Abstract
The objective of this study was to determine the tissue density, in vitro expansion and differentiation of canine adipose tissue-derived (ASC) and bone marrow-derived (BMSC) stromal cells. Primary (P0) and cell passages 1–6 (P1–6) cell doubling numbers (CD) and doubling times (DT) were determined in fresh cells. The P0, P3, and P6 adipogenic (CFU-Ad), osteogenic (CFU-Ob), and fibroblastic (CFU-F) colony forming unit frequencies, lineage specific mRNA levels in differentiated P3 cells and composition of P3 and P6 chondrogenic pellets were assessed in cryogenically preserved cells.
Cell yields from bone marrow were significantly higher than adipose tissue. Overall ASC and BMSC CDs and DTs and P3 and P6 CFU-F, CFU-Ad, and CFU-Ob were comparable. The P0 BMSC CFU-Ob was significantly higher than ASC. Lineage specific mRNA levels were higher in differentiated versus control cells, but similar between cell types. Protein was significantly greater in P3 versus P6 ASC chondrogenic pellets. Based on these findings, fresh and revitalized canine ASCs are viable alternatives to BMSCs for stromal cell applications.
Keywords: Canine, Multipotent stromal cells, Bone, Adipose tissue, Cryopreservation
Introduction
Undifferentiated cells in adult tissues are often referred to as multi-potent stromal cells (MSCs). Characteristics distinct to MSCs include immune privilege, long-term viability, multipotential differentiation, inhibition of apoptosis, and suppression of inflammation (Bocelli-Tyndall et al., 2007; Gimble et al., 2007). There are reported advantages of adipose tissue-derived stromal cells (ASCs) over bone marrow-derived stromal cells (BMSCs), including easier cell isolation, greater stromal cell density, and lower harvest site morbidity (Neupane et al., 2008). Stromal cell therapy has promising early results (Black et al., 2008; Black et al., 2007; Smith et al., 2003), but one of the greatest challenges of regenerative medicine is creation of stromal cell banks with characterized cell stores. A comprehensive evaluation of subcultivated fresh and cryopreserved adult canine MSCs is necessary to facilitate adult stromal cell applications in the dog.
The tissue density and multi-potentiality of adult canine ASCs and BMSCs dictate the volume of tissue required for specific therapies and tissue regeneration. In turn, the time between tissue harvest and clinical application is determined by in vitro cell doubling rates. This study was designed to address these interrelated scientific questions with fresh and cryopreserved canine MSCs to enhance and optimize translation of adult canine MSC treatments. To this end, freshly harvested adult canine ASC and BMSC in vitro expansion and revitalized MSC differentiation were compared to test the three part hypothesis that the two cell types have indistinguishable in vivo tissue density and in vitro cell expansion characteristics and multi-potentiality.
Materials and methods
The study was performed in accordance with Institutional and National Institutes of Health regulations governing the treatment of vertebrate animals after Institutional Animal Care Committee approval.
The MSCs were isolated from femoral and tibial bone marrow and patellar fat pads harvested immediately post-mortem from 10 male and 2 female mixed-breed hounds (mean ± SEM: 7.0 ± 2.0 months, 14 ± 1.8 kg) euthanased for reasons unrelated to this study. Freshly harvested cells were used to determine cell doubling numbers and doubling times for primary cells (P0) and cell passages 1–6 (P1–6) after 2, 4, and 6 days of culture. Subsequent assays were performed with revitalized ASCs and BMSCs subcultured from previously frozen P0 aliquots.
Adipogenic, osteogenic, and chondrogenic differentiation capacity was confirmed and adipogenic and osteogenic colony forming units (CFUs) were quantified for cell passages P0, P3, and P6. The mRNA levels of adipose and bone target genes were determined in P3 cells cultured in control, adipogenic or osteogenic medium. Collagen, DNA, glycosaminoglycan (GAG), and total protein were quantified in P3 and P6 chondrogenic pellets. Outcome measures were compared between cell types and among passages. All reagents used in this study were purchased from Sigma-Aldrich unless otherwise stated.
MSC isolation, expansion, and cryopreservation
Bone marrow aspirate was diluted 1:1 with stromal medium (DMEM-Ham’s F12 [Hyclone], 1% antibiotic/antimycotic solution [MP Biomedical], 20% characterized fetal bovine serum [FBS, Hyclone]), layered over Ficoll-Paque PLUS (Stem Cell Technologies) and centrifuged (350 g, 30 min). The MSC-enriched layer was centrifuged (260 g, 5 min) and the pellet was resuspended in stromal medium. Total nucleated cell numbers per unit volume of bone marrow was calculated.
Adipose tissue was minced and then washed with phosphate buffered saline (PBS) (Aust et al., 2004) The mixture was allowed to separate into two phases, and the infranatant was digested for 2 h at 37 °C in an equal volume of PBS containing 1% bovine serum albumin (BSA) and 0.1% type I collagenase. After addition of 1% BSA, the mixture was centrifuged (260 g, 5 min) and the resulting SVF pellet resuspended in stromal medium. An equal volume of red cell lysis buffer (9.93 g of ammonium chloride in 0.01 M Tris-HCL) was added to an aliquot of the cell suspension and total nucleated cell number per unit volume of adipose tissue was calculated.
The cell doubling time (DT) and cell doubling numbers (CD) were determined with replicate cultures of P0–P6 cells according to standard methods (Vidal et al., 2006). Cells were seeded in 12-well plates (Nunclon) at 3,400 cells/cm2 and cultured in stromal medium at 37 °C and 5% CO2. After 2, 4, and 6 days of culture, DT and CD were calculated with the following formulae (Rainaldi et al., 1991; Vidal et al., 2006; Vidal et al., 2007):
where CT = culture time, Nf = final cell number, and Ni = initial seeding density.
Aliquots (1 × 106 cells/mL) of primary and P1–P6 were frozen in cryopreservation medium (80% FBS, 10% DMEM, and 10% dimethylsulfoxide).
CFU assays
A total of 5 × 103, 2.5 × 103, 1.25 × 103, 6.25 × 102, 3.12 × 102, or 1.56 × 102 cells were placed in each of 6 wells in a 96-well plate, with each row containing wells of the same cell number. Cells were cultured in stromal medial for 6 days. Fibroblastic (CFU-F) wells were then fixed with 1% paraformaldehyde and stained with 0.1% toluidine blue. Adipogenesis (CFU-Ad) wells were subsequently cultured in adipogenic induction medium (DMEM-Ham’s F12, 3%FBS, 1% antibiotic/antimycotic solution, biotin [33 μmol/L], pantothenate [17 μmol/L], insulin [1 μmol/L], isobutylmethylxanthanine [0.5 mmol/L], dexamethasone [1mM], and rosiglitazone [5 μmol/L] for 6 days and then adipogenic maintenance medium (induction medium without isobutylmethylxanthanine and rosiglitazone) for 6 more days. Cells were fixed as above and stained with Oil Red O.
Osteogenesis wells were cultured in osteogenic medium (DMEM-Ham’s F12, 20% FBS, 1% antibiotic/antimycotic solution, β-glycerophosphate [10 μmol/L], dexamethasone [20 nmol/L], and sodium 2-phosphate ascorbate [50 μg/mL]) for 14 days after initial culture period. They were then fixed in 70% ETOH and stained with 2% Alizarin Red. Wells were considered positive for stromal colonies, adipogenesis, or osteogenesis when there were ≥ 10 toluidine blue-stained colonies, ≥ 10 Oil Red O-stained colonies, ≥ 1 Alizarin Red-stained colony, respectively. The frequency of CFUs was calculated as F=e− x, where F is the ratio of negative to positive wells within a row, e is the natural logarithm constant 2.71, and x is the number of CFUs per well (Wu et al., 2000).
Chondrogenesis and adipogenesis
After 6 days in stromal medium, cell pellets containing 2.5 × 105 cells were prepared according to the manufacturer’s instructions (R&D Systems), and then cultured in chondrogenic differentiation medium for 21 days with media changes every 2–3 days. Pellets were formalin-fixed, paraffin-embedded, and stained with Alcian blue (5 μm sections).
Eight wells of 24-well plates were seeded with 1.0 × 104 cells/cm2 of P3 ASCs or BMSCs from each dog. When cells reached 75% confluence, adipogenic induction medium was added to half of the wells, while the other half was maintained in stromal medium. One pair of wells was fixed and stained with Oil Red O 3, 6, 9, and 12 days after induction. Cell morphology was recorded with polarized light photomicrographs (magnification 200×).
Genetic expression
Levels of lineage-specific target genes were evaluated in P3 MSCs cultured in stromal, adipogenic or osteogenic conditions identical to those described for CFU assays. Total RNA was isolated with Trizol (Invitrogen), DNase-treated (DNase I, Turbo DNA free, Ambion), and reverse-transcribed using oligo(dT) primers and Moloney murine leukemia virus (M-MLV) reverse transcriptase. Target gene mRNA levels were quantified with qRT-PCR using SYBR Green technology (MJ Research Chromo4 Detector, Bio-Rad Laboratories). Canine-specific primers for adipogenic (leptin, peroxisome proliferator activated receptor gamma [PPAR-γ]) and osteogenic lineages (collagen type 1α1 [COL1α1], osteoprotegerin [OPG]) were used for all analyses (Table 1). Values were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Relative gene expression was quantified with the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Real time RT-PCR primer sequences.
| Lineage | Gene | Primer sequence |
|---|---|---|
| Housekeeping | GAPDH | 5′-TGG CAA AGT GGA TAT TGT CG 3′-AGA TGG ACT TCC CGT TGA TG |
| Adipogenic | PPAR-γ | 5′-TTC TCC AGC ATT TCC ACT CC 3′-AGG CTC CAC TTT GAT TGC AC |
| Leptin | 5′-TGT GTT GAA GCT GTG CCA AT 3′-CCC TCT GTT TGG AGG AGA CA |
|
| Osteogenic | COL1α1 | 5′-GTG TGT ACA GAA CGG CCT CA 3′-TCG CAA ATC ACG TCA TCG |
| OPG | 5′-TGT CTA TAC TGC AGG CCG GTG 3′-TCA GGC AGA ACT CAA GCT CCA |
PPAR-γ, peroxisome proliferator-activated receptor gamma; COL1α1, collagen type I alpha 1; OPG, osteoprotegerin.
Compositional analysis
Chondrogenic pellets were digested overnight at 60 °C (25 mg/mL papain, 0.4 M sodium acetate, 10 mM di-sodium EDTA, and 200 mM L-cysteine). Total collagen (Stegemann and Stalder, 1967), DNA (Labarca and Paigen, 1980), protein (Lowry et al., 1951), and sulfated glycosaminoglycan (GAG) (Ratcliffe et al., 1992; Ratcliffe et al., 1988) were then quantified in P3 and P6 pellets using standard assays adapted to microtiter plates as described below.
To determine collagen content, samples were incubated overnight in an equal volume of 6N HCl at 110 °C. Equal volumes of distilled water and 17% NaCl solution were then added followed by 250 μL of oxidant solution (chloramines T, isopropanol, citrate/acetate buffer, distilled water) and then an equal volume of Ehrlich’s reagent (dimethylaminobenzaldehyde, isopropanol, perchloric acid, distilled water). Total collagen was determined from absorbance at 550 nm on a trans-4-hydroxyproline standard curve. Samples were mixed with PicoGreen Dye solution (Molecular Probes) and incubated in darkness for 5 min in a black 96-well plate to determine DNA content. Absorbance at 480 and 520 nm was determined and total DNA quantified on a calf thymus DNA standard curve.
Samples and GAG assay buffer (sodium formate, formic acid, 1, 9-dimethylmethylene blue, ethyl alcohol, distilled water) were mixed to determine GAG content. Absorbance was read immediately at 540 and 600 nm and GAG content determined on a chondroitin sulfate standard curve. Samples were mixed with Biuret’s reagent and Folin-Ciocalteu’s phenol reagent to determine total protein. Absorbance was read at 650 nm after 30 min and protein quantified on a bovine serum albumin standard curve.
Statistical analysis
Statistical analyses were performed with commercially available software (SAS 9.1.2, SAS Institute). ANOVA models were used to evaluate CD, DT, limit dilution and composition data. Tukey’s post-hoc tests were performed to assess multiple group comparisons. Normality and homogeneity of variance were assessed with Shapiro-Wilk test and residual plots. Significance was set at P < 0.05.
Results
MSC isolation
There were 1.6 × 107 ± 4.8 × 106 nucleated cells/mL of bone marrow and 7.7 × 105 cells/fat pad. A heterogeneous population of primary bone marrow cells formed distinct colonies after 5 days in culture and reached 70–80% confluence after 8–9 days. The SVF (P0) adipose cells assumed a uniform, mononuclear, spindle-shaped appearance after 2–4 days in culture and were 70–80% confluent after 7 days in culture at the same seeding density. Subcultured ASCs and BMSCs became progressively more homogenous and fibroblast-like with increasing passages until P5 when cells assumed a broad, flat appearance (Figs. 1A–D). After P0, cells became confluent 4–5 days after seeding. About 26 ± 9.3% of bone marrow P0 cells adhered to plastic after 48 h, which was significantly greater than the 20 ± 9.6% of adipose P0 cells that adhered.
Fig. 1.
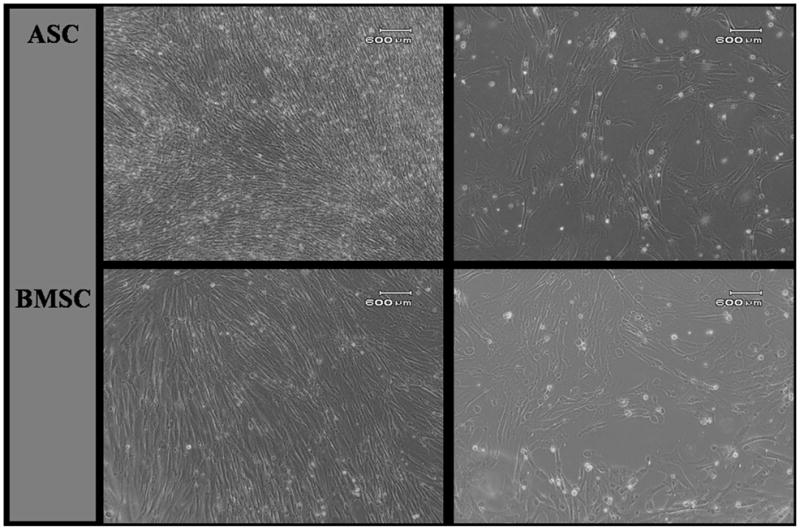
Photomicrographs of P3 (A, C) and P5 (B, D) cells from canine adipose tissue-derived (ACSs; A, B) and bone marrow-derived (BMSCs; C, D) stromal cells. P3 ASCs and BMSCs displayed a homogeneous phenotype of spindle-shaped cells. By P5, the majority of cells had changed from spindle-shaped to a wide, flat appearance. Scale bar = 600 μm. (Polarized light, 10X).
MSC expansion
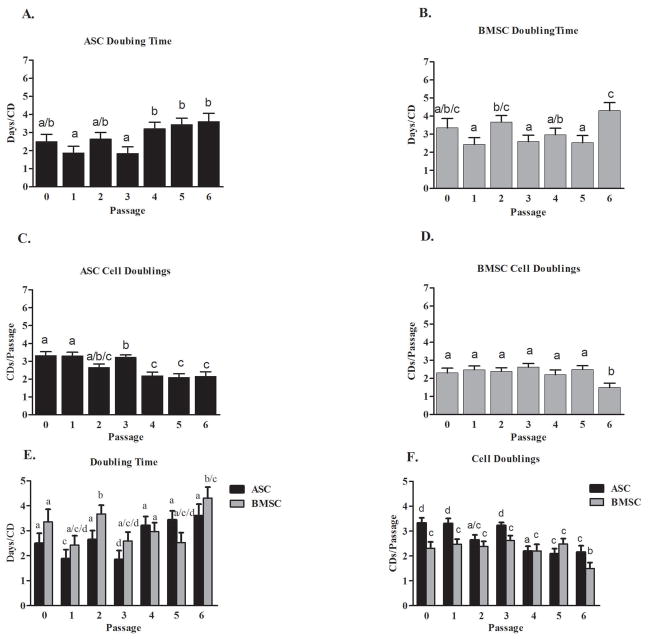
Day 2 cell counts were used as the initial seeding density to calculate expansion rates for days 4 and 6 to allow for cellular adherence. The P0 DT for ASCs (2.4 ± 0.3 days/CD) was not significantly different from that for BMSCs (3.3 ± 0.6 days/CD). The overall DTs (P1–6) were not significantly different between ASCs (2.8 ± 0.3 days/CD) and BMSCs (3.1 ± 0.2 days/CD). The total CDs by P6 were 19 ± 0.7 CDs for ASCs and 16 ± 0.6 CDs for BMSCs. There were trends for DTs to increase and CDs to decrease for both ASCs and BMSCs with increasing cell passages (Figs. 2A–F)
Fig. 2.
Canine adipose tissue-derived (ASCs) and bone marrow-derived stromal cells (BMSCs) doubling times (A, B) and cell doubling number (C, D) for P0–6 (mean ± SEM) within cell types and with cell types shown together (E, F). Columns with different letters within each graph are significantly different from one another (P < 0.05).
Colony-forming unit (CFU) assays, adipogenesis and osteogenesis
Adipogenic differentiation for both cell types was evident by Oil Red O staining in all induced cultures while no differentiation occurred in cells cultured in stromal medium (Figs. 3A and D). The ASCs had larger and more abundant vacuole formation (Figs. 3B and E). Small intracellular granules that coalesced into lipid droplets after about 6 days appeared 2–3 days earlier in ASCs (around 3 days in culture).
Fig. 3.
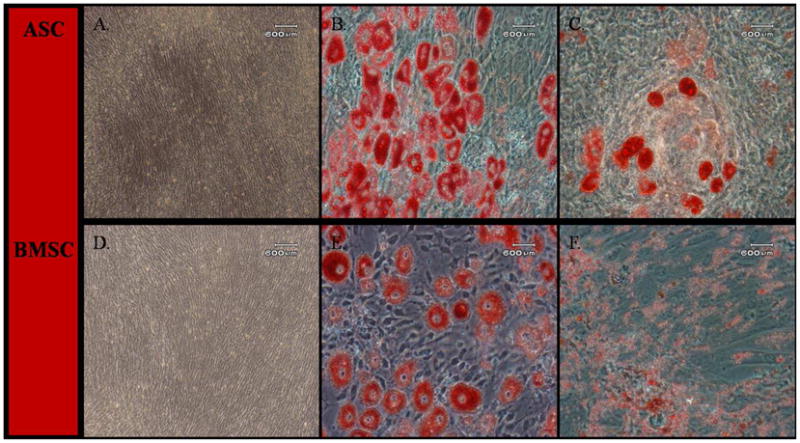
Light photomicrographs of canine adipose tissue-derived (ASCs; A–C) and bone marrow-derived stromal cells (BMSCs; D–F) following culture in stromal medium (A, D) or after adipogenic induction for P3 (B, E) and P6 (C, F). The ASCs (A) and BMSCs (D) cultured in stromal medium did not undergo any morphological changes. Following adipogenic induction, P3 and P6 ASCs and BMSCs formed adipocytes with intracellular lipid vacuoles confirmed by Oil Red O staining. P6 ASCs and BMSCs had less lipid accumulation than P3 cells. Scale bar = 600 μm.
Overall, the P6 cells had less robust adipogenesis compared to P3. Cellular morphology of all MSCs changed from spindle-shaped to cuboidal and formed cell aggregates 4–6 days after osteogenic induction. After 14 days in osteogenic medium, both cell types had comparable calcium phosphate mineralization based on alizarin red staining intensity (Figs. 4B and E). Alizarin red staining was less intense in P6 MSCs (Figs. 4C and F). There was no adipogenic or osteogenic differentiation in parallel stromal media cultures (Figs. 4A and D).
Fig. 4.
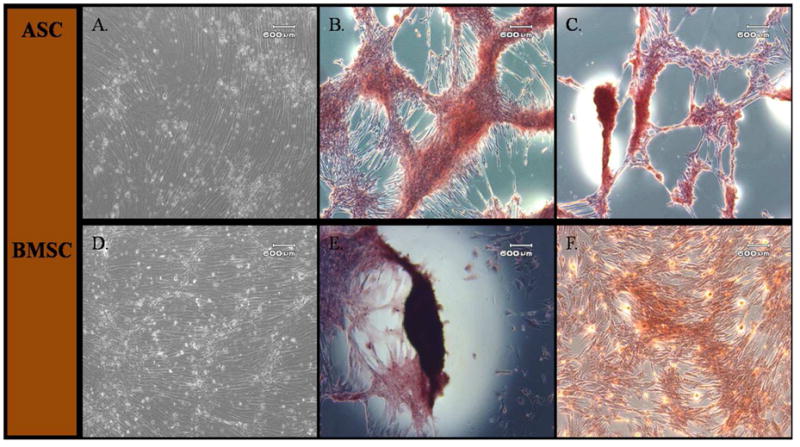
Light photomicrographs of canine adipose tissue-derived (ASCs; A–C) and bone marrow-derived stromal cells (BMSCs; D–F) following culture in stromal medium (A, D) or after osteogenic induction at P3 (B, E) and P6 (C, F). Both P3 and P6 ASCs and BMSCs formed calcified cell aggregates as shown by Alizarin Red staining after osteogenic induction for all passages. Both cell types showed greater calcium deposition after induction in P3 compared to P6 cells. Scale bar = 600 μm.
There were no significant differences in BMSC and ASC CFU-F and BMSC CFU-Ad frequencies among P0, P3, or P6 (Table 2). The CFU-Ad for P3 ASCs was significantly higher than for P6 ASCs. The P0 BMSCs had significantly higher CFU-Ob frequencies than P0 ASCs. Additionally, P6 BMSC CFU-Ob frequencies were significantly lower than for P0 and P3 BMSCs.
Table 2.
CFU frequency for canine adipose tissue-derived (ASCs) and bone marrow-derived stromal cells (BMSCs) following fibroblastic (CFU-F), adipogenic (CFU-Ad), or osteogenic (CFU-Ob) induction.
| CFU Frequency (%, mean ± SEM) | ||||
|---|---|---|---|---|
| Passage | Cell Type | CFU-F | CFU-Ad | CFU-Ob |
| 0 | ASC | 5.10 ± 0.69% | 9.12 ± 1.28%*/** | 9.96 ± 1.40%a |
| BMSC | 6.13 ± 1.33% | 6.86 ± 3.35% | 26.3 ± 0.37%**,b | |
| 3 | ASC | 7.06 ± 2.71% | 12.5 ± 1.06%** | 17.4 ± 0.92% |
| BMSC | 5.93 ± 3.28% | 10.2 ± 1.21% | 22.9 ± 1.26%** | |
| 6 | ASC | 8.16 ± 2.37% | 7.65 ± 1.42%* | 13.4 ± 2.00% |
| BMSC | 5.20 ± 2.31% | 5.54 ± 3.40% | 7.58 ± 2.70%* | |
a and b, significant differences between cell types for the indicated lineage (P < 0.05);
significant differences between passages within cell type for the indicated lineage (P < 0.05).
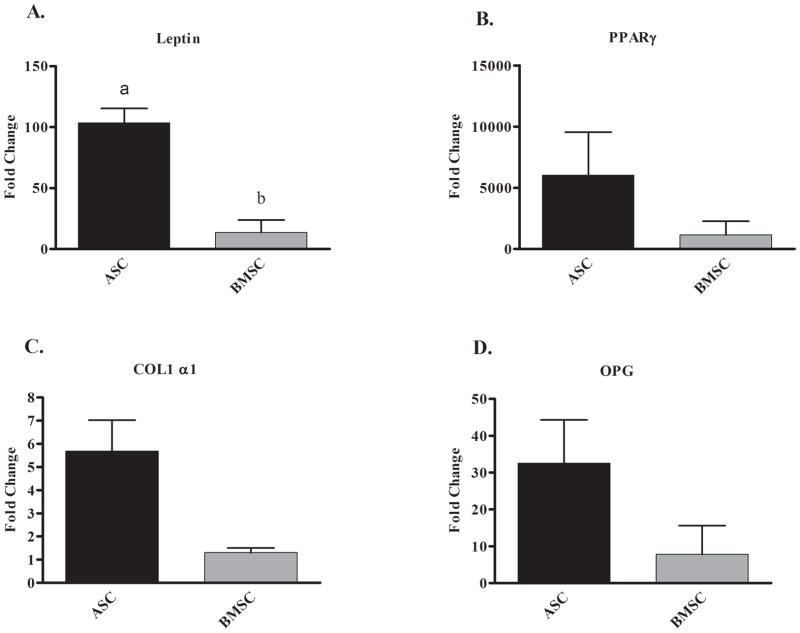
There were significantly greater fold increases in leptin mRNA levels in ASCs following adipogenic induction compared to BMSCs (102-fold and 13-fold, respectively; Fig. 5A), but increases in PPAR-γ mRNA were not significantly different between the two cell types (Fig. 5B). Osteogenic induction resulted in 5.6 and 1.3-fold increases in COL1α1 mRNA levels and 32.6 and 7.8-fold increases in OPG mRNA levels in ASCs and BMSCs, respectively (Figs. 5C and D). Values were not significantly different between cell types.
Fig. 5.
Fold increase (mean ± SEM) of adipogenic (A, B) and osteogenic (C, D) specific target gene mRNA levels in canine adipose tissue-derived (ASCs) and bone marrow-derived stromal cells (BMSCs) following adipogenic or osteogenic induction compared to parallel cultures maintained in stromal medium.
Chondrogenesis and compositional analysis
The P3 and P6 ASCs and BMSCs formed cell aggregates after 24 h and were maintained through the 21 day culture period. Extracellular matrix staining with Alcian blue was similar for P3 and P6 ASC and BMSC pellets (Figs. 6A – D). The DNA content was significantly higher in P3 ASC compared to P6 BMSC chondrogenic pellets (Fig. 7A). The P3 ASC pellets had significantly more protein than the P3 and P6 BMSC and P6 ASC pellets (Fig. 7B). There were no significant differences in collagen or GAG between cell types within passages or between passages within cell types (Figs. 7C and D).
Fig. 6.
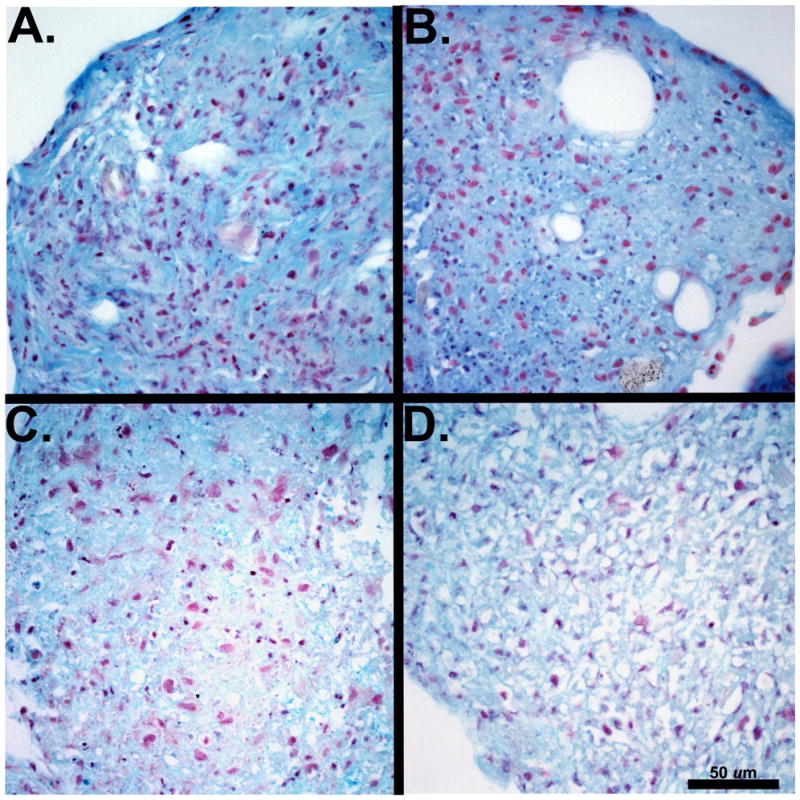
Light micrographs of canine adipose tissue-derived (ASCs) and bone marrow-derived stromal cells (BMSCs) following chondrogenic induction. Extracellular matrix formation was evident in chondrogenic pellets from P3 ASCs (A) and P3 BMSCs (B) and P6 ASCs (C) and P6 BMSCs (D). Scale bar = 50 μm. (Alcian blue, 400X).
Fig. 7.
Mean ± SEM % wet weight of DNA (A), protein (B), collagen (C), and sulfated glycosaminoglycan (GAG; D) in P3 and P6 canine adipose tissue-derived (ASC) and bone marrow-derived stromal cell (BMSC) chondrogenic pellets. Columns with different letters are significantly different from one another (P < 0.05).
Discussion
Based on direct comparisons of individual MSCs, canine ASCs and BMSCs have comparable in vitro expansion and multi-potentiality. Additionally, both cell types retain similar cellular proliferation and differentiation capacity following cryopreservation, essential considerations for cell banking. The intra-animal comparisons and post-cryopreservation results of this investigation contribute unique, vital translational information about canine MSCs. These findings support fresh or cryopreserved canine ASCs as an alternative to BMSCs for musculoskeletal therapeutic and regenerative purposes.
Nucleated cell number varies between species, individuals, tissues, and culture protocols and lack of standardized MSC isolation procedures contributes to differences (Bakker et al., 2004; Mochizuki et al., 2006; Neupane et al., 2008). The high variability between individuals in the number of nucleated cells from bone marrow in this study is therefore not surprising (Digirolamo et al., 1999). The number of nucleated cells harvested from patellar adipose tissue (7.7 × 105 cells/fat pad) was considerably lower than the 5.5 × 106 cells/fat pad reported in humans (Dragoo et al., 2003). This may have translated to the lower ASC P0 adherent cell number compared to BMSCs in contrast to previous reports (Vidal et al., 2007). There are significant differences in MSC numbers between fat depots within species and in the same fat depots between species (Mochizuki et al., 2006; Neupane et al., 2008). The results of this study possibly reflect these differences.
Both BMSCs and ASCs are similar in overall expansion rates as reported in other species (Izadpanah et al., 2006), though BMSCs had slightly longer cell doubling times for subcultured cells compared to ASCs. Decreasing cell doubling numbers with increasing passages suggest that canine MSCs from early passages may have greater potential for therapeutic use. The overall ASC DT in this study (around 2.5 days) is similar to that of human infrapatellar fat pad cells (2.0–2.5 days) (Dragoo et al., 2003), equine ASCs (2.0 days) (Vidal et al., 2007), and adult murine preadipocytes (2.0–2.5 days) (Doi et al., 2005).
Despite these similarities, published ASC DTs are highly variable within and between species (Mitchell et al., 2006). The DT of primary canine BMSCs was not significantly longer than that of subsequent passages in this study, in contrast to published reports. (Vidal et al., 2006) The overall BMSC DT of about 3 days was longer than that of human and equine BMSCs, which have been reported to be approximately 24 h (Montjovent et al., 2004; Vidal et al., 2006), but comparable to that reported previously for canine BMSCs (Kamishina et al., 2008). Along with species variation, differences in culture techniques affect proliferation rates (Kamishina et al., 2008; Neuhuber et al., 2008). Initial MSC seeding densities can significantly affect doubling times and cell doublings (Neuhuber et al., 2008). Additionally, FBS preparation and lot differences contribute to variability in cell expansion rates (Caplan, 2005; Lennon, 1996). Differences in culture conditions must be considered when comparing results between studies.
Limit dilution assays indicate the minimal number of cells for 1 colony forming clone (CFU-F). Assays performed with induction media show the number of cells required for 1 clone capable of tissue specific colony formation (CFU-Ad, CFU-Ob). The P0, P3 and P6 CFU-F frequencies for canine ASCs and BMSCs in this study were similar. Neupane et al. (2008) found an almost identical CFU-F frequency (17.8%) for canine ASCs subcultured in low calcium medium supplemented with antioxidants, growth factors and 10% FBS to our P3 CFU-F (17.4%). In contrast, the CFU-F frequency of primary canine BMSCs cultured in Dulbecco’s Modified Eagle’s Medium with 20% FBS has been reported as 0.0042% (range 0.0014–0.0057%), a frequency significantly lower than in the current study (Kamishina et al., 2008).
Donor characteristics, passage, isolation procedures, seeding density, and culture conditions contribute to variations in MSC frequency (Vidal et al., 2006). In addition, reported canine MSC CFU frequencies are derived from freshly harvested and subcultured MSCs, and not from MSCs revitalized from cryopreservation. It is thus difficult to compare CFU results between studies. In vitro characteristics and osteogenic differentiation of fresh and cryopreserved MSCs are similar in other species (Arrigoni et al., 2009). This information surrounding the multi-potentiality and expansion rates of revitalized MSCs is vital to clinical translation of stromal cell technology.
The MSC tissue sources selected for this study (patellar fat pad and femoral and tibial bone marrow) were selected since both can be accessed during routine surgery for stifle stabilization after cranial cruciate ligament rupture (Lopez et al., 2004). The harvested MSCs were heterogenous populations of cells in varying degrees of differentiation. Cell selection based on cell surface markers may have contributed to homogenous MSC populations with more uniform multipotentiality. Plastic adherence was used to select MSCs based on recent data showing that over 95% of MSCs isolated by plastic adherence express MSC-specific cell surface markers and do not express markers for hematopoietic progenitors (Jang et al, 2010). The heterogenous MSC populations from adipose and bone marrow had similar multipotentiality after cryopreservation. Future studies are necessary to evaluate whether the same is true for MSC subpopulations that differ in cell surface markers.
The adipogenic differentiation protocol in this investigation was modified from a published human protocol (Mitchell et al., 2006). Addition of rabbit serum is often necessary for adipogenesis in other species (Neupane et al., 2008; Vidal et al., 2007). Adipogenesis by canine ASCs has been previously limited without the addition of rabbit serum. Culture of MSCs in adipogenic induction medium for 6 days supported adipogenesis without rabbit serum in the current study. Lipid vacuole formation occurred in both cell types through P6, though BMSCs consistently formed lipids later than ASCs, and P6 lipid formation was less robust in both MSCs. The CFU-Ob of P6 BMSCs was significantly lower than at earlier passages consistent with reports in other species (Izadpanah et al., 2006). Less robust lipid vacuole formation in P6 cells and lower CFU-Ob in P6 BMSCs may indicate waning multi-potentiality in increasing passages of revitalized cells.
Upregulation of PPAR-γ and leptin in induced ASCs and BMSCs confirmed adipogenic induction. Initiation and maintenance of adipogenesis is supported by PPAR-γ and leptin modulates adipocyte metabolism (Gimble et al., 2006; Gregoire et al., 1998). Higher upregulation of PPAR-γ in ASCs suggests the presence of preadipocytes in the patellar fat stromal cell population. The osteogenic markers (COL1α1 and OPG) were both upregulated in MSCs induced to osteogenesis, although COL1α1 increases were modest. COL1α1 is the most abundant collagen found in bone and OPG promotes osteogenesis, but both are typically observed in the later stages of osteogenesis. A longer period of osteogenesis may have resulted in higher mRNA levels of these genes and a larger panel of target genes may have provided a more comprehensive evaluation of differences between the cell types. It is also likely that optimization of culture and induction conditions for each cell type may have influenced the outcomes assessed in this investigation.
In the current study, the chondrogenic potential of ASCs and BMSCs was similar in contrast to previous reports indicating less chondrogenic potential of ASCs (Huang et al., 2005; Vidal et al., 2008). These differences could be due to variations in chondrogenic medium. Chondrogenic induction is enhanced by the addition of supplements such as growth factors and the addition or exclusion of certain factors could mediate differences in chondrogenic differentiation. Chondrogenic differentiation was confirmed by evaluation of extracellular matrix with Alcian blue staining. The P3 and P6 MSCs had similar staining intensity. Human BMSCs and rhesus macaque ASCs lose chondrogenic potential by P5, although human ASCs and rhesus macaque BMSCs appear to retain their chondrogenic potential up to P10 (Izadpanah et al., 2006).
The compositional analysis of the chondrogenic pellets supported the light microscopic findings. The significantly higher protein in P3 ASC chondrogenic pellets is consistent with the greater matrix observed in the same pellets, although total collagen and GAG were not significantly different between groups. Albeit not significant, all components were lower in P6 compared to P3 pellets. Based on these observations, the significantly lower amount of DNA in P6 BMSC chondrogenic pellets may be due to cellular senescence. Overall, there were few differences between ASC and BMSC chondrogenic pellet composition but extracellular matrix production by both cell types decreased with increasing passages.
Conclusions
Stromal cell technology has significant potential to enhance treatment of canine injury and disease. Cell banking will facilitate maintenance of characterized cell stores, obviate cell harvest from sick and injured patients, and promote ready access to cells for therapy. The current results establish fresh and revitalized ASCs as a viable alternative to BMSCs for stromal cell applications and provide a novel foundation for development of therapeutic intervention with cryopreserved canine MSCs.
Acknowledgments
The authors would like to thank Lin Xie for her statistical analysis assistance and Sandra Robinson and Yanru Zhang for assistance with sample collection and tissue culture procedures. This research was supported by National Institutes of Health-National Institute of Arthritis and Musculoskeletal and Skin Disease, Grant K01-AR02714.
Footnotes
Conflict of interest statement
None of the authors have any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell and Tissue Research. 2009;338:401–411. doi: 10.1007/s00441-009-0883-x. [DOI] [PubMed] [Google Scholar]
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Bakker AH, Van Dielen FM, Greve JW, Adam JA, Buurman WA. Preadipocyte number in omental and subcutaneous adipose tissue of obese individuals. Obesity Research. 2004;12:488–498. doi: 10.1038/oby.2004.55. [DOI] [PubMed] [Google Scholar]
- Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Veterinary Therapeutics. 2008;9:192–200. [PubMed] [Google Scholar]
- Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Veterinary Therapeutics. 2007;8:272–284. [PubMed] [Google Scholar]
- Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, Pistoia V, Martin I, Tyndall A. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology. 2007;46:403–408. doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Engineering. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. British Journal of Haematology. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- Doi H, Masaki N, Takahashi H, Komatsu H, Fujimori K, Satomi S. A new preadipocyte cell line, AP-18, established from adult mouse adipose tissue. Tohoku Journal of Experimental Medicine. 2005;207:209–216. doi: 10.1620/tjem.207.209. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, Hedrick MH, Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. Journal of Bone and Joint Surgery, British Volume. 2003;85:740–747. [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation Research. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. Journal of Cellular Biochemistry. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiological Reviews. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. Journal of Orthopaedic Research. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of Cellular Biochemistry. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biology. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishina H, Farese JP, Storm JA, Cheeseman JA, Clemmons RM. The frequency, growth kinetics, and osteogenic/adipogenic differentiation properties of canine bone marrow stromal cells. In Vitro Cellular and Developmental Biology Animal. 2008;44:472–479. doi: 10.1007/s11626-008-9137-6. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Analytical Biochemistry. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Haynesworth SE, Bruder SP, Jaiswall N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. In Vitro Cellular and Developmental Biology. 1996:32. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez MJ, Markel MD, Kalscheur V, Lu Y, Manley PA. Hamstring graft technique for stabilization of carnine cranial cruciate ligament deficient stifles. Veterinary Surgery. 2003;32:390–401. doi: 10.1053/jvet.2003.50042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis and Rheumatism. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- Montjovent MO, Burri N, Mark S, Federici E, Scaletta C, Zambelli PY, Hohlfeld P, Leyvraz PF, Applegate LL, Pioletti DP. Fetal bone cells for tissue engineering. Bone. 2004;35:1323–1333. doi: 10.1016/j.bone.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Experimental Hematology. 2008;36:1176–1185. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and Characterization of Canine Adipose-Derived Mesenchymal Stem Cells. Tissue Engineering Part A. 2008;14:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- Rainaldi G, Pinto B, Piras A, Vatteroni L, Simi S, Citti L. Reduction of proliferative heterogeneity of CHEF18 Chinese hamster cell line during the progression toward tumorigenicity. In Vitro Cellular and Developmental Biology. 1991;27A:949–952. doi: 10.1007/BF02631122. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Billingham ME, Saed-Nejad F, Muir H, Hardingham TE. Increased release of matrix components from articular cartilage in experimental canine osteoarthritis. Journal of Orthopaedic Research. 1992;10:350–358. doi: 10.1002/jor.1100100307. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Doherty M, Maini RN, Hardingham TE. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Annals of the Rheumatic Diseases. 1988;47:826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RK, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Veterinary Journal. 2003;35:99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clinica Chimica Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Kilroy GE, Johnson JR, Lopez MJ, Moore RM, Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Veterinary Surgery. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Veterinary Surgery. 2007;36:613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Veterinary Surgery. 2008;37:713–724. doi: 10.1111/j.1532-950X.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Peters JM, Gonzalez FJ, Prasad HS, Rohrer MD, Gimble JM. Frequency of stromal lineage colony forming units in bone marrow of peroxisome proliferator-activated receptor-alpha-null mice. Bone. 2000;26:21–26. doi: 10.1016/s8756-3282(99)00238-0. [DOI] [PubMed] [Google Scholar]