/500) were from Santa Cruz Biotechnology. The antibody against LARG was a kind gift of Kozo Kaibuchi (Nagoya University, Japan, 1/1000). Anti-Net1 (1/500) was purchased from Abcam, anti–tubulin (1/2500) was purchased from SIGMA. Anti-phospho Src (Tyr416, 1/1000), anti-GEF-H1 (1/500) and anti-phospho-Threonine-proline (1/500) were purchased from Cell Signaling. Anti Pan Ras antibody (OP40, 1/800) was from EMB Chemicals. Anti-Fyn (610163, 1/1000) was from BD Transduction laboratories. Function-blocking anti β1 integrin (P4C10) was from Millipore.
Purification of recombinant proteins
Construction of the pGEX4T-1 prokaryotic expression constructs containing RhoA(G17A)11 and the Rho-binding domain (RBD) of Rhotekin have been described previously38. Plasmid containing the Raf1-GST construct is a kind gift from Dr Der (University of North Carolina at Chapel Hill). Briefly, expression of the fusion proteins in Escherichia coli was induced with 100 μM IPTG for 12–16 hours at room temperature. Bacterial cells were lysed in buffer containing 50 mM Tris pH 7.6 (for GST-RBD) or 20 mM HEPES pH 7.6 [for GST-RhoA(17A)], 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10 μg/ml each of aprotinin and leupeptin, and 1 mM phenylmethylsulfonyl fluoride, and the proteins purified by incubation with glutathione-sepharose 4B beads (GE Healthcare) at 4°C.
Bead coating and force application
2.8 mm tosyl-activated magnetic dynabeads (invitrogen) were washed with phosphate buffer and incubated 24h with FN or RGD at 37°C. After 3 washes with PBS the beads were sonicated and incubated with cells for 40 min. Coating with antibodies were performed according to the manufacturer’s recommendations (invitrogen). A ceramic permanent magnet was used to generate perpendicular, tensile forces on beads attached to the dorsal surface of cells. For all experiments the pole face was parallel with and .6 cm from the culture dish surface. At this distance the force on a single bead was 10 pN. A constant force of varying duration was used for all experiments.
Isolation of adhesion complexes
FN-coated beads were incubated with cells for 40 min and the bound adhesion complexes were isolated in ice-cold lysis buffer (20 mM Tris pH 7.6 NaCl 150 mM, 0.1% NP-40, 2 mM MgCl2, 20 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin). Beads were isolated from the lysate using a magnetic separation stand and denatured and reduced in Laemmli buffer.
GST-RBD, GST-Raf1 and GST-RhoA(G17A) pulldowns
Active RhoA pulldown experiments were performed as described elsewhere13. REF52 cells were lysed in 50 mM Tris (pH 7.6), 500 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10 mM MgCl2, 200 μM orthovanadate and protease inhibitors. After removal of the magnetic beads using the magnetic separator (invitrogen), lysates were clarified by centrifugation, equalized for total volume and protein concentration, and rotated for 30 minutes with 30 μg of purified GST-RBD bound to glutathione-sepharose beads. The bead pellets were washed in 50 mM Tris (pH 7.6), 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 200 μM orthovanadate, with protease inhibitors, and subsequently processed for SDS-PAGE. For active Ras pulldown experiments, cells were lysed in 25 mM Tris (pH 7.6), 150 mM NaCl, 5 mM MgCl2, 1% NP40, 5% glycerol and protease inhibitors. Affinity precipitation of exchange factors with the nucleotide-free RhoA mutant (G17A) has been described in detail in previous work from our laboratory11. Briefly, cells were lysed in 20 mM HEPES (pH 7.6), 150 mM NaCl, 1% Triton X-100, 5 mM MgCl2, 200 μM orthovanadate plus protease inhibitors. Equalized and clarified lysates were incubated with 20 μg of purified RhoA(17A) bound to glutathione-sepharose beads for 45 minutes at 4°C. Samples were then washed in lysis buffer and processed for SDS-PAGE.
Immunopecipitation
Cells were lysed directly in hot gel sample buffer (200 mM Tris (pH 6.8), 20% glycerol, 4% SDS, 5% 2-ME), and boiled for 10 min. Samples were then diluted with 20 volumes of 1% Triton X-100, 1% DOC in Tris-buffered saline. A total of 2 μg of PY-20 monoclonal anti-phosphotyrosine antibody and protein G-Sepharose were added and samples were incubated 4 h at 4°. Samples were then washed five times in 1% Triton X-100 and 1% DOC in TBS, and analyzed by Western blot using anti-LARG.
RNA interference
siRNAs were purchased from the UNC Nucleic Acid Core Facility-Sigma-Genosys (Sigma-Aldrich). The following siRNAs were used in this study: negative control 5′-UCACUCGUGCCGCAUUUCCTT-3′; RhoA targeted sequence: 5′-GACATGCTTGCTCATAGTCTTC-3′; LARG-Arhgef12 first duplex targeted sequence: 5′-GGACGGAGCTGTAATTGCA-3′; LARG-Arhgef12 second duplex targeted sequence: 5′-TGAAAGAACCTCGAAACTT-3′; p115-Arhgef1 (first duplex) targeted sequence: 5′ GGGCTGAGCAGTATCCTAG-3′; p115-Arhgef1 (second duplex) targeted sequence: 5′–GGCAAGAGGTCATCAGTGA-3′; Gef-H1-Arhgef2 (first duplex) targeted sequence:–5′-CACGTTTCCTTAGTCAGCT-3′; Gef-H1-Arhgef2 (second duplex) targeted sequence: 5′-CACCAAGGCCTTAAAGCTC-3′; Ect2 targeted sequence: 5′-TGCTGAGAATCTTATGTAC-3′. SiRNAs were transfected with Lipofectamine 2000 (Invitrogen).
Magnetic force assay
The UNC 3D Force Microscopy39 (3DFM) was used for applying controlled and precise 60–100pN local force on the magnetic beads. Cells were plated on coverslips for 24 h and incubated for 40 min after addition of beads. On force application, bead displacements were recorded with high speed video camera (Pulnix, JAI, Ca) and tracked using Video Spot Tracker (Center for Computer Integrated Systems for Microscopy and manipulation, http://cismm.cs.unc.edu). The spring constants were derived by fitting bead displacements and applied force to a Jeffrey’s model for viscoelastic liquid (supplementary figure 1).
Calculation of spring constant
The UNC 3DFM system was calibrated prior to experiments using a fluid of known viscosity. Displacement of individual beads attached to cells was tracked using video spot tracker software (Supplementary figure 2 a and b). Beads that showed displacements less than 10 nm (detection resolution) and loosely bound beads were not selected for analysis. Custom-made Matlab codes were used to calculate the creep compliance (also referred to as deformability) which is defined as the average time dependent deformation normalized by the constant stress applied (Jmax = rmax × 6πa/F, where a is the radius of the bead) (Supplementary figure 2c). Each compliance curve was then fitted to a Jeffrey’s model for viscoelastic materials, shown in supplementary figure 2d using a least squares method. Stiffness was reported as the value of k in Pascal. Subsequent pulses were fitted in the same manner and the average k for each cell type and pulse number was obtained and reported as mean±sem. All statistical analysis including two-tailed Student's t-tests for p values reported were done in Excel.
Calibration of permanent magnet system
Calibration of permanent system was done using previously described methods40. Briefly, the magnetic beads were diluted in a fluid of known viscosity and placed in a closed well to eliminate drift. The well was then placed at a known distance from the face of the permanent magnet. Particle velocities were obtained using Video spot tracker and in-house Matlab programs from which the applied force was calculated via Stokes’ formula.
Statistical analysis
Statistical differences between two groups of data were analysed with a two-tailed unpaired Student’s t-test.
Supplementary Material
Acknowledgments
The authors would like to thank Lisa Sharek for her technical support. This study was supported by National Institutes of Health Grants #GM029860 and GM029860-28S (to K.B.), P41-EB002025-23A1 (RS) and R01-HL077546-03A2 (RS), and a grant from the University Cancer Research Fund from the Lineberger Comprehensive Cancer Center. CG is supported by a Marie Curie Outgoing International Fellowship from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 254747.
Footnotes
AUTHOR CONTRIBUTIONS: C.G and V.S designed and performed experiments. R.G.M and E.T.O helped with experimental design and procedures. C.G and K.B wrote the manuscript. K.B and R.S directed the project and revised the manuscript. All authors provided detailed comments.
References
- 1.Hoffman BD, Crocker JC. Cell mechanics: dissecting the physical responses of cells to force. Annu Rev Biomed Eng. 2009;11:259–288. doi: 10.1146/annurev.bioeng.10.061807.160511. [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 3.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 4.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 6.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol. 2002;156:609–615. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 12.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 13.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim Y, Lim ST, Tomar A, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tim O’Brien E, Cribb J, Marshburn D, Taylor RM, 2nd, Superfine R. Chapter 16: Magnetic manipulation for force measurements in cell biology. Methods Cell Biol. 2008;89:433–450. doi: 10.1016/S0091-679X(08)00616-X. [DOI] [PubMed] [Google Scholar]
- 16.Bausch AR, Moller W, Sackmann E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J. 1999;76:573–579. doi: 10.1016/S0006-3495(99)77225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thoumine O, Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci. 1997;110 (Pt 17):2109–2116. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]
- 18.Na S, Collin O, Chowdhury F, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 20.Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitrossilis D, Fouchard J, Guiroy A, et al. Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci U S A. 2009;106:18243–18248. doi: 10.1073/pnas.0903994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 24.Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–219. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Fujishiro SH, Tanimura S, Mure S, Kashimoto Y, Watanabe K, Kohno M. ERK phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem Biophys Res Commun. 2008;368:162–167. doi: 10.1016/j.bbrc.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 26.Kakiashvili E, Speight P, Waheed F, et al. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem. 2009;284:11454–11466. doi: 10.1074/jbc.M805933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 28.Lin TH, Aplin AE, Shen Y, et al. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 31.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070–4082. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riveline D, Zamir E, Balaban NQ, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Ren XD, et al. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18(3):578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher JK, Cribb J, Desai KV, et al. Thin-foil magnetic force system for high-numerical-aperture microscopy. Rev Sci Instrum. 2006;77 doi: 10.1063/1.2166509. nihms8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair L, Ford K, Alam MR, Kole R, Fisher M, Superfine R. Size-uniform 200 nm particles: fabrication and application to magnetofection. J Biomed Nanotechnol. 2009;5:182–191. doi: 10.1166/jbn.2009.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.