Abstract
CD40 induces B cells to switch to IgE in the presence of IL-4 and up-regulates their expression of the low-affinity receptor for IgE, CD23, which promotes the immune response to allergen complexed with IgE antibody. CD40 binds to CD40L and to the C4b-binding protein (C4BP) using distinct sites. CD46 is a receptor for the product of activated complement C4b. Some microbial antigens bind both C4BP and CD46, potentially bridging CD40 to CD46. In addition, immune complexes containing both C4b and C4BP may cross-link CD40 to CD46. We demonstrate that cross-linking CD46 to CD40 on B cells inhibits CD40-mediated up-regulation of surface CD23 expression and induction of IL-4-dependent IgE isotype switching. This was associated with inhibition of induction of Cε germ line transcripts and of activation-induced cytidine deaminase mRNA expression. Furthermore, co-ligation of CD46 to CD40 blocked CD40-mediated NF-κB activation. These observations suggest that complement components may play an important role in regulating CD40 activation of B cells and the allergic response.
Keywords: CD23, IgE isotype switching
Introduction
CD40 is a member of the tumor necrosis factor receptor superfamily expressed on all mature B cells. CD40 ligation induces Ig isotype switching to IgE in the presence of the cytokines IL-4 or IL-13 (1). CD40 plays an important role in allergic inflammation not only by virtue of induction of IgE synthesis but also because of its up-regulation of CD23 expression on B cells (2). Capture of allergen bound to IgE antibody via CD23 potentiates allergen presentation by B cells to Th cells and results in the amplification of the IgE antibody response (3, 4). Furthermore, CD23 can up-regulate IgE synthesis (5, 6).
Antigen–antibody containing immune complexes activate the classical complement pathway. This involves activation of C1qrs and subsequent proteolytic cleavage of C4 and C2. C4 cleavage generates a small fragment, the fluid phase C4a component and a major fragment, C4b (200 kDa). C4b binds covalently to free NH2 groups in the immune complexes and, following C1s cleavage of C2, leads to the formation of the C4b2a complex. C4b2a cleaves C3 into the fluid phase C3a component and C3b, which is retained on the antigen–antibody complex. Both C3b and C4b are recognized by the cellular receptors CD46 and CD35, which function as co-factors for factor I-mediated C3b and C4b cleavage (7–9).
CD46 is expressed on many cells, including B cells. CD46 also serves as the receptor for several human pathogens. These include measles virus (10), adenoviruses (11), human herpes virus 6 (12), group A streptococcus M proteins (13) and Neisseria gonorrhea (14). The extracellular domain of CD46 is composed of 60 amino acids short consensus repeats, a characteristic of many C3/C4-binding proteins that include CD21 and CD35. CD46 exists in two isoforms that arise by alternative splicing and that differ in their intracytoplasmic region (15). Mutations in CD46 predispose to development of familial hemolytic uremic syndrome (16).
The C4b-binding protein (C4BP) is a regulatory component of the classical complement pathway. It circulates in human plasma in three isoforms based upon different combinations of α- (70 kDa) and β- (45 kDa) chains that are covalently linked by disufide bonds between their C-terminal regions, conferring a spider-like configuration (17). The predominant isoform contains seven α-chains and one β-chain (α7b1). Two minor isoforms consist of α7β0 and α6β1. Initially discovered as a widely expressed C3b- and C4b-binding protein, C4BP was subsequently shown to be a co-factor for the serine protease factor I, to inactivate by limited proteolysis these two components of the C3 convertase (7). C4BP also binds serum amyloid protein A (18), protein S (19) and a number of microbial agents. These include Streptococcus pyogenes M proteins (20, 21), Bordetella pertussis (22), N. gonorrhea outer membrane porin (Por) proteins (23), Candida albicans (24), Moraxella catarrhalis (25) and the fiber knob domain A of adenovirus (26). C4BP has been also shown to bind to CD40 on human B cells at a site distinct from that used by CD40L (27). Since B cells express the C4b receptor CD46, antigens, microorganisms and immune complexes that contain both C4BP and C4b have the potential to cross-link CD40 to CD46 on B cells. We show that cross-linking CD40 to CD46 significantly inhibits CD40-mediated up-regulation of CD23 expression, induction of IL-4-dependent IgE isotype switching and NF-κB activation in B cells.
Methods
Reagents
The anti-CD40 IgG1 mAb 626.1 was a gift of Dr S. M. Fu (University of Virginia, Charlottesville, VA, USA). Human CD40L:mouse CD8 fusion protein (sCD40L) was purchased from Ancell (Bayport, MN, USA). Anti-CD46 mAb GB-24 (IgG1 isotype) was a kind gift of Dr J. Atkinson (Washington University, St Louis, MO, USA). Mouse mAb to human CD21 clone BL-B21/3 (IgG1 isotype) was from Monosan (PB Uden, The Netherlands). Goat anti-mouse IgG (Fab)′2 (GAMIG) was obtained from Jackson Research Laboratories (Bar Harbor, ME, USA).
Cells
Human PBMCs were purified from normal donors after informed consent, using Ficoll-Paque (GE Healthcare, Boston, MA, USA) density gradient centrifugation as previously described (28). B cells were prepared from PBMCs by negative selection using a Dynal kit (Dynal Inc., Oslo, Norway) and contained >96% CD19+ cells. Cells were suspended in RPMI-1640 medium containing 10% FCS. The human Burkitt lymphoma cell line Ramos was obtained from American Type Culture Collection ( (Manassas, VA, USA).
Flow cytometry
To examine CD40-mediated up-regulation of CD23 expression, 1 × 106 cells were stimulated with anti-CD40 mAb (0.5 μg ml−1) or with sCD40L (0.5 μg ml−1) in the presence or absence of mAbs to CD46 or CD21 (0.5 μg ml−1) followed by addition of GAMIG at 0.5 μg ml−1. After 24 h, the cells were stained with mouse anti-human CD23-PE and FITC-conjugated mouse anti-human CD20 (PharMingen, San Diego, CA, USA). A minimum of 10 000 events were acquired and analyzed using a FACScalibur flow cytometer (Becton Dickinson Biosciences, Mountain View, CA, USA). Percent inhibition of CD23 expression was calculated as follows:
Proliferation and in vitro IgE synthesis
PBMCs (2 × 105 cells per well) were cultured in triplicates in 96-well plates (Nunc; Thermo Fisher Scientific, Rochester, NY, USA) with anti-CD40 (5 μg ml−1), recombinant human IL-4 (5 ng ml−1; R&D Systems, Minneapolis, MN, USA) or both in the presence or in the absence of anti-CD46 mAb (5 μg ml−1) or anti-CD21mAb (5 μg ml−1), with or without GAMIG. Proliferation was measured at day 4 using [3H]-thymidine incorporation. For IgE synthesis, supernatants were harvested from 14-day cultures and assayed for IgE production by ELISA as previously described (29).
Reverse transcription–PCR for Cϵ germ line transcripts and activation-induced cytidine deaminase
RNA was extracted from cultured B cells on day 4 using TRIzol (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed by Supercript II RT (Invitrogen) according to manufacturer's instructions. PCR primers used for Cε germ line transcripts (GLT), activation-induced cytidine deaminase (AID) and the housekeeping gene GAPDH were as previously described (27, 30). Reverse transcription (RT)–PCRs were performed on various dilutions to ensure that the products intensities were compared within the exponential phase of the reaction.
Electromobility shift assay for NF-κB
A single-stranded oligonucleotide corresponding to the NF-κB site in the Ig κ light chain enhancer sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was 5′-end-labeled with γ-[32P]-ATP, annealed and purified by PAGE. Nuclear extracts were prepared as previously described (31) and their protein concentration was estimated by the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA). For each reaction, 1 × 103 c.p.m. (∼0.1 ng) of radiolabeled oligonucleotide probe was incubated with 1–5 μg of nuclear extract in 20 μl of binding buffer for 20 min on ice or at room temperature. Samples were run on 4% polyacrylamide gel in Tris–glycine buffer. The densitometric analysis of the scanned bands was evaluated using the NIH Image program 1.63 f.
Western blot analysis
Purified B cells were stimulated with medium or anti-CD40 with or without anti-CD46 or anti-CD21 in the presence of GAMIG for 5 and 10 min. Total cell lysates were analyzed on 12% Tris–HCl acrylamide gel and the membrane was probed with anti-phospho-IκBα (Cell Signaling, Danvers, MA, USA) and reprobed with actin (Chemicon International, Temecula, CA, USA) as loading control.
Statistical analysis
The Students's t-test was used to compare differences between groups.
Results
Cross-linking CD46 to CD40 inhibits up-regulation of CD23 expression
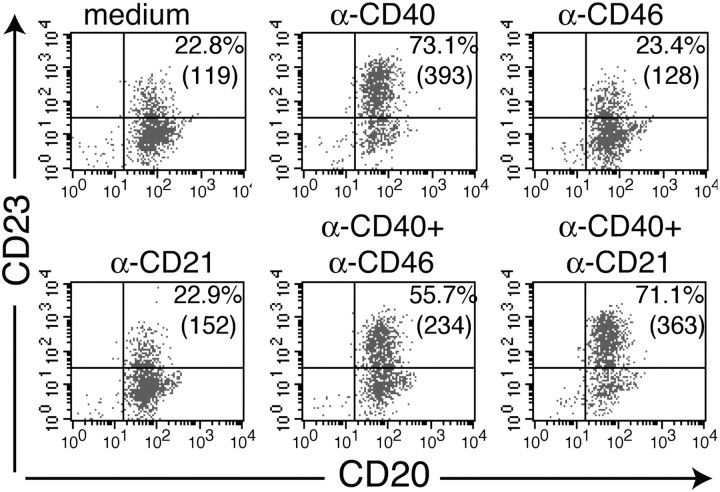
We examined the effect of cross-linking CD40 to CD46 on CD23 expression in PBMCs. Figure 1(A) shows that CD40 ligation up-regulates the expression of the low-affinity receptor for IgE, CD23, on CD20+ B cells. Cross-linking CD40 and CD46 using mouse mAbs, followed by goat anti-mouse IgG (GAMIG), inhibited CD40-mediated up-regulation of CD23 expression on B cells as assessed by measuring the percentage of B cells that expressed CD23 and the mean fluorescence intensity (MFI) of CD23 expression on the CD23+ cells (Fig. 1A). In four experiments, cross-linking of CD40 with CD46 resulted in 70 ± 11% inhibition (P < 0.05) of the percentage of B cells that were induced to express CD23 and in a 48 ± 12% decrease (P < 0.05) in the MFI of CD23 expression by CD23+ cells. In contrast, cross-linking of CD21 to CD40 had no significant effect on the percentage of B cells that were induced to express CD23 (7 ± 6% change, n = 4, P > 0.05) or on the MFI of CD23+ B cells (10 ± 11% change, n = 4, P > 0.05). Cross-linking of CD46 or CD21 by itself had no effect on CD23 expression (Fig. 1A).
Fig. 1.
Cross-linking CD40 to CD46 on PBMCs inhibits CD40 up-regulation of CD23 expression in B cells. PBMCs were stimulated with mAb to CD40 (0.5 μg ml−1) (A) or with sCD40L:muCD8 (sCD40L) (B) in the absence or presence of 0.5 μg ml−1 mAb to CD46 or mAb to CD21, followed by cross-linking with GAMIG. After 24 h, cells were analyzed by FACS for CD23 and CD20 expressions. Percentages of CD20+ cells that are CD23+ are given in the inset. Results are representative of four experiments in (A) and of three experiments in (B).
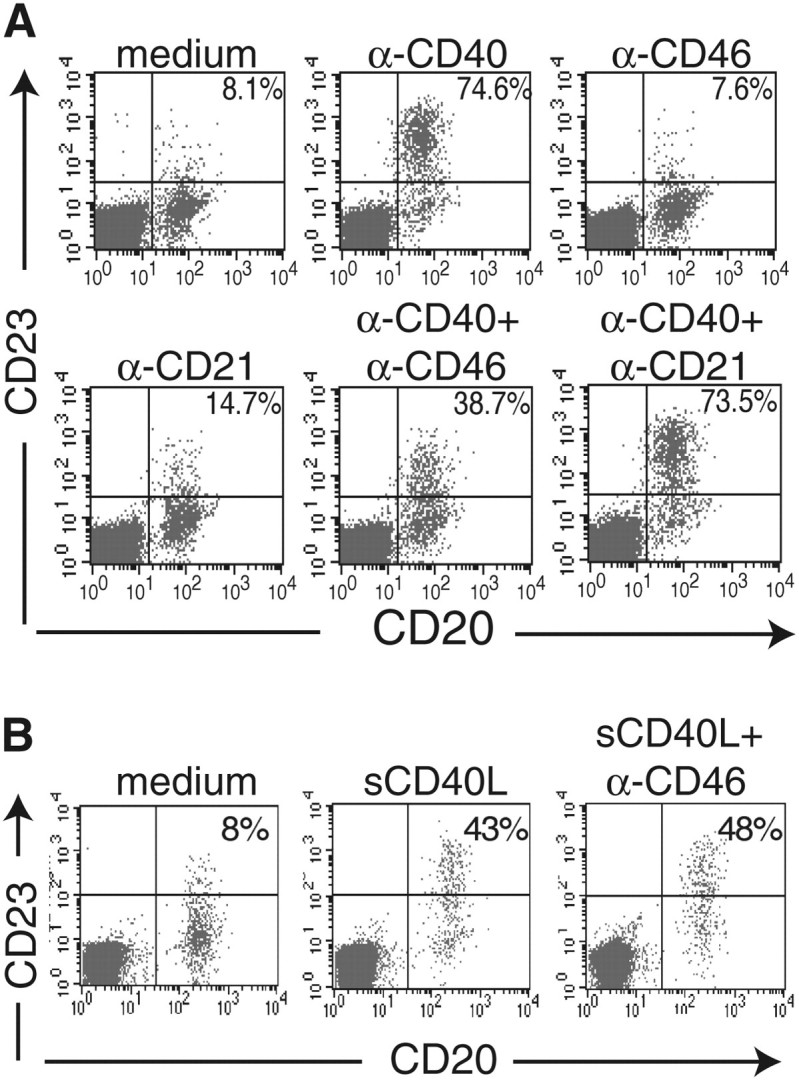
Inhibition of CD40 up-regulation of CD23 expression upon cross-linking CD40 to CD46 with mAbs followed by GAMIG could have been due to negative signals generated by homologous cross-linking of CD46. Alternatively, it may have been strictly dependent on the co-ligation of CD40 with CD46. To distinguish between these possibilities, we independently ligated CD40 and CD46 using a soluble CD40L:CD8 fusion protein (sCD40L) and mAb to CD46 followed by GAMIG, respectively. Cross-linking CD46 independently of CD40 caused no inhibition of CD23 up-regulation induced by CD40 ligation (Fig. 1B). This suggests that co-ligation of CD46 with CD40 is necessary to inhibit CD40 signaling.
To test whether the inhibitory effect of CD40/CD46 ligation is directly exerted on B cells, we examined up-regulation of CD23 in highly purified peripheral blood B cells (>96% CD19+ cells). Figure 2 shows that co-ligation of CD46 to CD40 on purified B cells caused inhibition of CD40 up-regulation of CD23 expression as assessed by measuring the percentage of CD23+ cells and the MFI of CD23 expression on these cells. In three experiments, co-ligation of CD46 and CD40 on purified B cells significantly decreased the percentage of B cells that expressed CD23 by 61 ± 12% (P < 0.05) and reduced the MFI of CD23 expression on the positive cells by 41 ± 14% (P < 0.05). Co-ligation of CD40 and CD21 had no significant effect on the percentage of B cells that expressed CD23 or on the MFI of CD23 expression. As in PBMCs, cross-linking of CD46 or CD21 did not have an effect on CD23 expression by purified B cells (Fig. 2). These results indicate that cross-linking of CD46 to CD40 inhibits CD40 signaling in a B-cell autonomous fashion.
Fig. 2.
Co-ligation of CD40 and CD46 on B cells inhibits CD40 up-regulation of CD23 expression. Purified peripheral blood B-cell populations (>96% CD20+ cells) were stimulated with mAb to CD40 in the absence or presence of mAb to CD46 or to CD21, followed by cross-linking with GAMIG and analyzed as described for PBMCs in Fig. 1. Percentages of CD20+ cells that are CD23+ are given in the inset. Numbers in parentheses refer to MFI. Results are representative of three experiments.
Cross-linking CD46 to CD40 inhibits IgE synthesis
We examined whether CD40 cross-linking to CD46 inhibits IgE synthesis in response to anti-CD40+IL-4. Figure 3A shows that co-ligation of CD40 to CD46 significantly inhibited IgE synthesis in PBMCs stimulated with anti-CD40+IL-4. In three experiments, co-ligation of CD40 to CD46 resulted in 51 ± 18% inhibition of IgE synthesis (P < 0.05). In contrast, ligation of CD40 to CD21 had no significant effect on IgE synthesis. In the absence of GAMIG, CD46 ligation by itself had no effect on IgE synthesis induced by anti-CD40 + IL-4 (Fig. 3A). Stimulation of B cells with anti-CD46 + IL-4 or anti-CD21 + IL-4 caused no detectable IgE production (data not shown).
Fig. 3.
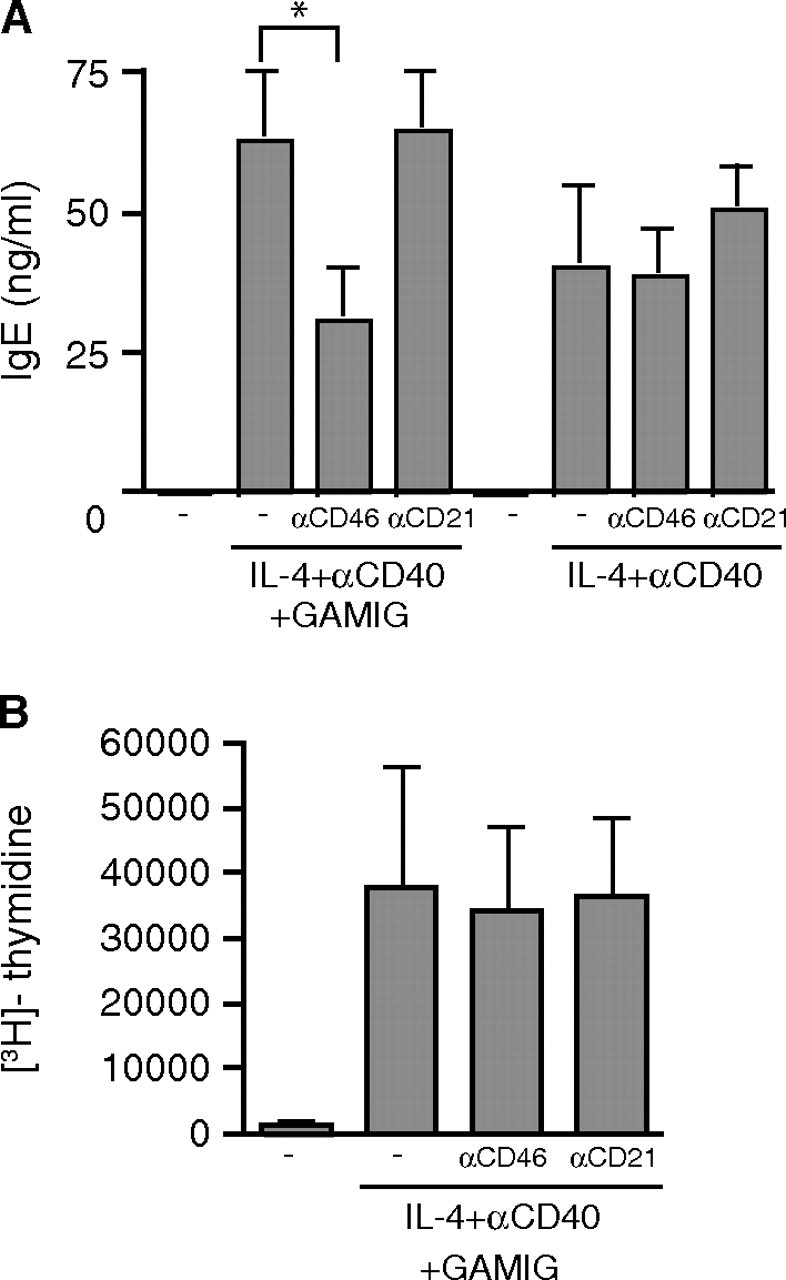
Cross-linking CD40 to CD46 inhibits CD40-mediated IgE synthesis. (A) B cells were stimulated as indicated with IL-4 and anti-CD40 mAb in the absence or presence of mAb to CD46 or CD21 and GAMIG. (B) Proliferation was measured at 4 days. Results are expressed as mean ± SD of four experiments. *P < 0.05.
Class switching by CD40 ligation and IL-4 is linked to cell division (32). Cross-linking CD46 to CD40 had no significant effect on B-cell proliferation in response to anti-CD40+IL-4 as measured by 3H-thymidine incorporation (Fig. 3B). Inhibition of IgE synthesis by co-ligation of CD40 and CD46 was not associated with increased apoptosis or cell death, as assessed by annexin V and propidium iodide staining (data not shown). CD46 ligation by itself had no detectable effect on the increased survival of B cells in response to stimulation with IL-4 (data not shown).
Cross-linking CD46 to CD40 inhibits Cϵ GLT and AID expression
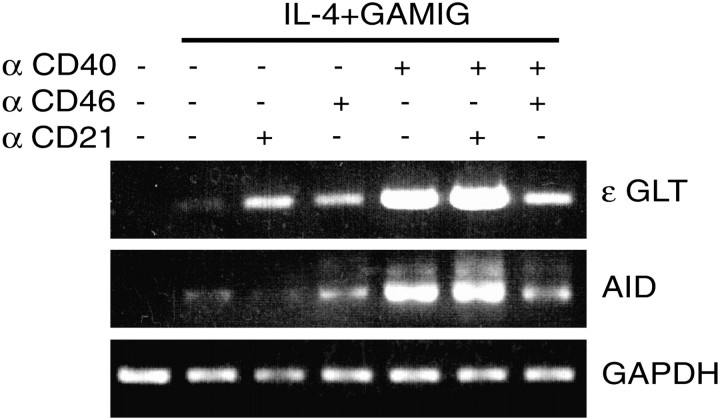
CD40 ligation synergizes with IL-4 to induce Cϵ GLT and expression of AID, which is essential for deletional switch recombination (33). Figure 4 shows that, as expected, CD40 ligation and IL-4 synergized to induce Cε GLT and AID mRNA expression in purified B cells. Cross-linking CD40 to CD46, but not CD21, inhibited Cϵ GLT and AID mRNA expression. Cross-linking of CD46 or CD21 by itself had no effect on Cϵ GLT and AID mRNA expression (data not shown) but had a modest enhancing effect on IL-4 induction of Cε GLT (Fig. 4).
Fig. 4.
Cross-linking of CD46 to CD40 inhibits induction of Cε germ line and AID mRNA expression in B cells by CD40 and IL-4. Purified human B cells were incubated with the indicated stimuli and GAMIG. Four days later, mRNA was extracted and analyzed by RT–PCR for the presence of Cε germ line and AID mRNA. GAPDH mRNA was used as loading control. Similar results were obtained in two other experiments.
Cross-linking CD46 to CD40 inhibits CD40-driven nuclear NF-κB-binding activity
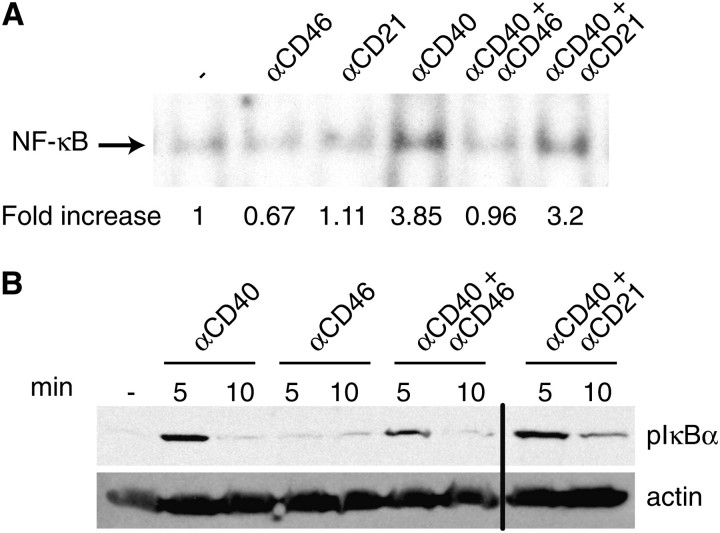
CD40 ligation induces nuclear translocation and activation of the transcription factor NF-κB (34). NF-κB plays an important role in CD40-mediated up-regulation of CD23 expression and in anti-CD40 + IL-4-mediated induction of Cε GLT and AID mRNA expression in B cells (35, 36). As expected, cross-linking of CD40 on Ramos B cells induced nuclear NF-κB-binding activity as determined by increased intensity of the broad high-molecular weight gel-shifted band on electromobility shift assay (Fig. 5A). The gel-shifted band was specific for NF-κB because it was competed out by a 100-fold excess of cold NF-κB oligonucleotide but not OCT-1 oligonucleotide (data not shown). Cross-linking of CD46 to CD40 inhibited nuclear NF-κB-binding activity. In contrast, cross-linking of CD21 and CD40 had no discernible effect (Fig. 5A). Ligation of CD46 or CD21 by itself did not induce nuclear NF-κB-binding activity (data not shown).
Fig. 5.
Cross-linking of CD46 to CD40 inhibits NF-κB activation. (A) Electromobility shift assay analysis of nuclear NF-κB complexes from the human B-cell line Ramos stimulated for 2 h with mAb to CD40 in the absence or presence of mAb to CD46 or CD21 followed by cross-linking with GAMIG. The shifted band represents nuclear NF-κB complexed to a radiolabeled NF-κB-binding oligonucleotide derived from the Ig κ light chain enhancer. Results of densitometry scans of the gel bands are shown as fold increase over the intensity band in control cells incubated with GAMIG alone. Similar results were obtained in another experiment. (B) Inhibition of CD40-mediated phopshorylation of IκBα by cross-linking with CD46 in primary B cells. Actin was used as loading control. The vertical line denotes where irrelevant lanes were deleted.
Inhibition of CD40-driven NF-κB activation by cross-linking with CD46 was confirmed in primary B cells. NF-κB activation by CD40 involves phopshorylation of IκBα, which leads to its ubiquination and degradation. This results in release of NF-κB and its translocation to the nucleus. CD40 cross-linking caused phopshorylation of IκBα in purified B cells. Cross-linking CD40 to CD46, but not to CD21, inhibited CD40-mediated IκBα phopshorylation (Fig. 5B).
Discussion
We demonstrate that cross-linking of CD46 to CD40 inhibits CD40-mediated up-regulation of CD23 expression and IgE isotype switching in B cells.
Inhibition of CD40-mediated up-regulation of CD23 by cross-linking CD46 to CD40 was specific because it was not observed when CD40 was cross-linked to the C3d/C3dg receptor CD21 (Fig. 1A). Inhibition was observed with purified B cells (Fig. 2), indicating that the inhibitory effect can be exerted directly on B cells. Inhibition of CD23 expression required co-ligation of CD40 and CD46 because it was not observed when these two receptors were ligated independently, using sCD40L and mAb to CD46 (Fig. 1B). This suggests that clustering of CD46 with CD40 is important for the inhibition of CD23 expression.
Cross-linking of CD40 to CD46 inhibited CD40-mediated IL-4-dependent IgE isotytpe switching by purified B cells (Fig. 3A). This inhibition was specific, because cross-linking of CD40 to CD21 had no effect on IgE synthesis, and was not due to interference with either the viability or the proliferation of the B cells in response to stimulation with anti-CD40 + IL-4 (Fig. 3B and data not shown). At the molecular level, co-ligation of CD46 to CD40 strongly inhibited both Cϵ GLT and AID mRNA expression in response to anti-CD40+IL-4. Cross-linking of CD46 by itself modestly synergized with IL-4 in inducing Cϵ GLT, in agreement with a previous study in Ramos B cells (37). Thus, it is likely that the inhibition of Cε GLT mRNA expression by cross-linking CD46 to CD40 in IL-4-stimulated B cells was exerted at the level of the CD40 signal. The modest synergy of CD46 ligation with IL-4 in inducing Cϵ GLT is unlikely to be explained by activation of NF-κB, as CD46 ligation did not induce nuclear NF-κB-binding activity (data not shown); its mechanism is at present not known. Cross-linking of CD21 also modestly synergized with IL-4 in the induction of Cϵ GLT, consistent with previous observations (38). Despite their synergy with IL-4 in inducing Cε GLT, ligation of CD46 or CD21 failed to induce AID mRNA expression and did not cause IgE isotype switching in IL-4-stimulated B cells (data not shown).
CD40 ligation activates NF-κB (39). There are NF-κB sites in the CD23, Iϵ and AID promoters. Induction of Cϵ GLT and AID expression, as well as IgE isotype switching, is impaired in p50-deficient mice (40). More importantly, NF-κB plays an important role in CD40-mediated CD23 gene transcription (35, 41), as well as in CD40-mediated amplification of IL-4-induced Cϵ GLT (42) and induction of AID mRNA expression (43). Our results demonstrate that co-ligation of CD46, but not CD21, inhibits CD40-mediated induction of NF-κB activation in B cells, as evidenced by inhibition of DNA-binding activity in B-cell lines and of IκBα phopshorylation in primary B cells (Fig. 5). Inhibition of NF-κB activation is likely to underlie the inhibition of CD23, Cϵ GLT and AID mRNA expression following cross-linking of CD46 to CD40. We cannot rule out inhibitory effects of CD46 cross-linking on other CD40 signaling events that are important for the expression of these genes and/or on the expression of other genes or activation of molecules that are important for class switching to IgE.
Protein tyrosine kinases play an essential role in CD40 signaling (44–46). CD46 ligation in monocytes causes recruitment of the SH2-containing tyrosine phosphatase-1 (SHP-1) (47). A similar finding was observed in purified blood B cells (data not shown). Cross-linking of CD46 to CD40 might result in SHP-1-mediated tyrosine dephosphorylation of signaling molecules that are critical for CD40-mediated NF-κB activation, CD23 up-regulation and isotype switching. Further work is needed to address the mechanism by which ligation of CD46 to CD40 inhibits CD40 signaling.
Streptococcal M protein N. gonorrhea and adenovirus may directly bridge CD40 to CD46 since they bind both molecules(11, 12, 14, 20, 21, 23, 26). Immune complexes that contain C4b and C4BP may also bridge CD46 and CD40. There are data to suggest that the binding sites on C4b for CD46 and C4BP may be distinct (48, 49). The binding site for CD40 on C4BP has not been precisely mapped but is likely to be at the C-terminal end of the C4BPα chain (27) and thus is probably distinct from the binding site for C4b, located at the N-terminal end of C4BPα (50). Even if the binding sites for C4b and CD40 overlap, different C4BPα subunits in the multimeric C4BP molecule may individually bind CD40 or C4b, allowing C4BP and C4b to bridge CD40 to CD46.
CD40-driven isotype switching occurs in germinal centers, which contain C4BP (27). Cross-linking of CD40 to CD46 on B cells may serve as a feedback mechanism to down-regulate T cell-dependent CD40-driven antibody responses. In the case of IgE antibody, this inhibition may be accentuated by inhibition of CD23 expression because CD23 potentiates allergen-specific T cell and B cell interaction by focusing allergen–IgE complexes on B cells (4). Cross-linking CD46 to CD40 may provide a novel strategy to inhibit IgE antibody responses and a potential therapy for allergic diseases.
Funding
This work was supported by USPHS grants AI-31136 and AI-07512. F.A. was the recipient of grants from the Ospedale Bambino Gesu in Rome, Italy and from the American Academy of Allergy, Asthma and Immunology.
Acknowledgments
We thank Drs S.M. Fu, P. Lane and J.P. Atkinson for their gifts of reagents and Drs M Carroll and M. Gadjeva for useful discussions.
The authors have no conflicting financial interests.
References
- 1.Oettgen HC. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts. Curr. Opin. Immunol. 2000;12:618. doi: 10.1016/s0952-7915(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 3.Kehry MR, Yamashita LC. Role of the low-affinity Fc epsilon receptor in B-lymphocyte antigen presentation. Res. Immunol. 1990;141:77. doi: 10.1016/0923-2494(90)90106-9. discussion 105–8. [DOI] [PubMed] [Google Scholar]
- 4.Getahun A, Hjelm F, Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J. Immunol. 2005;175:1473. doi: 10.4049/jimmunol.175.3.1473. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefoy JY, Gauchat JF, Life P, Graber P, Aubry JP, Lecoanet-Henchoz S. Regulation of IgE synthesis by CD23/CD21 interaction. Int. Arch. Allergy Immunol. 1995;107:40. doi: 10.1159/000236924. [DOI] [PubMed] [Google Scholar]
- 6.Fremeaux-Bacchi V, Fischer E, Lecoanet-Henchoz S, Mani JC, Bonnefoy JY, Kazatchkine MD. Soluble CD21 (sCD21) forms biologically active complexes with CD23: sCD21 is present in normal plasma as a complex with trimeric CD23 and inhibits soluble CD23-induced IgE synthesis by B cells. Int. Immunol. 1998;10:1459. doi: 10.1093/intimm/10.10.1459. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson JP, Krych M, Nickells M, et al. Complement receptors and regulatory proteins: immune adherence revisited and abuse by microorganisms. Clin. Exp. Immunol. 1994;97(Suppl. 2):1. doi: 10.1111/j.1365-2249.1994.tb06251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszewski MK, Kemper C, Price JD, Atkinson JP. Emerging roles and new functions of CD46. Springer Semin. Immunopathol. 2005;27:345. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 9.Masaki T, Matsumoto M, Nakanishi I, Yasuda R, Seya T. Factor I-dependent inactivation of human complement C4b of the classical pathway by C3b/C4b receptor (CR1, CD35) and membrane cofactor protein (MCP, CD46) J. Biochem. 1992;111:573. doi: 10.1093/oxfordjournals.jbchem.a123799. [DOI] [PubMed] [Google Scholar]
- 10.Dorig RE, Marcil A, Richardson CD. CD46, a primate-specific receptor for measles virus. Trends Microbiol. 1994;2:312. doi: 10.1016/0966-842x(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 11.Marttila M, Persson D, Gustafsson D, et al. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 2005;79:14429. doi: 10.1128/JVI.79.22.14429-14436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro F, Greenstone HL, Insinga A, et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 2003;278:25964. doi: 10.1074/jbc.M302373200. [DOI] [PubMed] [Google Scholar]
- 13.Giannakis E, Jokiranta TS, Ormsby RJ, et al. Identification of the streptococcal M protein binding site on membrane cofactor protein (CD46) J. Immunol. 2002;168:4585. doi: 10.4049/jimmunol.168.9.4585. [DOI] [PubMed] [Google Scholar]
- 14.Kallstrom H, Blackmer Gill D, Albiger B, Liszewski MK, Atkinson JP, Jonsson AB. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell. Microbiol. 2001;3:133. doi: 10.1046/j.1462-5822.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Liszewski MK, Chan AC, Atkinson JP. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 2000;164:1839. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- 16.Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA. 2003;100:12966. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Corral P, Criado Garcia O, Rodriguez de Cordoba S. Isoforms of human C4b-binding protein. I. Molecular basis for the C4BP isoform pattern and its variations in human plasma. J. Immunol. 1995;155:4030. [PubMed] [Google Scholar]
- 18.Sorensen IJ, Nielsen EH, Andersen O, Danielsen B, Svehag SE. Binding of complement proteins C1q and C4bp to serum amyloid P component (SAP) in solid contra liquid phase. Scand. J. Immunol. 1996;44:401. doi: 10.1046/j.1365-3083.1996.d01-326.x. [DOI] [PubMed] [Google Scholar]
- 19.Linse S, Hardig Y, Schultz DA, Dahlback B. A region of vitamin K-dependent protein S that binds to C4b binding protein (C4BP) identified using bacteriophage peptide display libraries. J. Biol. Chem. 1997;272:14658. doi: 10.1074/jbc.272.23.14658. [DOI] [PubMed] [Google Scholar]
- 20.Johnsson E, Thern A, Dahlback B, Heden LO, Wikstrom M, Lindahl G. Human C4BP binds to the hypervariable N-terminal region of many members in the streptococcal M protein family. Adv. Exp. Med. Biol. 1997;418:505. doi: 10.1007/978-1-4899-1825-3_120. [DOI] [PubMed] [Google Scholar]
- 21.Johnsson E, Thern A, Dahlback B, Heden LO, Wikstrom M, Lindahl G. A highly variable region in members of the streptococcal M protein family binds the human complement regulator C4BP. J. Immunol. 1996;157:3021. [PubMed] [Google Scholar]
- 22.Berggard K, Lindahl G, Dahlback B, Blom AM. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur. J. Immunol. 2001;31:2771. doi: 10.1002/1521-4141(200109)31:9<2771::aid-immu2771>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Ngampasutadol J, Ram S, Blom AM, et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Natl Acad. Sci. USA. 2005;102:17142. doi: 10.1073/pnas.0506471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect. Immun. 2004;72:6633. doi: 10.1128/IAI.72.11.6633-6641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordstrom T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J. Immunol. 2004;173:4598. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- 26.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodeur SR, Angelini F, Bacharier LB, et al. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 28.Jabara HH, Ahern DJ, Vercelli D, Geha RS. Hydrocortisone and IL-4 induce IgE isotype switching in human B cells. J. Immunol. 1991;147:1557. [PubMed] [Google Scholar]
- 29.Jabara HH, Brodeur SR, Geha RS. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J. Clin. Invest. 2001;107:371. doi: 10.1172/JCI10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Zhang L, Zhu D, Bae D, Nel A, Saxon A. CD40-mediated p38 mitogen-activated protein kinase activation is required for immunoglobulin class switch recombination to IgE. J. Allergy Clin. Immunol. 2002;110:421. doi: 10.1067/mai.2002.126382. [DOI] [PubMed] [Google Scholar]
- 31.Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J. Biol. Chem. 1996;271:3763. doi: 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- 32.Hasbold J, Lyons AB, Kehry MR, Hodgkin PD. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur. J. Immunol. 1998;28:1040. doi: 10.1002/(SICI)1521-4141(199803)28:03<1040::AID-IMMU1040>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003;3:721. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 34.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 35.Hsing Y, Hostager BS, Bishop GA. Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes. J. Immunol. 1997;159:4898. [PubMed] [Google Scholar]
- 36.Jabara H, Laouini D, Tsitsikov E, et al. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 2002;17:265. doi: 10.1016/s1074-7613(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 37.Imani F, Proud D, Griffin DE. Measles virus infection synergizes with IL-4 in IgE class switching. J. Immunol. 1999;162:1597. [PubMed] [Google Scholar]
- 38.Henchoz S, Gauchat JF, Aubry JP, Graber P, Pochon S, Bonnefoy JY. Stimulation of human IgE production by a subset of anti-CD21 monoclonal antibodies: requirement of a co-signal to modulate epsilon transcripts. Immunology. 1994;81:285. [PMC free article] [PubMed] [Google Scholar]
- 39.Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-kB. J. Immunol. 1994;153:4357. [PubMed] [Google Scholar]
- 40.Snapper CM, Zelazowski P, Rosas FR, et al. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 1996;156:183. [PubMed] [Google Scholar]
- 41.Tinnell SB, Jacobs-Helber SM, Sterneck E, Sawyer ST, Conrad DH. STAT6, NF-kappaB and C/EBP in CD23 expression and IgE production. Int. Immunol. 1998;10:1529. doi: 10.1093/intimm/10.10.1529. [DOI] [PubMed] [Google Scholar]
- 42.Iciek LA, Delphin SA, Stavnezer J. CD40 cross-linking induces Ig epsilon germline transcripts in B cells via activation of NF-kappaB: synergy with IL-4 induction. J. Immunol. 1997;158:4769. [PubMed] [Google Scholar]
- 43.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int. Immunol. 2004;16:395. doi: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 44.Loh RK, Jabara HH, Ren CL, Fu SM, Geha RS. Role of protein tyrosine kinases in CD40/interleukin-4-mediated isotype switching to IgE. J. Allergy Clin. Immunol. 1994;94:784. doi: 10.1016/0091-6749(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 45.Worm M, Geha RS. CD40-mediated lymphotoxin alpha expression in human B cells is tyrosine kinase dependent. Eur. J. Immunol. 1995;25:2438. doi: 10.1002/eji.1830250905. [DOI] [PubMed] [Google Scholar]
- 46.Knox KA, Gordon J. Protein tyrosine phosphorylation is mandatory for CD40-mediated rescue of germinal center B cells from apoptosis. Eur. J. Immunol. 1993;23:2578. doi: 10.1002/eji.1830231030. [DOI] [PubMed] [Google Scholar]
- 47.Kurita-Taniguchi M, Fukui A, Hazeki K, et al. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J. Immunol. 2000;165:5143. doi: 10.4049/jimmunol.165.9.5143. [DOI] [PubMed] [Google Scholar]
- 48.Hessing M, van 't Veer C, Hackeng TM, Bouma BN, Iwanaga S. Importance of the alpha 3-fragment of complement C4 for the binding with C4b-binding protein. FEBS Lett. 1990;271:131. doi: 10.1016/0014-5793(90)80389-z. [DOI] [PubMed] [Google Scholar]
- 49.Lambris JD, Lao Z, Oglesby TJ, Atkinson JP, Hack CE, Becherer JD. Dissection of CR1, factor H, membrane cofactor protein, and factor B binding and functional sites in the third complement component. J. Immunol. 1996;156:4821. [PubMed] [Google Scholar]
- 50.Blom AM, Webb J, Villoutreix BO, Dahlback B. A cluster of positively charged amino acids in the C4BP alpha-chain is crucial for C4b binding and factor I cofactor function. J. Biol. Chem. 1999;274:19237. doi: 10.1074/jbc.274.27.19237. [DOI] [PubMed] [Google Scholar]