Abstract
Approximately 20 of the 30 mammalian transient receptor potential (TRP) channel subunits are expressed by specific neurons and cells within the alimentary canal. They subserve important roles in taste, chemesthesis, mechanosensation, pain and hyperalgesia and contribute to the regulation of gastrointestinal motility, absorptive and secretory processes, blood flow, and mucosal homeostasis. In a cellular perspective, TRP channels operate either as primary detectors of chemical and physical stimuli, as secondary transducers of ionotropic or metabotropic receptors, or as ion transport channels. The polymodal sensory function of TRPA1, TRPM5, TRPM8, TRPP2, TRPV1, TRPV3 and TRPV4 enables the digestive system to survey its physical and chemical environment, which is relevant to all processes of digestion. TRPV5 and TRPV6 as well as TRPM6 and TRPM7 contribute to the absorption of Ca2+ and Mg2+, respectively. TRPM7 participates in intestinal pacemaker activity, and TRPC4 transduces muscarinic acetylcholine receptor activation to smooth muscle contraction. Changes in TRP channel expression or function are associated with a variety of diseases/disorders of the digestive system, notably gastro-esophageal reflux disease, inflammatory bowel disease, pain and hyperalgesia in heartburn, functional dyspepsia and irritable bowel syndrome, cholera, hypomagnesemia with secondary hypocalcemia, infantile hypertrophic pyloric stenosis, esophageal, gastrointestinal and pancreatic cancer, and polycystic liver disease. These implications identify TRP channels as promising drug targets for the management of a number of gastrointestinal pathologies. As a result, major efforts are put into the development of selective TRP channel agonists and antagonists and the assessment of their therapeutic potential.
Keywords: Chemesthesis, Chemosensation, Gastrointestinal cancer, Gastrointestinal motility, Hypersensitivity, Hyperalgesia, Inflammation, Inflammatory bowel disease, Mechanosensation, Pain, Taste, Transducers, TRPA1, TRPC4, TRPC6, TRPM5, TRPM6, TRPV1, TRPV4, TRPV6
Abbreviations: AITC, allyl isothiocyanate; CCK, cholecystokinin; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; DSS, dextran sulfate sodium; GI, gastrointestinal; GPCR, G protein-coupled receptor; 5-HT, 5-hydroxytryptamine; ICC, interstitial cell of Cajal; mRNA, messenger ribonucleic acid; PAR, protease-activated receptor; PKD, polycystic kidney disease; RNA, ribonucleic acid; siRNA, small interfering ribonucleic acid; TNBS, trinitrobenzene sulfonic acid; TRP, transient receptor potential; TRPA, transient receptor potential ankyrin; TRPC, transient receptor potential canonical (or classical); TRPM, transient receptor potential melastatin; TRPP, transient receptor potential polycystin; TRPV, transient receptor potential vanilloid
1. Introduction
The selection of useful food and its effective digestion are essential for survival. To accomplish this goal, the digestive tract possesses a large array of molecular sensors and transducers to make the right choice and guide the process of digestion by appropriate feed-forward and feed-back mechanisms. The physiological task of the alimentary canal is highly challenging because, depending on the nature of ingested food, the gut needs to carry out programs with opposite aims: digestion of food and absorption of nutrients and water, on the one hand, and the elimination of toxins, antigens, pathogens and indigestible waste, on the other hand. Research in the past decade has revealed an elaborate system of molecular sensors serving taste, analyzing the chemical composition of food, monitoring the motor and secretory activity of the gastrointestinal (GI) tract, and detecting adverse conditions in the lumen and wall of the alimentary canal. Transient receptor potential (TRP) channels, so named after the role they play in Drosophila phototransduction, take a prominent position among these sensors and transducers in the digestive system. A number of their implications in GI physiology and pathology were discovered subsequently to the analysis of the mechanism of action of pungent spices (Bandell et al., 2007; Holzer, 2008a).
The search for and the intake of nutrients are governed by the appearance, smell and taste of food. There is anthropological evidence that the adoption of cooking food has been a key feature in human evolution (Wrangham & Conklin-Brittain, 2003; Wobber et al., 2008). Along with cooking, mankind also learned that the taste of food can be heightened by seasoning, and food prepared with the right mixture of spices is one of the major human pleasures. The chemicals responsible for the gustatory and olfactory pleasures of spices are secondary metabolites of plants (Max, 1992; Cromer & McIntyre, 2008; Vriens et al., 2008). Recognition of the chemical qualities of spices must have driven the co-evolution of TRP channels as appropriate sensors in the animal kingdom. The functional implications of TRP channels are, in the first place, congruent with the strategy of plants to discourage predators by the unusual sensory quality of spices. However, by perversion of this principle, humans have learned to enjoy low doses of those deterrent chemicals (Max, 1992). This argument is supported by the fact that capsaicin is pungent for mammals, but not birds which are supposed to help distributing the seeds of red pepper, given that the avian orthologue of the TRP vanilloid-1 channel (TRPV1) lacks the binding site for capsaicin (Jordt & Julius, 2002).
The implication of TRP channels in the sensation of deterrent chemicals was first heralded when, in 1997, TRPV1 (then named the vanilloid receptor-1) was identified as the sensor for capsaicin at the genetic and functional level (Caterina et al., 1997). Responsible for the piquancy of red pepper (Capsicum spp.), the vanilloid capsaicin in its pure form is one of the most painful chemicals we know, yet is also widely used for food seasoning. TRPV1 was soon joined by other TRP channels with unique transduction properties relevant to chemo-, thermo- and mechanosensation as well as sweet, bitter, sour, salt and umami taste sensation (Zhang et al., 2003; DeSimone & Lyall, 2006; Huang et al., 2006; Bandell et al., 2007; Kaske et al., 2007; Montell & Caterina, 2007; Vriens et al., 2008; Ishimaru & Matsunami, 2009; Wu et al., 2010). In addition, these molecular sensors can detect specific chemical entities including unpleasant and/or painful toxins, whereby they subserve chemesthesis, the chemical sense distinct from taste and smell (Bandell et al., 2007).
Currently, some 30 different TRP subunit genes have been identified in mammals, these subunits being grouped in 6 families (Wu et al., 2010). Members of 5 subunit families, specifically of the vanilloid TRP (TRPV), melastatin TRP (TRPM), ankyrin TRP (TRPA), polycystin TRP (TRPP) and canonical or classical TRP (TRPC) family, are relevant to spice sensing, chemo-, thermo- and/or mechanosensation (Table 1). The TRP channel subunits are made of 6 transmembrane domains with a pore between transmembrane domains 5 and 6 (Clapham et al., 2005; Wu et al., 2010). Functional TRP channels, thought to be composed as tetramers of four subunits, are opened or closed by conformational changes in the channel protein (Dhaka et al., 2006; Bandell et al., 2007). TRP channels are only weakly sensitive to depolarization but open in response to changes in temperature, binding of ligands or other alterations of the channel protein (Clapham et al., 2005; Matta & Ahern, 2007; Nilius et al., 2007; Wu et al., 2010). The ion selectivity differs markedly among the family of TRP channels, most of them being non-selective cation channels.
Table 1.
Overview of TRP channels with a chemosensory role in the alimentary canal.
| TRP channel | Sensory modalities in general | Select chemical agonists | References (regarding chemical agonists) |
|---|---|---|---|
| TRPV1 | Heat (>42 °C) | Spices | |
| Pungent spices | Allicin (garlic, onion) | Macpherson et al., 2005 | |
| Salt taste | Allyl isothiocyanate (mustard, horseradish, wasabi) | Everaerts et al., 2011 | |
| Acidosis | Camphora | Xu et al., 2005 | |
| Alkalosis | Cannabidiol (Cannabis sativa) | Bisogno et al., 2001 | |
| Chemesthesis | Capsaicin (red pepper, Capsicum ssp.) | Caterina et al., 1997 | |
| Chemical pain | Citral (lemongrass) | Stotz et al., 2008 | |
| Distension | Eugenol (clove) | Bandell et al., 2007 | |
| Evodiamine (Evodia rutaecarpa) | Pearce et al., 2004 | ||
| Geraniol (citronella grass, Geranium ssp.) | Stotz et al., 2008 | ||
| Gingerol (ginger) | Bandell et al., 2007 | ||
| Isovelleral (Lactarius vellereus) | Szallasi et al., 1996; Ralevic et al., 2003 | ||
| 6-Paradol (Sichuan pepper) | Riera et al., 2009 | ||
| Piperine and other black pepper constituents (dehydropipernonaline, isochavicine, isopiperine, piperanine, pipernonaline, piperolein A and B, retrofractamide C) | Andrè et al., 2006; Okumura et al., 2010 | ||
| Polygodial (Polygonum hydropiper) | Szallasi & Blumberg, 1999 | ||
| 6-Shogaol (Sichuan pepper) | Riera et al., 2009 | ||
| Zingerone (ginger) | Bandell et al., 2007 | ||
| Natural toxins | |||
| Brevetoxin (ciguatera and shellfish toxin) | Cuypers et al., 2007 | ||
| Gambierol (ciguatera and shellfish toxin) | Cuypers et al., 2007 | ||
| Resiniferatoxin (Euphorbia resinifera) | Bandell et al., 2007 | ||
| Vanillotoxin DkTx (Earth Tiger tarantula venom) | Bohlen et al., 2010 | ||
| Vanillotoxins VaTx1, VaTx2, VaTx3 (Trinidad Chevron tarantula venom) | Cromer & McIntyre, 2008 | ||
| Endogenous and exogenous chemicals | |||
| Acesulfame-K | Riera et al., 2007 | ||
| Acid (pH < 6) | Tominaga et al., 1998 | ||
| 2-Aminoethoxydiphenyl borate | Hu et al., 2004 | ||
| Ammonia (pH > 8) | Dhaka et al., 2009 | ||
| Anandamide | Zygmunt et al., 1999 | ||
| Clotrimazole | Meseguer et al., 2008 | ||
| CuSO4 | Riera et al., 2007 | ||
| Ethanol | Trevisani et al., 2002 | ||
| FeSO4 | Riera et al., 2007 | ||
| 12-(S)-Hydroperoxy eicosatetraenoic acid | Hwang et al., 2000 | ||
| 15-(S)-Hydroperoxy eicosatetraenoic acid | Hwang et al., 2000 | ||
| Leukotriene B4 | Hwang et al., 2000 | ||
| N-arachidonoyl-dopamine | Huang et al., 2002 | ||
| Nitric oxide | Miyamoto et al., 2009 | ||
| Nitro-oleic acid (antiinflammatory nitric oxide derivative) | Sculptoreanu et al., 2010 | ||
| N-oleoyldopamine | Chu et al., 2003 | ||
| Oleoylethanolamide | Wang et al., 2005 | ||
| Polyamines (putrescine, spermidine, spermine) | Ahern et al., 2006 | ||
| Propofol | Fischer et al., 2010 | ||
| Saccharin | Riera et al., 2007 | ||
| ZnSO4 | Riera et al., 2007 | ||
| TRPV2 | Heat (>52 °C) Hypotonicity | Natural compounds | |
| Cannabidiol (Cannabis sativa) | Qin et al., 2008 | ||
| Δ9-Tetrahydrocannabinol (Cannabis sativa) | Qin et al., 2008 | ||
| Synthetic chemicals | |||
| 2-Aminoethoxydiphenyl borate | Hu et al., 2004; Stotz et al., 2008 | ||
| Probenecid | Bang et al., 2007b | ||
| TRPV3 | Warmth (22–40 °C) | Spices | |
| Spices | (+)-Borneol (Borneo camphor) | Vogt-Eisele et al., 2007 | |
| Chemesthesis | Camphora | Macpherson et al., 2006; Vogt-Eisele et al., 2007 | |
| Chemical pain | Carvacrol (oregano) | Xu et al., 2006; Vogt-Eisele et al., 2007 | |
| Carveol (spearmint oil) | Vogt-Eisele et al., 2007 | ||
| Citral (lemongrass) | Stotz et al., 2008 | ||
| Dihydrocarveol (caraway and other plants) | Vogt-Eisele et al., 2007 | ||
| Eugenol (clove) | Xu et al., 2006 | ||
| Geraniol (citronella grass, Geranium ssp.) | Stotz et al., 2008 | ||
| Menthol (mint) | Macpherson et al., 2006 | ||
| Thymol (thyme) | Xu et al., 2006; Vogt-Eisele et al., 2007 | ||
| Vanillin (vanilla) | Xu et al., 2006 | ||
| Synthetic chemicals | |||
| 2-Aminoethoxydiphenyl borate | Hu et al., 2004; Stotz et al., 2008 | ||
| Ethyl vanillin | Xu et al., 2006 | ||
| 6-Tert-butyl-m-cresol | Vogt-Eisele et al., 2007 | ||
| TRPV4 | Warmth (>25–34 °C) | Endogenous and exogenous chemicals | |
| Acidosis | Acid (pH < 6) | Suzuki et al., 2003 | |
| Hypotonicity | Bisandrographolide A (Andrographis paniculata) | Smith et al., 2006 | |
| Distension | Citrate | Suzuki et al., 2003 | |
| 5′,6′-Epoxy eicosatrienoic acid | Watanabe et al., 2003 | ||
| 4α-Phorbol 12,13-didecanoate | Watanabe et al., 2002 | ||
| TRPM5 | Sweet, umami and bitter taste | Sweet, umami and bitter tastants | Zhang et al., 2003; Damak et al., 2006; Kaske et al., 2007 |
| TRPM8 | Cold (<25 °C) | Spices | |
| Spices | L-Carvone (Mentha spicata) | Bandell et al., 2007 | |
| Cold pain | Citral (lemongrass) | Stotz et al., 2008 | |
| Chemesthesis | Eucalyptol (Eucalyptus polybractea) | Behrendt et al., 2004 | |
| Chemical pain | Eugenol (clove) | Behrendt et al., 2004 | |
| Geraniol (citronella grass, Geranium ssp.) | Behrendt et al., 2004; Stotz et al., 2008 | ||
| Isopulegol (Mentha pulegium) | Bandell et al., 2007 | ||
| Linalool (Onagraceae ssp.) | Behrendt et al., 2004 | ||
| Menthol (mint)b | McKemy et al., 2002; Peier et al., 2002 | ||
| Synthetic chemical | |||
| Icilin | McKemy et al., 2002 | ||
| TRPP2 | Sour taste | Acid | Chandrashekar et al., 2006; Huang et al., 2006; Ishimaru et al., 2006 |
| TRPA1 | Cold (<17 °C) | Spices | |
| Pungent spices | 1′-Acetoxychavicol acetate (galangal) | Narukawa et al., 2010 | |
| Alkalosis | Allicin (garlic, onion) | Bautista et al., 2005; Macpherson et al., 2005 | |
| Chemesthesis | Allyl isothiocyanate (mustard, horseradish, wasabi) | Story et al., 2003; Jordt et al., 2004 | |
| Chemical pain | Benzyl isothiocyanate (yellow mustard) | Jordt et al., 2004 | |
| Distension | Carvacrol (oregano) | Xu et al., 2006; Lee et al., 2008 | |
| Cinnamaldehyde (cinnamon)c | Bandell et al., 2004 | ||
| Citral (lemongrass) | Stotz et al., 2008 | ||
| Diallyl disulfide (garlic, onion) | Bautista et al., 2005; Macpherson et al., 2005 | ||
| Eugenol (clove) | Bandell et al., 2004 | ||
| Geraniol (citronella grass, Geranium ssp.) | Stotz et al., 2008 | ||
| Gingerol (ginger) | Bandell et al., 2004 | ||
| Isopropyl isothiocyanate (Nasturtium seeds) | Jordt et al., 2004 | ||
| Linalool (Sichuan pepper) | Riera et al., 2009 | ||
| Methyl isothiocyanate (Capparis spinosa) | Jordt et al., 2004 | ||
| Methyl salicylate (wintergreen) | Bandell et al., 2004 | ||
| 6-Paradol (Sichuan pepper) | Riera et al., 2009 | ||
| Phenylethyl isothiocyanate (Brussels sprouts) | Jordt et al., 2004 | ||
| Piperine and other black pepper constituents (isochavicine, isopiperine, piperanine, piperolein A and B) | Okumura et al., 2010 | ||
| 6-Shogaol (Sichuan pepper) | Riera et al., 2009 | ||
| Thymol (thyme) | Lee et al., 2008 | ||
| Natural toxin | |||
| GsMTx-4 (tarantula toxin) | Hill & Schaefer, 2007 | ||
| Endogenous and exogenous chemicals | |||
| Acetaldehyde | Bang et al., 2007a, 2007b | ||
| Acrolein | Bautista et al., 2006 | ||
| 2-Aminoethyl methanethiosulfonate | Macpherson et al., 2007a | ||
| Ammonia | Fujita et al., 2008 | ||
| Bradykinin | Bandell et al., 2004; Bautista et al., 2006 | ||
| Carbon dioxide | Wang et al., 2010 | ||
| Croton aldehyde (cigarette smoke) | Andrè et al., 2008 | ||
| Cyclopentenone prostaglandin metabolites | Andersson et al., 2008; Materazzi et al., 2008; Maher et al., 2008 | ||
| Farnesyl thiosalicylic acid | Maher et al., 2008 | ||
| Formaldehyde | Macpherson, Xiao, et al., 2007; McNamara et al., 2007 | ||
| H2S | Streng et al., 2008 | ||
| H2O2 (oxidative insult) | Andersson et al., 2008; Bessac et al., 2008; Sawada et al., 2008 | ||
| 4-Hydroxy-2-nonenal (oxidative insult) | Macpherson et al., 2007b; Trevisani et al., 2007 | ||
| Hypochlorite (oxidative insult) | Bessac et al., 2008 | ||
| Icilin | Bandell et al., 2007 | ||
| Iodoacetamide | Macpherson et al., 2007a | ||
| Methyl isocyanate | Bessac et al., 2009 | ||
| Methyl p-hydroxybenzoate | Fujita et al., 2007 | ||
| Naphthalene metabolites | Lanosa et al., 2010 | ||
| Nicotine | Talavera et al., 2009 | ||
| Nitric oxide | Miyamoto et al., 2009 | ||
| Nitro-oleic acid (antiinflammatory nitric oxide derivative) | Sculptoreanu et al., 2010 | ||
| 4-Oxo-nonenal (oxidative insult) | Andersson et al., 2008; Taylor-Clark et al., 2008 | ||
| Ozone | Taylor-Clark and Undem, 2010 | ||
| Tear gas (morphanthridine analogs) | Bessac et al., 2009; Gijsen et al., 2010 | ||
| Δ9-Tetrahydrocannabinol | Jordt et al., 2004 | ||
| Toluene diisocyanate | Taylor-Clark et al., 2009 | ||
| Drugs | |||
| N-Acetyl-p-benzo-quinoneimine (metabolite of acetaminophen) | Nassini et al., 2011 | ||
| Clioquinol | Andersson et al., 2009 | ||
| Chlordantoin | Maher et al., 2008 | ||
| Clotrimazoled | Meseguer et al., 2008 | ||
| Diclofenac | Hu et al., 2010 | ||
| 1,4-Dihydropyridines (nicardipine, nifedipine, nimodipine) | Fajardo et al., 2008 | ||
| Disulfiram | Maher et al., 2008 | ||
| Flufenamic acide | Hu et al., 2010 | ||
| Flurbiprofen | Hu et al., 2010 | ||
| Indomethacin | Hu et al., 2010 | ||
| Isoflurane | Eilers et al., 2010 | ||
| Ketoprofen | Hu et al., 2010 | ||
| Mefenamic acid | Hu et al., 2010 | ||
| Niflumic acid | Hu et al., 2010 | ||
| Propofol | Lee et al., 2008; Fischer et al., 2010 | ||
Camphor activates TRPV1 and TRPV3 but inhibits TRPA1 (Xu et al., 2005).
Menthol activates TRPM8 but inhibits TRPA1 (Macpherson et al., 2006).
Cinnamaldehyde activates TRPA1 but inhibits TRPM8 (Macpherson et al., 2006).
Clotrimazole activates both TRPA1 and TRPV1 but blocks TRPM8 (Meseguer et al., 2008).
Flufenamic acid activates TRPA1 but inhibits TRPV1, TRPV3 and TRPM8 (Hu et al., 2010). For further details see Geppetti and Trevisani (2004), Vriens et al. (2008, 2009), Baraldi et al. (2010), and Boesmans et al. (2011).
The objective of this article is to review the occurrence and function of TRP channels in the digestive tract, with a particular emphasis on their roles in health and disease. To keep the wealth of the available information in perspective, overview panels guide the reader to keep track of the functional implications of TRP channels in the alimentary canal (Figs. 1 and 2) and their associations with disorders of the digestive system (Fig. 3). Alterations in their expression and function in disease have raised enormous interest in TRP channels being attractive targets for new therapeutic strategies in gastroenterology. Accordingly, several TRP channel ligands have been developed, and it is a further objective of this article to summarize and discuss the pharmacological opportunities which TRP channel ligands offer to gastroenterology (Fig. 4).
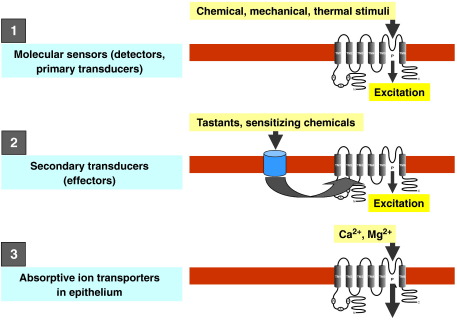
Fig. 1.
Diagram portraying 3 different molecular roles of TRP channels in the digestive system: (1) TRP channels as molecular sensors (detectors or primary transducers) of chemical and physical stimuli, (2) TRP channels as secondary transducers (downstream transducers or effectors) of cell activation induced by G protein-coupled receptors or ion channel receptors, and (3) TRP channels as Ca2+ or Mg2+ transport channels.
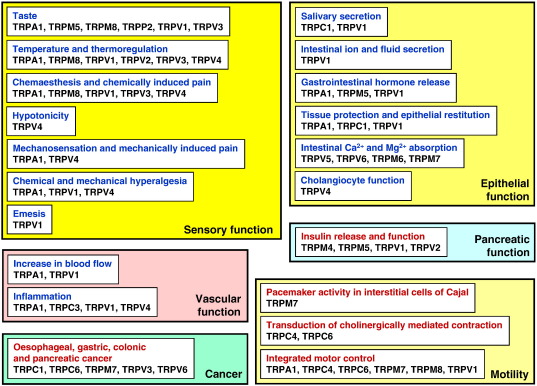
Fig. 2.
Overview of the functional implications of TRP channels in the digestive system.
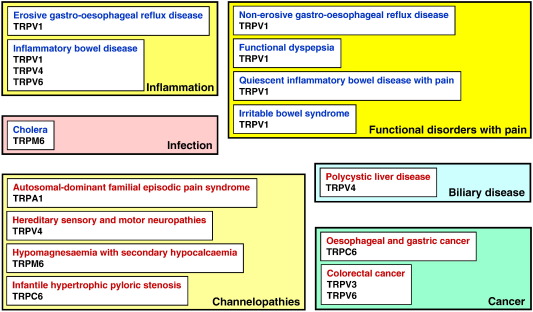
Fig. 3.
Overview of the associations of TRP channels with diseases and disorders of the digestive system.
Fig. 4.
Overview of the therapeutic potential of TRP channel ligands in diseases and disorders of the digestive system.
2. Expression and functional implications of TRP channels in the digestive tract
2.1. General considerations
Although various TRP channels, notably TRPV1, TRPV3, TRPA1, TRPM5 and TRPM8, enable humans to enjoy seasoned food, they subserve a much wider spectrum of functions in the alimentary canal. TRP channels survey the environment of the gut for a large variety of chemicals and toxins that either come in with the food, are formed in the GI tissue, are constituents of the digestive juice, are produced by the GI microbiome, or are generated by tissue injury and inflammation (Table 1). In addition, some TRP channels are involved in mechanosensation or can sense a specific range of temperatures. Apart from their sensory surveillance function, TRP channels in the alimentary canal are relevant to the control of membrane potential and excitability of neurons, epithelial cells, muscle cells and interstitial cells of Cajal (ICCs), play a role in Ca2+ and Mg2+ absorption, govern blood flow, pacemaker activity, motor activity, secretory processes and mucosal homeostasis, and impact on the development of GI cancer.
In reviewing the functional implications of TRP channels in the alimentary canal, 3 distinct roles come to light (Fig. 1). (1) TRP channels operate as molecular sensors (detectors or primary transducers) of chemical and physical stimuli; (2) TRP channels play a role as downstream or secondary transducers (or effectors) of cell activation mediated by G protein-coupled receptors (GPCRs) or ion channel receptors; and (3) TRP channels function as ion transport channels responsible, e.g., for Ca2+ and Mg2+ homeostasis. Within primary afferent neurons, translation of signals detected by TRP channel detectors into effector responses is carried by two distinct mechanisms: (1) by the local release of neuropeptides from the peripheral fibers of TRP-expressing afferent neurons, which causes changes in local tissue function (Holzer, 1988; Maggi & Meli, 1988); and (2) by transmission to the central nervous system resulting in autonomic reflex responses and sensation.
2.2. TRPV1 channels
2.2.1. Occurrence of TRPV1 in the alimentary canal
The existence of TRPV1, the specific sensor for capsaicin, has been envisaged more than 30 years ago, given that most pharmacological effects of capsaicin have been attributed to a specific action on capsaicin-sensitive primary afferent neurons (Jancsó et al., 1987; Holzer, 1991; Szallasi & Blumberg, 1999; Szolcsányi, 2004). The role of capsaicin-sensitive afferent neurons and, by indirect inference, the distribution of capsaicin receptors have been traced with two experimental paradigms: stimulation of afferent neurons by acute application of capsaicin, and pretreatment of animals or tissues with high doses of capsaicin (“capsaicin desensitization”) to defunctionalize afferent neurons via excess influx of Ca2+ following stimulation of TRPV1. Capsaicin-sensitive nerve cells may even degenerate if, e.g., a high dose of capsaicin is administered to newborn rats (Jancsó et al., 1977) or micromolar concentrations of capsaicin are added to dorsal root ganglion (DRG) cultures (Wood et al., 1988). Since the whole sensory neuron is defunctionalized by this approach, referral to a capsaicin-sensitive structure or function in its historical connotation does not necessarily point to an implication of TRPV1. In addition, capsaicin at higher concentrations can act on ion channels unrelated to TRPV1 and on cellular structures lacking TRPV1 (Holzer, 1991) such as ICCs (Choi et al., 2010). Treatment of adult rats with a neurotoxic dose of capsaicin reduces the number of nodose ganglion neurons that express TRPV1 and project to the stomach as observed 10 days post-treatment. By 67 days post-treatment, much of the vagal afferent innervation of the stomach is restored, mainly because of sprouting of capsaicin-insensitive nerve fibers (Ryu et al., 2010).
In the alimentary canal, TRPV1 occurs in extrinsic sensory neurons, intrinsic enteric neurons as well as epithelial and endocrine cells. The major cellular sources of TRPV1 in the rat, guinea-pig and murine alimentary canal are spinal and vagal primary afferent neurons (Patterson et al., 2003; Ward et al., 2003; Holzer, 2004; Horie et al., 2004; Kadowaki et al., 2004; Robinson et al., 2004; Schicho et al., 2004; Banerjee et al., 2007; Ryu et al., 2010; Zhao et al., 2010). Both in the DRG and nodose ganglion cells, TRPV1 is restricted to small and medium-sized somata that are known to give rise to unmyelinated (C-) and thinly myelinated (Aδ-) fibers (Caterina et al., 1997; Guo et al., 1999; Michael & Priestley, 1999; Banerjee et al., 2007; Tan et al., 2008, 2009). There are distinct regional differences in the relative proportion of sensory neurons that stain positive for TRPV1. Thus, TRPV1-immunoreactive fibers are considerably more prevalent in visceral than in somatic afferent neurons (Robinson et al., 2004; Brierley et al., 2005; Hwang et al., 2005; Christianson et al., 2006a). In fact, the majority of nodose ganglion neurons projecting to the rodent stomach and of DRG neurons projecting to the rodent gut express TRPV1 (Robinson et al., 2004; Schicho et al., 2004; Zhang et al., 2004; Brierley et al., 2005; Hwang et al., 2005; Christianson et al., 2006a, 2006b; Tan et al., 2008; Zhong et al., 2008), while only 32% of the vagal afferent neurons supplying the murine jejunum contain TRPV1 (Tan et al., 2009).
Within the rodent and human alimentary canal, TRPV1-positive nerve fibers occur in the musculature, enteric nerve plexuses, arterioles and mucosa (Facer et al., 2001; Yiangou et al., 2001; Chan et al., 2003; Patterson et al., 2003; Ward et al., 2003; Holzer, 2004; Horie et al., 2004; Kadowaki et al., 2004; Matthews et al., 2004; Robinson et al., 2004; Schicho et al., 2004; Zhang et al., 2004; Faussone-Pellegrini et al., 2005; Bhat & Bielefeldt, 2006; Akbar et al., 2008; Matsumoto et al., 2009, 2011). In addition, TRPV1 is present in nerve fibers supplying the taste papillae of the tongue (Caterina et al., 1997; Tominaga et al., 1998; Ishida et al., 2002; DeSimone & Lyall, 2006).
There are regional and species differences in the chemical coding of primary afferent neurons expressing TRPV1. Thus, calcitonin gene-related peptide (CGRP), substance P, somatostatin and other neuropeptides are messenger molecules characteristic of capsaicin-sensitive DRG neurons (Green & Dockray, 1988; Holzer, 1991; Sternini, 1992; Szallasi & Blumberg, 1999; Hwang et al., 2005; Price & Flores, 2007), while CGRP is absent from vagal afferent neurons containing TRPV1 (Tan et al., 2009). DRG neurons can be largely differentiated by their binding of isolectin B4 and their responsiveness to different neurotrophins (Guo et al., 1999; Michael & Priestley, 1999; Liu et al., 2004; Hwang et al., 2005; Price & Flores, 2007). In adult rodents, the isolectin B4-negative cell population responds to nerve growth factor, while isolectin B4-positive cells respond to the glial cell line-derived family of neurotrophins. However, there is no clear distinction between these groups of DRG neurons in their expression of TRPV1 and the neuropeptides substance P, CGRP and somatostatin (Price & Flores, 2007). In the rat, TRPV1 is found in both populations of DRG neurons but is more prevalent in isolectin B4-positive cells (Guo et al., 1999; Michael & Priestley, 1999; Liu et al., 2004; Hwang et al., 2005; Banerjee et al., 2007; Price & Flores, 2007), whereas in the mouse TRPV1 is largely absent from isolectin B4-positive DRG cells (Zwick et al., 2002; Woodbury et al., 2004; Price & Flores, 2007). In both rat and mouse, however, TRPV1 abounds in visceral DRG neurons that bind little isolectin B4 but are rich in CGRP and substance P (Ward et al., 2003; Robinson et al., 2004; Schicho et al., 2004; Brierley et al., 2005; Hwang et al., 2005; Christianson et al., 2006a, 2006b; Tan et al., 2008).
The presence of TRPV1 in the enteric nervous system has been less extensively studied than that in primary afferent neurons and has remained a matter of controversy. While some authors have localized TRPV1 immunoreactivity to somata of the guinea-pig, porcine and human enteric nervous system (Nozawa et al., 2001; Poonyachoti et al., 2002; Anavi-Goffer & Coutts, 2003; Chan et al., 2003), other authors have failed to confirm this location (Patterson et al., 2003; Ward et al., 2003; Holzer, 2004; Schicho et al., 2004). Importantly, the chemical coding of TRPV1-positive DRG neurons innervating the murine gut and of TRPV1-positive nerve fibers in the murine colon and rectum is distinct from that of enteric neurons (Tan et al., 2008; Matsumoto et al., 2011). Furthermore, TRPV1 messenger ribonucleic acid (mRNA) disappears from the rat stomach following extrinsic denervation (Schicho et al., 2004). Enteric neurons that issue projections from the rat colon to the spinal cord are likewise TRPV1-negative (Suckow & Caudle, 2008). The inconsistencies in the localization of TRPV1 to enteric neurons could in part be explained by the existence of several splice variants of TRPV1 (Wang et al., 2004; Faussone-Pellegrini et al., 2005; Szallasi et al., 2007) that may substantially differ in their immunoreactivity. For instance, two splicing products of the murine TRPV1 gene have been identified in the DRGs and several tissues including the gut. One of them, TRPV1-α, is equivalent to the rat and human TRPV1, whereas the other one, TRPV1-β, encodes a dominant negative subunit of the TRPV1 channel (Wang et al., 2004).
In addition to its prominent location in neurons, TRPV1 has been localized to non-neural cells of the alimentary canal. Thus, TRPV1-like immunoreactivity is found in the human submandibular gland where it occurs in serous acinar and ductal cells, but not in mucous acinar cells (Ding et al., 2010). Furthermore, TRPV1-like immunoreactivity has been localized to gastrin and parietal cells of the stomach as well as to epithelial cells of the esophageal, gastric and small intestinal mucosa of several species including man (Nozawa et al., 2001; Kato et al., 2003; Zhang et al., 2004; Faussone-Pellegrini et al., 2005; Kechagias et al., 2005; Cheng et al., 2009; Ericson et al., 2009; Ma et al., 2010). With respect to the presence of TRPV1 in these tissues a word of caution is in place, given that TRPV1 antisera can yield false positive staining in non-neural tissues, which persists in TRPV1 knockout mice (Everaerts et al., 2009).
2.2.2. Functional implications of TRPV1 in the digestive system
2.2.2.1. Sensory modalities of TRPV1
TRPV1 is a sensor that is stimulated by several spices, noxious heat, and a number of endogenous stimuli, some of which are particularly relevant to the GI tract (Table 1). Among the spices acting on TRPV1, capsaicin (red pepper), piperine (black pepper), gingerol and zingerone (ginger), pungent compounds from onion and garlic, eugenol (clove) and camphor figure most prominently. TRPV1 is also activated by allyl isothiocyanate present in mustard, horseradish and wasabi (Everaerts et al., 2011), resiniferatoxin, a toxin of tropical Euphorbia plants (Szallasi & Blumberg, 1999), and “vanillotoxins” present in the venoms of the Trinidad Chevron tarantula and Earth Tiger tarantula (Cromer & McIntyre, 2008; Bohlen et al., 2010). A taste variant of TRPV1 is thought to be involved in nonspecific salt taste perception (DeSimone & Lyall, 2006). Furthermore, TRPV1 contributes to the aversive bitter aftertaste sensation evoked by high concentrations of artificial sweeteners such as saccharin and acesulfame-K and to the metallic taste sensation evoked by high concentrations of CuSO4, ZnSO4 and FeSO4 (Riera et al., 2007). The burning sensation evoked by ethanol in the mouth and throat is also most likely mediated by TRPV1 (Trevisani et al., 2002). The endogenous stimuli that TRPV1 responds to include acid (pH < 6), ammonia (pH > 8), anandamide and other arachidonic acid-derived metabolites such as N-arachidonoyl-dopamine, N-oleoyldopamine, oleoylethanolamide, 12-(S)-hydroperoxy eicosatetraenoic acid, 15-(S)-hydroperoxy eicosatetraenoic acid and leukotriene B4 (Tominaga et al., 1998; Caterina & Julius, 2001; Holzer, 2007; Szallasi et al., 2007; Dhaka et al., 2009).
2.2.2.2. Role of TRPV1 in abdominal pain and hypersensitivity
Several lines of evidence indicate that TRPV1 is involved in pain and hyperalgesia throughout the alimentary canal. In addition, TRPV1-expressing vagal afferents in the esophagus may contribute to the cough evoked by gastro-esophageal reflux of gastric contents (Kollarik & Brozmanova, 2009). In keeping with its nociceptive role, activation of TRPV1 on primary afferent neurons innervating the gut elicits pain in humans and pain-related behavior in rodents (Table 2). Intraesophageal instillation of capsaicin in human volunteers evokes symptoms of heartburn, while the sensitivity to balloon distension or acid exposure remains unchanged (Kindt et al., 2009; Chen et al., 2010). Intragastric administration of capsaicin in humans increases the sensitivity to proximal gastric distension (Lee et al., 2004), and ingestion of capsaicin capsules induces gastric sensations of pressure, heartburn and warmth (Hammer & Vogelsang, 2007). Infusion of capsaicin into the proximal small intestine of human volunteers likewise evokes sensations of pain, cramps, pressure, warmth and nausea (Schmidt et al., 2004; Hammer & Vogelsang, 2007). The capsaicin-induced sensations are most intense in the duodenum and appear to decrease along the intestine (Hammer & Vogelsang, 2007), although local capsaicin instillation in ileostomy and colostomy patients is also painful (Drewes et al., 2003; Arendt-Nielsen et al., 2008). Ingestion of capsaicin for 7 days leads to a decrease in jejunal mechanosensation and to an increase in the jejunal sensitivity to capsaicin (Hammer, 2006a), and oral chili intake for 3 days has been reported to increase rectal sensitivity to urgency (Gonlachanvit et al., 2007). In contrast, ingestion of capsaicin (0.25 mg) capsules three times per day for 4 weeks leads to desensitization of the duodenum to both acute capsaicin administration and balloon distension (Führer & Hammer, 2009).
Table 2.
Select implications of TRPV1 in the physiology and pathophysiology of the digestive tract.
| Tissue | Physiological or pathophysiological process | Type of evidence | References |
|---|---|---|---|
| Submandibular salivary gland | Acinar cell activation and salivary secretion in response to TRPV1 stimulation in humans | Induction by TRPV1 agonism | Ding et al., 2010; Zhang et al., 2010 |
| Esophagus, stomach and \intestine | Stimulation of vagal and spinal afferent nerve fibers by capsaicin, acid and/or distension in rodents | Induction by TRPV1 agonism and prevention by TRPV1 knockout and/or antagonism | Maubach & Grundy, 1999; Su et al., 1999; Blackshaw et al., 2000; Rong et al., 2004; Sugiura et al., 2007; Bielefeldt & Davis, 2008; Peles et al., 2009 |
| Esophagus, stomach and intestine | Hyperemia, bicarbonate secretion and protection of the mucosa from chemical injury (acid, ethanol, nosteroidal antiinflammatory drugs) in rats and humans | Induction by TRPV1 agonism | Hamid et al., 1981; Szolcsányi & Barthó, 1981; Holzer & Lippe, 1988; Holzer et al., 1989; Yeoh et al., 1995; Holzer, 1998, 2002; Aihara et al., 2005; Akiba et al., 2006a, 2008; Massa et al., 2006 |
| Esophagus, stomach and intestine | Patients with erosive gastro-esophageal reflux disease, non-erosive reflux disease, irritable bowel syndrome, inflammatory bowel disease, idiopathic rectal hypersensitivity and fecal urgency, and Hirschsprung's disease | Upregulation of TRPV1 in the mucosa, with a correlation to pain sensitivity in irritable bowel syndrome and in inflammatory bowel disease with irritable bowel syndrome-like symptoms | Facer et al., 2001; Yiangou et al., 2001; Chan et al., 2003; Matthews et al., 2004; Bhat & Bielefeldt, 2006; Akbar et al., 2008, 2010; Guarino et al., 2010; Shieh et al., 2010 |
| Esophagus, stomach, intestine and pancreas | Acid-induced oesophagitis, gastric acid-evoked injury of the stomach, trinitrobenzene sulfonic acid-induced pancreatitis and colitis in rodents | Upregulation of TRPV1 in vagal and spinal afferent neurons | Schicho et al., 2004; Banerjee et al., 2007; Miranda et al., 2007; Xu et al., 2007 |
| Esophagus, stomach and small intestine | Abdominal pain in human volunteers | Induction by intraluminal capsaicin | Schmidt et al., 2004; Hammer, 2006a, 2006b; Hammer & Vogelsang, 2007; Hammer et al., 2008; Führer & Hammer, 2009; Kindt et al., 2009; Chen et al., 2010; van Boxel et al., 2010 |
| Stomach | Behavioral pain response to intragastric acid challenge in rats | Prevention by TRPV1 antagonism | Lamb et al., 2003 |
| Stomach and upper small intestine | Functional dyspepsia | Hypersensitivity to the algesic effect of capsaicin | Hammer et al., 2008 |
| Stomach and upper small intestine | Functional dyspepsia | Beneficial effect of 5 week ingestion of capsaicin capsules | Bortolotti et al., 2002 |
| Duodenum | Pain sensitivity to capsaicin exposure and balloon distension in humans | Beneficial effect of 4 week ingestion of capsaicin capsules | Führer & Hammer, 2009 |
| Jejunum | Cardiovascular pain response to noxious distension in rats | Attenuation by TRPV1 ablationa | Lembeck & Skofitsch, 1982 |
| Ileum | Ileitis induced by Clostridium difficile toxin A in rodents | Attenuation by TRPV1 antagonism | McVey & Vigna, 2001 |
| Ileum and colon | Abdominal pain and viscerosomatic reflexes in humans | Intraluminal administration of capsaicin to ileostomy and colostomy patients | Drewes et al., 2003; Arendt-Nielsen et al., 2008 |
| Colon | Abdominal pain in rodents | Induction by intraluminal capsaicin | Laird et al., 2001; Mansikka et al., 2004; Christoph et al., 2006 |
| Colon | Hypersensitivity to balloon distension in humans | Ingestion of capsaicin capsules | Gonlachanvit et al., 2007 |
| Colon | Abdominal pain and burning in patients with diarrhea-predominant irritable bowel syndrome | Hypersensitivity to ingestion of chili capsules | Gonlachanvit et al., 2009 |
| Colon | Hyperalgesia caused by repetitive colorectal distension in rats | Prevention by TRPV1 antagonism | Ravnefjord et al., 2009 |
| Colon | Sensitization of murine afferent nerve fibers to heat, acid and capsaicin by 5-hydroxytryptamine | Attenuation by TRPV1 knockout | Sugiura et al., 2004 |
| Colon | Hyperalgesia to intraluminal acid and colorectal distension in rodents with acute experimental colitis | Attenuation by TRPV1 ablation, knockout and antagonism | Plourde et al., 1997; Delafoy et al., 2003; Jones et al., 2005, 2007; Miranda et al., 2007 |
| Colon | Acute stress-evoked hyperalgesia to colorectal distension in rats subjected to maternal separation as neonates | Prevention by TRPV1 antagonism | van den Wijngaard et al., 2009b |
| Colon | Water avoidance stress-induced upregulation of TRPV1 in rat DRG neurons and hypersensitivity to colorectal distension | Prevention by TRPV1 antagonism | Hong et al., 2009 |
| Colon | Post-inflammatory chemical and mechanical hyperalgesia in rodents | Attenuation by TRPV1 knockout and antagonism | Eijkelkamp et al., 2007; Jones et al., 2007; Winston et al., 2007; Wiskur et al., 2010 |
| Colon | Colitis induced by dextran sulfate or trinitrobenzene sulfonic acid in rodents | Attenuation by TRPV1 knockout and antagonism | Kihara et al., 2003; Kimball et al., 2004; Miranda et al., 2007; Szitter et al., 2010 |
| Colon | Colitis induced by trinitrobenzene sulfonic acid in rats | Upregulation of TRPV1 in and sensitization of colon-specific pelvic DRG neurons | De Schepper et al., 2008 |
| Colon | Colitis-induced inhibition of gastric emptying in rats | Prevention by TRPV1 antagonism | De Schepper et al., 2008 |
| Colon | Colitis induced by T-cell transfer in mice | Prevention by TRPV1 ablation | Gad et al., 2009 |
| Colon | Secretory responses induced by distension or H2S in guinea-pig and human preparations | Attenuation by TRPV1 ablation and antagonism | Weber et al., 2001; Krueger et al., 2010 |
| Anus | Intractable idiopathic pruritus ani | Attenuation by TRPV1 ablation | Lysy et al., 2003 |
| Pancreas | Chronic pancreatitis induced by trinitrobenzene sulfonic acid in rats | Upregulation and sensitization of TRPV1 in pancreas-specific DRG neurons | Xu et al., 2007 |
| Pancreas | Pancreatitis induced by caerulein in mice | Attenuation by TRPV1 antagonism | Nathan et al., 2001 |
| Pancreas | Acid-evoked injury in a rat model of post-endoscopic cholangiopancreatography pancreatitis | Attenuation by TRPV1 ablation | Noble et al., 2008 |
| Pancreas | Islet inflammation in non-obese diabetic mice (genetic model of type I diabetes) | Prevention by TRPV1 ablation | Razavi et al., 2006; Suri & Szallasi, 2008 |
| Pancreas | Pain behavior, referred allodynia/hyperalgesia and spinal c-Fos expression associated with experimental pancreatitis in rodents | Attenuation by TRPV1 antagonism | Wick et al., 2006; Xu et al., 2007; Nishimura et al., 2010 |
| Peritoneal cavity | Behavioral pain response to intraperitoneal injection of acetic acid or oleoylethanolamide in rodents | Attenuation by TRPV1 antagonism | Urban et al., 2000; Ikeda et al., 2001; Rigoni et al., 2003; Wang et al., 2005; Tang et al., 2007 |
TRPV1 ablation refers to pretreatment with capsaicin or resiniferatoxin to defunctionalize afferent neurons.
TRPV1 can be sensitized by many proalgesic factors (Caterina & Julius, 2001; Geppetti & Trevisani, 2004; Szallasi et al., 2007; Holzer, 2008b), and there is increasing evidence that GI inflammation causes chemical and mechanical hyperalgesia at least in part by upregulation and sensitization of TRPV1 (Table 2). Neurotrophins, various inflammatory mediators as well as endocrine factors of the GI mucosa are thought to be involved in this process. Thus, the ability of 5-hydroxytryptamine (5-HT) to sensitize GI afferent neurons to heat, acid and capsaicin is absent in TRPV1 knockout mice (Sugiura et al., 2004). The effect of 5-HT to sensitize colonic DRG neurons is mediated by metabotropic 5-HT2 and 5-HT4 receptors which appear to enhance TRPV1 activity by downstream phosphorylation pathways (Sugiura et al., 2004). Vice versa, depletion of 5-HT from the colon by pretreatment of rats with p-chlorophenylalanine reduces the excitability of DRG neurons by capsaicin and elevates the threshold of distension-induced pain behavior (Qin et al., 2010). Referred hyperalgesia associated with caerulein-induced pancreatitis in mice depends on both protease-activated receptor-2 (PAR-2) and TRPV1, TRPV1 being a downstream transducer of the pronociceptive action of PAR-2 stimulation (Fukushima et al., 2010; Nishimura et al., 2010).
TRPV1 is upregulated not only in inflammation, but also in the absence of overt inflammation (Table 2) as is typical of functional GI disorders (Holzer, 2008a). This is true for patients with irritable bowel syndrome in which the increased density of TRPV1 in the rectosigmoid colon correlates with pain severity (Akbar et al., 2008). A similar correlation between pain intensity and number of mucosal TRPV1-positive nerve fibers is found in patients with quiescent inflammatory bowel disease who continue to complain of abdominal pain (Akbar et al., 2010). Non-erosive reflux disease (Bhat & Bielefeldt, 2006; Guarino et al., 2010), idiopathic rectal hypersensitivity and fecal urgency (Chan et al., 2003) are other instances of TRPV1 upregulation in the absence of appreciable inflammation. In addition, patients with functional dyspepsia (Hammer et al., 2008) and diarrhea-predominant irritable bowel syndrome (Gonlachanvit et al., 2009) exhibit hypersensitivity to the painful sensations evoked by capsaicin-containing capsules. Experimental findings have likewise shown that TRPV1 has a bearing on post-inflammatory colonic hyperalgesia in rodents, given that upregulation of TRPV1 expression and function persists long after the initial inflammatory insult has subsided (Eijkelkamp et al., 2007; Jones et al., 2007; Winston et al., 2007; Holzer, 2008b).
Although the participation of TRPV1 in chemical nociception figures most prominently, there is evidence that TRPV1 also contributes to mechanical hyperalgesia in the inflamed gut (Table 2). As shown for pelvic afferents innervating the murine colon, TRPV1 is preferentially expressed by mechanoreceptors that respond to distension with a low frequency of firing, the distension responses of these fibers being sensitized by capsaicin or acidosis (Malin et al., 2009). Pelvic afferents innervating the smooth muscle and myenteric plexus of the murine rectum are likewise sensitive to low-threshold distension and capsaicin (Spencer et al., 2008). Accordingly, capsaicin enhances the visceromotor response to colorectal distension (an indirect measure of abdominal pain), this effect being inhibited by the TRPV1 blocker SB-705,498 (van den Wijngaard et al., 2009a). Similarly, the hypersensitivity to colorectal distension, which is seen after acute stress exposure of adult rats that have been subjected to maternal separation as neonates, is reversed by TRPV1 blockade (van den Wijngaard et al., 2009b). This finding is consistent with a report that chronic exposure of adult rats to water avoidance stress for 10 days causes a significant upregulation of TRPV1 (and TRPA1) in DRG neurons supplying the colon, along with an increase in the abdominal withdrawal reflex to colorectal distension (Yu et al., 2010). Furthermore, TRPV1 blockade prevents the development of mechanical hyperalgesia that is evoked by repetitive colorectal distension in rats, but does not cause hypoalgesia (Ravnefjord et al., 2009). High-threshold splanchnic afferents innervating the mesentery and serosa of the colon fail to be sensitized in rats with dextran sulfate sodium (DSS)-induced colitis, yet the response of these fibers to mechanical probing is attenuated by a TRPV1 blocker in inflammation but not health (Phillis et al., 2009). The role of TRPV1 in mechanical pain seems to be that of a secondary transducer, because a mechanodetector role of TRPV1 has not yet been substantiated. The implication of TRPV1 in inflammatory hypersensitivity is likewise that of a secondary transducer, TRPV1 channel activity being enhanced by multiple mechanisms triggered by receptors for inflammatory mediators and neurotrophins.
2.2.2.3. Role of TRPV1 in emesis
A prominent role of vagal afferent neurons supplying the upper GI tract is to cause nausea and vomiting if the ingested food is deemed potentially or actually toxic. In view of their broad spectrum of sensory modalities, distinct TRP channels are likely to play a role in these processes. There is indeed experimental evidence that activation of TRPV1 has an impact on emesis. Thus, subcutaneous or intracerebroventricular administration of capsaicin or resiniferatoxin has been reported to cause retching and vomiting in the dog, ferret and Suncus marinus, the house musk shrew (Shiroshita et al., 1997; Andrews et al., 2000; Rudd & Wai, 2001; Cheng et al., 2005). The proemetic action of TRPV1 activation appears to reverse quickly to an antiemetic action, given that vomiting caused by stimulation of the medial solitary nucleus in the brainstem, radiation, systemic administration of cisplatin, loperamide or apomorphine or intragastric administration of CuSO4 is depressed by capsaicin and resiniferatoxin (Andrews & Bhandari, 1993; Shiroshita et al., 1997; Andrews et al., 2000; Rudd & Wai, 2001; Yamakuni et al., 2002). Since the mechanism behind the dual proemetic and antiemetic action of TRPV1 agonists is not understood, the existence of a novel vanilloid receptor has been envisaged (Cheng et al., 2005). Non-pungent agonists at TRPV1 such as arvanil, olvanil, anandamide and N-arachidonoyl-dopamine fail to evoke emesis in the ferret but are able to blunt vomiting evoked by morphine-6-glucuronide, apomorphine, cisplatin and CuSO4 (Sharkey et al., 2007; Chu et al., 2010).
2.2.2.4. Thermoregulation by abdominal TRPV1
TRPV1 is a heat sensor (Caterina et al., 1997; Tominaga et al., 1998), which explains why the sensation caused by capsaicin is described as “hot” and “burning”. Although capsaicin and related TRPV1 agonists evoke a thermoregulatory response (Szallasi & Blumberg, 1999; Masamoto et al., 2009), TRPV1 knockout mice have a normal body temperature and do not seem to have a deficit in heat sensing (Szelényi et al., 2004; Woodbury et al., 2004; Iida et al., 2005), except that heat hyperalgesia in response to inflammation (Caterina et al., 2000; Davis et al., 2000) or heat injury (Bölcskei et al., 2005) and fever in response to the bacterial pyrogen lipopolysaccharide (Iida et al., 2005) are attenuated. The maintenance of basal thermoregulation in TRPV1 knockout mice is probably due to developmental compensations, given that many TRPV1 blockers cause substantial hyperthermia in mice, rats, dogs, monkeys and humans (Gavva et al., 2007, 2008; Steiner et al., 2007; Holzer, 2008b; Garami et al., 2010). It need be inferred, therefore, that TRPV1 is tonically active to control core body temperature (Gavva et al., 2007; Montell & Caterina, 2007), lowering body temperature through suppression of autonomic cold-defense effectors (Gavva, 2008; Romanovsky et al., 2009).
The hyperthermic action of TRPV1 blockers involves cutaneous vasoconstriction and shivering-related thermogenesis, but not warmth-seeking behavior, and is inhibited by the antipyretic drug acetaminophen (Steiner et al., 2007; Gavva et al., 2008). Because the magnitude of the hyperthermic effect of the TRPV1 blocker AMG0347 is independent of the baseline temperature, it has been concluded that the rise of body temperature results from blockade of tonic TRPV1 activation by non-thermal factors (Steiner et al., 2007). Further analysis suggests that the hyperthermic response to TRPV1 antagonists depends on blockade of the proton mode of TRPV1 activation, either alone or together with the capsaicin mode of TRPV1 activation (Romanovsky et al., 2009; Garami et al., 2010). However, this issue has not yet been settled because some TRPV1 antagonists that do not block the proton mode of TRPV1 activation can elicit hyperthermia (Gavva et al., 2007) and other TRPV1 blockers that do not cause hyperthermia affect the proton mode of TRPV1 stimulation (Lehto et al., 2008; Garami et al., 2010; Voight & Kort, 2010). It thus awaits to be confirmed that hyperthermia is in fact driven by a low tissue pH, either alone or together with endovanilloid agonists (Garami et al., 2010) and that the TRPV1-mediated thermoregulatory action takes place in the abdominal cavity (Steiner et al., 2007) in which the stomach and colon have an acidic environment (Holzer, 2007). The increase in metabolic rate and thermogenesis evoked by intrajejunal administration of non-pungent capsaicin analogs (capsinoids) to mice also seems to be mediated by TRPV1 within the GI tract (Kawabata et al., 2009).
2.2.2.5. Control of digestive functions by TRPV1
2.2.2.5.1. Vasodilatation, tissue protection and inflammation
When TRPV1-expressing sensory nerve fibers are activated, they release peptide transmitters from their peripheral endings and in this way modify GI vascular, immune and smooth muscle functions (Holzer, 1998; Barthó et al., 2004, 2008; Holzer, 2004; Mózsik et al., 2007). Following tissue irritation or injury, some of these reactions (e.g., vasodilatation and plasma protein extravasation) contribute to the process of neurogenic inflammation. The TRPV1 agonist capsaicin and mustard oil (a TRPA1 and TRPV1 agonist) have been instrumental in discovering and analyzing this phenomenon (Holzer, 1988). The messengers involved in the efferent-like mode of operation include CGRP, somatostatin and the tachykinins substance P and neurokinin A (Maggi, 1995; Holzer & Maggi, 1998; Pintér et al., 2006).
Administration of capsaicin to the esophageal, gastric and intestinal mucosa increases mucosal blood flow, a response which is mimicked by exposure to excess acid (Holzer, 1998, 2004). The acid- and carbon dioxide-evoked hyperemia in the esophageal and duodenal mucosa is inhibited by the TRPV1 antagonist capsazepine, which indicates that acid activates TRPV1 on sensory nerve fibers (Akiba et al., 2006a, 2008). Through this mechanism, which also includes increases in bicarbonate and mucus secretion, TRPV1-positive sensory nerve fibers are able to protect the esophageal, gastric and intestinal mucosa from a variety of injurious insults (Holzer, 1998). Paradoxically, knockout of TRPV1 has been reported to ameliorate acid-induced injury in the esophagus and stomach (Akiba et al., 2006b; Fujino et al., 2006). Analysis of this observation in the stomach suggests that disruption of the TRPV1 gene causes a compensatory upregulation of several protective mechanisms in the gastric mucosa (Akiba et al., 2006b). These counterregulatory mechanisms may also explain why acid-induced release of CGRP from the murine stomach remains unchanged in TRPV1 knockout mice (Auer et al., 2010).
Apart from protecting the GI mucosa (Holzer, 1998; Massa et al., 2006; Martelli et al., 2007), TRPV1 activation has been found to exacerbate inflammation and injury in certain models of ileitis, colitis and pancreatitis (Table 2). In addition, capsaicin- as well as acid-evoked stimulation of epithelial TRPV1 in the feline and human esophagus has the potential to give rise to esophagitis (Cheng et al., 2009; Ma et al., 2010). In the murine gastric mucosa, ethanol-induced injury appears to involve TRPV1-mediated release of neuronal substance P and subsequent formation of reactive oxygen species (Gazzieri et al., 2007). Experimental colitis in the mouse is attenuated by TRPV1 antagonism or knockout (Table 2). Emerging evidence indicates that TRPV1 contributes to pancreatic islet inflammation associated with type I diabetes and, in addition, plays a role in insulin-dependent glucose regulation, type II diabetes, adipogenesis and obesity (Razavi et al., 2006; Gram et al., 2007; Zhang L.L. et al., 2007; Suri & Szallasi, 2008). The observation that stimulation of TRPV1, under some conditions, reduces and, under other conditions, exaggerates tissue inflammation and injury may reflect stimulus- and tissue-dependent differences in the process of neurogenic inflammation. In particular, the peptide mediators of TRPV1-positive afferent neurons cover a wide spectrum of actions: Tachykinins facilitate inflammation, while CGRP promotes vasodilatation but not necessarily inflammation, and somatostatin is capable of inhibiting inflammatory processes (Pintér et al., 2006).
2.2.2.5.2. Motor activity and secretory processes
The messengers released from capsaicin-sensitive afferent nerve fibers in the gut act on enteric nervous system, GI smooth muscle and epithelium to modify motility and secretion (Weber et al., 2001; Holzer, 1998, 2002; Benkó et al., 2005; Geber et al., 2006; Schicho et al., 2006; Sibaev et al., 2006; Boudaka et al., 2007; Barthó et al., 2008; de Man et al., 2008; Matsumoto et al., 2011). Tachykinins and adenosine triphosphate stimulate motor activity, while CGRP, vasoactive intestinal polypeptide and nitric oxide account for motor inhibition caused by TRPV1 activation (Benkó et al., 2005; Barthó et al., 2008; de Man et al., 2008; Matsumoto et al., 2009). Local motor effects mediated by TRPV1 may become operative when the GI tract is disturbed by endogenous or exogenous irritants. For instance, TRPV1 and substance P are involved in the acid-evoked contraction of opossum esophageal longitudinal muscle (Paterson et al., 2007). The circular muscle of the isolated human gut is relaxed by capsaicin, and this effect appears to be mediated by nitric oxide and, to some extent, vasoactive intestinal polypeptide (Barthó et al., 2008). Despite these motor effects of TRPV1 stimulation in vitro, the motor activity of the small and large intestine of experimental animals is maintained at a physiological level following functional ablation of capsaicin-sensitive afferent neurons (Barthó et al., 2008). Likewise, no obvious changes in GI motor function have thus far been reported to occur in mice deficient in TRPV1.
Ingestion of capsaicin by humans increases amplitude and velocity of esophageal pressure waves and facilitates secondary peristalsis due to air injection, decreases proximal gastric tone, inhibits phasic contractions of the proximal stomach and inhibits gastric emptying, but does not significantly alter orocecal transit time (Gonzalez et al., 1998; Lee et al., 2004; Chen et al., 2010). The capsaicin-induced improvement of esophageal motility has been observed in healthy volunteers as well as in patients with gastro-esophageal reflux disease (Gonzalez et al., 1998; Grossi et al., 2006; Chen et al., 2010). The relevance of TRPV1 to GI motor control may be most pronounced under pathopysiological conditions when stimulation of TRPV1 on sensory nerve fibers causes local messenger release and activation of sympathetic reflexes. In this way, laparotomy or peritoneal irritation slows GI transit and may even cause ileus (Holzer et al., 1986; Barquist et al., 1996; Zittel et al., 2001). This contention is also borne out by the observation that the gastroparesis associated with experimental colitis is relieved by TRPV1 blockers (De Schepper et al., 2008).
Secretory functions in the alimentary canal are subject to regulation by TRPV1 expressed in neurons as well as epithelial cells. In the human submandibular gland, TRPV1 has been found to activate acinar cells, to stimulate the trafficking of aquaporin-5 to the cell membrane and to cause salivary secretion (Ding et al., 2010; Zhang et al., 2010). Acid- or capsaicin-evoked stimulation of epithelial TRPV1 in the feline and human esophagus induces the formation of platelet-activating factor (Cheng et al., 2009; Ma et al., 2010). Exposure of human isolated antral glands to capsaicin causes release of gastrin and somatostatin, an effect that is thought to be mediated by TRPV1 expressed by gastrin cells, because it is blunted by a chili-rich diet ingested for 3 weeks (Ericson et al., 2009). Distension of the guinea-pig colon (Weber et al., 2001) or exposure of the guinea-pig and human colon to H2S (Schicho et al., 2006; Krueger et al., 2010) in vitro evokes a prosecretory effect which depends on activation of TRPV1-expressing sensory nerve fibers, release of substance P and stimulation of cholinergic secretomotor neurons.
2.2.3. Association of TRPV1 with gastrointestinal disease
Upregulation of TRPV1 tissue levels has been observed in a number of inflammatory and functional GI disorders (Table 2). Thus, both erosive and non-erosive reflux diseases are associated with increased levels of TRPV1 in the esophageal mucosa (Matthews et al., 2004; Bhat & Bielefeldt, 2006; Guarino et al., 2010; Shieh et al., 2010). The upregulation of TRPV1 in the mucosa of patients with erosive esophagitis is associated with elevated expression of nerve growth factor and glial cell line-derived neurotrophic factor (Shieh et al., 2010). Idiopathic rectal hypersensitivity and fecal urgency (Chan et al., 2003) and Hirschsprung's disease (Facer et al., 2001) are instances of TRPV1 upregulation in the absence of inflammation. Patients with uninvestigated dyspepsia have been found hypersensitive to intrajejunal capsaicin infusion (Hammer, 2006b), and a proportion of patients with functional dyspepsia are more responsive to ingestion of capsaicin capsules than healthy controls (Hammer et al., 2008). A role of TRPV1 in upper GI pain is also suggested by the antinociceptive effect which prolonged treatment with capsaicin-containing capsules has in healthy volunteers and patients with functional dyspepsia (Bortolotti et al., 2002; Führer & Hammer, 2009). Furthermore, the homozygous G315C polymorphism of the TRPV1 gene has been found to be inversely related to symptom severity in functional dyspepsia patients (Tahara et al., 2010).
Patients suffering from diarrhea-predominant irritable bowel syndrome exhibit hypersensitivity to the painful and burning sensations which chili-containing capsules and food elicit in the GI tract (Gonlachanvit et al., 2009). The TRPV1-positive innervation of the mucosa in the rectosigmoid colon is increased in patients with inflammatory bowel disease, in patients with irritable bowel syndrome as well as in patients with quiescent inflammatory bowel disease who continue to complain of irritable bowel syndrome-like symptoms (Yiangou et al., 2001; Akbar et al., 2008, 2010). Importantly, the density of TRPV1-positive nerve fibers in the rectosigmoid colon correlates with pain severity in both patients with irritable bowel syndrome and patients with symptomatic but quiescent inflammatory bowel disease (Akbar et al., 2008, 2010).
2.2.4. Therapeutic potential of TRPV1 ligands in the digestive system
Recognition of TRPV1 as a multimodal nocisensor, its sensitization by a number of proinflammatory and proalgesic pathways, its upregulation under conditions of hyperalgesia and its apparent implication in experimental colitis have made this TRP channel an attractive target for novel antinociceptive and antiinflammatory drugs (Szallasi et al., 2007; Holzer, 2008a, 2008b; Voight & Kort, 2010). Pharmacologically, the function of TRPV1 can be manipulated by two principal approaches: stimulant/defunctionalizing TRPV1 agonists and TRPV1 antagonists (Roberts & Connor, 2006; Gharat & Szallasi, 2008; Gunthorpe & Szallasi, 2008; Baraldi et al., 2010). The mechanism and result of the two approaches are profoundly different. While TRPV1 antagonists specifically modify the function of the ion channel, stimulant/defunctionalizing TRPV1 agonists target the cellular function of capsaicin-sensitive afferent neurons (Holzer, 1991; Szallasi et al., 2007). The “desensitization” that is brought about by capsaicin, resiniferatoxin or non-pungent TRPV1 ligands reflects “defunctionalization” of the whole afferent neuron expressing TRPV1 for a prolonged period of time.
At present, there is only evidence for a beneficial effect of stimulant/defunctionalizing TRPV1 agonists in GI disease. Acute ingestion of capsaicin has a protective effect against aspirin-induced erosions in the gastric mucosa of human volunteers (Yeoh et al., 1995). In addition, capsaicin can improve esophageal motility in patients with gastro-esophageal reflux disease (Grossi et al., 2006). Prolonged intake of capsaicin appears to desensitize afferent nerve fibers against noxious stimulation of the upper GI tract. Thus, ingestion of capsaicin (0.25 mg) capsules by healthy volunteers 3 times per day for 4 weeks blunts the pain response evoked by duodenal capsaicin administration and balloon distension (Führer & Hammer, 2009). Similarly, treatment of patients suffering from functional dyspepsia with capsaicin-containing capsules for 5 weeks leads to a significant reduction of pain symptoms (Bortolotti et al., 2002). Since there is some information that the prevalence of heartburn symptoms is low in Asian countries in which the intake of chili-rich food is common, it has been proposed that chronic ingestion of capsaicin may be beneficial in patients with gastro-esophageal reflux disease and functional dyspepsia (Gonlachanvit, 2010).
A great deal of effort has been put into developing compounds that block TRPV1 activation in a competitive or noncompetitive manner (Gharat & Szallasi, 2008; Gunthorpe & Szallasi, 2008; Kym et al., 2009; Wong & Gavva, 2009; Voight & Kort, 2010). Some TRPV1 blockers exhibit species differences in their activity and/or act in a stimulus-specific manner, i.e., differentially inhibit the activation of TRPV1 by capsaicin, heat, and acid (Gavva et al., 2005; Lehto et al., 2008). The design of stimulus-specific blockers is of particular importance because a number of TRPV1 antagonists turned out to have a pronounced hyperthermic effect that limits their clinical usefulness (Caterina, 2008; Gavva, 2008; Holzer, 2008b; Romanovsky et al., 2009; Wong & Gavva, 2009). Apart from their thermoregulatory perils (Caterina, 2008), TRPV1 antagonists are liable to impair the perception of potentially injurious heat (Voight & Kort, 2010). Furthermore, blockade of TRPV1 will interfere with the physiological role of this nocicensor in surveying the physical and chemical environment and, if necessary, in initiating protective responses. Such a role is obvious in the GI tract in which capsaicin-sensitive afferent neurons constitute a neural alarm system which helps maintaining mucosal homeostasis in the face of pending injury (Holzer, 1998, 2004; Akiba et al., 2006b). Other caveats are posed by the incomplete analysis of the involvement of TRPV1 in GI disease. For instance, the upregulation of TRPV1 in gut disorders does not necessarily reflect a causal implication of TRPV1 but may equally well mirror an epiphenomenon of the disease process (Holzer, 2008a).
There are several opportunities to design TRPV1 blockers such that they can be specifically targeted at aberrantly expressed or aberrantly operating TRPV1 channels (Holzer, 2008b) while sparing their physiological function. These opportunities include the development of modality-specific TRPV1 blockers, of uncompetitive TRPV1 antagonists, of compounds that selectively address certain TRPV1 splice variants that may be particularly disease-relevant, compounds that prevent TRPV1 sensitization, and compounds that prevent the recruitment and trafficking of TRPV1 to the cell membrane (Gavva, 2008; Holzer, 2008b; Romanovsky et al., 2009). If these approaches turn out to be successful, TRPV1 blockers may enjoy a wide spectrum of usefulness in GI disease which includes, pain, hyperalgesia and inflammation associated with gastro-esophageal reflux disease, inflammatory bowel disease, functional Gi disorders including functional dyspepsia and irritable bowel syndrome, disturbances of GI motor activity, and nausea and emesis.
2.3. TRPV2 and TRPV3 channels
2.3.1. Occurrence of TRPV2 and TRPV3 in the alimentary canal
TRPV2 has been localized to rodent DRG and nodose ganglion neurons supplying the GI tract (Caterina et al., 1999; Kashiba et al., 2004; Zhang et al., 2004; Stotz et al., 2008; Zhao & Simasko, 2010; Zhao et al., 2010). In contrast to TRPV1, TRPV2 occurs primarily in medium and large diameter afferent neurons (Zhang et al., 2004). In the rat oral mucosa, TRPV2 is not only present in subepithelial nerve fibers, but also in junctional epithelial cells surrounding each tooth, Langerhans cells, dendritic cells, macrophages and endothelial cells of venules, but not in oral epithelial cells (Shimohira et al., 2009). In the rat and guinea-pig small intestine, TRPV2 is found in nerve fibers within the muscularis and myenteric plexus as well as in myenteric cell bodies (Kashiba et al., 2004; Zhang et al., 2004). In addition, TRPV2 occurs in the β-cells of murine pancreatic islets and in the insulinoma cell line MIN6 (Hisanaga et al., 2009). Immunohistochemistry of human tissues has localized TRPV2 to epithelial cells of the salivary glands, pancreatic duct, stomach, duodenum and colon (Kowase et al., 2002).
TRPV3 is highly expressed by epithelial cells in the nose and tongue (Xu et al., 2006). It likewise occurs in DRG and nodose ganglion neurons (Zhang et al., 2004; Stotz et al., 2008; Zhao & Simasko, 2010; Zhao et al., 2010), but retrograde labeling has failed to identify TRPV3 in vagal afferent neurons supplying the murine stomach (Zhang et al., 2004). However, the muscle and mucosa of the murine stomach and small intestine do contain TRPV3 mRNA (Zhang et al., 2004). In addition, TRPV3 is expressed by superficial epithelial cells of the murine distal colon, but not by superficial epithelial cells of the stomach, duodenum and proximal colon (Ueda et al., 2009).
2.3.2. Functional implications of TRPV2 and TRPV3 in the digestive system
2.3.2.1. Sensory modalities of TRPV2 and TRPV3
In terms of their thermosensory modalities, TRPV2 and TRPV3 belong to the group of thermo-TRP channels because they are activated by specific temperatures (Dhaka et al., 2009). While TRPV2 is typically stimulated by temperatures above 52 °C (Caterina et al., 1999), TRPV3 responds to temperatures in the innocuous physiological range (Bandell et al., 2007). TRPV2 and in particular TRPV3 are sensitive to a variety of chemical entities and—together with TRPA1, TRPM8 and TRPV1—TRPV3 is one of the multimodal spice sensors (Table 1). Noteworthy, TRPV3 is activated by a range of monoterpenoid compounds (Vogt-Eisele et al., 2007) including carvacrol (oregano), thymol (thyme) and menthol (mint), although these compounds are also recognized by other TRP channels such as TRPA1 and TRPM8.
2.3.2.2. Emerging role of TRPV2 and TRPV3 in digestion
The expression of TRPV2 and TRPV3 in the oral cavity, nose and tongue together with their sensory modalities suggests that these TRP channels play a role in probing food for its thermal and chemical qualities. Specific implications of TRPV2 and TRPV3 in the GI tract have not yet been explored. There is evidence, however, that TRPV2 plays a role in the autocrine regulation of pancreatic β-cells, since insulin causes translocation and insertion of TRPV2 into the plasma membrane and the insulin-evoked calcium entry is prevented by TRPV2 inhibition or knockdown (Hisanaga et al., 2009). In addition, the release of insulin induced by a high glucose concentration depends on TRPV2.
2.3.3. Association of TRPV3 with gastrointestinal disease
A study analyzing a possible association between genetic variability (as examined for 392 single-nucleotide polymorphisms) in 43 fatty acid metabolism-related genes and risk for colorectal cancer in 1225 patients with cancer and 2032 controls has shown that TRPV3 is associated with a higher risk for development of colorectal cancer (Hoeft et al., 2010).
2.4. TRPV4 channels
2.4.1. Occurrence of TRPV4 in the alimentary canal
In the GI tract, TRPV4 occurs primarily in fibers of extrinsic primary afferent neurons, although some epithelial and other cells have also been reported to stain positively for this TRPV channel subunit. TRPV4 is present in the nodose ganglion, DRG, stomach, small intestine and colon of rodents (Zhang et al., 2004; Grant et al., 2007; Cenac et al., 2008; Brierley et al., 2008; Zhao & Simasko, 2010; Zhao et al., 2010). Retrograde labeling shows that vagal afferent neurons projecting to the murine forestomach contain TRPV4 and that, in these neurons, TRPV4 is coexpressed with TRPV1, TRPV2 and TRPA1 to various degrees (Zhang et al., 2004). Similarly, DRG neurons projecting to the murine pancreas synthesize both TRPV4 and TRPA1 (Ceppa et al., 2010). The expression of TRPV4 in the DRG neurons that project to the murine and human intestine via mesenteric and pelvic nerves exhibits pronounced regional differences (Brierley et al., 2008; Cenac et al., 2008; Sipe et al., 2008). Thus, TRPV4 is particularly abundant in DRG neurons supplying the colon, in which it is coexpressed with CGRP (Brierley et al., 2008; Cenac et al., 2008; Sipe et al., 2008). In the human colon, TRPV4 is found in fine nerve fibers associated with blood vessels in the submucosa and serosa, while the myenteric plexus, the circular and the longitudinal smooth muscles are largely negative (Brierley et al., 2008).
In the murine colon, TRPV4 occurs in brush-bordered epithelial cells, but not in mucus-secreting epithelial cells, as well as in mucosal glial cells and unidentified cells of the submucosal and muscular layers (Cenac et al., 2008; D'Aldebert et al., 2011). TRPV4 is likewise expressed by epithelial cells of the human colon and by the human colon carcinoma cell line Caco-2 (D'Aldebert et al., 2011).
A further location of TRPV4 is in the hepatobiliary tract in which it is expressed by ciliated cholangiocytes, as shown for both the rat, murine and human liver and bile system (Gradilone et al., 2007, 2010). In these cells, TRPV4 occurs specifically in the apical membrane and the cilia which are thought to subserve a mechano- and osmosensory role (Gradilone et al., 2007).
2.4.2. Functional implications of TRPV4 in the digestive system
2.4.2.1. Sensory modalities of TRPV4
The sensory modalities of TRPV4 (Table 1) include strong acidosis, hypotonicity, warmth, mechanical stimuli such as distension of the gut, 5,6-epoxyeicosatrienoic acid (an endogenously formed metabolite of arachidonic acid), and the synthetic phorbol ester 4-α-phorbol 12,13-didecanoate (Güler et al., 2002; Mizuno et al., 2003; Suzuki et al., 2003; Nilius et al., 2007). Although it has been surmised that the mechanosensory function of TRPV4 reflects a secondary transducer role, a patch-clamp study indicates that TRPV4 is a primary detector of mechanical forces (Loukin et al., 2010).
2.4.2.2. Role of TRPV4 in gastrointestinal pain and hyperalgesia
TRPV4 has turned out to play a major role in mechanical pain and hyperalgesia in the colon. TRPV4 agonists excite colonic afferent neurons in the mouse (Sipe et al., 2008) and enhance the mechanosensory responses of colonic serosal and mesenteric afferent nerve fibers, while the mechanosensitivity of these high-threshold afferent nerve fibers is substantially attenuated in TRPV4 knockout mice (Brierley et al., 2008). The TRPV4 agonist-evoked sensitization of colonic afferent nerve fibers to mechanical stimuli is associated with mechanical hyperalgesia, as the visceromotor response to colorectal distension is enhanced (Cenac et al., 2008; Sipe et al., 2008). Vice versa, TRPV4 knockout or intravertebral pretreatment of mice with TRPV4-directed small interfering RNA (siRNA) reduces the visceromotor response to colonic distension in the noxious range (Brierley et al., 2008; Cenac et al., 2008). Taken these findings together, the specific contribution to colonic high-threshold mechanosensory function makes TRPV4 currently the only nociceptor-specific TRP channel in the gut (Blackshaw et al., 2010).
TRPV4 and PAR-2 are colocalized in spinal afferent neurons (Grant et al., 2007; Sipe et al., 2008), in which these two entities interact with each other to cause mechanical hyperalgesia in the murine colon (Cenac et al., 2008; Sipe et al., 2008). Thus, the excitatory effect of TRPV4 stimulation on firing of colonic afferents is increased by a PAR-2 agonist (Sipe et al., 2008), and a subnociceptive dose of a PAR-2 agonist is able to sensitize colonic afferents to a subnociceptive dose of a TRPV4 agonist (Cenac et al., 2008). Activation of PAR-2 likewise evokes discharge of action potentials in colonic afferent nerve fibers and enhances the visceromotor response to colorectal distension. These effects of PAR-2 agonism are absent in TRPV4 knockout mice (Sipe et al., 2008) as well as after pretreatment with TRPV4 siRNA (Cenac et al., 2008).
Based on these findings it is inferred that TRPV4 plays two distinct roles in colonic pain and hyperalgesia. Under basal conditions, TRPV4 is involved in mechanical nociception, and in this capacity is likely to operate as primary mechanodetector. In addition, TRPV4 mediates the mechanical hyperalgesia evoked by PAR-2 agonism. In this instance, TRPV4 seems to act as secondary transducer of PAR-2, a conclusion that is consistent with the observation that this secondary transducer role of TRPV4 is not confined to PAR-2. Thus, 5-HT and histamine can likewise enhance the expression of TRPV4 in DRG neurons projecting to the murine colon and are able to increase the ability of TRPV4 agonism to induce mechanical hyperalgesia (Cenac et al., 2010). Importantly, the colonic hypersensitivity to colorectal distension evoked by 5-HT or histamine is attenuated by TRPV4 siRNA (Cenac et al., 2010).
In DRG neurons projecting to the murine colon, TRPV4 is not only colocalized with PAR-2 but also with PAR-4 (Augé et al., 2009). Unlike PAR-2 agonism, activation of PAR-4 counteracts mechanical pain and hyperalgesia. Thus, the excitation of DRG neurons by PAR-2 and TRPV4 agonists is reduced and the ensuing mechanical hypersensitivity to colonic distension is attenuated by a PAR-4 agonist (Augé et al., 2009).
TRPV4 is also likely to contribute to the pain associated with pancreatitis. Intraductal administration of a TRPV4 agonist to the murine pancreas causes expression of c-Fos in the spinal cord (Ceppa et al., 2010). Deletion of the TRPV4 gene inhibits the input to the spinal cord and the pain behavior associated with experimental pancreatitis due to caerulein (Ceppa et al., 2010).
2.4.2.3. Role of TRPV4 in the gastrointestinal mucosa and hepatobiliary system
Emerging evidence attributes TRPV4 a proinflammatory role in the colonic mucosa. Exposure of Caco-2 cells and the human intestinal epithelial cell line T84 to the TRPV4 agonist 4-α-phorbol 12,13-didecanoate increases the intracellular calcium concentration and causes release of chemokines (D'Aldebert et al., 2011). In keeping with these effects, intraluminal administration of the TRPV4 agonist to the murine colon evokes a transient increase in paracellular permeability and colitis (D'Aldebert et al., 2011). Furthermore, the expression of TRPV4 in epithelial cells of the colon is significantly enhanced in mice with DSS-induced colitis (D'Aldebert et al., 2011).
Hypotonicity causes an increase in the intracellular calcium in cultured murine cholangiocytes and rat intrahepatic bile duct units, a response that is mimicked by a TRPV4 agonist and impaired by TRPV4 knockdown (Gradilone et al., 2007). The hypotonicity-induced activation of cholangiocytes leads to TRPV4-dependent bicarbonate secretion, the main determinant of ductal bile formation. This mechanism operates as well in vivo, since intrabiliary infusion of a TRPV4 agonist increases luminal bicarbonate secretion and bile flow in the rat, this effect being prevented by TRPV4 blockade (Gradilone et al., 2007). In polycystic kidney rats in which mutations in the polycystic kidney and hepatic disease 1 gene cause cholangiocytes to undergo hyperproliferation and to form cysts, TRPV4 is overexpressed and mislocalized in the cholangiocytes, given that TRPV4 is present intracellularly rather than at the apical membrane and the cilia (Gradilone et al., 2010). TRPV4 agonists attenuate the growth of cultured cholangiocytes from polycystic kidney rats and retard the formation of cysts in culture, whereas the growth of cholangiocytes from normal rats is not altered (Gradilone et al., 2010).
2.4.3. Association of TRPV4 with gastrointestinal disease
TRPV4 channelopathies are responsible for a number of hereditary sensory and motor neuropathies as well as diseases with defects in bone development (Nilius & Owsianik, 2010). Thus, mutations of TRPV4 are found in congenital distal spinal muscular atrophy and scapuloperoneal spinal muscular atrophy (two hereditary motor neuropathies), hereditary motor and sensory neuropathy of type 2C (also known as Charcot–Marie–Tooth disease type 2C) (Auer-Grumbach et al., 2010; Deng et al., 2010; Landouré et al., 2010) and other rare motor neuropathies (Zimoń et al., 2010). Diseases with defective bone development such as metatropic dysplasia, brachyolmia, spondylo-epiphyseal dysplasia of Kozlowski type, spondylo-epiphyseal dysplasia of Maroteaux type (pseudo-Morquio syndrome type 2) and parastremmatic dysplasia are also associated with TRPV4 channelopathies (Camacho et al., 2010; Loukin et al., 2010; Nishimura G. et al., 2010).
Although the GI tract is not the prime victim of these diseases, gastro-esophageal reflux has been observed in hereditary sensory neuropathy of type 1B (Auer-Grumbach, 2008). Little is known, however, whether the hereditary sensory neuropathies of types 1A, 1B and 1D (Auer-Grumbach, 2008) impact on GI sensation and nociception, although the available reports do not mention any conspicuous GI disease phenotype.
TRPV4 in sensory neurons is upregulated in inflammatory bowel disease, and serosal blood vessels in tissues with active colitis are more densely innervated by TRPV4-positive nerve fibers than in biopsies from healthy controls (Brierley et al., 2008). Expression of TRPV4 in colonic epithelial cells of mice with experimental colitis is likewise upregulated, while the levels of TRPV4 mRNA in mucosal biopsies taken from patients with inflammatory bowel disease have been found unaltered (D'Aldebert et al., 2011). However, the colonic mucosa of inflammatory bowel disease patients is infiltrated by TRPV4-positive CD45-positive leukocytes which are absent from the mucosa of healthy controls (D'Aldebert et al., 2011).
In patients with polycystic liver diseases (autosomal-recessive polycystic kidney disease and autosomal-dominant polycystic kidney disease) TRPV4 is overexpressed in cholangiocytes, a finding that is reproduced in polycystic kidney rats, a model for autosomal-recessive polycystic kidney disease (Gradilone et al., 2010).
2.4.4. Therapeutic potential of TRPV4 ligands in the digestive system
Taken together, the experimental findings related to TRPV4 function in the digestive system attribute this TRP channel a pathophysiological role in inflammation, pain, hyperalgesia and polycystic liver disease. This inference if supported by the overexpression of TRPV4 in experimental colitis, inflammatory bowel disease and polycystic liver disease. Since TRPV4 appears to be a common mediator of mechanical hyperalgesia in the colon, it presents itself as a novel therapeutic target in patients with abdominal pain. In addition to their antinociceptive and antihyperalgesic potential, TRPV4 blockers may be useful as antiinflammatory drugs. To the contrary, TRPV4 agonists seem to be beneficial in polycystic liver disease in which they can inhibit cholangiocyte proliferation and cyst formation (Gradilone et al., 2010). Indeed, TRPV4 agonists and blockers are already on the horizon (Vincent et al., 2009) and await to be assessed for their therapeutic efficacy and safety.
2.5. TRPV5 and TRPV6 channels
2.5.1. Occurrence of TRPV5 and TRPV6 in the alimentary canal
TRPV5 and TRPV6 are expressed in the mucosa of the human and rodent small and large intestine (Clapham et al., 2005; Nijenhuis et al., 2005; Schoeber et al., 2007; Benn et al., 2008; Suzuki et al., 2008; Fukushima et al., 2009; Peleg et al., 2010). While TRPV5 occurs primarily in the kidneys, TRPV6 is predominantly expressed in intestinal epithelial cells and has also been localized to human colon cancer (Caco-2) cells (Schoeber et al., 2007; Fukushima et al., 2009; Bartik et al., 2010). In addition, TRPV5 mRNA occurs in rat nodose ganglion neurons expressing cholecystokinin (CCK) CCK1 receptors (Zhao & Simasko, 2010), which are likely to project to the GI tract.
2.5.2. Functional implications of TRPV5 and TRPV6 in the digestive system
TRPV5 and TRPV6 are the most Ca2+-selective members of the TRP ion channel family, play an important role in intestinal Ca2+ absorption (Clapham et al., 2005; Nijenhuis et al., 2005; Schoeber et al., 2007; Benn et al., 2008; Suzuki et al., 2008), and undergo calcium-induced inactivation via a phospholipase C-mediated pathway (Thyagarajan et al., 2009). The functional roles of TRPV5 and TRPV6 are interconnected with each other, given that TRPV5 knockout mice upregulate their intestinal TRPV6 expression to compensate for the negative Ca2+ balance caused by the loss of TRPV5-mediated Ca2+ reabsorption in the kidney (Suzuki et al., 2008). In contrast, TRPV6 knockout mice do not display any compensatory mechanism, which results in secondary hyperparathyroidism (Benn et al., 2008; Suzuki et al., 2008).
The expression and function of both TRPV5 and TRPV6 are subject to regulation by dietary conditions and multiple calciotropic hormones (Nijenhuis et al., 2005; Schoeber et al., 2007; Suzuki et al., 2008). Calcium deficiency in the diet is an important factor that increases the expression of TRPV6 in the duodenal mucosa of mice (Ko et al., 2009). Short chain fatty acids, the fermentation products of fructooligosaccharides, likewise increase expression of TRPV6 and calcium absorption in the rat colorectal epithelium as well as in Caco-2 cells (Fukushima et al., 2009). Curcumin, a polyphenol present in turmeric, is a ligand of the nuclear vitamin D receptor, activation of which enhances the expression of TRPV6 mRNA in Caco-2 cells (Bartik et al., 2010).
Parathyroid hormone and 1,25-dihydroxyvitamin D3 are particularly relevant to the expression of TRPV5 and TRPV6 in the intestinal mucosa (Nijenhuis et al., 2005; Schoeber et al., 2007). In chicken intestinal epithelial cells, 1,25-dihydroxyvitamin D3 and parathyroid hormone increase calcium uptake by stimulating a protein kinase A-mediated pathway to release β-glucuronidase, which in turn activates TRPV6 (Khanal et al., 2008). Consistent with this secondary transducer role of TRPV6 is the finding that the 1,25-dihydroxyvitamin D3-induced calcium uptake is abolished by TRPV6 siRNA (Khanal et al., 2008). In normal human duodenal mucosa obtained at endoscopy, TRPV6 expression is likewise increased following exposure to 1,25-dihydroxyvitamin D3 (Balesaria et al., 2009). Although there is a close functional interconnection between vitamin D and intestinal TRPV6, these factors can also operate independently of each other. Thus, the 1,25-dihydroxyvitamin D3-induced intestinal calcium transport in the murine duodenum does not entirely depend on TRPV6, because intestinal calcium uptake also occurs in TRPV6 knockout mice (Benn et al., 2008). In addition, intestinal calcium absorption and duodenal TRPV6 expression are upregulated in pregnant and lactating mice even in the absence of the nuclear vitamin D receptor (Fudge & Kovacs, 2010). Other hormones that impact on intestinal calcium absorption include prolactin which induces TRPV6 mRNA in the rat duodenal epithelium, synergizes with 1,25-dihydroxyvitamin D3 in enhancing TRPV6 expression and stimulates duodenal calcium transport (Ajibade et al., 2010).
Calcium homeostasis is disturbed in patients with Crohn's disease (Huybers et al., 2008). Similarly, heterozygous mice carrying a deletion in the adenine- and uridine-rich elements of the tumor necrosis factor gene, considered to be a model for Crohn's disease, display an increase in bone resorption along with a decrease in the duodenal expression of TRPV6 mRNA (Huybers et al., 2008). TRPV6 in the intestinal epithelium also appears to have a bearing on colon cancer development. Thus, TRPV6 expression is significantly enhanced in the Citrobacter rodentium-induced transmissible murine colonic hyperplasia model (Peleg et al., 2010). Whereas in the normal colon TRPV6 is restricted to the apical membrane of absorptive enterocytes, in the hyperplasia model TRPV6 is also distributed to the proliferating zone of the colonic crypts, in which it occurs in the basolateral membrane and perinuclear area of many epithelial cells (Peleg et al., 2010). When the animals are fed with a calcium-rich diet, the overexpression of TRPV6 in the hyperplasia model is reversed.
2.5.3. Association of TRPV6 with gastrointestinal disease
Increased levels of colonic TRPV6 are associated with early-stage colon cancer. Thus, TRPV6 has been found to be overexpressed in 66% of stage I tumors and in 17% of stage II tumors but is barely detectable in stages III and IV tumors (Peleg et al., 2010). These results suggest that aberrant function of TRPV6 is associated with early colon carcinogenesis but not with frank malignancy (Peleg et al., 2010). This inference is supported by experiments with the human colon carcinoma cell line Caco-2, which constitutively express high levels of TRPV6. Treatment of these cells with TRPV6-specific siRNA significantly reduces TRPV6 mRNA levels by about 50%, which is associated with a 40% reduction of cell proliferation and a more than twofold increase in apoptosis (Peleg et al., 2010). Together with experiments in mice with colon crypt hyperplasia (Peleg et al., 2010), these findings indicate that TRPV6 promotes the proliferation of colonic epithelial cells and may be a pathogenetic factor in the early stages of colon cancer development.
2.5.4. Therapeutic potential of TRPV6 ligands in the digestive system
Pharmacological interference with TRPV6 in the digestive system is likely to interfere with calcium absorption and bone mineralization as well as with epithelial cell hyperplasia and early stages of malignancies. Thus, blockade of TRPV6 can be considered as a measure to prevent and inhibit the development of colon cancer, but this effect needs to be balanced against any negative impact on calcium homeostasis.
2.6. TRPA1 channels
2.6.1. Occurrence of TRPA1 in the alimentary canal
In the GI tract, TRPA1 occurs in 3 distinct cellular systems: extrinsic primary afferent neurons, intrinsic enteric neurons and endocrine cells of the mucosa.
Primary sensory neurons constitute the most significant source of TRPA1 in the body, given that TRPA1 is abundantly expressed by trigeminal ganglion, nodose ganglion and DRG neurons (Story et al., 2003; Zhang et al., 2004; Kobayashi et al., 2005; Anand et al., 2008; Yang et al., 2008; Brierley et al., 2009; Kondo et al., 2009, 2010; Yu et al., 2009; Cattaruzza et al., 2010; Ceppa et al., 2010; Hondoh et al., 2010; Zhao et al., 2010). In rodent and human DRG neurons, especially in those projecting to the gut, TRPA1 is preferentially expressed by small and medium diameter cell bodies in which it is colocalized with TRPV1 and CGRP (Bautista et al., 2005; Kobayashi et al., 2005; Anand et al., 2008; Brierley et al., 2009; Kondo et al., 2009, 2010; Cattaruzza et al., 2010; Hondoh et al., 2010; Zhao et al., 2010). Similarly, TRPA1 on nodose ganglion neurons in the guinea-pig is primarily associated with small to medium diameter somata and co-expressed with PAR-2 (Yu et al., 2009). In the periphery, TRPA1 has been found in the stomach, small intestine, colon and pancreas of the rat and mouse, the major source being peripheral nerve fibers of DRG and nodose ganglion neurons (Zhang et al., 2004; Penuelas et al., 2007; Brierley et al., 2009; Kondo et al., 2009, 2010; Cattaruzza et al., 2010; Ceppa et al., 2010; Dong Y. et al., 2010). The distribution of TRPA1 in the rodent gut is similar to that in the canine gut, given that TRPA1 is strongly expressed in the stomach, pancreas, small intestine and colon of the dog (Doihara et al., 2009a).
The other sources of TRPA1 in the GI tract are neurons of the human and murine enteric nervous system (Penuelas et al., 2007; Anand et al., 2008) as well as 5-HT-releasing enterochromaffin cells and CCK-releasing endocrine cells of the human, rat and murine GI mucosa (Purhonen et al., 2008; Nozawa et al., 2009). The murine neuroendocrine cell line STC-1, the rat endocrine cell line RIN14B and the human pancreatic endocrine cell line QGP-1, which contain enterochromaffin cell markers, likewise express TRPA1 (Purhonen et al., 2008; Doihara et al., 2009c; Nozawa et al., 2009).
2.6.2. Functional implications of TRPA1 in the digestive system
2.6.2.1. Sensory modalities of TRPA1
TRPA1 was originally identified as a cold-activated channel (Story et al., 2003), a role that later provoked some controversy as TRPM8 is attributed a greater role in this respect than TRPA1 (Kwan et al., 2006; Bandell et al., 2007; Karashima et al., 2009; Knowlton et al., 2010). Most remarkable, however, is that TRPA1 turned out to be a multimodal sensor not only for various spices but also for an extensive list of endogenous and exogenous irritant chemicals including a number of drugs in clinical use (Table 1). Many of these pungent and irritating chemicals are reactive electrophiles which need to be recognized and avoided because of their potential to damage tissues through modification of nucleic acids, proteins and other biomolecules (Kang et al., 2010).
Its sensory modalities (Table 1) place TRPA1 specifically in a position (1) to taste spicy compounds present in mustard, horseradish, wasabi, galangal, black pepper, garlic, onion, cinnamon, ginger, oregano, wintergreen and clove (Table 1), (2) to detect toxic environmental stimuli (Table 1) such as ozone, tear gas, nicotine, formaldehyde, acrolein, iodoacetamide, methyl p-hydroxybenzoate (an antibacterial added to food, pharmaceutical and cosmetic products), irritating drugs (e.g., acetaminophen metabolites, fenamate nonsteroidal antiinflammatory drugs, isoflurane, propofol, dihydropyridines, and clotrimazole), and industrial chemicals (e.g., methyl isothiocyanate, and styrene), and (3) to survey the alimentary canal for potentially deleterious conditions arising from the presence of alkalosis (Fujita et al., 2008), H2S, nitric oxide, and oxidative insult products such as 4-hydroxy-2-nonenal, H2O2, and acetaldehyde (Table 1).
Although there is emerging evidence that TRPA1 contributes to mechanosensation in the gut (Brierley et al., 2009), it appears that TRPA1 does not operate as primary mechanosensor. The slowly adapting currents which indentation of the soma membrane causes primarily in isolectin B4-negative DRG neuron cultures are suppressed by the TRPA1 blocker HC-030031 and are absent in TRPA1 knockout mice (Vilceanu & Stucky, 2010). Analysis of TRPA1-transfected HEK293 cells, however, shows that TRPA1 alone is not sufficient to confer mechanical sensitivity (Vilceanu & Stucky, 2010). It would appear, therefore, that TRPA1 functions as a secondary transducer of primary mechanosensors that await to be identified.
2.6.2.2. Sensory function of TRPA1 in the digestive system
In the oral cavity, TRPA1 mediates the taste of many spices (Table 1) and appears to contribute to the stinging and pungent sensations caused by carbon dioxide and carbonated beverages (Wang et al., 2010). Once it enters sensory nerve fibers, carbon dioxide causes intracellular acidification, which in turn activates TRPA1 (Wang et al., 2010).
Many studies have shown that activation of TRPA1 in the esophagus and GI tract causes excitation of primary afferent neurons in the vagus, splanchnic and pelvic nerves. TRPA1 agonists such as allyl isothiocyanate (AITC) excite vagal afferent C-fibers, but not Aδ-fibers, in the guinea-pig esophagus (Yu & Ouyang, 2009) and spinal afferent neurons in the murine colon (Cattaruzza et al., 2010). Accordingly, intracolonic administration of TRPA1 agonists to rodents induces c-Fos expression in the spinal cord (Cattaruzza et al., 2010; Mitrovic et al., 2010) and elicits nociception as deduced from the occurrence of a visceromotor response (Yang et al., 2008) and referred pain (Laird et al., 2001). Intraductal administration of a TRPA1 agonist to the murine pancreas likewise causes expression of c-Fos in the spinal cord (Ceppa et al., 2010). These nociceptive reactions to TRPA1 stimulation in the digestive system are inhibited by the TRPA1 blocker HC-030031 (Mitrovic et al., 2010), by intrathecal pretreatment with a TRPA1 antisense oligodeoxynucleotide (Yang et al., 2008) or by TRPA1 gene knockout (Brierley et al., 2009; Cattaruzza et al., 2010; Ceppa et al., 2010).
TRPA1 is required for normal mechano- and chemosensory function in distinct subsets of vagal, splanchnic and pelvic afferent neurons supplying the murine GI tract (Brierley et al., 2009). Specifically, spike discharges in response to punctate mechanical stimulation in splanchnic afferents with receptive fields in the mesentery and serosa and in pelvic afferents with receptive fields in the mucosa, muscularis and serosa are attenuated in TRPA1 knockout mice, while the responses of pelvic afferents to muscle stretch remain unabated (Brierley et al., 2009). Despite these findings there is at present uncertainty as to whether TRPA1 contributes to normal mechanonociception in the colon, given that the visceromotor response to noxious colorectal distension has been reported to be reduced (Brierley et al., 2009) or to remain unchanged (Cattaruzza et al., 2010) in TRPA1 knockout mice. In contrast, there is pharmacological evidence that in the rat stomach TRPA1 plays a role in mechanical nociception, given that the pseudoaffective pain reaction (contraction of the acromiotrapezius muscle) to gastric distension is attenuated by intrathecal administration of a TRPA1 antisense oligodeoxynucleotide as well as by intrathecal or intraperitoneal injection of HC-030031 (Kondo et al., 2009, 2010).
TRPA1 is colocalized with TRPV1 in many afferent neurons, and there is evidence that the two TRP channels interact with each other in the sensory function of peripheral nerve fibers (Salas et al., 2009; Patil et al., 2010). For instance, the mechanical desensitization which capsaicin induces in splanchnic, but not pelvic, colonic afferents is absent in TRPA1 knockout mice, while the excitatory effect of capsaicin on these nerve fibers remains unaltered by TRPA1 gene deletion (Brierley et al., 2009). The interaction between TRPV1 and TRPA1 is thought to be related to phosphatidyl 4,5-bisphosphate which is a factor relevant to the function of both TRP channels (Brierley et al., 2009).
2.6.2.3. Role of TRPA1 in visceral hypersensitivity
In addition to stimulating afferent neurons, TRPA1 agonists sensitize colonic sensory neurons to mechanical stimulation (Brierley et al., 2009) and enhance the visceromotor response to colorectal distension (Cattaruzza et al., 2010), these effects being prevented by TRPA1 gene knockout or HC-030031. The ability of AITC to sensitize mechanosensitive afferent neurons may be related to its effect to enhance the level of TRPA1 in the colon (Kimball et al., 2007). This argument is supported by a study in which AITC-induced irritation of the colon in newborn mouse pups results in a long-lasting hypersensitivity to colorectal distension as examined in the grown-up animals (Christianson et al., 2010). The enhanced visceromotor response to colorectal distension seen in these experiments is thought to be driven by an early increase in colonic neurotrophin expression, which leads to a permanent increase in the percentage of TRPA1-positive DRG neurons, whereas the number of TRPV1-positive DRG cells remains unaltered (Christianson et al., 2010).
There is substantial evidence that TRPA1 contributes to the GI mechanical hypersensitivity that is associated with GI inflammation and stress. For instance, colitis induced by trinitrobenzene sulfonic acid (TNBS) in rats causes upregulation of TRPA1 in DRG neurons innervating the rat colon and enhances the visceromotor response to colorectal distension, this effect being reduced by intrathecal pretreatment with a TRPA1 antisense oligodeoxynucleotide (Yang et al., 2008). Likewise, the effect of TNBS-induced colitis to enhance spinal neuron activation and the visceromotor pain reaction to colorectal distension is absent in TRPA1 knockout mice (Cattaruzza et al., 2010). This observation is consistent with the ability of TNBS-induced colitis to increase the ability of TRPA1 agonists to sensitize mechanosensitive afferents in the splanchnic and pelvic nerves (Brierley et al., 2009). Apart from GI inflammation, stress is also known to give rise to visceral hypersensitivity. The abdominal withdrawal reflex to colorectal distension, which is enhanced following chronic exposure to water avoidance stress for 10 days, is associated with a significant upregulation of TRPA1 in DRG neurons supplying the colon (Yu et al., 2010), but it has not yet been demonstrated whether TRPA1 is causally involved in stress-evoked visceral hyperalgesia.
Under conditions of inflammation, TRPA1 appears to take over a significant role as secondary transducer of several proinflammatory mediators such as bradykinin and endogenous PAR-2 agonists. Pharmacological and gene knockout experiments indicate that TRPA1 mediates the bradykinin-induced mechanical sensitization of splanchnic afferents innervating the colonic serosa of the mouse (Brierley et al., 2009) and the bradykinin-evoked mechanical hypersensitivity of vagal afferent neurons in the guinea-pig esophagus (Yu & Ouyang, 2009). Importantly, the activation of vagal and splanchnic afferents by bradykinin itself is independent of TRPA1 (Brierley et al., 2009; Yu & Ouyang, 2009). Mast cell activation and activation of PAR-2 are further stimuli that cause mechanical hypersensitivity of vagal afferent neurons in the guinea-pig esophagus via a TRPA1-dependent mechanism (Yu et al., 2009). Similarly, the ability of a PAR-2 agonist to enhance the visceromotor response to colorectal distension is abrogated by TRPA1 gene deletion (Cattaruzza et al., 2010), while the effect of a PAR-2 agonist to stimulate colonic afferents in the splanchnic nerves per se is unaltered in TRPA1 knockout mice (Brierley et al., 2009).
The role of TRPA1 is not limited to mechanical hypersensitivity, as TRPA1 also participates in inflammation-evoked chemical hypersensitivity in the digestive system. Induction of mild colitis with DSS causes colonic hypersensitivity to AITC as revealed by increased expression of c-Fos in the murine spinal cord (Mitrovic et al., 2010). This hyperresponsiveness to colonic TRPA1 stimulation is prevented by HC-030031 as well as by morphine, which suggests that the spinal c-Fos response to colonic TRPA1 stimulation reflects colonic chemonociception (Mitrovic et al., 2010). Similarly, TNBS-induced colitis in the rat amplifies the visceromotor response to intracolonic AITC in a TRPA1 antisense oligodeoxynucleotide-sensitive manner (Yang et al., 2008). TNBS-evoked colitis is associated with an upregulation of TRPA1 in DRG neurons supplying the colon (Yang et al., 2008), an effect that may involve neurotrophic factors formed under conditions of irritation and inflammation (Anand et al., 2008; Christianson et al., 2010).
2.6.2.4. Role of TRPA1 in inflammation within the digestive system
AITC has long been used to elicit and examine neurogenic inflammation (Holzer, 1988), and there is evidence that activation of TRPA1 on visceral sensory neurons gives rise to both vasodilatation (Pozsgai et al., 2010) and inflammation (Kimball et al., 2007; Ceppa et al., 2010). Introduction of excess AITC into the murine colon induces colitis which is associated with an upregulation of TRPA1 and other factors of the intestinal nervous and immune systems (Kimball et al., 2007). Similarly, intraductal administration of an AITC agonist to the murine pancreas causes inflammation and activation of spinal neurons, these effects being markedly attenuated in TRPA1 knockout mice (Ceppa et al., 2010). The pancreatitis evoked by caerulein is likewise ameliorated by TRPA1 deletion, as is the activation of spinal neurons and the pain behavior that accompany pancreatitis (Ceppa et al., 2010). Neurogenic inflammation does not necessarily have a deleterious effect on the tissue, given that AITC has a protective effect against experimentally induced gastric lesions in the rat, an effect that appears to involve endogenous prostaglandins (Matsuda et al., 2007). The inhibitory effect which AITC and icilin have on the restitution of wounded monolayers of the rat gastric epithelial cell line RGM1 does not seem to be mediated by TRPA1 (Hayashi et al., 2008).
2.6.2.5. Role of TRPA1 in the gastrointestinal endocrine system
Stimulation of TRPA1 on the murine neuroendocrine cell line STC-1 causes cell activation and release of CCK (Purhonen et al., 2008), and activation of TRPA1 on rat enterochromaffin cells, on the rat endocrine cell line RIN14B and on the human pancreatic endocrine cell line QGP-1 leads to release of 5-HT (Doihara et al., 2009c; Nozawa et al., 2009). These effects of TRPA1 stimulation may be of functional relevance if the broad sensory spectrum of both TRPA1 and GI endocrine cells and the functional implications of CCK and 5-HT in digestion and GI signaling to the brain are considered. However, these implications of TRPA1 await to be explored and analyzed both in vitro and in vivo.
2.6.2.6. Role of TRPA1 in gastrointestinal motor function
Stimulation of TRPA1 in the gut has distinct effects on GI motor activity, the effects of TRPA1 stimulation being region- and species-dependent. Although intracolonic AITC (2.5%) does not alter compliance of the murine colon in vivo (Cattaruzza et al., 2010), isolated segments of the murine colon, but not small intestine, contract in response to AITC (30–100 μM) and allicin, the response being depressed by tetrodotoxin throughout the colon, whereas atropine inhibits the AITC-induced contraction in the distal colon only (Penuelas et al., 2007). In the guinea-pig ileum, AITC is able to evoke contraction via a mechanism that involves 5-HT and activation of 5-HT3 receptors, which suggests that the AITC-induced release of 5-HT from enterochromaffin cells has an impact on the control of intestinal motility (Nozawa et al., 2009).
While the effect of TRPA1 stimulation in the intestine is to facilitate motor activity, gastric emptying in the rat in vivo is delayed by the TRPA1 agonists AITC and cinnamaldehyde through an action that also involves 5-HT and activation of 5-HT3 receptors (Doihara et al., 2009b). Administration of TRPA1 agonists to the dog in vivo stimulates motor activity in the gastric antrum and jejunum, and elicits giant migrating contractions in the colon (Doihara et al., 2009a). Activation of TRPA1 also has a prokinetic effect in the murine colon, in which AITC is able to antagonize atonic constipation induced by clonidine as well as spastic constipation induced by loperamide (Kojima et al., 2009). However, the physiological implications of TRPA1 in the nerve circuits and smooth muscle mechanisms governing GI motility remain largely unexplored. Indirect evidence suggests that TRPA1 participates in the cold-induced contraction of the rat colon (Dong Y. et al., 2010), but the physiological relevance of this finding is not yet possible to judge.
2.6.3. Association of TRPA1 with gastrointestinal disease
A point mutation in the S4 transmembrane segment of the TRPA1 gene appears to be responsible for an autosomal-dominant familial episodic pain syndrome that is triggered by fasting and physical stress (Kremeyer et al., 2010). Functional analysis shows that this mutation in the TRPA1 channel is associated with a 5-fold increase in inward current following activation and with enhanced secondary hyperalgesia caused by AITC (Kremeyer et al., 2010). It awaits to be explored whether function-relevant mutations in the TRPA1 gene are relevant to GI disease.
2.6.4. Therapeutic potential of TRPA1 ligands in the digestive system
The properties of TRPA1 as a multimodal sensor for a huge variety of irritant chemicals (Table 1) indicate that TRPA1 blockers could be used to manage chemical irritation and pain. In addition, the emerging pathophysiological implications of TRPA1 suggest that both TRPA1 agonists and blockers hold therapeutic potential in particular disease entities. There is good reason to surmise that TRPA1 blockers could be of particular benefit in inflammation-induced hyperalgesia in the GI tract. TRPA1 blockers may also be of use in pancreatitis (Ceppa et al., 2010) and inflammatory bowel disease. The discovery that activation of TRPA1 by some drugs contributes to their adverse effect profile (Table 1), which may include the alimentary canal, provides a rational basis for developing drugs that lack this unwanted property.
TRPA1 blockers such as HC-030031 have already been characterized, and many other compounds are in the pipeline (Baraldi et al., 2010). The interaction between TRPA1 and TRPV1 in the excitation and/or sensitization of nociceptive nerve fibers (Salas et al., 2009; Patil et al., 2010) hints at the possibility that combined TRPA1 plus TRPV1 blockers may have superior efficacy compared with blockers that target TRPA1 or TRPV1 alone. It awaits to be explored whether interference with TRPA1 disturbs temperature homeostasis, as it has been found with TRPV1 blockers. This limitation cannot be neglected, given that intragastric administration of the TRPA1 agonists AITC and cinnamaldehyde increases thermogenesis and reduces heat diffusion in mice (Masamoto et al., 2009).
Apart from TRPA1 blockers, TRPA1 agonists also may have therapeutic potential. The large variety of TRPA1 agonists available to date (Table 1) can be roughly categorized in two classes: (1) agonists that stimulate TRPA1 via a transient and reversible interaction with the channel, and (2) agonists with an electrophilic carbon or sulfur that bind covalently to cysteine residues of the cytoplasmatic N-terminus of TRPA1 (Baraldi et al., 2010). A specific application of TRPA1 agonists is suggested by their prokinetic effect in the murine colon, which—if translated to humans—could be utilized in the management of both atonic and spastic motor stasis of the gut (Kojima et al., 2009). TRPA1 agonists that will be useful in therapy certainly require optimization in their pharmacological profile, since the utility of the currently available TRPA1 agonists is limited by their irritative effects and high reactivity.
2.7. TRPM4 and TRPM5 channels
2.7.1. Expression of TRPM4 and TRPM5 in the alimentary canal
Expression of TRPM4 and, particularly, TRPM5, is limited to taste, epithelial and endocrine cells. TRPM4 transcripts have been identified in pancreatic α- and β-cell lines of rodent origin (Cheng et al., 2007; Marigo et al., 2009), and TRPM5 has been localized to pancreatic islets of the mouse in which it is expressed by the insulin-secreting β cells (Colsoul et al., 2010). TRPM5 abounds on the receptor cells of the lingual taste buds as well as on other chemosensory cells, notably on epithelial taste cells of the gut (Zhang et al., 2003; Bezençon et al., 2007; Kaske et al., 2007; Kokrashvili et al., 2009). Both TRPM4 and TRPM5 mRNA are found in the human digestive system (Fonfria et al., 2006). TRPM5 occurs in the stomach, small intestine and colon of mice and humans, the channel occurring in solitary brush (also termed tufted or caveolated) cells at the surface epithelium of the small and large intestine and in endocrine cells of the duodenal glands (Fonfria et al., 2006; Bezençon et al., 2007, 2008; Kaske et al., 2007; Kokrashvili et al., 2009; Young et al., 2009). Endocrine cells isolated from the rat stomach also stain positively for TRPM5 (Nakamura et al., 2010).
2.7.2. Functional implications of TRPM4 and TRPM5 in the digestive system
Functional evidence suggests that TRPM4 contributes to the membrane depolarization and increase in the intracellular calcium concentration that are relevant to glucose-, arginine vasopressin- and glibenclamide-evoked secretion of insulin from pancreatic β-cells and perhaps glucagon from pancreatic α-cells (Cheng et al., 2007; Marigo et al., 2009). TRPM5 is likewise thought to play a major role in the glucose-dependent release of insulin from pancreatic β-cells (Brixel et al., 2010; Colsoul et al., 2010). Specifically, the effect of glucose to release insulin is significantly reduced in TRPM5 knockout mice, which impairs glucose tolerance (Brixel et al., 2010; Colsoul et al., 2010).
The Ca2+-gated TRPM5 channel on the receptor cells of the taste buds in the tongue has been established to contribute to signal transduction in the sweet, umami and bitter tastes (Zhang et al., 2003; Damak et al., 2006). Bitter, sweet, and umami tastants bind to GPCRs which initiate a second-messenger signaling cascade involving activation of phospholipase Cβ2 and hydrolysis of phosphatidylinositol 4,5-bisphosphate to diacylglycerol and inositol trisphosphate. The subsequent increase in the intracellular Ca2+ concentration gates TRPM5 and causes depolarization of the taste cells and chemical transmission to gustatory afferent neurons (Zhang Z. et al., 2007). Real life gustatory experiences are often evoked by taste mixtures and determined by multiple interactions between different taste stimuli (Talavera et al., 2008). One such interaction is the suppression of sweet taste transduction by bitter tastants such as quinine. Quinine and its stereoisomer quinidine have been found to block TRPM5 currents and to inhibit the nerve impulses of sucrose-stimulated gustatory nerves in mice (Talavera et al., 2008). Since this quinidine-evoked inhibition of sweet taste signaling is absent in TRPM5 knockout mice, it would appear that TRPM5 plays an important role in bitter–sweet taste interactions (Talavera et al., 2008).
Apart from their occurrence in lingual taste cells, TRPM5 is widely expressed by other chemosensory cells, notably by the tufted epithelial cells of the GI tract (Kaske et al., 2007; Rozengurt & Sternini, 2007). Given that the TRPM5-positive cells of the gut express most genes encoding proteins implicated in sweet, bitter, and umami taste signaling (Bezençon et al., 2008), it would appear that TRPM5 exert taste functions throughout the alimentary canal and contribute to the surveillance of the chemical composition in its lumen. This functional implication is corroborated by the finding that the expression of TRPM5 in the upper small intestine is inversely correlated with the blood glucose concentration in type 2 diabetes patients (Young et al., 2009).
Another task of TRPM5-positive taste cells in the digestive system may be to play local effector roles because they express markers of neuronal and inflammatory cells (Bezençon et al., 2008) and can release biologically active messengers in response to stimulation. Solitary chemosensory cells in the upper small intestine of the murine coexpress TRPM5, β-endorphin, methionine enkephalin and uroguanylin (Kokrashvili et al., 2009). Stimulation of these cells with hyperosmolar solutions or glucose causes release of β-endorphin into the intestinal lumen but not blood serum, an effect that is blunted in TRPM5 knockout mice (Kokrashvili et al., 2009). In contrast, the intestinal release of β-endorphin evoked by acidic solutions or lipid exposure appears to be independent of TRPM5 (Kokrashvili et al., 2009).
2.7.3. Therapeutic potential of TRPM5 ligands in the digestive system
Being a transducer of the sweet taste, TRPM5 has been suggested to have therapeutic potential in the management of obesity and related metabolic dysfunctions, given that taste signaling is a critical determinant of ingestive behavior (Sprous & Palmer, 2010). How efficacious such an approach may be is difficult to judge, however, because deletion of TRPM5 does not completely abolish the preference for sweets and energy-rich food. The reason for this finding is that there are TRPM5-independent transduction mechanisms for glucose tasting (Ohkuri et al., 2009) and sweet and calorie-rich nutrients can directly influence brain reward circuits that control food intake independently of functional taste transduction (de Araujo et al., 2008; Ren et al., 2010).
As TRPM5 appears to be involved in glucose-dependent insulin secretion, TRPM5 dysfunction has been suggested to be a factor in the etiology of some forms of type 2 diabetes (Brixel et al., 2010). If so, normalization of TRPM5 function may be envisaged as a target in diabetes management.
2.8. TRPM6 and TRPM7 channels
2.8.1. Occurrence of TRPM6 and TRPM7 in the alimentary canal
TRPM6 and TRPM7 occur in GI epithelial cells and, as is true for TRPM7, in ICCs. In the murine gut, TRPM6 and TRPM7 abound in the brush-border membrane of the colon (Schlingmann et al., 2002; Voets et al., 2004; Fonfria et al., 2006; Groenestege et al., 2006; Rondón et al., 2008a, 2008b; Woudenberg-Vrenken et al., 2010). Furthermore, TRPM7 mRNA and protein are expressed by AGS cells, the most common human gastric adenocarcinoma cell line (Kim et al., 2008). Apart from their association with epithelial cells, TRPM7 is present on ICCs of the myenteric plexus and circular muscle throughout the GI tract of mice and humans (Kim et al., 2005, 2006, 2009).
2.8.2. Functional implications of TRPM6 and TRPM7 in the digestive system
Several data suggest that the formation of functional TRPM6 channels in epithelial cell membranes requires co-expression with TRPM7. There is indeed evidence for the formation of heteromeric ion channels consisting of both TRPM6 and TRPM7 subunits, but there is still controversy as to whether TRPM6 can also function on its own and TRPM7 is required only for trafficking of TRPM6 to the plasma membrane (Schlingmann et al., 2007; Quamme, 2008). TRPM6 and TRPM7 possess an atypical α-kinase domain and are involved in Mg2+ homeostasis, given that genetic and functional data indicate that both channel kinases play a critical role in the cellular Mg2+ absorption within the GI mucosa (especially in the colon) and in the reabsorption of Mg2+ in the kidney (Schlingmann et al., 2007; Quamme, 2008; Woudenberg-Vrenken et al., 2010). Homozygous deletion of TRPM6 in mice is lethal, while heterozygous deletion of TRPM6 results in mild hypomagnesemia, a deficit that cannot be compensated for by a magnesium-rich diet (Woudenberg-Vrenken et al., 2010).
TRPM6 and TRPM7 channel function and colonic Mg2+ absorption are in fact controlled by many factors. For instance, the hypomagnesemia associated with dietary Mg2+ restriction in mice causes upregulation of TRPM6 expression both in the intestine and kidney (Rondón et al., 2008a). The expression of TRPM6, but not TRPM7, mRNA in the colon is likewise enhanced by a Mg2+-rich diet (Groenestege et al., 2006). Feeding of mice with long-chain inulin for 2 weeks, which increases Mg2+ absorption and enhances bone stores of Mg2+, results in a rise of TRPM6 and TRPM7 expression in the hindgut, while TRPM7 in the kidney is down-regulated (Rondón et al., 2008b).
Mg2+ is critical for the growth and survival of certain cancer cells, notably the human gastric adenocarcinoma cell line AGS (Kim et al., 2008). Blockade of TRPM7 channels with La3+ or suppression of TRPM7 expression by siRNA inhibits the growth and survival of AGS cells (Kim et al., 2008).
In addition to its epithelial function, TRPM7 plays a role in controlling intestinal motor activity. Electrophysiological, molecular biological, and immunohistochemical evidence indicates that TRPM7 participates in the nonselective cation current that underlies the pacemaker activity of ICCs in the GI tract (Kim et al., 2006). Thus, the electrophysiological and pharmacological properties of the nonselective cation current in ICCs of the mouse are identical to those of TRPM7, and the pacemaker activity of ICCs is inhibited by TRPM7-specific siRNA (Kim et al., 2005). TRPM7 expressed on ICCs is also likely to contribute to pacemaker activity in the human gut, since slow waves in the isolated muscle of the human small and large intestine are attenuated by blockade of TRPM7 channels with La3+ (Kim et al., 2009).
2.8.3. Association of TRPM6 with gastrointestinal disease
Non-functional mutations of TRPM6 are responsible for a primary disorder termed hypomagnesemia with secondary hypocalcemia (Schlingmann et al., 2002; Voets et al., 2004). The expression of TRPM6 mRNA in the intestinal epithelium is reduced in patients suffering from cholera, a change that is thought to counteract the secretory response to the infection (Flach et al., 2007).
2.8.4. Therapeutic potential of TRPM7 blockers in the digestive system
The ability of TRPM7 channel blockade to inhibit the growth and survival of AGS cells suggests TRPM7 to be a potential target for the treatment of gastric cancer (Kim et al., 2008).
2.9. TRPM8 channels
2.9.1. Occurrence of TRPM8 in the alimentary canal
TRPM8 is distributed to several cell types in the digestive system. Being menthol-sensitive, the TRPM8 subunit is expressed in the papillae of the tongue as well as by a distinct population of primary afferent neurons originating in the nodose-petrosal ganglion and DRG (McKemy et al., 2002; Peier et al., 2002; Zhang et al., 2004; Kobayashi et al., 2005; Hondoh et al., 2010; Zhao et al., 2010). Unlike that of TRPA1, the colocalization of TRPM8 with TRPV1 in sensory neurons is limited (Bautista et al., 2005; Kobayashi et al., 2005; Kondo et al., 2009; Hondoh et al., 2010; Zhao et al., 2010). Within the GI tract, TRPM8 has been localized to the muscle of the rat stomach and colon (Mustafa & Oriowo, 2005) as well as to the liver (Fonfria et al., 2006). The expression of TRPM8 in the murine stomach and small intestine remains controversial, given that both positive (Zhang et al., 2004) and negative (Penuelas et al., 2007) results have been reported.
2.9.2. Functional implications of TRPM8 in the digestive system
TRPM8 is activated by temperatures in the cool to cold range and makes an important contribution to cold-induced pain (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010). Apart from low temperatures, TRPM8 is stimulated by various chemical entities including menthol, icilin, geraniol, L-carvone, isopulegol and linalool (Table 1). These sensory modalities of TRPM8 are likely to explain the refreshing taste of menthol. Also keeping with this property is the finding that intragastric administration of the TRPM8 agonist menthol induces thermogenesis in mice (Masamoto et al., 2009).
Expressed by extrinsic primary afferent neurons and intrinsic systems within the GI tract, TRPM8 may play a chemosensory role in the alimentary canal. This contention is supported by the observation that the mixed TRPA1/TRPM8 agonist icilin is able to stimulate murine nodose ganglion neurons in culture (Zhang et al., 2004). However, systematic studies of the chemosensory role of TRPM8-bearing primary afferent neurons in the gut have not yet been reported, and the precise functional implications of TRPM8 in the digestive system await to be explored. In the murine colon, menthol induces a long lasting relaxation of smooth muscle, which remains unaffected by tetrodotoxin (Penuelas et al., 2007). It is questionable if this motor effect of menthol is due to activation of TRPM8 because the presence of TRPM8 in the murine gut has not unequivocally been established (Zhang et al., 2004; Penuelas et al., 2007). Whether the cooling-induced contraction of the rat gastric fundus (Mustafa & Oriowo, 2005) and guinea-pig ileum (Holzer & Lembeck, 1979) involves TRPM8 has not conclusively been investigated.
2.10. TRPP2 channels
2.10.1. Occurrence of TRPP2 in the alimentary canal
The polycystin TRP channel TRPP2 (also known as polycystic kidney disease-like ion channel, PKD2L1) and the related PKD1 polycystin L3 (PKD1L3) are expressed by a select class (type III) of taste chemoreceptor cells in the murine tongue, which are distinct from those mediating sweet, umami and bitter tastes (Huang et al., 2006; Ishimaru et al., 2006; LopezJimenez et al., 2006; Kataoka et al., 2008; Chandrashekar et al., 2009; Kawaguchi et al., 2010). While TRPP2 has been reported to occur in the circumvallate, foliate and fungiform papillae of the tongue and in the palate, PKD1L3 has been localized to the circumvallate and foliate papillae only (Huang et al., 2006; Ishimaru et al., 2006). PKD1, the gene that is mutated in ~85% of autosomal dominant polycystic kidney disease patients, has been described to occur in many tissues including the intestine (Geng et al., 1997).
2.10.2. Functional implications of TRPP2 in the digestive system
Although several members of the TRPV, TRPA and TRPC channel subfamilies are sensitive to alterations in extracellular pH (Holzer, 2009), TRPP2 (PKD2L1) is the TRP channel involved in sour taste transduction (Chandrashekar et al., 2006; Ishimaru & Matsunami, 2009). In order to form functional channels, TRPP2 needs to associate as heteromer with related proteins of the PKD1 polycystin family such as PKD1L3 (Ishimaru et al., 2006; Kawaguchi et al., 2010). The PKD1 polycystins are not included in the TRP channel family because they are large proteins with a very long N-terminal extracellular domain and 11 transmembrane domains that include a 6-transmembrane TRP-like channel domain at the C terminus (Clapham et al., 2005).
Both genetic and functional studies corroborate that TRPP2 plays an important role as sour taste receptor (Huang et al., 2006; Ishimaru et al., 2006; LopezJimenez et al., 2006; Kataoka et al., 2008; Kawaguchi et al., 2010). In the taste buds of the murine tongue, TRPP2 is accumulated at the taste pore region where taste chemicals are detected. Coexpression of TRPP2 and PKD1L3 is necessary for the positioning of functional sour taste receptors at the cell surface, which is the case in the circumvallate and foliate papillae of the murine tongue (Huang et al., 2006; Ishimaru et al., 2006; Chandrashekar et al., 2009; Kawaguchi et al., 2010). In native circumvallate taste cells expressing TRPP2 the pH threshold for acid-induced increases in the intracellular calcium concentration is around 5.0 (Kawaguchi et al., 2010). Targeted ablation of TRPP2-expressing taste receptor cells results in a specific and total loss of sour taste transduction, whereas responses to sweet, umami, bitter and salty tastants remain unchanged (Huang et al., 2006). The relevance of PKD1, which occurs in the intestine (Geng et al., 1997), to GI function remains unexplored.
The TRPP2-expressing sour taste cells also appear to contribute to the taste of carbonation. While the principal CO2 taste sensor is carbonic anhydrase-4 (Chandrashekar et al., 2009), it remains to be determined whether TRPP2 plays a role in the downstream signaling of the carbonation taste.
2.11. TRPC channels
2.11.1. Occurrence of TRPC in the alimentary canal
Distinct members of the classical or canonical TRP channels (TRPC) are widely distributed in the GI tract, the cellular sources being vagal afferent neurons, enteric neurons, smooth muscle cells, ICCs and epithelial cells. TRPC1, TRPC3, TRPC5 and TRPC6 have been localized to nodose ganglion neurons in which TRPC1 and TRPC6 are, at least in part, coexpressed with TRPV1 and TRPA1. The evidence for this colocalization rests on the finding that systemic pretreatment of rats with a neurotoxic dose of capsaicin causes a loss of TRPV1, TRPA1, TRPC1 and TRPC6 from vagal afferent neurons (Zhao et al., 2010). In the mouse DRG, mRNA for all 7 types of TRPC subunits (TRPC1–TRPC7) has been found, with TRPC1, TRPC3 and TRPC6 being the most abundant (Elg et al., 2007).
All 7 types of TRPC subunits have likewise been identified in the murine stomach (Lee et al., 2003; So & Kim, 2003). TRPC6 is expressed in human esophageal and gastric epithelial cells (Cai et al., 2009; Shi et al., 2009), while TRPC1 and TRPC5 have been localized to intestinal epithelial cells (Rao et al., 2006). In addition, TRPC4, TRPC6 and TRPC7 as well as splice variants of TRPC4 (TRPC4-α and TRPV-β) and TRPC7 are present in GI and vascular smooth muscle of the murine and canine alimentary canal, TRPC4 being the most abundant subunit (Walker et al., 2001, 2002; Torihashi et al., 2002; Lee et al., 2005; Kim et al., 2006; Tsvilovskyy et al., 2009). TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6 mRNA have likewise been localized to circular muscle cells of the human esophageal body and lower esophageal sphincter, the levels of TRPC3 and TRPC4 mRNA being higher in the sphincter than in the body (Wang et al., 2003).
The guinea-pig enteric nervous system expresses TRPC1, TRPC3, TRPC4 and TRPC6, which are differentially distributed to the myenteric and submucosal plexuses (Liu et al., 2008). While TRPC1 abounds in myenteric neurons with cholinergic and nitrergic phenotypes as well as in submucosal cholinergic and noncholinergic secretomotor neurons, TRPC3 is restricted to submucosal neurons containing neuropeptide Y (Liu et al., 2008). TRPC4 and TRPC6 are likewise preferentially expressed by noncholinergic secretomotor neurons of the submucosal plexus (Liu et al., 2008).
2.11.2. Functional implications of TRPC in the digestive system
The abundant distribution of TRPC channels to vagal afferent neurons, enteric neurons, smooth muscle cells, ICCs and epithelial cells in the gut points to multiple functional implications of this class of TRP channels. However, their role in the neural control of digestive functions has not yet been examined. There is, however, increasing evidence that TRPC channels are involved in signal transduction within the epithelium and smooth muscle of the GI tract. In addition, distinct TRPC channels play a physiological role in GI epithelial cell homeostasis and in the development of malignancies.
The phenotype of TRPC1 knockout mice is characterized by a pronounced impairment of neurotransmitter-stimulated salivary gland fluid secretion, which suggests that TRPV1 is relevant to the secretory process in salivary gland acinar cells (Liu et al., 2007). In human colonic epithelial cells TRPC1 is involved in the signal transduction of the extracellular calcium-sensing receptor which is relevant to both intracellular calcium oscillation and inhibition of proliferation (Rey et al., 2010). Wounding of the mucosa causes translocation of stromal interaction molecule-1 to the plasma membrane, which in turn activates TRPC1-mediated calcium influx and promotes intestinal epithelial restitution (Rao et al., 2010). Induced TRPC1 expression facilitates, while inhibition of its expression by TRPC1-directed siRNA inhibits epithelial cell migration after wounding (Rao et al., 2006, 2010). In addition, induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis via calcium influx-mediated inhibition of nuclear factor-κB activity (Marasa et al., 2006, 2008).
TRPC1 and TRPC6 play a role in pancreatic and GI cancer development. In the pancreatic cancer cell line BxPc3, TRPC1 is a transducer of the action of tumor growth factor-β to stimulate cell motility, a process that is of relevance to tumor cell invasion (Dong H. et al., 2010). TRPC6, which can be activated both by receptor tyrosine kinases and GPCRs, appears to have a role in human esophageal and gastric cancer development (Cai et al., 2009; Shi et al., 2009). Treatment of the human gastric cancer cell lines AGS and MKN45 with the TRPC channel blocker SKF-96,365 arrests the cell cycle in the G2/M phase and suppresses the formation of gastric tumors in nude mice (Cai et al., 2009). Inhibition of TRPC6 likewise arrests the cell cycle of human esophageal squamous carcinoma cells in the G2 phase and inhibits tumor formation in nude mice injected with esophageal squamous carcinoma cells (Shi et al., 2009).
In the exocrine pancreas, TRPC3-mediated calcium influx is attributed an adverse role in the acute inflammation induced by supramaximal stimulation or exposure to toxic bile acids and palmitoleic acid ethyl ester (Kim M.S. et al., 2009). Consequently, deletion of TRPC3 has been found to attenuate experimentally evoked acute pancreatitis (Kim M.S. et al., 2009).
TRPC channels have been proposed to participate in intestinal pacemaker activity of ICCs which through electrical coupling drive slow wave activity in intestinal smooth muscle. The pacemaker current that initiates each slow wave derives from a Ca2+-inhibited, voltage-independent, nonselective cation channel in the ICCs. This channel exhibits properties similar to that reported for TRPC channels, notably TRPC4 (Walker et al., 2002). However, slow waves in intestinal smooth muscle remain unchanged in TRPC4 knockout mice (Lee et al., 2005). Thus, there is no conclusive evidence that TRPC channels, notably TRPC4, play a physiological role in the pacemaker activity of ICCs.
In contrast, distinct TRPC channels have been shown to operate as downstream transducers (effectors) of stimulated GPCRs such as muscarinic acetylcholine receptors. This mechanism has been suggested to apply to TRPC4, TRPC5 and TRPC6 which are activated by cholinergic stimulation of ICCs and smooth muscle in the murine stomach and intestine (Lee et al., 2003, 2005; Kim et al., 2006, 2007; Tsvilovskyy et al., 2009). Based on electrophysiological and pharmacological similarities, both TRPC4 (Lee et al., 2005) and TRPC5 (Lee et al., 2003) have originally been proposed to mediate the nonselective cation current evoked by carbachol in the murine stomach. A role of TRPC4 has been firmly established by the finding that the nonselective cation current evoked by muscarinic receptor stimulation is abolished by genetic deletion of TRPC4 (Lee et al., 2005; Kim et al., 2006).
Further studies using both TRPC4 and TRPC6 knockout mice indicate that TRPC4 underlies more than 80% of the muscarinic receptor-induced cation current in intestinal smooth muscle, while the residual current is mediated by TRPC6 (Tsvilovskyy et al., 2009). Following TRPC4 knockout, the carbachol-induced membrane depolarization and the atropine-sensitive contraction elicited by acetylcholine release from excitatory motor neurons are inhibited, these effects being aggravated by additional deletion of TRPC6. In addition, intestinal transit is slowed down in mice lacking TRPC4 and TRPC6 (Tsvilovskyy et al., 2009). From these studies it is evident that TRPC4 and TRPC6 couple muscarinic acetylcholine receptors to depolarization and contraction of intestinal smooth muscle. This is true for both M2 and M3 muscarinic receptors which use different second messenger mechanisms owing to their coupling to Gi/o and Gq/11 signaling pathways, respectively (Tsvilovskyy et al., 2009).
2.11.3. Association of TRPC6 with gastrointestinal disease
An implication of TRPC6 in human GI motor control is exemplified by the observation that a single nucleotide polymorphism in the promoter region of the TRPC6 gene and a missense variant in exon 4 of the TRPC6 gene may contribute to infantile hypertrophic pyloric stenosis (Everett et al., in press). TRPC6 appears to have a role in cancer development, given that there is a marked upregulation of TRPC6 expression in esophageal squamous cell carcinoma (Shi et al., 2009) and in epithelial cells of human gastric cancers (Cai et al., 2009).
2.11.4. Therapeutic potential of TRPC blockers in the digestive system
Cholinergic contraction of smooth muscle is a key determinant of GI motility. Recognition that TRPC4 and, to a minor degree, TRPC6 are transducers of the depolarizing action of M2 and M3 muscarinic acetylcholine receptors in GI smooth muscle raises the possibility to attenuate cholinergic motor effects while sparing other cholinergically controlled tissue functions. This opportunity would be particularly useful if pathological hypermotility in the GI tract is shown to be due to exaggerated TRPC4 and TRPC6 transduction (Ambudkar, 2009).
Blockade of TRPC1 and TRPC6 also holds considerable potential in GI oncology, given that TRPC1 may promote pancreatic cancer cell invasion (Dong H. et al., 2010) and TRPC6 seems to play a significant role in human esophageal and gastric cancer development (Cai et al., 2009; Shi et al., 2009).
3. Conclusions
Distinct TRP channels have emerged as pleiotropic signal transducers in the digestive tract. They play physiological roles in taste, chemo- and mechanosensation, thermoregulation, pain and hyperalgesia, epithelial cell function and homeostasis, control of motility by neurons, ICCs and muscle cells, vascular function, and cancer development (Fig. 2). While the implications of some TRP channels, notably TRPA1, TRPC4, TRPM5, TRPM6, TRPM7, TRPV1, TRPV4, and TRPV6, have been elucidated to a considerable degree, the relevance of other TRP channels to digestive function awaits to be explored. The polymodal chemo- and mechanosensory function of TRPA1, TRPM5, TRPV1 and TRPV4 is of particular pathophysiological relevance, but there are several other aspects of GI function that are under the critical control of TRP channels, such as Ca2+ and Mg2+ absorption, transduction of muscarinic acetylcholine receptor activation to smooth muscle contraction, and cancer development (Fig. 2).
TRP channels in the digestive system can operate (1) as molecular sensors (detectors or primary transducers) of chemical and physical stimuli, (2) as downstream or secondary transducers (or effectors) of cell activation induced by GPCRs, receptor tyrosine kinases or ion channels, and (3) as ion transport channels (Fig. 1). While some TRP channels operate only in one of these capacities, other TRP channels can act both as primary and secondary transducers. This dual role is seen with TRPA1, TRPV1 and TRPV4 which, on the one hand, are detectors of thermal, chemical and mechanical stimuli and, on the other hand, mediate cell responses to other stimuli, notably distension and proinflammatory mediators (Blackshaw et al., 2010). A secondary transducer role is also typical of TRPM5 in its taste function and of TRPC4 in its role to transduce acetylcholine-evoked contraction.
Pathophysiologically, TRP channels participate in many disturbances of GI function (Fig. 3). Changes in TRP channel expression and function are associated with GI inflammation, hyperalgesia and other disease identities, some of which are due to specific TRP channelopathies (Nilius et al., 2007). These implications have raised enormous interest in the therapeutic exploitation of TRP channels as new drug targets in GI disease. As a result, extensive drug development programs have been initiated, especially with regard to TRPV1, TRPA1 and TRPV4 channel blockers (Gharat & Szallasi, 2008; Gunthorpe & Szallasi, 2008; Kym et al., 2009; Vincent et al., 2009; Wong & Gavva, 2009; Baraldi et al., 2010; Voight & Kort, 2010). Fig. 4 summarizes the potential indications of TRP channel agonists and antagonists in the management of GI diseases and disorders. While the results of many preclinical tests involving TRP channel agonism, antagonism, knockdown or knockout nourish these expectations, clinical proof-of-concept data are not yet available, except for TRPV1 desensitization strategies. In the absence of conclusive information it is not possible to predict which TRP channels are the most promising drug targets. The translation from the preclinical validation to the clinical application of TRP channel-directed drugs is liable to meet many obstacles (Hicks, 2006; Storr, 2007; Holzer, 2008a, 2008b; Wong & Gavva, 2009), given that TRP channels are widely distributed throughout the body and play many important physiological roles within and outside the GI system (Wu et al., 2010). The challenge, therefore, is to design TRP channel-directed drugs with a pharmacological profile that limits their action to the alimentary canal and specifically addresses the pathological functions of TRP channels while the physiological implications are spared (Holzer, 2008b).
Acknowledgments
Work performed in the author's laboratory was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Science Funds (FWF grant L25-B05).
References
- Ahern G.P., Wang X., Miyares R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- Aihara E., Hayashi M., Sasaki Y., Kobata A., Takeuchi K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: comparison with mucosal acidification. J Pharmacol Exp Ther. 2005;315:423–432. doi: 10.1124/jpet.105.087619. [DOI] [PubMed] [Google Scholar]
- Ajibade D.V., Dhawan P., Fechner A.J., Meyer M.B., Pike J.W., Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D3 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151:2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A., Yiangou Y., Facer P., Brydon W.G., Walters J.R., Anand P. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- Akbar A., Yiangou Y., Facer P., Walters J.R., Anand P., Ghosh S. Increased capsaicin receptor TRPV1 expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y., Ghayouri S., Takeuchi T., Mizumori M., Guth P.H., Engel E. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006;131:142–152. doi: 10.1053/j.gastro.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Akiba Y., Takeuchi T., Mizumori M., Guth P.H., Engel E., Kaunitz J.D. TRPV-1 knockout paradoxically protects mouse gastric mucosa from acid/ethanol-induced injury by upregulating compensatory protective mechanisms. Gastroenterology. 2006;130(Suppl. 2):A-106. [Google Scholar]
- Akiba Y., Mizumori M., Kuo M., Ham M., Guth P.H., Engel E. CO2 chemosensing in rat oesophagus. Gut. 2008;57:1654–1664. doi: 10.1136/gut.2007.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar I.S. Unraveling smooth muscle contraction: the TRP link. Gastroenterology. 2009;137:1211–1214. doi: 10.1053/j.gastro.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Otto W.R., Facer P., Zebda N., Selmer I., Gunthorpe M.J. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S., Coutts A.A. Cellular distribution of vanilloid VR1 receptor immunoreactivity in the guinea-pig myenteric plexus. Eur J Pharmacol. 2003;458:61–71. doi: 10.1016/s0014-2999(02)02653-5. [DOI] [PubMed] [Google Scholar]
- Andersson D.A., Gentry C., Moss S., Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D.A., Gentry C., Moss S., Bevan S. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+ Proc Natl Acad Sci USA. 2009;106:8374–8379. doi: 10.1073/pnas.0812675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrè E., Campi B., Trevisani M., Ferreira J., Malheiros A., Yunes R.A. Pharmacological characterisation of the plant sesquiterpenes polygodial and drimanial as vanilloid receptor agonists. Biochem Pharmacol. 2006;71:1248–1254. doi: 10.1016/j.bcp.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Andrè E., Campi B., Materazzi S., Trevisani M., Amadesi S., Massi D. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.L., Bhandari P. Resinferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferret. Neuropharmacology. 1993;32:799–806. doi: 10.1016/0028-3908(93)90189-a. [DOI] [PubMed] [Google Scholar]
- Andrews P.L., Okada F., Woods A.J., Hagiwara H., Kakaimoto S., Toyoda M. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol. 2000;130:1247–1254. doi: 10.1038/sj.bjp.0703428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L., Schipper K.P., Dimcevski G., Sumikura H., Krarup A.L., Giamberardino M.A. Viscero-somatic reflexes in referred pain areas evoked by capsaicin stimulation of the human gut. Eur J Pain. 2008;12:544–551. doi: 10.1016/j.ejpain.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Auer J., Reeh P.W., Fischer M.J. Acid-induced CGRP release from the stomach does not depend on TRPV1 or ASIC3. Neurogastroenterol Motil. 2010;22:680–687. doi: 10.1111/j.1365-2982.2009.01459.x. [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M. Hereditary sensory neuropathy type I. Orphanet J Rare Dis. 2008;3:7. doi: 10.1186/1750-1172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach M., Olschewski A., Papić L., Kremer H., McEntagart M.E., Uhrig S. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé C., Balz-Hara D., Steinhoff M., Vergnolle N., Cenac N. Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01310.x. 1189-e107. [DOI] [PubMed] [Google Scholar]
- Balesaria S., Sangha S., Walters J.R. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol. 2009;297:G1193–G1197. doi: 10.1152/ajpgi.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bandell M., Macpherson L.J., Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee B., Medda B.K., Lazarova Z., Bansal N., Shaker R., Sengupta J.N. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Bang S., Kim K.Y., Yoo S., Kim Y.G., Hwang S.W. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci. 2007;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- Bang S., Kim K.Y., Yoo S., Lee S.H., Hwang S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Baraldi P.G., Preti D., Materazzi S., Geppetti P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem. 2010;53:5085–5107. doi: 10.1021/jm100062h. [DOI] [PubMed] [Google Scholar]
- Barquist E., Bonaz B., Martinez V., Rivier J., Zinner M.J., Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- Barthó L., Benkó R., Patacchini R., Pethö G., Holzer-Petsche U., Holzer P. Effects of capsaicin on visceral smooth muscle: a valuable tool for sensory neurotransmitter identification. Eur J Pharmacol. 2004;500:143–157. doi: 10.1016/j.ejphar.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Barthó L., Benkó R., Holzer-Petsche U., Holzer P., Undi S., Wolf M. Role of extrinsic afferent neurons in gastrointestinal motility. Eur Rev Med Pharmacol Sci. 2008;12(Suppl. 1):21–31. [PubMed] [Google Scholar]
- Bartik L., Whitfield G.K., Kaczmarska M., Lowmiller C.L., Moffet E.W., Furmick J.K., Hernandez Z. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21:1153–1161. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D.M., Movahed P., Hinman A., Axelsson H.E., Sterner O., Högestätt E.D. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista D.M., Siemens J., Glazer J.M., Tsuruda P.R., Basbaum A.I., Stucky C.L. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Behrendt H.J., Germann T., Gillen C., Hatt H., Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkó R., Lazar Z., Undi S., Illenyi L., Antal A., Horvath O.P. Inhibition of nitric oxide synthesis blocks the inhibitory response to capsaicin in intestinal circular muscle preparations from different species. Life Sci. 2005;76:2773–2782. doi: 10.1016/j.lfs.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Benn B.S., Ajibade D., Porta A., Dhawan P., Hediger M., Peng J.B. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac B.F., Sivula M., von Hehn C.A., Escalera J., Cohn L., Jordt S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac B.F., Sivula M., von Hehn C.A., Caceres A.I., Escalera J., Jordt S.E. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezençon C., Le Coutre J., Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Bezençon C., Fürholz A., Raymond F., Mansourian R., Métairon S., Le Coutre J. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- Bhat Y.M., Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K., Davis B.M. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw L.A., Page A.J., Partosoedarso E.R. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407–416. doi: 10.1016/s0306-4522(99)00547-3. [DOI] [PubMed] [Google Scholar]
- Blackshaw L.A., Brierley S.M., Hughes P.A. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- Boesmans W., Owsianik G., Tack J., Voets T., Berghe P.V. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011;162:18–37. doi: 10.1111/j.1476-5381.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen C.J., Priel A., Zhou S., King D., Siemens J., Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölcskei K., Helyes Z., Szabó A., Sándor K., Elekes K., Németh J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Bortolotti M., Coccia G., Grossi G., Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075–1082. doi: 10.1046/j.1365-2036.2002.01280.x. [DOI] [PubMed] [Google Scholar]
- Boudaka A., Wörl J., Shiina T., Neuhuber W.L., Kobayashi H., Shimizu Y. Involvement of TRPV1-dependent and -independent components in the regulation of vagally induced contractions in the mouse esophagus. Eur J Pharmacol. 2007;556:157–165. doi: 10.1016/j.ejphar.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Brierley S.M., Carter R., Jones W., Xu L., Robinson D.R., Hicks G.A. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol (London) 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley S.M., Page A.J., Hughes P.A., Adam B., Liebregts T., Cooper N.J. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley S.M., Hughes P.A., Page A.J., Kwan K.Y., Martin C.M., O'Donnell T.A. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixel L.R., Monteilh-Zoller M.K., Ingenbrandt C.S., Fleig A., Penner R., Enklaar T. TRPM5 regulates glucose-stimulated insulin secretion. Pflügers Arch. 2010;460:69–76. doi: 10.1007/s00424-010-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R., Ding X., Zhou K., Shi Y., Ge R., Ren G. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009;125:2281–2287. doi: 10.1002/ijc.24551. [DOI] [PubMed] [Google Scholar]
- Camacho N., Krakow D., Johnykutty S., Katzman P.J., Pepkowitz S., Vriens J. Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am J Med Genet A. 2010;152A:1169–1177. doi: 10.1002/ajmg.a.33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J. On the thermoregulatory perils of TRPV1 antagonism. Pain. 2008;136:3–4. doi: 10.1016/j.pain.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F., Spreadbury I., Miranda-Morales M., Grady E.F., Vanner S.J., Bunnett N.W. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N., Altier C., Chapman K., Liedtke W., Zamponi G., Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Cenac N., Altier C., Motta J.P., d'Aldebert E., Galeano S., Zamponi G.W. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- Ceppa E., Cattaruzza F., Lyo V., Amadesi S., Pelayo J.C., Poole D.P. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.L., Facer P., Davis J.B., Smith G.D., Egerton J., Bountra C. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Hoon M.A., Ryba N.J., Zuker C.S. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N.J. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Liu T.T., Yi C.H., Orr W.C. Effects of capsaicin-containing red pepper sauce suspension on esophageal secondary peristalsis in humans. Neurogastroenterol Motil. 2010;22:1177–1182. doi: 10.1111/j.1365-2982.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- Cheng F.H., Andrews P.L., Moreaux B., Ngan M.P., Rudd J.A., Sam T.S. Evaluation of the anti-emetic potential of anti-migraine drugs to prevent resiniferatoxin-induced emesis in Suncus murinus (house musk shrew) Eur J Pharmacol. 2005;508:231–238. doi: 10.1016/j.ejphar.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Cheng H., Beck A., Launay P., Gross S.A., Stokes A.J., Kinet J.P. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., de la Monte S., Ma J., Hong J., Tong M., Cao W. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol. 2009;297:G135–G143. doi: 10.1152/ajpgi.90386.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Sun J.M., Shahi P.K., Zuo D.C., Kim H.I., Jun J.Y. Capsaicin inhibits the spontaneous pacemaker activity in interstitial cells of Cajal from the small intestine of mouse. J Neurogastroenterol Motil. 2010;16:265–273. doi: 10.5056/jnm.2010.16.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J.A., Bielefeldt K., Malin S.A., Davis B.M. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J.A., McIlwrath S.L., Koerber H.R., Davis B.M. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christianson J.A., Traub R.J., Davis B.M. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Christoph T., Grünweller A., Mika J., Schäfer M.K., Wade E.J., Weihe E. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chu C.J., Huang S.M., De Petrocellis L., Bisogno T., Ewing S.A., Miller J.D. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Chu K.M., Ngan M.P., Wai M.K., Yeung C.K., Andrews P.L., Percie du Sert N. Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret. Neuropharmacology. 2010;58:383–391. doi: 10.1016/j.neuropharm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Clapham D.E., Julius D., Montell C., Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure–function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Colburn R.W., Lubin M.L., Stone D.J., Wang Y., Lawrence D., D'Andrea M.R. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Colsoul B., Schraenen A., Lemaire K., Quintens R., Van Lommel L., Segal A. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci USA. 2010;107:5208–5213. doi: 10.1073/pnas.0913107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer B.A., McIntyre P. Painful toxins acting at TRPV1. Toxicon. 2008;51:163–173. doi: 10.1016/j.toxicon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cuypers E., Yanagihara A., Rainier J.D., Tytgat J. TRPV1 as a key determinant in ciguatera and neurotoxic shellfish poisoning. Biochem Biophys Res Commun. 2007;361:214–217. doi: 10.1016/j.bbrc.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aldebert E., Cenac N., Rousset P., Martin L., Rolland C., Chapman K. Transient receptor potential vanilloid-4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Damak S., Rong M., Yasumatsu K., Kokrashvili Z., Pérez C.A., Shigemura N. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Davis J.B., Gray J., Gunthorpe M.J., Hatcher J.P., Davey P.T., Overend P. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E., Oliveira-Maia A.J., Sotnikova T.D., Gainetdinov R.R., Caron M.G., Nicolelis M.A. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- De Man J.G., Boeckx S., Anguille S., De Winter B.Y., De Schepper H.U., Herman A.G. Functional study on TRPV1-mediated signalling in the mouse small intestine: involvement of tachykinin receptors. Neurogastroenterol Motil. 2008;20:546–556. doi: 10.1111/j.1365-2982.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- De Schepper H.U., De Man J.G., Ruyssers N.E., Deiteren A., Van Nassauw L., Timmermans J.P. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am J Physiol. 2008;294:G245–G253. doi: 10.1152/ajpgi.00351.2007. [DOI] [PubMed] [Google Scholar]
- Delafoy L., Raymond F., Doherty A.M., Eschalier A., Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105:489–497. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- Deng H.X., Klein C.J., Yan J., Shi Y., Wu Y., Fecto F. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet. 2010;42:165–169. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J.A., Lyall V. Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol. 2006;291:G1005–G1010. doi: 10.1152/ajpgi.00235.2006. [DOI] [PubMed] [Google Scholar]
- Dhaka A., Viswanath V., Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dhaka A., Murray A.N., Mathur J., Earley T.J., Petrus M.J., Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A., Uzzell V., Dubin A.E., Mathur J., Petrus M., Bandell M. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q.W., Zhang Y., Wang Y., Wang Y.N., Zhang L., Ding C. Functional vanilloid receptor-1 in human submandibular glands. J Dent Res. 2010;89:711–716. doi: 10.1177/0022034510366841. [DOI] [PubMed] [Google Scholar]
- Doihara H., Nozawa K., Kawabata-Shoda E., Kojima R., Yokoyama T., Ito H. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur J Pharmacol. 2009;617:124–129. doi: 10.1016/j.ejphar.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Doihara H., Nozawa K., Kawabata-Shoda E., Kojima R., Yokoyama T., Ito H. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn-Schmiedebergs Arch Pharmacol. 2009;380:353–357. doi: 10.1007/s00210-009-0435-7. [DOI] [PubMed] [Google Scholar]
- Doihara H., Nozawa K., Kojima R., Kawabata-Shoda E., Yokoyama T., Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem. 2009;331:239–245. doi: 10.1007/s11010-009-0165-7. [DOI] [PubMed] [Google Scholar]
- Dong Y., Shim K.N., Li J.R., Estrema C., Orneles T., Nguyen F. Molecular mechanisms underlying Ca2+-mediated motility of human pancreatic duct cells. Am J Physiol. 2010;299:C1493–C1503. doi: 10.1152/ajpcell.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Shi H.L., Shi J.R., Wu D.Z. Transient receptor potential A1 is involved in cold-induced contraction in the isolated rat colon smooth muscle. Sheng Li Xue Bao. 2010;62:349–356. [PubMed] [Google Scholar]
- Drewes A.M., Schipper K.P., Dimcevski G., Petersen P., Gregersen H., Funch-Jensen P. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain. 2003;104:333–341. doi: 10.1016/s0304-3959(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N., Kavelaars A., Elsenbruch S., Schedlowski M., Holtmann G., Heijnen C.J. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am J Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- Eilers H., Cattaruzza F., Nassini R., Materazzi S., Andrè E., Chu C. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology. 2010;112:1452–1463. doi: 10.1097/ALN.0b013e3181d94e00. [DOI] [PubMed] [Google Scholar]
- Elg S., Marmigere F., Mattsson J.P., Ernfors P. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J Comp Neurol. 2007;503:35–46. doi: 10.1002/cne.21351. [DOI] [PubMed] [Google Scholar]
- Ericson A., Nur E.M., Petersson F., Kechagias S. The effects of capsaicin on gastrin secretion in isolated human antral glands: before and after ingestion of red chilli. Dig Dis Sci. 2009;54:491–498. doi: 10.1007/s10620-008-0400-1. [DOI] [PubMed] [Google Scholar]
- Everaerts W., Sepúlveda M.R., Gevaert T., Roskams T., Nilius B., De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn-Schmiedebergs Arch Pharmacol. 2009;379:421–425. doi: 10.1007/s00210-008-0391-7. [DOI] [PubMed] [Google Scholar]
- Everaerts W., Gees M., Alpizar Y.A., Farre R., Leten C., Apetrei A. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Everett K.V., Chioza B.A., Georgoula C., Reece A., Gardiner R.M., Chung E.M. Infantile hypertrophic pyloric stenosis: evaluation of three positional candidate genes, TRPC1, TRPC5 and TRPC6, by association analysis and re-sequencing. Hum Genet. 2009;126:819–831. doi: 10.1007/s00439-009-0735-5. [DOI] [PubMed] [Google Scholar]
- Facer P., Knowles C.H., Tam P.K., Ford A.P., Dyer N., Baecker P.A. Novel capsaicin (VR1) and purinergic (P2X3) receptors in Hirschsprung's intestine. J Pediatr Surg. 2001;36:1679–1684. doi: 10.1053/jpsu.2001.27959. [DOI] [PubMed] [Google Scholar]
- Fajardo O., Meseguer V., Belmonte C., Viana F. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin) 2008;2:429–438. doi: 10.4161/chan.2.6.7126. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini M.S., Taddei A., Bizzoco E., Lazzeri M., Vannucchi M.G., Bechi P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem Cell Biol. 2005;124:61–68. doi: 10.1007/s00418-005-0025-9. [DOI] [PubMed] [Google Scholar]
- Fischer M.J., Leffler A., Niedermirtl F., Kistner K., Eberhardt M., Reeh P.W. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285:34781–34792. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach C.F., Qadri F., Bhuiyan T.R., Alam N.H., Jennische E., Holmgren J. Differential expression of intestinal membrane transporters in cholera patients. FEBS Lett. 2007;581:3183–3188. doi: 10.1016/j.febslet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Fonfria E., Murdock P.R., Cusdin F.S., Benham C.D., Kelsell R.E., McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- Fudge N.J., Kovacs C.S. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886–895. doi: 10.1210/en.2009-1010. [DOI] [PubMed] [Google Scholar]
- Führer M., Hammer J. Effect of repeated, long term capsaicin ingestion on intestinal chemo- and mechanosensation in healthy volunteers. Neurogastroenterol Motil. 2009;21:521–527. doi: 10.1111/j.1365-2982.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- Fujino K., de la Fuente S.G., Takami Y., Takahashi T., Mantyh C.R. Attenuation of acid induced oesophagitis in VR-1 deficient mice. Gut. 2006;55:34–40. doi: 10.1136/gut.2005.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita F., Moriyama T., Higashi T., Shima A., Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita F., Uchida K., Moriyama T., Shima A., Shibasaki K., Inada H. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A., Aizaki Y., Sakuma K. Short-chain fatty acids induce intestinal transient receptor potential vanilloid type 6 expression in rats and Caco-2 cells. J Nutr. 2009;139:20–25. doi: 10.3945/jn.108.096230. [DOI] [PubMed] [Google Scholar]
- Fukushima O., Nishimura S., Matsunami M., Aoki Y., Nishikawa H., Ishikura H. Phosphorylation of ERK in the spinal dorsal horn following pancreatic pronociceptive stimuli with proteinase-activated receptor-2 agonists and hydrogen sulfide in rats: evidence for involvement of distinct mechanisms. J Neurosci Res. 2010;88:3198–3205. doi: 10.1002/jnr.22480. [DOI] [PubMed] [Google Scholar]
- Gad M., Pedersen A.E., Kristensen N.N., de Felipe Fernandez C., Claesson M.H. Blockage of the neurokinin 1 receptor and capsaicin-induced ablation of the enteric afferent nerves protect SCID mice against T-cell-induced chronic colitis. Inflamm Bowel Dis. 2009;15:1174–1182. doi: 10.1002/ibd.20902. [DOI] [PubMed] [Google Scholar]
- Garami A., Shimansky Y.P., Pakai E., Oliveira D.L., Gavva N.R., Romanovsky A.A. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci. 2010;30:1435–1440. doi: 10.1523/JNEUROSCI.5150-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva N.R. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gavva N.R., Tamir R., Klionsky L., Norman M.H., Louis J.C., Wild K.D. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol. 2005;68:1524–1533. doi: 10.1124/mol.105.015727. [DOI] [PubMed] [Google Scholar]
- Gavva N.R., Bannon A.W., Surapaneni S., Hovland D.N., Lehto S.G., Gore A. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva N.R., Treanor J.J., Garami A., Fang L., Surapaneni S., Akrami A. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gazzieri D., Trevisani M., Springer J., Harrison S., Cottrell G.S., Andrè E. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med. 2007;43:581–589. doi: 10.1016/j.freeradbiomed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Geber C., Mang C.F., Kilbinger H. Facilitation and inhibition by capsaicin of cholinergic neurotransmission in the guinea-pig small intestine. Naunyn-Schmiedebergs Arch Pharmacol. 2006;372:277–283. doi: 10.1007/s00210-005-0021-6. [DOI] [PubMed] [Google Scholar]
- Geng L., Segal Y., Pavlova A., Barros E.J., Löhning C., Lu W. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol. 1997;272:F451–F459. doi: 10.1152/ajprenal.1997.272.4.F451. [DOI] [PubMed] [Google Scholar]
- Geppetti P., Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharat L.A., Szallasi A. Advances in the design and therapeutic use of capsaicin receptor TRPV1 agonists and antagonists. Expert Opin Ther Pat. 2008;18:159–209. [Google Scholar]
- Gijsen H.J., Berthelot D., Zaja M., Brône B., Geuens I., Mercken M. Analogues of morphanthridine and the tear gas dibenz[b, f][1,4]oxazepine (CR) as extremely potent activators of the human transient receptor potential ankyrin 1 (TRPA1) channel. J Med Chem. 2010;53:7011–7020. doi: 10.1021/jm100477n. [DOI] [PubMed] [Google Scholar]
- Gonlachanvit S. Are rice and spicy diet good for functional gastrointestinal disorders? J Neurogastroenterol Motil. 2010;16:131–138. doi: 10.5056/jnm.2010.16.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonlachanvit S., Fongkam P., Wittayalertpanya S., Kullavanijaya P. Red chili induces rectal hypersensitivity in healthy humans: possible role of 5HT-3 receptors on capsaicin-sensitive visceral nociceptive pathways. Aliment Pharmacol Ther. 2007;26:617–625. doi: 10.1111/j.1365-2036.2007.03396.x. [DOI] [PubMed] [Google Scholar]
- Gonlachanvit S., Mahayosnond A., Kullavanijaya P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol Motil. 2009;21:23–32. doi: 10.1111/j.1365-2982.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- Gradilone S.A., Masyuk T.V., Huang B.Q., Banales J.M., Lehmann G.L., Radtke B.N. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology. 2010;139:304–314. doi: 10.1053/j.gastro.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Dunkel R., Koletzko B., Schusdziarra V., Allescher H.D. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig Dis Sci. 1998;43:1165–1171. doi: 10.1023/a:1018831018566. [DOI] [PubMed] [Google Scholar]
- Gradilone S.A., Masyuk A.I., Splinter P.L., Banales J.M., Huang B.Q., Tietz P.S. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram D.X., Ahrén B., Nagy I., Olsen U.B., Brand C.L., Sundler F. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- Grant A.D., Cottrell G.S., Amadesi S., Trevisani M., Nicoletti P., Materazzi S. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol (London) 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T., Dockray G.J. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse, and guinea-pig. Neuroscience. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Groenestege W.M., Hoenderop J.G., van den Heuvel L., Knoers N., Bindels R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol. 2006;17:1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- Grossi L., Cappello G., Marzio L. Effect of an acute intraluminal administration of capsaicin on oesophageal motor pattern in GORD patients with ineffective oesophageal motility. Neurogastroenterol Motil. 2006;18:632–636. doi: 10.1111/j.1365-2982.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- Guarino M.P., Cheng L., Ma J., Harnett K., Biancani P., Altomare A. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol Motil. 2010;22:746–751. doi: 10.1111/j.1365-2982.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- Güler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe M.J., Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- Guo A., Vulchanova L., Wang J., Li X., Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamid M.R., Hedayet A., Elkhamy D.T., Almankoush A.M. Abstracts of the 41st International Congress of Pharmaceutical Sciences, Vienna, Austria. 1981. A protective effect of capsaicin against experimental induction of gastric ulcers in rats; p. 126. [Google Scholar]
- Hammer J. Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation. Aliment Pharmacol Ther. 2006;24:679–686. doi: 10.1111/j.1365-2036.2006.03022.x. [DOI] [PubMed] [Google Scholar]
- Hammer J. Hyperalgesia against capsaicin in persons with un-investigated dyspepsia: potential as a new diagnostic test. Wien Klin Wochenschr. 2006;118:43–48. doi: 10.1007/s00508-005-0474-0. [DOI] [PubMed] [Google Scholar]
- Hammer J., Führer M., Pipal L., Matiasek J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol Motil. 2008;20:125–133. doi: 10.1111/j.1365-2982.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hammer J., Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol Motil. 2007;19:279–287. doi: 10.1111/j.1365-2982.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Nakamura E., Kubo Y., Takahashi N., Yamaguchi A., Matsui H. Impairment by allyl isothiocyanate of gastric epithelial wound repair through inhibition of ion transporters. J Physiol Pharmacol. 2008;59:691–706. [PubMed] [Google Scholar]
- Hicks G.A. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol Motil. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Hill K., Schaefer M. TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J Biol Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- Hisanaga E., Nagasawa M., Ueki K., Kulkarni R.N., Mori M., Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes. 2009;58:174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft B., Linseisen J., Beckmann L., Müller-Decker K., Canzian F., Hüsing A. Polymorphisms in fatty-acid-metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis. 2010;31:466–472. doi: 10.1093/carcin/bgp325. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Sensory neurone responses to mucosal noxae in the upper gut: relevance to mucosal integrity and gastrointestinal pain. Neurogastroenterol Motil. 2002;14:459–475. doi: 10.1046/j.1365-2982.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol. 2007;292:G699–G705. doi: 10.1152/ajpgi.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. TRPV1: a new target for treatment of visceral pain in IBS? Gut. 2008;57:882–884. doi: 10.1136/gut.2008.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155:1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009;194:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Lembeck F. Longitudinal contraction of isolated guinea-pig ileum induced by rapid cooling. Naunyn-Schmiedebergs Arch Pharmacol. 1979;310:169–174. doi: 10.1007/BF00500281. [DOI] [PubMed] [Google Scholar]
- Holzer P., Lippe I.T. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience. 1988;27:981–987. doi: 10.1016/0306-4522(88)90201-1. [DOI] [PubMed] [Google Scholar]
- Holzer P., Lippe I.T., Holzer-Petsche U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology. 1986;91:360–363. doi: 10.1016/0016-5085(86)90569-x. [DOI] [PubMed] [Google Scholar]
- Holzer P., Maggi C.A. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Holzer P., Pabst M.A., Lippe I.T. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology. 1989;96:1425–1433. doi: 10.1016/0016-5085(89)90508-8. [DOI] [PubMed] [Google Scholar]
- Hondoh A., Ishida Y., Ugawa S., Ueda T., Shibata Y., Yamada T. Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res. 2010;1319:60–69. doi: 10.1016/j.brainres.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Hong S., Fan J., Kemmerer E.S., Evans S., Li Y., Wiley J.W. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S., Yamamoto H., Michael G.J., Uchida M., Belai A., Watanabe K. Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol. 2004;39:303–312. doi: 10.1080/00365520310008647. [DOI] [PubMed] [Google Scholar]
- Hu H.Z., Gu Q., Wang C., Colton C.K., Tang J., Kinoshita-Kawada M. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hu H., Tian J., Zhu Y., Wang C., Xiao R., Herz J.M. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflügers Arch. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.L., Chen X., Hoon M.A., Chandrashekar J., Guo W., Tränkner D. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybers S., Apostolaki M., van der Eerden B.C., Kollias G., Naber T.H., Bindels R.J. Murine TNFΔARE Crohn's disease model displays diminished expression of intestinal Ca2+ transporters. Inflamm Bowel Dis. 2008;14:803–811. doi: 10.1002/ibd.20385. [DOI] [PubMed] [Google Scholar]
- Hwang S.W., Cho H., Kwak J., Lee S.Y., Kang C.J., Jung J. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.J., Oh J.M., Valtschanoff J.G. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Iida T., Shimizu I., Nealen M.L., Campbell A., Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Ueno A., Naraba H., Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69:2911–2919. doi: 10.1016/s0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Ugawa S., Ueda T., Murakami S., Shimada S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Mol Brain Res. 2002;107:17–22. doi: 10.1016/s0169-328x(02)00441-2. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y., Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J Dent Res. 2009;88:212–218. doi: 10.1177/0022034508330212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Király E., Such G., Joó F., Nagy A. Neurotoxic effect of capsaicin in mammals. Acta Physiol Hung. 1987;69:295–313. [PubMed] [Google Scholar]
- Jones R.C., Xu L., Gebhart G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.C., Otsuka E., Wagstrom E., Jensen C.S., Price M.P., Gebhart G.F. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Högestätt E.D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jordt S.E., Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Kadowaki M., Kuramoto H., Takaki M. Combined determination with functional and morphological studies of origin of nerve fibers expressing transient receptor potential vanilloid 1 in the myenteric plexus of the rat jejunum. Auton Neurosci. 2004;116:11–18. doi: 10.1016/j.autneu.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kang K., Pulver S.R., Panzano V.C., Chang E.C., Griffith L.C., Theobald D.L. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–601. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y., Talavera K., Everaerts W., Janssens A., Kwan K.Y., Vennekens R. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiba H., Uchida Y., Takeda D., Nishigori A., Ueda Y., Kuribayashi K. TRPV2-immunoreactive intrinsic neurons in the rat intestine. Neurosci Lett. 2004;366:193–196. doi: 10.1016/j.neulet.2004.05.069. [DOI] [PubMed] [Google Scholar]
- Kaske S., Krasteva G., König P., Kummer W., Hofmann T., Gudermann T. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S., Yang R., Ishimaru Y., Matsunami H., Sévigny J., Kinnamon J.C. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Aihara E., Nakamura A., Xin H., Matsui H., Kohama K. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- Kawabata F., Inoue N., Masamoto Y., Matsumura S., Kimura W., Kadowaki M. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem. 2009;73:2690–2697. doi: 10.1271/bbb.90555. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H., Yamanaka A., Uchida K., Shibasaki K., Sokabe T., Maruyama Y. Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J Biol Chem. 2010;285:17277–17281. doi: 10.1074/jbc.C110.132944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechagias S., Botella S., Petersson F., Borch K., Ericson A.C. Expression of vanilloid receptor-1 in epithelial cells of human antral gastric mucosa. Scand J Gastroenterol. 2005;40:775–782. doi: 10.1080/00365520510015782. [DOI] [PubMed] [Google Scholar]
- Khanal R.C., Peters T.M., Smith N.M., Nemere I. Membrane receptor-initiated signaling in 1,25(OH)2D3-stimulated calcium uptake in intestinal epithelial cells. J Cell Biochem. 2008;105:1109–1116. doi: 10.1002/jcb.21913. [DOI] [PubMed] [Google Scholar]
- Kihara N., De La Fuente S.G., Fujino K., Takahashi T., Pappas T.N., Mantyh C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Kim B.J., So I., Kim K.W. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J Smooth Muscle Res. 2006;42:1–7. doi: 10.1540/jsmr.42.1. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Jeon J.H., Kim S.J., So I. Role of calmodulin and myosin light chain kinase in the activation of carbachol-activated cationic current in murine ileal myocytes. Can J Physiol Pharmacol. 2007;85:1254–1262. doi: 10.1139/Y07-118. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Park E.J., Lee J.H., Jeon J.H., Kim S.J., So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99:2502–2509. doi: 10.1111/j.1349-7006.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Hong J.H., Li Q., Shin D.M., Abramowitz J., Birnbaumer L. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Park K.J., Kim H.W., Choi S., Jun J.Y., Chang I.Y. Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J Gastroenterol. 2009;15:5799–5804. doi: 10.3748/wjg.15.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball E.S., Wallace N.H., Schneider C.R., D'Andrea M.R., Hornby P.J. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- Kimball E.S., Prouty S.P., Pavlick K.P., Wallace N.H., Schneider C.R., Hornby P.J. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19:390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- Kindt S., Vos R., Blondeau K., Tack J. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01332.x. 1032-e82. [DOI] [PubMed] [Google Scholar]
- Knowlton W.M., Bifolck-Fisher A., Bautista D.M., McKemy D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.H., Lee G.S., Vo T.T., Jung E.M., Choi K.C., Cheung K.W. Dietary calcium and 1,25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J Reprod Dev. 2009;55:137–142. doi: 10.1262/jrd.20139. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Fukuoka T., Obata K., Yamanaka H., Dai Y., Tokunaga A. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kojima R., Doihara H., Nozawa K., Kawabata-Shoda E., Yokoyama T., Ito H. Characterization of two models of drug-induced constipation in mice and evaluation of mustard oil in these models. Pharmacology. 2009;84:227–233. doi: 10.1159/000236524. [DOI] [PubMed] [Google Scholar]
- Kokrashvili Z., Rodriguez D., Yevshayeva V., Zhou H., Margolskee R.F., Mosinger B. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology. 2009;137:598–606. doi: 10.1053/j.gastro.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M., Brozmanova M. Cough and gastroesophageal reflux: insights from animal models. Pulm Pharmacol Ther. 2009;22:130–134. doi: 10.1016/j.pupt.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Obata K., Miyoshi K., Sakurai J., Tanaka J., Miwa H. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–1352. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- Kondo T., Oshima T., Obata K., Sakurai J., Knowles C.H., Matsumoto T. Role of transient receptor potential A1 in gastric nociception. Digestion. 2010;82:150–155. doi: 10.1159/000310836. [DOI] [PubMed] [Google Scholar]
- Kowase T., Nakazato Y., Yoko-O H., Morikawa A., Kojima I. Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr J. 2002;49:349–355. doi: 10.1507/endocrj.49.349. [DOI] [PubMed] [Google Scholar]
- Kremeyer B., Lopera F., Cox J.J., Momin A., Rugiero F., Marsh S. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D., Foerster M., Mueller K., Zeller F., Slotta-Huspenina J., Donovan J. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol Motil. 2010;22:1224–1231. doi: 10.1111/j.1365-2982.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- Kwan K.Y., Allchorne A.J., Vollrath M.A., Christensen A.P., Zhang D.S., Woolf C.J. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kym P.R., Kort M.E., Hutchins C.W. Analgesic potential of TRPV1 antagonists. Biochem Pharmacol. 2009;78:211–216. doi: 10.1016/j.bcp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Laird J.M., Martinez-Caro L., Garcia-Nicas E., Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Lamb K., Kang Y.M., Gebhart G.F., Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Landouré G., Zdebik A.A., Martinez T.L., Burnett B.G., Stanescu H.C., Inada H. Mutations in TRPV4 cause Charcot–Marie–Tooth disease type 2 C. Nat Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanosa M.J., Willis D.N., Jordt S., Morris J.B. Role of metabolic activation and the TRPA1 receptor in the sensory irritation response to styrene and naphthalene. Toxicol Sci. 2010;115:589–595. doi: 10.1093/toxsci/kfq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.M., Kim B.J., Kim H.J., Yang D.K., Zhu M.H., Lee K.P. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lee K.J., Vos R., Tack J. Effects of capsaicin on the sensorimotor function of the proximal stomach in humans. Aliment Pharmacol Ther. 2004;19:415–425. doi: 10.1046/j.1365-2036.2004.01823.x. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Jun J.Y., Chang I.Y., Suh S.H., So I., Kim K.W. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cells. 2005;20:435–441. [PubMed] [Google Scholar]
- Lee S.P., Buber M.T., Yang Q., Cerne R., Cortés R.Y., Sprous D.G. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto S.G., Tamir R., Deng H., Klionsky L., Kuang R., Le A. Antihyperalgesic effects of (R, E)-N-(2-hydroxy-2,3-dihydro-1 H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326:218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Skofitsch G. Visceral pain reflex after pretreatment with capsaicin and morphine. Naunyn-Schmiedebergs Arch Pharmacol. 1982;321:116–122. doi: 10.1007/BF00518478. [DOI] [PubMed] [Google Scholar]
- Liu M., Willmott N.J., Michael G.J., Priestley J.V. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience. 2004;127:659–672. doi: 10.1016/j.neuroscience.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Liu X., Cheng K.T., Bandyopadhyay B.C., Pani B., Dietrich A., Paria B.C. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Qu M.H., Ren W., Hu H.Z., Gao N., Wang G.D. Differential expression of canonical (classical) transient receptor potential channels in guinea pig enteric nervous system. J Comp Neurol. 2008;511:847–862. doi: 10.1002/cne.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LopezJimenez N.D., Cavenagh M.M., Sainz E., Cruz-Ithier M.A., Battey J.F., Sullivan S.L. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- Loukin S., Zhou X., Su Z., Saimi Y., Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysy J., Sistiery-Ittah M., Israelit Y., Shmueli A., Strauss-Liviatan N., Mindrul V. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomised, placebo controlled, crossover study. Gut. 2003;52:1323–1326. doi: 10.1136/gut.52.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Harnett K.M., Behar J., Biancani P., Cao W. Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells. Am J Physiol. 2010;298:G233–G240. doi: 10.1152/ajpgi.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L.J., Geierstanger B.H., Viswanath V., Bandell M., Eid S.R., Hwang S. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Hwang S.W., Miyamoto T., Dubin A.E., Patapoutian A., Story G.M. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Dubin A.E., Evans M.J., Marr F., Schultz P.G., Cravatt B.F. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Xiao B., Kwan K.Y., Petrus M.J., Dubin A.E., Hwang S. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- Maggi C.A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Maher M., Ao H., Banke T., Nasser N., Wu N.T., Breitenbucher J.G. Activation of TRPA1 by farnesyl thiosalicylic acid. Mol Pharmacol. 2008;73:12251234. doi: 10.1124/mol.107.042663. [DOI] [PubMed] [Google Scholar]
- Malin S.A., Christianson J.A., Bielefeldt K., Davis B.M. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansikka H., Lähdesmäki J., Scheinin M., Pertovaara A. Alpha2A adrenoceptors contribute to feedback inhibition of capsaicin-induced hyperalgesia. Anesthesiology. 2004;101:185–190. doi: 10.1097/00000542-200407000-00029. [DOI] [PubMed] [Google Scholar]
- Marasa B.S., Rao J.N., Zou T., Liu L., Keledjian K.M., Zhang A.H. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-kappaB activation through Ca2+ influx. Biochem J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasa B.S., Xiao L., Rao J.N., Zou T., Liu L., Wang J. Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-kappaB activation. Am J Physiol. 2008;294:C1277–C1287. doi: 10.1152/ajpcell.90635.2007. [DOI] [PubMed] [Google Scholar]
- Marigo V., Courville K., Hsu W.H., Feng J.M., Cheng H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic beta-cells. Mol Cell Endocrinol. 2009;299:194–203. doi: 10.1016/j.mce.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Martelli L., Ragazzi E., di Mario F., Martelli M., Castagliuolo I., Dal Maschio M. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil. 2007;19:668–674. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Masamoto Y., Kawabata F., Fushiki T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci Biotechnol Biochem. 2009;73:1021–1027. doi: 10.1271/bbb.80796. [DOI] [PubMed] [Google Scholar]
- Massa F., Sibaev A., Marsicano G., Blaudzun H., Storr M., Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med. 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- Materazzi S., Nassini R., Andrè E., Campi B., Amadesi S., Trevisani M. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Ochi M., Nagatomo A., Yoshikawa M. Effects of allyl isothiocyanate from horseradish on several experimental gastric lesions in rats. Eur J Pharmacol. 2007;561:172–181. doi: 10.1016/j.ejphar.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Hosoya T., Tashima K., Namiki T., Murayama T., Horie S. Distribution of transient receptor potential vanilloid 1 channel-expressing nerve fibers in mouse rectal and colonic enteric nervous system: relationship to peptidergic and nitrergic neurons. Neuroscience. 2011;172:518–534. doi: 10.1016/j.neuroscience.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Kurosawa E., Terui H., Hosoya T., Tashima K., Murayama T. Localization of TRPV1 and contractile effect of capsaicin in mouse large intestine: high abundance and sensitivity in rectum and distal colon. Am J Physiol. 2009;297:G348–G360. doi: 10.1152/ajpgi.90578.2008. [DOI] [PubMed] [Google Scholar]
- Matta J.A., Ahern G.P. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol (London) 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P.J., Aziz Q., Facer P., Davis J.B., Thompson D.G., Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- Maubach K.A., Grundy D. The role of prostaglandins in the bradykinin-induced activation of serosal afferents of the rat jejunum in vitro. J Physiol (London) 1999;515:277–285. doi: 10.1111/j.1469-7793.1999.277ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max B. This and that: the essential pharmacology of herbs and spices. Trends Pharmacol Sci. 1992;13:15–20. doi: 10.1016/0165-6147(92)90010-4. [DOI] [PubMed] [Google Scholar]
- McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNamara C.R., Mandel-Brehm J., Bautista D.M., Siemens J., Deranian K.L., Zhao M. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D.C., Vigna S.R. The capsaicin VR1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides. 2001;22:1439–1446. doi: 10.1016/s0196-9781(01)00463-6. [DOI] [PubMed] [Google Scholar]
- Meseguer V., Karashima Y., Talavera K., D'Hoedt D., Donovan-Rodríguez T., Viana F. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci. 2008;28:576–586. doi: 10.1523/JNEUROSCI.4772-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.J., Priestley J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A., Nordstrom E., Mannem A., Smith C., Banerjee B., Sengupta J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic M., Shahbazian A., Bock E., Pabst M.A., Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br J Pharmacol. 2010;160:1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Dubin A.E., Petrus M.J., Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A., Matsumoto N., Imai M., Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- Montell C., Caterina M.J. Thermoregulation: channels that are cool to the core. Curr Biol. 2007;17:R885–R887. doi: 10.1016/j.cub.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Mózsik G., Szolcsányi J., Dömötör A. Capsaicin research as a new tool to approach of the human gastrointestinal physiology, pathology and pharmacology. Inflammopharmacol. 2007;15:232–245. doi: 10.1007/s10787-007-1584-2. [DOI] [PubMed] [Google Scholar]
- Mustafa S., Oriowo M. Cooling-induced contraction of the rat gastric fundus: mediation via transient receptor potential (TRP) cation channel TRPM8 receptor and Rho-kinase activation. Clin Exp Pharmacol Physiol. 2005;32:832–838. doi: 10.1111/j.1440-1681.2005.04273.x. [DOI] [PubMed] [Google Scholar]
- Nakamura E., Hasumura M., San Gabriel A., Uneyama H., Torii K. New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharmacol Sci. 2010;112:13–18. doi: 10.1254/jphs.09r16fm. [DOI] [PubMed] [Google Scholar]
- Narukawa M., Koizumi K., Iwasaki Y., Kubota K., Watanabe T. Galangal pungent component, 1′-acetoxychavicol acetate, activates TRPA1. Biosci Biotechnol Biochem. 2010;74:1694–1696. doi: 10.1271/bbb.100133. [DOI] [PubMed] [Google Scholar]
- Nassini R., Materazzi S., Andrè E., Sartiani L., Aldini G., Trevisani M. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010;24:4904–4916. doi: 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- Nathan J.D., Patel A.A., McVey D.C., Thomas J.E., Prpic V., Vigna S.R. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol. 2001;281:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- Nijenhuis T., Hoenderop J.G., Bindels R.J. TRPV5 and TRPV6 in Ca2+ (re)absorption: regulating Ca2+ entry at the gate. Pflügers Arch. 2005;451:181–192. doi: 10.1007/s00424-005-1430-6. [DOI] [PubMed] [Google Scholar]
- Nilius B., Owsianik G. Transient receptor potential channelopathies. Pflügers Arch. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- Nilius B., Owsianik G., Voets T., Peters J.A. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nishimura G., Dai J., Lausch E., Unger S., Megarbané A., Kitoh H. Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations. Am J Med Genet A. 2010;152A:1443–1449. doi: 10.1002/ajmg.a.33414. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Ishikura H., Matsunami M., Shinozaki Y., Sekiguchi F., Naruse M. The proteinase/proteinase-activated receptor-2/transient receptor potential vanilloid-1 cascade impacts pancreatic pain in mice. Life Sci. 2010;87:643–650. doi: 10.1016/j.lfs.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Noble M.D., Romac J., Vigna S.R., Liddle R.A. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut. 2008;57:1566–1571. doi: 10.1136/gut.2008.148551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K., Kawabata-Shoda E., Doihara H., Kojima R., Okada H., Mochizuki S. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa Y., Nishihara K., Yamamoto A., Nakano M., Ajioka H., Matsuura N. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci Lett. 2001;309:33–36. doi: 10.1016/s0304-3940(01)02021-3. [DOI] [PubMed] [Google Scholar]
- Ohkuri T., Yasumatsu K., Horio N., Jyotaki M., Margolskee R.F., Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura Y., Narukawa M., Iwasaki Y., Ishikawa A., Matsuda H., Yoshikawa M. Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem. 2010;74:1068–1072. doi: 10.1271/bbb.90964. [DOI] [PubMed] [Google Scholar]
- Paterson W.G., Miller D.V., Dilworth N., Assini J.B., Lourenssen S., Blennerhassett M.G. Intraluminal acid induces oesophageal shortening via capsaicin-sensitive neurokinin neurons. Gut. 2007;56:1347–1352. doi: 10.1136/gut.2006.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.J., Jeske N.A., Akopian A.N. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+ Neuroscience. 2010;171:1109–1119. doi: 10.1016/j.neuroscience.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson L.M., Zheng H., Ward S.M., Berthoud H.R. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Pearce L.V., Petukhov P.A., Szabo T., Kedei N., Bizik F., Kozikowski A.P. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org Biomol Chem. 2004;2:2281–2286. doi: 10.1039/B404506H. [DOI] [PubMed] [Google Scholar]
- Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peleg S., Sellin J.H., Wang Y., Freeman M.R., Umar S. Suppression of aberrant transient receptor potential cation channel, subfamily V, member 6 expression in hyperproliferative colonic crypts by dietary calcium. Am J Physiol. 2010;299:G593–G601. doi: 10.1152/ajpgi.00193.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles S., Medda B.K., Zhang Z., Banerjee B., Lehmann A., Shaker R. Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience. 2009;161:515–525. doi: 10.1016/j.neuroscience.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas A., Tashima K., Tsuchiya S., Matsumoto K., Nakamura T., Horie S. Contractile effect of TRPA1 receptor agonists in the isolated mouse intestine. Eur J Pharmacol. 2007;576:143–150. doi: 10.1016/j.ejphar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Phillis B.D., Martin C.M., Kang D., Larsson H., Lindström E.A., Martinez V. Role of TRPV1 in high-threshold rat colonic splanchnic afferents is revealed by inflammation. Neurosci Lett. 2009;459:57–61. doi: 10.1016/j.neulet.2009.04.051. [DOI] [PubMed] [Google Scholar]
- Pintér E., Helyes Z., Szolcsányi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther. 2006;112:440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Plourde V., St.-Pierre S., Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273:G191–G196. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- Poonyachoti S., Kulkarni-Narla A., Brown D.R. Chemical coding of neurons expressing delta- and kappa-opioid receptor and type I vanilloid receptor immunoreactivities in the porcine ileum. Cell Tissue Res. 2002;307:23–33. doi: 10.1007/s00441-001-0480-0. [DOI] [PubMed] [Google Scholar]
- Pozsgai G., Bodkin J.V., Graepel R., Bevan S., Andersson D.A., Brain S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. 2010;87:760–768. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- Price T.J., Flores C.M. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen A.K., Louhivuori L.M., Kiehne K., Kerman K.E., Herzig K.H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008;582:229–232. doi: 10.1016/j.febslet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Qin N., Neeper M.P., Liu Y., Hutchinson T.L., Lubin M.L., Flores C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H.Y., Luo J.L., Qi S.D., Xu H.X., Sung J.J., Bian Z.X. Visceral hypersensitivity induced by activation of transient receptor potential vanilloid type 1 is mediated through the serotonin pathway in rat colon. Eur J Pharmacol. 2010;647:75–83. doi: 10.1016/j.ejphar.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Quamme G.A. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol. 2008;24:230–235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Jerman J.C., Brough S.J., Davis J.B., Egerton J., Smart D. Pharmacology of vanilloids at recombinant and endogenous rat vanilloid receptors. Biochem Pharmacol. 2003;65:143–151. doi: 10.1016/s0006-2952(02)01451-x. [DOI] [PubMed] [Google Scholar]
- Rao J.N., Platoshyn O., Golovina V.A., Liu L., Zou T., Marasa B.S. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol. 2006;290:G782–G792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- Rao J.N., Rathor N., Zou T., Liu L., Xiao L., Yu T.X. STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-mediated Ca2+ signaling after wounding. Am J Physiol. 2010;299:C579–C588. doi: 10.1152/ajpcell.00066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnefjord A., Brusberg M., Kang D., Bauer U., Larsson H., Lindström E. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur J Pharmacol. 2009;611:85–91. doi: 10.1016/j.ejphar.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Razavi R., Chan Y., Afifiyan F.N., Liu X.J., Wan X., Yantha J. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Ren X., Ferreira J.G., Zhou L., Shammah-Lagnado S.J., Yeckel C.W., de Araujo I.E. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey O., Young S.H., Jacamo R., Moyer M.P., Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol. 2010;225:73–83. doi: 10.1002/jcp.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera C.E., Vogel H., Simon S.A., le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am J Physiol. 2007;293:R626–R634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- Riera C.E., Menozzi-Smarrito C., Affolter M., Michlig S., Munari C., Robert F. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009;157:1398–1409. doi: 10.1111/j.1476-5381.2009.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoni M., Trevisani M., Gazzieri D., Nadaletto R., Tognetto M., Creminon C. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.A., Connor M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Pat CNS Drug Discov. 2006;1:65–76. doi: 10.2174/157488906775245309. [DOI] [PubMed] [Google Scholar]
- Robinson D.R., McNaughton P.A., Evans M.L., Hicks G.A. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Romanovsky A.A., Almeida M.C., Garami A., Steiner A.A., Norman M.H., Morrison S.F. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondón L.J., Groenestege W.M., Rayssiguier Y., Mazur A. Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. Am J Physiol. 2008;294:R2001–R2007. doi: 10.1152/ajpregu.00153.2007. [DOI] [PubMed] [Google Scholar]
- Rondón L.J., Rayssiguier Y., Mazur A. Dietary inulin in mice stimulates Mg2+ absorption and modulates TRPM6 and TRPM7 expression in large intestine and kidney. Magnes Res. 2008;21:224–231. [PubMed] [Google Scholar]
- Rong W., Hillsley K., Davis J.B., Hicks G., Winchester W.J., Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol (London) 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007;7:557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd J.A., Wai M.K. Genital grooming and emesis induced by vanilloids in Suncus murinus, the house musk shrew. Eur J Pharmacol. 2001;422:185–195. doi: 10.1016/s0014-2999(01)01041-x. [DOI] [PubMed] [Google Scholar]
- Ryu V., Gallaher Z., Czaja K. Plasticity of nodose ganglion neurons after capsaicin- and vagotomy-induced nerve damage in adult rats. Neuroscience. 2010;167:1227–1238. doi: 10.1016/j.neuroscience.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Salas M.M., Hargreaves K.M., Akopian A.N. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Hosokawa H., Matsumura K., Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- Schicho R., Florian W., Liebmann I., Holzer P., Lippe I.T. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schicho R., Krueger D., Zeller F., Von Weyhern C.W., Frieling T., Kimura H. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Schlingmann K.P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schlingmann K.P., Waldegger S., Konrad M., Chubanov V., Gudermann T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Hammer J., Holzer P., Hammer H.F. Chemical nociception in the jejunum induced by capsaicin. Gut. 2004;53:1109–1116. doi: 10.1136/gut.2003.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeber J.P., Hoenderop J.G., Bindels R.J. Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans. 2007;35:115–119. doi: 10.1042/BST0350115. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Kullmann F.A., Artim D.E., Bazley F.A., Schopfer F., Woodcock S. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. J Pharmacol Exp Ther. 2010;333:883–895. doi: 10.1124/jpet.109.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey K.A., Cristino L., Oland L.D., Van Sickle M.D., Starowicz K., Pittman Q.J. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Shi Y., Ding X., He Z.H., Zhou K.C., Wang Q., Wang Y.Z. Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut. 2009;58:1443–1450. doi: 10.1136/gut.2009.181735. [DOI] [PubMed] [Google Scholar]
- Shieh K.R., Yi C.H., Liu T.T., Tseng H.L., Ho H.C., Hsieh H.T. Evidence for neurotrophic factors associating with TRPV1 gene expression in the inflamed human esophagus. Neurogastroenterol Motil. 2010;22:971–977. doi: 10.1111/j.1365-2982.2010.01530.x. [DOI] [PubMed] [Google Scholar]
- Shimohira D., Kido M.A., Danjo A., Takao T., Wang B., Zhang J.Q. TRPV2 expression in rat oral mucosa. Histochem Cell Biol. 2009;132:423–433. doi: 10.1007/s00418-009-0616-y. [DOI] [PubMed] [Google Scholar]
- Shiroshita Y., Koga T., Fukuda H. Capsaicin in the 4th ventricle abolishes retching and transmission of emetic vagal afferents to solitary nucleus neurons. Eur J Pharmacol. 1997;339:183–192. doi: 10.1016/s0014-2999(97)01370-8. [DOI] [PubMed] [Google Scholar]
- Sibaev A., Massa F., Yüce B., Marsicano G., Lehr H.A., Lutz B. CB1 and TRPV1 receptors mediate protective effects on colonic electrophysiological properties in mice. J Mol Med. 2006;84:513–520. doi: 10.1007/s00109-006-0040-x. [DOI] [PubMed] [Google Scholar]
- Sipe W.E., Brierley S.M., Martin C.M., Phillis B.D., Cruz F.B., Grady E.F. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Smith P.L., Maloney K.N., Pothen R.G., Clardy J., Clapham D.E. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- So I., Kim K.W. Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle. J Smooth Muscle Res. 2003;39:231–247. doi: 10.1540/jsmr.39.231. [DOI] [PubMed] [Google Scholar]
- Spencer N.J., Kerrin A., Singer C.A., Hennig G.W., Gerthoffer W.T., McDonnell O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience. 2008;153:518–534. doi: 10.1016/j.neuroscience.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprous D., Palmer K.R. The T1R2/T1R3 sweet receptor and TRPM5 ion channel taste targets with therapeutic potential. Prog Mol Biol Transl Sci. 2010;91:151–208. doi: 10.1016/S1877-1173(10)91006-5. [DOI] [PubMed] [Google Scholar]
- Steiner A.A., Turek V.F., Almeida M.C., Burmeister J.J., Oliveira D.L., Roberts J.L. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C. Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann NY Acad Sci. 1992;657:170–186. doi: 10.1111/j.1749-6632.1992.tb22766.x. [DOI] [PubMed] [Google Scholar]
- Storr M. TRPV1 in colitis: is it a good or a bad receptor? A viewpoint. Neurogastroenterol Motil. 2007;19:625–629. doi: 10.1111/j.1365-2982.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Stotz S.C., Vriens J., Martyn D., Clardy J., Clapham D.E. Citral sensing by transient receptor potential channels in dorsal root ganglion neurons. PLoS ONE. 2008;3:e2082. doi: 10.1371/journal.pone.0002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T., Axelsson H.E., Hedlund P., Andersson D.A., Jordt S.E., Bevan S. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Su X., Wachtel R.E., Gebhart G.F. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol. 1999;277:G1180–G1188. doi: 10.1152/ajpgi.1999.277.6.G1180. [DOI] [PubMed] [Google Scholar]
- Suckow S.K., Caudle R.M. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience. 2008;153:803–813. doi: 10.1016/j.neuroscience.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Bielefeldt K., Gebhart G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Bielefeldt K., Gebhart G.F. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol. 2007;292:C1768–C1774. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- Suri A., Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Mizuno A., Kodaira K., Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Landowski C.P., Hediger M.A. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu Rev Physiol. 2008;70:257–271. doi: 10.1146/annurev.physiol.69.031905.161003. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A., Jonassohn M., Acs G., Biro T., Acs P., Blumberg P.M. The stimulation of capsaicin-sensitive neurones in a vanilloid receptor-mediated fashion by pungent terpenoids possessing an unsaturated 1,4-dialdehyde moiety. Br J Pharmacol. 1996;119:283–290. doi: 10.1111/j.1476-5381.1996.tb15983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A., Cortright D.N., Blum C.A., Eid S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szelényi Z., Hummel Z., Szolcsányi J., Davis J.B. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19:1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- Szitter I., Pozsgai G., Sandor K., Elekes K., Kemeny A., Perkecz A. The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J Mol Neurosci. 2010;42:80–88. doi: 10.1007/s12031-010-9366-5. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J., Barthó L. Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat. In: Mózsik G., Hänninen O., Jávor T., editors. Gastrointestinal Defense Mechanisms. Akadémiai Kiadó; Budapest: 1981. pp. 39–51. [Google Scholar]
- Tahara T., Shibata T., Nakamura M., Yamashita H., Yoshioka D., Hirata I. Homozygous TRPV1 315C influences the susceptibility to functional dyspepsia. J Clin Gastroenterol. 2010;44:e1–e7. doi: 10.1097/MCG.0b013e3181b5745e. [DOI] [PubMed] [Google Scholar]
- Talavera K., Yasumatsu K., Yoshida R., Margolskee R.F., Voets T., Ninomiya Y. The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB J. 2008;22:1343–1355. doi: 10.1096/fj.07-9591com. [DOI] [PubMed] [Google Scholar]
- Talavera K., Gees M., Karashima Y., Meseguer V.M., Vanoirbeek J.A., Damann N. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Tan L.L., Bornstein J.C., Anderson C.R. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Tan L.L., Bornstein J.C., Anderson C.R. Neurochemical and morphological phenotypes of vagal afferent neurons innervating the adult mouse jejunum. Neurogastroenterol Motil. 2009;21:994–1001. doi: 10.1111/j.1365-2982.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Tang L., Chen Y., Chen Z., Blumberg P.M., Kozikowski A.P., Wang Z.J. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N′-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T.E., Kiros F., Carr M.J., McAlexander M.A. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2009;40:756–762. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T.E., McAlexander M.A., Nassenstein C., Sheardown S.A., Wilson S., Thornton J. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol (London) 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T.E., Undem B.J. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol (London) 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B., Benn B.S., Christakos S., Rohacs T. Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport. Mol Pharmacol. 2009;75:608–616. doi: 10.1124/mol.108.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M., Caterina M., Malmberg A.B., Rosen T.A., Gilbert H., Skinner K. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Torihashi S., Fujimoto T., Trost C., Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- Trevisani M., Smart D., Gunthorpe M.J., Tognetto M., Barbieri M., Campi B. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Trevisani M., Siemens J., Materazzi S., Bautista D.M., Nassini R., Campi B. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvilovskyy V.V., Zholos A.V., Aberle T., Philipp S.E., Dietrich A., Zhu M.X. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Yamada T., Ugawa S., Ishida Y., Shimada S. TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem Biophys Res Commun. 2009;383:130–134. doi: 10.1016/j.bbrc.2009.03.143. [DOI] [PubMed] [Google Scholar]
- Urban L., Campbell E.A., Panesar M., Patel S., Chaudhry N., Kane S. In vivo pharmacology of SDZ 249–665, a novel, non-pungent capsaicin analogue. Pain. 2000;89:65–74. doi: 10.1016/S0304-3959(00)00349-3. [DOI] [PubMed] [Google Scholar]
- van Boxel O.S., ter Linde J.J., Siersema P.D., Smout A.J. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010;105:803–811. doi: 10.1038/ajg.2010.100. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R.M., Klooker T.K., Welting O., Stanisor O.I., Wouters M.M., van der Coelen D. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01339.x. 1107-e94. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R.M., Welting O., Bulmer D.C., Wouters M.M., Lee K., de Jonge W.J. Possible role for TRPV1 in neomycin-induced inhibition of visceral hypersensitivity in rat. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2009.01287.x. 863-e60. [DOI] [PubMed] [Google Scholar]
- Vilceanu D., Stucky C.L. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS ONE. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F., Acevedo A., Nguyen M.T., Dourado M., DeFalco J., Gustafson A. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Voets T., Nilius B., Hoefs S., van der Kemp A.W., Droogmans G., Bindels R.J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Vogt-Eisele A.K., Weber K., Sherkheli M.A., Vielhaber G., Panten J., Gisselmann G. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight E.A., Kort M.E. Transient receptor potential vanilloid-1 antagonists: a survey of recent patent literature. Expert Opin Ther Pat. 2010;20:1107–1122. doi: 10.1517/13543776.2010.497756. [DOI] [PubMed] [Google Scholar]
- Vriens J., Nilius B., Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6:79–96. doi: 10.2174/157015908783769644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J., Appendino G., Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Walker R.L., Hume J.R., Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Walker R.L., Koh S.D., Sergeant G.P., Sanders K.M., Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- Wang J., Laurier L.G., Sims S.M., Preiksaitis H.G. Enhanced capacitative calcium entry and TRPC channel gene expression in human LES smooth muscle. Am J Physiol. 2003;284:G1074–G1083. doi: 10.1152/ajpgi.00227.2002. [DOI] [PubMed] [Google Scholar]
- Wang C., Hu H.Z., Colton C.K., Wood J.D., Zhu M.X. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004;279:37423–37430. doi: 10.1074/jbc.M407205200. [DOI] [PubMed] [Google Scholar]
- Wang X., Miyares R.L., Ahern G.P. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol (London) 2005;564:541–547. doi: 10.1113/jphysiol.2004.081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Chang R.B., Liman E.R. TRPA1 is a component of the nociceptive response to CO2. J Neurosci. 2010;30:12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S.M., Bayguinov J., Won K.J., Grundy D., Berthoud H.R. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Davis J.B., Smart D., Jerman J.C., Smith G.D., Hayes P. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Weber E., Neunlist M., Schemann M., Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol (London) 2001;536:741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick E.C., Hoge S.G., Grahn S.W., Kim E., Divino L.A., Grady E.F. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol. 2006;290:G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- Winston J., Shenoy M., Medley D., Naniwadekar A., Pasricha P.J. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Wiskur B.J., Tyler K., Campbell-Dittmeyer K., Chaplan S.R., Wickenden A.D., Greenwood-Van Meerveld B. A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats. Meth Find Exp Clin Pharmacol. 2010;32:557–564. doi: 10.1358/mf.2010.32.8.1507853. [DOI] [PubMed] [Google Scholar]
- Wobber V., Hare B., Wrangham R. Great apes prefer cooked food. J Hum Evol. 2008;55:340–348. doi: 10.1016/j.jhevol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Wong G.Y., Gavva N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wood J.N., Winter J., James I.F., Rang H.P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury C.J., Zwick M., Wang S., Lawson J.J., Caterina M.J., Koltzenburg M. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg-Vrenken T.E., Sukinta A., van der Kemp A.W., Bindels R.J., Hoenderop J.G. Transient receptor potential melastatin 6 knockout mice are lethal whereas heterozygous deletion results in mild hypomagnesemia. Nephron Physiol. 2010;117:11–19. doi: 10.1159/000320580. [DOI] [PubMed] [Google Scholar]
- Wrangham R., Conklin-Brittain N. Cooking as a biological trait. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:35–46. doi: 10.1016/s1095-6433(03)00020-5. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Sweet T.B., Clapham D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Blair N.T., Clapham D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Delling M., Jun J.C., Clapham D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu G.Y., Winston J.H., Shenoy M., Yin H., Pendyala S., Pasricha P.J. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Yamakuni H., Sawai-Nakayama H., Imazumi K., Maeda Y., Matsuo M., Manda T. Resiniferatoxin antagonizes cisplatin-induced emesis in dogs and ferrets. Eur J Pharmacol. 2002;442:273–278. doi: 10.1016/s0014-2999(02)01541-8. [DOI] [PubMed] [Google Scholar]
- Yang J., Li Y., Zuo X., Zhen Y., Yu Y., Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- Yeoh K.G., Kang J.Y., Yap I., Guan R., Tan C.C., Wee A. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig Dis Sci. 1995;40:580–583. doi: 10.1007/BF02064374. [DOI] [PubMed] [Google Scholar]
- Yiangou Y., Facer P., Dyer N.H., Chan C.L., Knowles C., Williams N.S. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Young R.L., Sutherland K., Pezos N., Brierley S.M., Horowitz M., Rayner C.K. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- Yu S., Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol. 2009;296:G255–G265. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]
- Yu S., Gao G., Peterson B.Z., Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol. 2009;297:G34–G242. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- Yu Y.B., Yang J., Zuo X.L., Gao L.J., Wang P., Li Y.Q. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res. 2010;35:797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hoon M.A., Chandrashekar J., Mueller K.L., Cook B., Wu D. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang L., Jones S., Brody K., Costa M., Brookes S.J. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol. 2004;286:G983–G991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Yan Liu D., Ma L.Q., Luo Z.D., Cao T.B., Zhong J. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhao Z., Margolskee R., Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cong X., Shi L., Xiang B., Li Y.M., Ding Q.W. Activation of transient receptor potential vanilloid subtype 1 increases secretion of the hypofunctional, transplanted submandibular gland. Am J Physiol. 2010;299:G54–G62. doi: 10.1152/ajpgi.00528.2009. [DOI] [PubMed] [Google Scholar]
- Zhao H., Simasko S.M. Role of transient receptor potential channels in cholecystokinin-induced activation of cultured vagal afferent neurons. Endocrinology. 2010;151:5237–5246. doi: 10.1210/en.2010-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Sprunger L.K., Simasko S.M. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol. 2010;298:G212–G221. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F., Christianson J.A., Davis B.M., Bielefeldt K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig Dis Sci. 2008;53:194–203. doi: 10.1007/s10620-007-9843-z. [DOI] [PubMed] [Google Scholar]
- Zimoń M., Baets J., Auer-Grumbach M., Berciano J., Garcia A., Lopez-Laso E. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain. 2010;133:1798–1809. doi: 10.1093/brain/awq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel T.T., Meile T., Jehle E.C., Becker H.D. Intraperitoneal capsaicin treatment reduces postoperative gastric ileus in awake rats. Langenbecks Arch Surg. 2001;386:204–211. doi: 10.1007/s004230100228. [DOI] [PubMed] [Google Scholar]
- Zwick M., Davis B.M., Woodbury C.J., Burkett J.N., Koerber H.R., Simpson J.F. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P.M., Petersson J., Andersson D.A., Chuang H., Sørgård M., Di Marzo V. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]