Abstract
Like many carcinomas, urothelial carcinoma (UroCa) is associated with chronic injury. A better understanding of this association could inform improved strategies for preventing and treating this disease. We investigated the expression, regulation, and function of the transcriptional regulator SRY-related HMG box 9 (Sox9) in urothelial development, injury repair, and cancer. In mouse bladders, Sox9 levels were high during periods of prenatal urothelial development and diminished with maturation after birth. In adult urothelial cells, Sox9 was quiescent but was rapidly induced by a variety of injuries, including exposure to the carcinogen cyclophosphamide, culture with hydrogen peroxide, and osmotic stress. Activation of extracellular signal-regulated kinases 1/2 (ERK1/2) was required for Sox9 induction in urothelial injury and resulted from activation of the epidermal growth factor receptor (Egfr) by several Egfr ligands that were dramatically induced by injury. In UroCa cell lines, SOX9 expression was constitutively upregulated and could be suppressed by EGFR or ERK1/2 blockade. Gene knockdown demonstrated a role for SOX9 in cell migration and invasion. Accordingly, SOX9 protein levels were preferentially induced in invasive human UroCa tissue samples (n=84) compared to noninvasive cancers (n=56) or benign adjacent urothelium (n=49). These results identify a novel, potentially oncogenic signaling axis linking urothelial injury to UroCa. Inhibiting this axis is feasible through a variety of pharmacologic approaches and may have clinical utility.
Keywords: Epidermal Growth Factor Receptor, SOX9, migration, invasion, urothelial carcinoma
Introduction
Cancer growth and spread requires coordinated cell migration, proliferation, and stromal remodeling. Similar programs operate in both organogenesis and in injury repair (1). Repeated injury repair vastly increases the risk of epithelial cancers (carcinomas), particularly bladder cancer (2;3). The process of injury repair recapitulates aspects of normal organogenesis (4;5), with transient reactivation of certain genes that are active in embryonic organogenesis and quiescent in mature tissues. Chronic injury, however, may lead to sustained activation of the these genes, and such perseverative signals may lead, in turn, to carcinogenesis (1).
In investigating the molecular links between injury and cancer, transcription factors are appealing targets because they have distinctive and dynamic expression profiles and can themselves coordinate complex genetic programs. These properties are illustrated by SRY-related high-mobility-group (HMG) box (Sox) 9 (SOX9). SOX9 belongs to group E (SOX8, SOX9, and SOX10) of the SOX transcription factor family (6) defined by a common HMG box domain originally identified in SRY, the sex-determining gene on the Y chromosome. SOX9 has roles in epithelial invasion, migration, and proliferation as demonstrated in developing prostate (7-9), and similar roles in prostate cancer (8). In chondrocyte development, Sox9 is a master chondrogenic factor whose expression is induced by receptor tyrosine kinase (RTK) signaling (10). Sox9 induction by RTKs requires activation of mitogen-activated protein kinase (p44/42 MAPK or Erk1/2) (10). In this study, we investigate RTK induction of Sox9 in urothelial development, regeneration, and cancer.
Here we identify Sox9 as a molecular link between urothelial injury and urothelial cancer. Sox9 expression coincides with urothelial proliferation during bladder organogenesis, is quiescent in adult urothelium, and is reactivated during acute bladder injury. Sox9 induction occurs through ligand-stimulated activation of epidermal growth factor receptor (EGFR) and subsequent MAPK pathway activation. In contrast to benign bladder, urothelial carcinomas show constitutive SOX9 induction through autonomous EGFR activation, and SOX9 was significantly upregulated in invasive carcinomas. SOX9 knockdown significantly impaired UroCa cell migration and invasion, suggesting its role in UroCa pathogenesis. These data identify a novel link between urothelial development, regeneration, and cancer.
Materials and Methods
Cell lines and cell cultures
BFTC905 (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO) and 1% penicillin/streptomycin (Invitrogen). Human SCaBER bladder cancer cells (American Type Culture Collection, ATCC, VA) were cultured in DMEM with 10% FBS. UROtsa (11) cells provided by S. H. Garrett (University of North Dakota), were cultured in DMEM with 2 g/L glucose and 5% FBS. The mouse urothelial carcinoma line MB49 (12) was provided by Dr. Yi Luo (University of Iowa) and cultured in RPMI1640 with 10% FBS. Cell line identity was assured by use within 6 months of receipt from ATCC or short tandem repeat (STR) confirmation using reference material provided by the contributor (UROtsa cells) or by an online database maintained by the Deutsche Sammlung von Mikroorganismen und Zellkulturen repository.
Compounds and reagents
Erlotinib (OSI-774, Tarceva) was purchased from Johns Hopkins Hospital Pharmacy. All other chemicals were purchased from Sigma, unless otherwise indicated. EGF and Matrigel were purchased from BD Pharmingen (San Diego, CA) and Collagen I from Invitrogen.
Antibodies and immunoblotting
Antibodies against EGFR, phospho-EGFR (Tyr1068), Akt, phospho-Akt (Ser473), p44/42 MAPK (Erk1/2), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), and anti-phospho-STAT3 were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Immunoblotting were performed as previously described (13). Antibodies against human EGFR, β-actin (#A5316) and GAPDH (#sc-32233) were purchased from Dako (Carpenteria, CA), Sigma, and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Anti-Sox9 antibody (#AB5535) was purchased from Chemicon (Temecula, CA). Cells were cultured in serum-free medium overnight (16 hours), pretreated with inhibitors for 3 hrs or 24 hrs, and then with EGF or HB-EGF for 10 or 24 hours.
Animals and mouse bladder injury model
C56BL6 mice (age: 6-8 weeks) were obtained from the Jackson Laboratory. The protocol was approved by the animal care and use committee of the Johns Hopkins University. Mice were randomly selected for a single 0.2 ml intraperitoneal (i.p.) injection of 250 mg/kg body weight of cyclophosphamide (CPA) or Phosphate Buffered Saline (PBS, control). This dose is similar to that used in humans receiving high dose CPA (14).
Explant culture
Bladder strips were laid flat on tissue culture inserts (Millicell-CM 0.4 μM, 30 mm, Millipore, Billicell, MA) floated in DMEM/F-12 (1:1) serum-free media with 1% ITS (10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml sodium selenite, Sigma) (15;16). Inhibitors of EGFR (erlotinib) or MEK1/2 (U0126) were added and tissues were cultured for 1 day before processing for histology.
RNA extraction, reverse transcription (RT) and real-time PCR
RNA was extracted using Trizol (Invitrogen) followed by RNAeasy mini kit cleanup (Qiagen, Valencia, CA). RNA was reverse transcribed with Superscript III (Invitrogen). Primer sequences are shown in Supplementary Table 1. iTaq SYBR green Supermix with Rox dye (Bio-Rad) was used for real-time PCR and reactions were performed in triplicate. Quantification of target transcripts was calculated relative to hypoxanthine phosphoribosyltransferase (Hprt1) using the ΔΔCt method with values from injured (CPA exposed) bladders normalized to values from uninjured (PBS) controls. Data were expressed as mean ± SEM and the student t-test was used to compare the difference in means between control and CPA-treated samples.
Immunohistochemistry (IHC)
was performed as previously described (13). Briefly, tissue sections were serially incubated in PBS/3% H2O2 (10 min at 22°C), PBS (rinse), 10% goat serum (block) (30 min at 22°C) rabbit anti-SOX9 antibody (1 hour at 22°C), PBS (rinse), goat anti-rabbit biotinylated secondary antibody (DAKO) (30 min at 22°C), PBS (rinse), streptavidin-HRP (DAKO) (30 min at 22°C), and PBS (rinse). Staining was visualized with 3,3’-diaminobenzidine (DAB) tetrahydrochloride (Zymed, CA).
Growth factor and inhibitor treatment
Cells cultured in fresh complete or serum-free media overnight (16 hours) were treated with inhibitors of one of the following targets: PI3K (LY294002), MEK1/2 (U0126), p38 MAPK (PD 169316), EGFR (Erlotinib), or vehicle control (DMSO) in fresh serum-free medium for 2 hours, then EGF (10 ng/ml), or HB-EGF (10 or 50 ng/ml) for an additional 24 hours.
SOX9 knockdown
Lentiviral vector-based SOX9 and control shRNAmir constructs were obtained from Open Biosystems (Huntsville, AL). shRNAs were transfected into human BFTC905 UroCa cells using Arrest-In™ reagent (Open Biosystems), followed by puromycin (10 μg/ml) selection for 4 weeks to generate stable clones. Clonal colonies isolated were validated by Western blot for SOX9.
Proliferation Assay, scratch wound healing and invasion assay
Proliferation assays were performed in 96-well plates according to manufacturer's instruction (ATCC, VA) using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) colorimetric assay kit. Plates were read using a SpectraMax plate reader (Molecular Devices Corp., Sunnyvale, CA) at 570 nm with a reference wavelength of 650 nm.
Wounds in 6-well plates were produced with a modified P1000 pipette tip and monitored daily. Wound area was measured using the Measurement function in the Analysis Tab of Adobe Photoshop CS4 Extended and then exported as an Excel file for statistical analysis.
In vitro invasion assays used 24-well transwell Boyden chambers (17). Polycarbonate membrane inserts (Costar, New York, NY) were pre-coated with a mixture of growth factor-reduced Matrigel (Invitrogen) and DMEM (1:1 ratio) or of Collagen I (Invitrogen) and DMEM (1:1 ratio). Bottom chambers were filled with DMEM containing 10% FBS as a chemoattractant. 5×104 cells were seeded on the top chamber and incubated for 24 hours. Invasion was quantified as described (13). Aliquots of the same culture were also plated in 24-well plate for MTT assay on the same day.
Tissue microarrays (TMAs), IHC staining and scoring
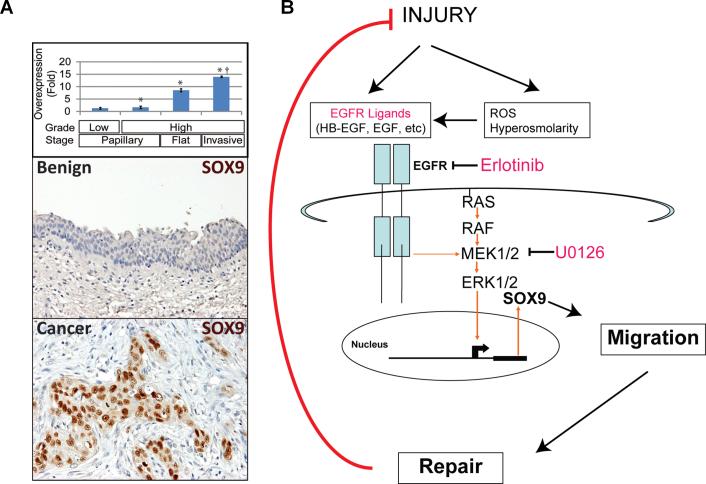
Construction and composition of the two TMAs used in this study have been previously described (18;19). Cases were included on the basis of available tissue and follow-up data. Control samples and cancer samples were deemed informative when they contained morphologically recognizable benign urothelium or cancer cells (respectively). The non-invasive cohort (18) included biopsies of benign urothelium, paired with corresponding low (n=30), and high (n=30) grade noninvasive papillary carcinomas evaluated at our institution between 1971 and 1995. Of these, 28 low grade and 28 high grade carcinomas were deemed informative. In addition, 14 cases had paired benign controls available for analysis. The invasive cohort (19) comprised 132 cystectomies performed in our institution between 1994 and 2002. Of these, 84 invasive and 15 adjacent CIS lesions were deemed informative. 4 micron TMA sections were stained for SOX9 by IHC as above. Pathologic stages for informative invasive urothelial carcinoma cases were pT1 (n=4), pT2 (n=28), pT3 (n=40), and pT4 (n=12). Intensity of SOX9 nuclear staining was evaluated and assigned an incremental 0, 1+, 2+, 3+ score. Distribution of staining was categorized as absent, focal (1-25% of cells), multifocal (25-75%), or diffuse (>75%). To integrate intensity and distribution of staining, an H-Score for SOX9 score was calculated by multiplying the intensity score and the distribution score as previously described (19). H-scores were compared using the 1-way ANOVA test with the Bonferroni's post-hoc pairwise comparison test. A 2-tailed P-value <0.05 was required for statistical significance. Data were analyzed using PASW Statistics version 18.0 (IBM Inc., Somers, NY). Upregulation (Fig. 6A) was calculated separately for each TMA as the ratio of H-Score in cancer to H-Score in benign.
Figure 6. SOX9 is overexpressed in human bladder cancer tissues.
A, Immunohistochemical SOX9 staining demonstrated undetectable or low levels in benign urothelium (n=49; representative section in A, top panel) Staining (both distribution and intensity) was slightly elevated in noninvasive carcinomas (n=56; staining not shown), more widespread and intense in the majority of high grade flat carcinoma in situ (Flat) lesions (n=15; staining not shown) and invasive human UroCas (n=84; A, middle panel). Overall, mean SOX9 scores for cancer exceeded those for than benign urothelium (* P=0.0001), and mean scores for invasive cancers exceeded those for noninvasive cancers († P<0.03). Compared to benign urothelium, SOX9 scores were slightly elevated in low grade papillary noninvasive cancers (n. s.) but became increasingly elevated in high grade and invasive tumors (* P<0.05). B, Model of EGF ligand-receptor-ERK1/2-SOX9 induction by injury. Note that the system turns off in response to completion of injury repair. With chronic injury, the system may remain activated and facilitate cancer formation.
Results
Induction of Sox9 during urothelial organogenesis
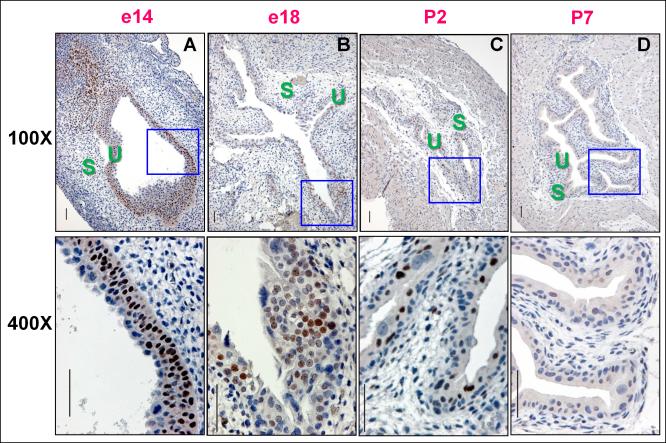
The rudiment of the mouse urinary bladder forms at embryonic day 14 (e14). Urothelium proliferates and differentiates until the perinatal period (e17-birth) (20), giving rise to the mature relatively quiescent trilaminar state consisting of basal stem cells, intermediate transit amplifying cells, and fully differentiated superficial/umbrellas cells (21;22). In rapidly growing epithelium (e14), Sox9 protein induction was detected by immunohistochemical staining in the basal and intermediate layers, but not in the superficial layer, with occasional staining seen in stromal cells (Fig. 1A). Sox 9 staining diminished near term (e18, Fig. 1B), was barely detectable by Postnatal day 2 (P2) (Fig. 1C), and undetectable by P7 (Fig. 1D). SOX9 was also quiescent in human urothelium from organ donors ranging from age 4 to 40 (data not shown).
Figure 1. Elevated Sox9 levels in embryonic urothelium diminish with maturation.
Immunohistochemical stains with a Sox9 specific antibody show A, Intense nuclear staining in basal and intermediate cells of mouse bladder mucosa at e14. B, By e18, Sox9 staining is reduced and mainly seen in the intermediate layer. Rare superficial and intermediate cells express Sox9 at postnatal day 2 (P2) (C) and Sox9 is undetectable at P7 (D). S, stroma; U, urothelium. The scale bar = 50 microns.
Urothelial injury reactivates Sox9 expression
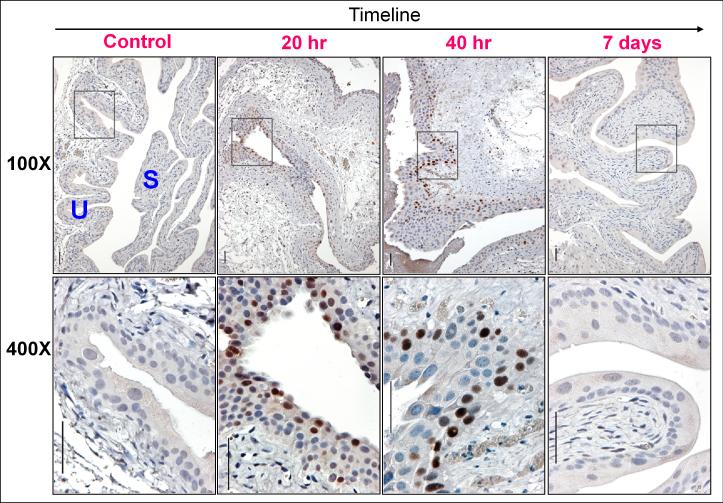
The chemotherapeutic agent cyclophosphamide (CPA) induces urothelial injury and can cause bladder cancer in humans. In modeling the onset of such injury in mice, we found that Sox9 levels rose and fell in parallel with the urothelial repair program. Urothelial necrosis, sloughing, and regeneration begin shortly after CPA administration, with peak urothelial proliferation at 36 hours and return to baseline by 7 days, when injury has healed (23;24) (Supplementary Fig. 1). Sox9 was undetectable by IHC in adult controls. Within 20 hours of CPA injection, Sox9 protein was readily detected in all three urothelial cell layers. By 40 hours, we observed Sox9 expression only in the basal cell layer, and by 7 days, the protein was undetectable (Fig. 2A). Thus, induction of Sox9 is a tightly regulated event with temporal kinetics that tracks closely with urothelial injury repair.
Figure 2. Sox9 expression is rapidly but transiently induced in urothelial injury/repair.
Sox9 was undetectable in the bladders of mice subcutaneously injected with PBS (control). Intense nuclear Sox9 staining was detected 20 and 40 hours after cyclophosphamide (CPA) injection, but not at 7 days when repair is complete (see Supplementary Fig. 1). Sox9 staining was trilaminar at 20 hrs and predominantly basal at the 40 hour time point.
EGFR pathway induction in urothelial injury repair
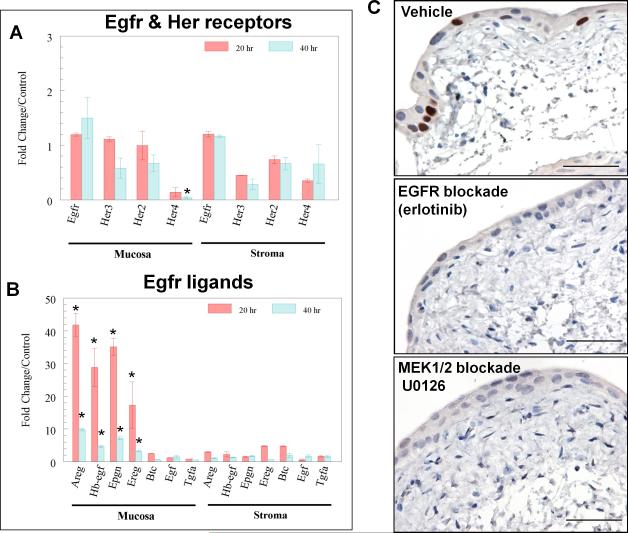
Given its known roles in injury repair (25;26) and proposed roles in the urothelium (26;27), EGFR and its family members are likely candidates to induce Sox9. In order to separately examine epithelial and stromal responses to injury, we rapidly separated the two compartments for analysis at the time of harvest after mice had been injected with PBS or CPA for 20 or 40 hrs. By RT-PCR analysis, Egfr, Her2, and Her3 were readily detected at equivalent levels in urothelium (mucosa) and in the muscular bladder wall (stroma), whereas Her4 was nearly undetectable in mucosa (Fig. 3A). Injury caused little change in receptor transcripts (Fig. 3A). In contrast, levels of Egfr ligands were markedly induced (up to 42-fold, P<0.001) in urothelial mucosa. Amphiregulin (Areg), heparin-binding EGF-like growth factor (Hb-egf), epiregulin, and epigen were the most highly induced ligands, with peak induction within 20 hours of injury (Fig. 3B, P<0.001). Since urothelial basal cells express EGFRs (27;28), the induction of EGFR ligands in urothelial cells by injury suggests an autocrine/paracrine mechanism through which injury could lead to Sox9 induction.
Figure 3. Upregulation of Sox9 in urothelial injury by Egfr family receptors and ligands.
A, Real-time RT-PCR demonstrates the levels of mRNA transcripts encoding Egfr, Her2, Her3, and Her4 receptors in the bladder wall (stroma, right) and urothelial mucosa (left), compared with controls. The inductions were not significant, except for Her4, which showed decreased expression in CPA-injured mouse urothelial mucosa. *: P<0.05. B, Transcripts encoding Egfr family ligands Areg, Hb-egf, Epigen (Epgn), and Epiregulin (Ereg), were markedly induced in urothelial mucosa (left) by CPA injury, but not in bladder wall (stroma, right). The quantitative data shown in A and B were averaged from 5 bladders in each condition (PBS control and CPA injury). Values are expressed as mean ± SEM. *: P <0.001. C, Photomicrographs (bar = 50 microns) show intense nuclear immunohistochemical staining for Sox9 in surgically injured bladder explants cultured in control media (vehicle). 24-hour culture with erlotinib or U0126 resulted in undetectable Sox9 levels. Representative images from 6 independent experiments (n=6).
EGFR induces Sox9 through ERK1/2 signaling
We confirmed that Egfr induces Sox9 expression through Erk1/2 signaling using cultured strips of mouse bladder (explant culture), with the cut tissue edges simulating urothelial injury (29). Unlike intact bladder, urothelial Sox9 expression was readily detected (Fig. 3C), but this induction was completely blocked by culture with erlotinib (Fig. 3C), confirming Egfr-dependent activation of the pathway. Sox9 induction was also blocked by a MEK1/2 inhibitor, U0126 (Fig. 3C), indicating that canonical Egfr signaling through the Erk1/2 pathway is required for induction of Sox9 by injury.
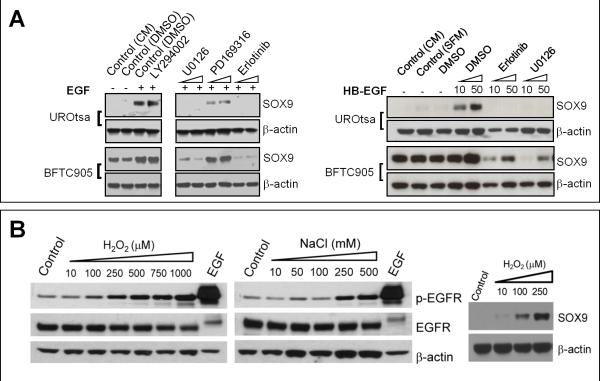
We confirmed and further investigated this pattern of SOX9 regulation in benign immortalized human UROtsa cells. This line phenocopies the basal cells from which it was derived, as evidenced by the expressions of EGFR and p63 (Supplementary Fig. 2) (30). Under normal culture conditions, SOX9 protein was undetectable (Fig. 4A; Supplementary Fig. 2B & 2C). Proteolysis through the ubiquitin-proteasome pathway likely contributes to such low SOX9 levels, since treatment with the proteosome blocker MG132 increased the levels of SOX9 protein (Supplementary Fig. 4A). SOX9 induction also resulted from culture with EGFR ligands, including EGF, which is physiologically enriched in urine (27), and HB-EGF, which is physiologically synthesized by urothelial cells (31) and upregulated in urothelial cells following injury (Fig. 3A). When mediated by EGF or HB-EGF, SOX9 induction was detectable within 6 hours of ligand exposure (Supplementary Fig. 4B), consistent with the kinetics seen with CPA exposure in vivo (data not shown). Induction in this case likely resulted from enhanced de novo synthesis of the SOX9 protein rather than enhanced protein stability since the protein translation inhibitor cycloheximide blocked SOX9 induction (Supplementary Fig. 4B). Consistent with results from organ culture (Fig. 3C), SOX9 induction in cell lines was completely blocked by ERK1/2 or EGFR inhibition (Fig. 4A).Thus, the urothelial EGFR-ERK1/2-SOX9 axis appears to operate similarly across mouse and human species and can do so in the absence of stromal-epithelial interactions.
Figure 4. Regulation of SOX9 by growth factors and injury.
A, SOX9 was induced in UROtsa and BFTC905 cells by treatment of EGF or HB-EGF Induction was blocked by small molecule inhibitors of ERK1/2 (U0126) or EGFR (erlotinib), but not PI3 kinase (LY294002) or p38 MAPK (PD169316). B, Immunoblots showing induction of EGFR phosphorylation and SOX9 protein levels in UROtsa cells following treatment with hydrogen peroxide or sodium chloride.
Injurious chemicals present in urine such as NaCl and urea (32) or released from injured cells such as H2O2 (33) and ATP (34), are known to activate EGFR. In UROtsa cells, treatment with H2O2, NaCl, or ATP-γ-S (a non-hydrolyzable form of ATP) induced coordinate EGFR phosphorylation and elevated levels of SOX9 (Fig. 4B and Supplementary Fig. 5A). Interestingly, treatment with urea affected neither EGFR phosphorylation (Supplementary Fig. 5B), nor SOX9 levels (data not shown), suggesting that distinct pathways may mediate responses to different injuries. Thus, a variety of physiologically relevant injuries induce SOX9 in urothelial cells and such induction may involve activation of EGFR.
Autocrine EGFR signaling maintains constitutive SOX9 elevation in carcinomas
In contrast to undetectable levels of SOX9 found in benign urothelial cells, SOX9 protein levels were high in 8 of 9 UroCa cell lines tested (Supplementary Fig. 2B). As demonstrated in human BFTC905 (Fig. 4A; Supplementary Fig. 6B), J82 (Supplementary Fig. 6A) and murine MB49 UroCa cells (Supplementary Fig. 6B), and confirmed in human SCaBER squamous bladder carcinoma cells (Supplementary Fig. 6A), treatment with the EGFR inhibitor or with the MEK1/2 inhibitor effectively suppressed SOX9 levels to undetectable levels. In contrast, effective pharmacologic inhibition (Supplementary Fig. 4) of Akt, p38 MAPK, STAT3, c-MET, IGF-1R, or PDGFR did not significantly alter SOX9 expression (Fig. 4; Supplementary Fig. 6A & 6B). SOX9 expression in urothelial cancer cells was further increased by treatment of EGF or HB-EGF, but not when cells were pre-treated with erlotinib or U0126 (Fig. 4A; Supplementary Fig. 6A). In addition to UroCa, we found evidence for an active EGFR/ERK1/2/SOX9 signaling axis in a variety of other human carcinomas, including those arising in lung, prostate, oropharyngeal mucosa, and skin (Supplementary Fig. 7A). These data indicate that constitutive activation of SOX9 expression through EGFR and ERK1/2 is a common feature of carcinomas. This constitutive activation is also present in vivo as demonstrated in the xenografts of UroCa and of a variety of other carcinomas (Supplementary Fig. 2D and 7B).
Constitutive SOX9 expression may result from autocrine/paracrine signaling by HB-EGF, a phenomenon previously observed to promote growth in urothelial cell cultures (31). Heparin binds EGFR ligands, HB-EGF and AREG with high affinity. Heparin reduced SOX9 expression (Supplementary Fig. 6C), suggesting that UroCa cells produce EGFR ligands, HB-EGF and AREG, to sustain constitutive SOX9 expression.
Requirement for SOX9 in cell migration and invasion, but not proliferation
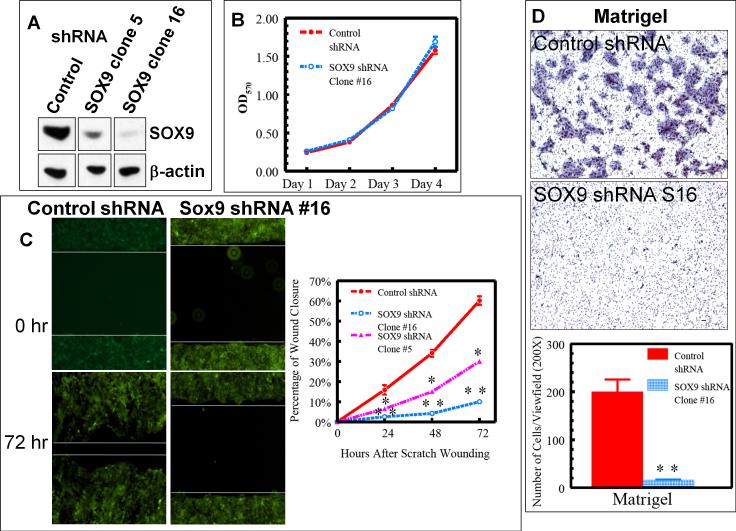
Urothelial injury repair likely requires coordinated proliferation and differentiation of basal cells as they migrate to cover the wound and reconstitute the urothelial barrier. To address potential roles of SOX9 in this process, we used stable expression of two different short hairpin small interfering RNAs (shRNAs) to generate human BFTC905 UroCa clones deficient in SOX9 (Supplementary Fig. 8A & 8B). Comparing clones with low (clone 16), intermediate (clone 5), and high (control) levels of SOX9 (Fig. 5A; Supplementary Fig. 8B), effective reduction of SOX9 elicited no significant change in growth rate (Fig. 5B). However, in proportion to the effectiveness of SOX9 reduction, SOX9-deficient BFTC905 cells showed markedly impaired abilities to migrate and cover a “wound” scraped across a culture dish (Fig. 5C). Consistent with a migration defect, SOX9-deficient BFTC905 cells displayed decreased abilities to invade Matrigel- and collagen-coated membranes in transwell (Boyden) chamber assays (Fig. 5D and Supplementary Fig. 9). This phenotype was confirmed using separate shRNAs targeting two distinct regions of the SOX9 transcript, indicating that the migration effect is an “on-target” effect. Using transient SOX9 knockdown, this effect was further confirmed in another human UroCa cell line, UM-UC-3 (Supplementary Fig. 10). T24 UroCa cells, in contrast, were unaffected, indicating that they use substitute or redundant pathways for migration. Although not universal, these results confirm a role for SOX9 in UroCa invasion and migration.
Figure 5. SOX9 is required for UroCa migration and invasion.
A, Immunoblot showing SOX9 protein levels in independent BFTC905 UroCa clones engineered to stably express control shRNA or shRNAs that target SOX9. Note that each immunoblot was done on the same membrane as the supplementary Fig. 8B with unrelated lanes removed and represented as gaps. B, MTT assay showing no effect of SOX9 knockdown on cell growth. C, Control clones covered wounds scratched more rapidly than SOX9 shRNA clones 5 or 16. D, Representative photomicrographs (top and middle panels) showing cells that invaded through the Matrigel layered onto a filter with 8 micron pores (modified Boyden chamber assay). The quantitative data shown are averaged across 3 independent experiments. Values are expressed as mean ± SEM. *: P<0.05; **: P<0.01.
Sox9 is re-expressed in urothelial carcinoma
Expression analysis in primary human samples suggests a general role for SOX9 in UroCa, particularly in invasive cancers. UroCa arises through two divergent pathways (reviewed in (35)). The majority originates in an indolent form with papillary formations that extend into the bladder lumen. A minority originates as flat or invasive forms, metastasize early and are often lethal. In analysis of SOX9 IHC by H-score (intensity × proportion of stained cells), cancers scored higher than benign urothelium (P=0.0001). Compared to noninvasive papillary cancers, SOX9 induction was more dramatic in more aggressive flat/invasive cancers (P<0.03). SOX9 induction was 7-fold for CIS and 14-fold for invasive cancers (Fig. 6A). Induction was observed frequently. Compared to benign urothelium, SOX9 staining was elevated in 75% of CIS cases and 89% of invasive cases (data not shown). However, SOX9 staining was as high in early stage (pathologic stage pT1) invasive carcinomas as in advanced (pT3 or greater) cases (data not shown). Thus, SOX9 induction appears to be a general property of UroCa, particularly of the more aggressive flat/invasive pathway, consistent with the notion that the protein is induced early in the course of urothelial carcinogenesis.
Discussion
In embryonic urothelium and urothelium undergoing injury repair, Sox9 is expressed in basal and intermediate cells, but not in terminally differentiated superficial cells. This expression pattern suggests that the transcription factor may antagonize urothelial differentiation, a role consonant with that seen in pre-adipocytes (36), chondrocytes (37), pyloric sphincter epithelial cells (38), and early differentiation of prostate bud epithelia (7).
Sox9 expression is limited to the invasive front of epithelial buds in developing prostate (7-9) and lung (39) as the buds enter surrounding mesenchyme. Tissue-specific knockout in the prostate prevents bud outgrowth, likely through defective growth, migration, or both. shRNA-mediated knockdown of SOX9 expression in UroCa cells can result in a migration defect without affecting tumor cell growth. While more studies would be needed to distinguish roles in embryonic growth versus injury repair, it may be that the proliferative role of SOX9 operates mainly in primitive embryonic cells whereas the migratory role is common to both organogenesis and injury repair.
In normal undamaged bladder, an overlying urine-blood barrier formed by superficial cells (20;40) is hypothesized to protect the EGFR-enriched basal cell layer (27;28;41) from contact with urinary EGFR ligands (especially EGF). In addition to the possibility that basal urothelial cells might respond to urinary ligands, urothelium and adjacent smooth muscle can produce EGFR ligands in response to injury, including TGFα (42), HB-EGF, and Epiregulin (43). It has not been previously demonstrated that injury to urothelial tissue activates EGFR signaling, and the sequelae of such activation have not been previously determined. Here we provide new evidence that HB-EGF and AREG are significantly induced by injury in urothelial tissue (Fig. 3A), supporting an autocrine mode of action in activating EGFR. Of particular interest, HB-EGF has been found to be an autocrine growth factor for human urothelial cells (31). This finding, coupled with the induction of this ligand by urothelial tissue injury (Fig. 3) and evidence that HB-EGF induces SOX9 in UroCa cells (Supplementary Fig. 6E & 6F), suggest a mechanism linking migration induced by injury to that operating in cancer.
We have discovered a role for SOX9 in cancer cell migration and invasion, indicating that SOX9 might mediate EGFR-induced cancer spread. We further provide evidence for this hypothesis by showing that SOX9 has significantly higher expression in the flat/invasive pathway of UroCa compared to non-invasive tumors or benign urothelium (Fig. 6A). The role of SOX9 in cell migration is also consistent with the notion that urothelial cells are very mobile during injury repair and need to migrate to the superficial layer and to differentiate to heal. In UroCa cells, aberrant expression of EGFR receptors and ligands that lead to constitutive induction of SOX9 also support the invasive migratory phenotype of these cells. This notion is further supported by our recent finding that EGFR ligands, HB-EGF and NRG2, were highly expressed in a highly tumorigenic basal cell compartment in urothelial carcinoma (44) that is also enriched for EGFR (Supplementary Fig. 2A) and SOX9 expression (Supplementary Fig. 2D).
Our findings have implications for bladder injury repair and carcinogenesis. Cancer is long been thought as a chronic wound that does not heal (45). The ultimate source of cells for repairing injured tissue is stem/progenitors cells. For tissues with a slow turnover like urothelium, stem/progenitor cells remain quiescent. However, when injury occurs, cells can rapidly migrate (a process known as epithelial restitution), proliferate, differentiate, and remodel to heal the wound.
A common trait of both cancer and repair is the activation of signaling pathways best known for their roles in embryonic growth and patterning. We (1;46) hypothesize that chronic injury acts on tissue stem cells/progenitors to permanently to activate survival, proliferation, and migration, all of which are prominent features of cancer. As part of this process, we propose that urothelial cells become cell-autonomous for EGFR activation during urothelial injury, and that such activation induces SOX9 expression to support epithelial migration and wound repair. In chronic injury, unknown genetic or epigenetic mechanisms could lock this signaling circuit in the active state, contributing to malignant transformation of urothelial cells. Future studies will be needed to expand this pathway upstream to identify mediators of sustained EGFR ligand expression and downstream to discover the molecular links between SOX9 and the migration machinery.
In the bladder, epidemiological and experimental evidence have linked human and animal bladder cancers, both squamous and urothelial (reviewed in (47)) to chronic injury through chronic parasitic infection (48), or environmental exposure to arsenic (49;50) or inorganic cadmium (50). Recent evidence indicates that arsenic can also induce EGFR ligands and activate urothelial EGFR and Erk1/2 (26). In these situations, aberrant activation of EGFR, as well as SOX9 is anticipated based on our data.
Effective treatment of any cancer will likely require combinations of targeted therapies that overcome resistance mechanisms and redundant signaling circuits. A better understanding of inducers and effectors of this newly recognized EGFR-ERK1/2-SOX9 pathway has the potential to aid in this effort.
Supplementary Material
Acknowledgements
We are grateful to Dr. Yi Luo (University of Iowa) and Dr. Scott H. Garrett (University of North Dakota) for MB49 and UROtsa cell lines, respectively, and to Paula Hurley and Will Brandt for critical reading of the manuscript.
This study is funded by NIH R01 grant (project number: 5R01DK072000-05).
Reference
- 1.Beachy PA, Karhadkar SS, Berman DM. Mending and malignancy. Nature. 2004 Sep 23;431(7007):402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 2.Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutation Research. 2008;659(1-2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Michaud DS. Chronic inflammation and bladder cancer. Urologic Oncology: Seminars and Original Investigations. 2007;25(3):260–8. doi: 10.1016/j.urolonc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. J Embryol Exp Morphol. 2004 Jul;131(13):3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 5.Ingber DE, Levin M. What lies at the interface of regenerative medicine and developmental biology? J Embryol Exp Morphol. 2007 Jul 15;134(14):2541–7. doi: 10.1242/dev.003707. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. The International Journal of Biochemistry & Cell Biology. 2007;39(12):2195–214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomsen MK, Francis JC, Swain A. The role of Sox9 in prostate development. Differentiation. 2008 Jul;76(6):728–35. doi: 10.1111/j.1432-0436.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Leav I, Ibaragi S, Wegner M, Hu GF, Lu ML, Balk SP, Yuan X. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008 Mar 15;68(6):1625–30. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, Yu W, Parmigiani G, Berman DM. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008 Sep 15;27(57):7180–91. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000 Feb 1;97(3):1113–8. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi MR, Masters JRW, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environmental Health Perspectives. 2001;109(8):801–8. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summerhayes IC, Franks LM. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979 Apr;62(4):1017–23. [PubMed] [Google Scholar]
- 13.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the Stem Cell Associated Intermediate Filament Nestin in Prostate Cancer Migration and Metastasis. Cancer Res. 2007 Oct 1;67(19):9199–206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein J, Rey P, Dansey R, Karanes C, Abella E, Cassells L, Hamm C, Flowers M, Couwlier C, Peters W, et al. Cyclophosphamide and paclitaxel as initial or salvage regimen for the mobilization of peripheral blood progenitor cells. Bone Marrow Transplant. 1999 Nov;24(9):959–63. doi: 10.1038/sj.bmt.1702009. [DOI] [PubMed] [Google Scholar]
- 15.Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, Beachy PA, Shen MM. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Developmental Biology. 2004 Mar 15;267(2):387–98. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Developmental Biology. 2005 Dec 15;288(2):334–47. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A Rapid in Vitro Assay for Quantitating the Invasive Potential of Tumor Cells. Cancer Res. 1987 Jun 15;47(12):3239–45. [PubMed] [Google Scholar]
- 18.Cheung WL, Albadine R, Chan T, Sharma R, Netto GJ. Phosphorylated H2AX in noninvasive low grade urothelial carcinoma of the bladder: correlation with tumor recurrence. J.Urol. 2009 Mar;181(3):1387–92. doi: 10.1016/j.juro.2008.10.146. [DOI] [PubMed] [Google Scholar]
- 19.Schultz L, Albadine R, Hicks J, Jadallah S, DeMarzo AM, Chen YB, Neilsen ME, Gonzalgo ML, Sidransky D, Schoenberg M, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010 Dec 1;116(23):5517–26. doi: 10.1002/cncr.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jezernik K, Pipan N. Blood-urine barrier formation in mouse urinary bladder development. Anat.Rec. 1993 Apr;235(4):533–8. doi: 10.1002/ar.1092350405. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000 Jun;278(6):F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 22.Brandt W, Matsui W, Rosenberg J, He X, Ling S, Schaeffer E, Berman D. Urothelial carcinoma: Stem cells on the edge. Cancer and Metastasis Reviews. 2009 Dec 10;28(3-4):291–304. doi: 10.1007/s10555-009-9187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farsund T. Cell kinetics of mouse urinary bladder epithelium. II. Changes in proliferation and nuclear DNA content during necrosis regeneration, and hyperplasia caused by a single dose of cyclophosphamide. Virchows Arch B Cell Pathol. 1976 Oct 1;21(4):279–98. [PubMed] [Google Scholar]
- 24.Anton E. Delayed toxicity of cyclophosphamide on the bladder of DBA/2 and C57BL/6 female mouse. Int.J Exp.Pathol. 2002 Feb;83(1):47–53. doi: 10.1046/j.1365-2613.2002.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of Functional EGF Receptors in Recovery from Acute Nephrotoxic Injury. J Am Soc Nephrol. 2003 Dec 1;14(12):3147–54. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]
- 26.Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic Signal Transduction Caused by Monomethylarsonous Acid in Human Bladder Cells: Role in Arsenic-Induced Carcinogenesis. Toxicol.Sci. 2007 Feb 1;95(2):321–30. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- 27.Messing EM, Hanson P, Ulrich P, Erturk E. Epidermal growth factor--interactions with normal and malignant urothelium: in vivo and in situ studies. J Urol. 1987 Nov;138(5):1329–35. doi: 10.1016/s0022-5347(17)43593-2. [DOI] [PubMed] [Google Scholar]
- 28.Chow NH, Liu HS, Yang HB, Chan SH, Su IJ. Expression patterns of erbB receptor family in normal urothelium and transitional cell carcinoma. An immunohistochemical study. Virchows Arch. 1997 Jun;430(6):461–6. doi: 10.1007/s004280050056. [DOI] [PubMed] [Google Scholar]
- 29.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol. 2006 Jul;291(1):F9–21. doi: 10.1152/ajprenal.00035.2006. [DOI] [PubMed] [Google Scholar]
- 30.Eblin KE, Bredfeldt TG, Gandolfi AJ. Immortalized human urothelial cells as a model of arsenic-induced bladder cancer. Toxicology. 2008 Mar 30; doi: 10.1016/j.tox.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Freeman MR, Yoo JJ, Raab G, Soker S, Adam RM, Schneck FX, et al. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Invest. 1997 Mar 1;99(5):1028–36. doi: 10.1172/JCI119230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lezama R, Diaz-Tellez A, Ramos-Mandujano G, Oropeza L, Pasantes-Morales H. Epidermal growth factor receptor is a common element in the signaling pathways activated by cell volume changes in isosmotic, hyposmotic or hyperosmotic conditions. Neurochem.Res. 2005 Dec;30(12):1589–97. doi: 10.1007/s11064-005-8837-5. [DOI] [PubMed] [Google Scholar]
- 33.Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, et al. EGF-Receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998 Nov;19(5):786–98. doi: 10.1165/ajrcmb.19.5.3249. [DOI] [PubMed] [Google Scholar]
- 34.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007 Mar 1;120(5):815–25. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat.Rev.Cancer. 2005 Sep;5(9):713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009 Mar;9(3):287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biology. 2000 Sep;19(5):389–94. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 38.Moniot B, Biau S, Faure S, Nielsen CM, Berta P, Roberts DJ, et al. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. J Embryol Exp Morphol. 2004 Aug 1;131(15):3795–804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okubo T, Knoepfler PS, Eisenman RN, Hogan BLM. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. J Embryol Exp Morphol. 2005 Mar 15;132(6):1363–74. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 40.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. American Journal Of Physiology.Renal Physiology. 2002 Aug;283(2):F242–F253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 41.Messing EM. Clinical implications of the expression of epidermal growth factor receptors in human transitional cell carcinoma. Cancer Res. 1990 Apr 15;50(8):2530–7. [PubMed] [Google Scholar]
- 42.Baskin LS, Sutherland RS, Thomson AA, Nguyen HT, Morgan DM, Hayward SW, et al. Growth Factors in Bladder Wound Healing. The Journal of Urology. 1997 Jun;157(6):2388–95. [PubMed] [Google Scholar]
- 43.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J.Biol.Chem. 2002 Feb 22;277(9):7412–9. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 44.He X, Marchionni L, Hansel DE, Yu W, Sood A, Yang J, et al. Differentiation of a Highly Tumorigenic Basal Cell Compartment in Urothelial Carcinoma. Stem Cells. 2009 Apr 9;27(7):1487–95. doi: 10.1002/stem.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 46.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004 Nov 18;432(7015):324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 47.Burin GJ, Gibb HJ, Hill RN. Human bladder cancer: evidence for a potential irritation-induced mechanism. Food Chem.Toxicol. 1995 Sep;33(9):785–95. doi: 10.1016/0278-6915(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 48.Gelfand M, Weinberg RW, Castle WM. Relation between carcinoma of the bladder and infestation with Schistosoma haematobium. Lancet. 1967 Jun 10;1(7502):1249–51. doi: 10.1016/s0140-6736(67)92714-6. [DOI] [PubMed] [Google Scholar]
- 49.Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of northern chile due to arsenic in drinking water. American Journal of Epidemiology. 1998;147(7):660–9. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 50.Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic Cadmiumand Arsenite-Induced Malignant Transformation of Human Bladder Urothelial Cells. Toxicol.Sci. 2004 May 1;79(1):56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.