Abstract
Background: We examined correlates of infant morbidity and mortality within the first 3 months of life among HIV-exposed infants receiving post-exposure antiretroviral prophylaxis in South Africa. Methods: We conducted a prospective cohort study of 848 mother–child dyads. Multivariable Cox proportional hazards models were used. Results: The main causes of infant morbidity were gastrointestinal and respiratory infections. Morbidity was higher with infant HIV infection (HR: 2.61; 95% CI: 1.40–4.85; p = 0.002) and maternal plasma viral load (PVL) >100 000 copies ml−1 (HR: 1.87; 95% CI: 1.01–3.48; p = 0.048), and lower with maternal age <20 years (HR: 0.25; 95% CI: 0.07–0.88; p = 0.031). Mortality was higher with infant HIV infection (HR: 4.10; 95% CI: 1.18–14.31; p = 0.027) and maternal PVL >100 000 copies ml−1 (HR: 6.93; 95% CI: 1.64–29.26; p = 0.008). Infant feeding status did not influence the risk of morbidity nor mortality. Conclusions: Future interventions that minimize pediatric HIV infection and reduce maternal viremia, which are the main predictors of child health soon after birth, will impact positively on infant health outcomes.
Keywords: South Africa, mother-to-child transmission, breast-feeding, infant HIV-1 infection, mortality, morbidity
Introduction
In South Africa, it is estimated that ∼3% of children are HIV infected, and most of these children have acquired HIV infection through mother-to-child transmission (MTCT) [1]. Breast milk transmission of HIV in sub-Saharan Africa is estimated to be responsible for 40% of perinatally acquired HIV infections [2], and over a third of women of childbearing age are HIV infected [3, 4]. HIV-infected mothers face a dilemma regarding how to feed their newborn infants due to the competing risk of HIV transmission associated with breast-feeding and the risk of increased morbidity and mortality associated with formula feeding [1, 5–7]. Postnatal transmission through breast-feeding remains an important source of possible infection [2, 8]. Several African studies have suggested that exclusive breast-feeding could decrease the cumulative risk of HIV transmission while maintaining the benefits of breast-feeding [9–11], but the risk of HIV infection does not exist if breast exposure is completely avoided [12]. However, non-breast-fed infants can be at increased risk of hospitalization and dying from infectious diseases [13, 14].
Antiretroviral (ARV) prophylaxis during pregnancy and delivery has been shown to be an effective means of reducing the risk of MTCT [15, 16]; however, <50% of South African women attending MTCT sites have been tested for HIV [17]. Despite expanding access to highly active antiretroviral therapy (HAART) in resource-limited settings, it is estimated that less than half of those in need are currently receiving treatment [18]. Major gaps remain in adequate coverage of MTCT services as well as HAART access in sub-Saharan Africa for women and their infants [19], highlighting the need for continued studies to examine the impact of HIV infection on infant health outcomes. Although evidence on the effect of HIV on childhood morbidity and mortality in sub-Saharan Africa is increasing [20, 21], to date use of large cohorts of mother–infant dyads with detailed measurement of clinical outcomes remains very limited.
The current study examines maternal and infant correlates of infant morbidity and mortality within the first 3 months of life in a cohort of HIV-exposed infants receiving ARV post-exposure prophylaxis in South Africa.
Methods
Setting
This study was conducted at three hospitals in South Africa: Chris Hani Baragwanath Hospital (Soweto), Coronation Hospital (Johannesburg) and Mombray Hospital (Cape Town) between October 2000 and September 2002. The primary study was a randomized, open-label, multi-center clinical trial to compare the use of single dose nevirapine to 6 weeks of zidovudine commenced within 24 h of delivery in infants of ART-naïve HIV-infected mothers [22]. The study was undertaken before HAART was readily available. Clean public drinking water was available in the urban South African settings of the study [23]. This study was approved by the Gauteng Department of Health Provincial Review Committee, the University of the Witwatersrand Committee for Research on Human Subjects, and the Mowbray Maternity Hospital Research Committee.
Participants
Women delivering without prior knowledge of their HIV status were offered postpartum voluntary counseling and rapid onsite testing within 24 h of delivery. Eligible women testing HIV positive were offered enrollment. Infants were excluded if they were preterm and weighing <1200 g, required ventilation, were unable to take oral medication, or had congenital abnormalities. Women with CD4 cell counts <200 cells µl−1 were started on co-trimoxazole prophylaxis. Infants found to be HIV infected received co-trimoxazole prophylaxis from 6 weeks of age. As was current practice in South Africa, enrolled women were counseled on infant feeding practices per current national guidelines and subsidized formula was provided to women who chose not to breast-feed [24]. Women who decided to breast-feed were encouraged to breast-feed exclusively for 3–6 months.
Among the enrolled 1051 mother–child dyads, 234 patients completed the first visit, but then did not attend any follow-up visits, and four patients were missing infant HIV status. The current analyses have been conducted only on the remaining 813 mother–child dyads that provided follow-up time, defined as a minimum of two clinical evaluations within the first 3 months of life. The final cohort of patients (N = 813) and excluded patients (N = 238) were of similar maternal age, maternal plasma viral load (PVL), maternal CD4 cell count, infant gender, infant weight and infant ARV prophylaxis status.
Clinical assessments
Baseline sociodemographic, medical and pregnancy history were collected from all enrolled mothers. Obstetric and neonatal data was collected after delivery. Follow-up visits at 10 days, 6 weeks and 3 months included a clinical examination and a blood sample for HIV-1 diagnosis. At each visit, infant-feeding method was ascertained via interviewer-administered structured standardized questionnaires. Mortality outcomes and hospitalization diagnoses were captured from patient records. Staff training, site visits and ongoing external monitoring were conducted to ensure uniformity of the study across all sites.
Laboratory procedures
Maternal blood samples were tested with Determine HIV-1/2 tests (Abbot Laboratories, Abbot Park, IL, USA), and, if reactive, a second confirmatory test using the Uni-Gold HIV test (Trinity Biotech, Wicklow, Ireland) was performed. Women who tested negative with the initial Determine test were considered uninfected. At study enrollment, maternal blood was sent for HIV-1 RNA quantitative HIV testing (viral load) and CD4 cell counts. Blood samples were collected from infants for HIV-1 DNA polymerase chain reaction (PCR) using the Roche Amplicor Monitor version 1.5 qualitative PCR assay (Roche Diagnostics, Basel, Switzerland).
Clinical definitions
Confirmed infant HIV-1 infection was defined as two consecutive blood samples testing positive for HIV-1 DNA by PCR. Infants who had a single documented positive result and were then lost to follow-up were considered infected. A child was considered to be uninfected when a Week 6 or later result was negative in the absence of breast-feeding. In breast-fed infants, retesting occurred 1 month after breast-feeding cessation. These infants were then considered uninfected if this sample was negative. For the Kaplan–Meier survival analysis, feeding status was assessed for infants who were hospitalized or died at the time of event, and for infants who did not experience either outcome, the feeding status of longest duration. For the multivariable analysis, feeding status was assessed as a time-dependent covariate as feeding status could change between clinic visits.
World Health Organization (WHO) International Classification of Disease (ICD-10) criteria were used to classify causes of hospitalization and mortality [25]. Hospitalizations and deaths were classified into the following criteria: birth related: birth asphyxia, neonatal jaundice, congenital pneumonia, acute respiratory distress syndrome; respiratory: pulmonary tuberculosis, Pneumocystis jiroveci pneumonia (PCP), pneumococcal pneumonia, other chest infection; gastrointestinal: acute infection, chronic infection; neurological: meningitis, encephalitis; other infections: infection unspecified, HIV, viral infection, septicemia, urinary tract infection; malnutrition: kwashiorkor, marasmus; and other: immunization/adverse event, anemia requiring transfusion, unknown.
Outcomes that occurred within the first 24 h of life as well as birth-related diagnoses were excluded as these events were neither associated with HIV status nor feeding status. Of 107 total hospitalizations, 48 (44.9%) birth related and two (1.9%) within the first 24 h of birth were excluded from the analysis. Of 16 total deaths, 4 (25%) birth-related deaths were excluded from the analysis. Deaths and hospitalizations with unknown dates were included in the 12-week analysis, and time to hospitalization was estimated as midpoint between baseline visit and last visit.
Statistical analysis
In the univariate analysis, mother–infant dyads were stratified by method of infant feeding and infant HIV status. Categorical variables were compared using chi-squared tests and continuous variables were compared using Student’s t-tests. Descriptive analyses of the incidence of hospital admissions and deaths were conducted using Kaplan–Meier survival analysis, and survival curves were compared using log-rank tests [26]. Univariate and multivariable Cox proportional hazards models were used to assess maternal and infant correlates associated with infant death and hospitalization. To check the proportional hazards assumption, a test based on estimation was used to verify that the squared linear predictor was insignificant. Confounding variables were included in the multivariable model based on a priori confounders identified from the literature and variables that were significant at a 0.1 level in the univariate analysis. Interaction effects between infant-feeding status and infant HIV status were examined; however these findings were not statistically significant and are not presented here. Risk factors that were included in both regression models for mortality and hospitalization included ARV prophylaxis status, time-varying infant-feeding exposure, infant HIV status, infant birth weight, mode of delivery, maternal PVL, maternal CD4 cell count and maternal age. Maternal death was only included in the hospitalization model as no deaths were documented among the mothers of infants who died. All data analyses were conducted using STATA (STATACORP, version 10.0, College Station, TX, USA) software. A 95% confidence interval (CI) and a 5% level of significance were used to assess statistical significance. All statistical tests were two tailed.
Results
Baseline characteristics of mothers and infants
Table 1 describes the demographic and clinical characteristics of the 813 mother–infant dyads stratified by infant-feeding method and HIV status. By the end of the study, among 179 breast-fed infants (22%), 18.4% of infants became HIV infected, and among 634 exclusively formula-fed infants (78%), 13.2% of infants became HIV infected (p = 0.081). Maternal PVL was higher among HIV-infected infants compared to HIV-uninfected infants among both breast-fed infants (89 250 copies ml−1 vs. 12 395 copies ml−1; p < 0.0001) and exclusively formula-fed infants (72 200 copies ml−1 vs. 16200 copies ml−1; p = <0.0001). Maternal CD4 cell count was lower among HIV-infected infants compared to HIV-uninfected infants among both breast-fed infants (401 cells µl−1 vs. 489 cells µl−1; p = 0.0447) and exclusively formula-fed infants (357 cells µl−1 vs. 491 cells µl−1; p = 0.0013). Among exclusively formula-fed infants, maternal death during study follow-up was more common among HIV-infected infants compared to HIV-uninfected infants (6.0% vs. 1.6%; p = 0.044). Women above 25 years of age were more likely to have a PVL >100 000 copies ml−1 compared to women under 25 years (11.7% vs. 9.4%; p = 0.03). Also, women above 25 years of age were less likely to have a CD4 cell count >350 cells µl−1 compared to women <25 years (63.5% vs. 73.3%; p = 0.004).
Table 1.
Baseline demographic and clinical characteristics of HIV-infected mothers and infants (N = 813)
| Breast milk (N = 179) |
Exclusive Formula fed (N = 634) |
|||||
|---|---|---|---|---|---|---|
| HIV-infected infants (N = 33) | HIV-uninfected infants (N = 146) | p-value | HIV-infected infants (N = 84) | HIV-uninfected infants (N = 550) | p-value | |
| Mothers | ||||||
| Median age (IQR) (years) | 24 (22–26) | 24 (21–28) | 0.5539 | 27 (22–30) | 25 (22–29) | 0.2585 |
| Median PVL (IQR) (copies ml−1) | 89 250 (38 550–198 500) | 12 395 (3520–39 900) | <0.0001 | 72 200 (18 800–21 9000) | 16 200 (3270–67 300) | <0.0001 |
| Median CD4 cell count (IQR) (cells µl−1) | 401 (303–527) | 489 (355–696) | 0.0447 | 357 (198–559) | 491 (312–687) | 0.0013 |
| Mode of delivery (%) | ||||||
| Normal vaginal delivery | 89.0 | 97.0 | 0.443 | 88.1 | 89.1 | 0.943 |
| Mother died (%) | 0.0 | 0.7 | 0.727 | 6.0 | 1.6 | 0.044 |
| Infants | ||||||
| Gender (%) | ||||||
| Male | 42.4 | 37.7 | 0.612 | 66.7 | 48.9 | 0.002 |
| Female | 57.6 | 62.3 | 33.3 | 51.1 | ||
| Gestational age (IQR) (weeks) | 40 (37–40) | 39 (37–40) | 0.4508 | 39 (37–40) | 39 (36–40) | 0.8159 |
| Median weight (IQR) (g) | 2850 (2500–3020) | 3000 (2700–3200) | 0.0232 | 2875 (2500–3150) | 2900 (2600–3200) | 0.4667 |
| Premature birth (%) | 21.2 | 19.9 | 0.703 | 22.6 | 26.0 | 0.224 |
| Prophylaxis status (%) | ||||||
| Nevirapine | 48.5 | 50.0 | 0.875 | 44.0 | 49.1 | 0.389 |
| Zidovudine | 51.5 | 50.0 | 56.0 | 50.9 | ||
Infant morbidity
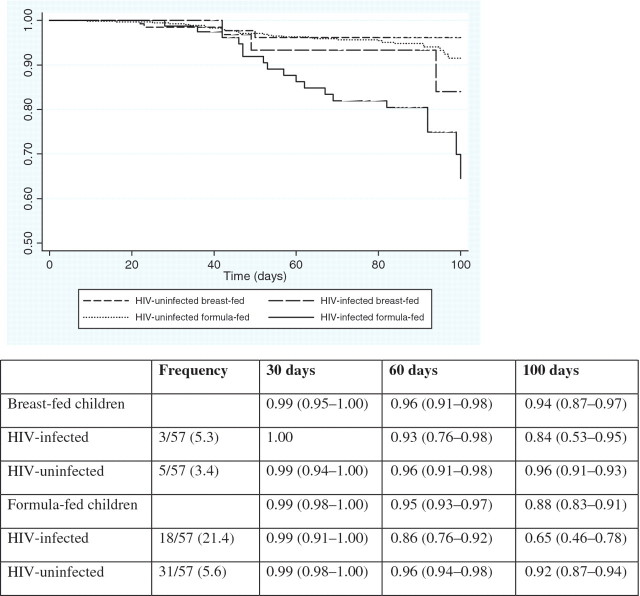
Table 2 presents the frequencies and causes of infant hospitalization stratified by infant HIV status and feeding method separately. Among the 57 infants (7.0%) who were hospitalized, the most frequent clinical diagnoses requiring hospitalization were respiratory (40.4%) and gastrointestinal infections (42.1%). The overall incidence rate of hospitalization was 0.87 (95% CI: 0.67–1.13) per 1000 child-days. Although the incidence rate of hospitalization among formula-fed infants (0.95 per 1000 child-days) was higher than among breast-fed infants (0.56 per 1000 child-days) (p = 0.4712), this was not statistically significant. The incidence rate of hospitalization among HIV-infected infants (2.33 per 1000 child-days) was over three times higher than among HIV-uninfected infants (0.64 per 1000 child-days) (p < 0.0001). The median number of days from birth to hospitalization was similar for all clinical diagnoses (range: 42–63 days). The median duration of hospitalization was 6 days (IQR: 2–8), and was broadly the same across all clinical diagnoses. Seven infants were hospitalized a second time within the first 3 months of life, five of which were due to gastrointestinal infections and four infants were hospitalized three times within the first 3 months of life. As shown in Fig. 1, among breast-fed infants, 84% of the HIV-infected infants and 96% of HIV-uninfected infants remained free of hospitalization (p = 0.1730), and among formula-fed infants, 65% of HIV-infected infants and 92% of HIV-uninfected infants remained free of hospitalization (p < 0.0001). The difference in overall probability of remaining free of hospitalization between HIV-infected and HIV-uninfected infants was also significant (p < 0.0001).
Table 2.
Pattern of infant hospitalizations within the first 100 days of lifea
| Median time to event (IQR) (days) | Breast milk N = 179 (%) | Formula fed N = 634 (%) | Hazard ratio (HR) (95% CI) | HIV-infected N = 117 (%) | HIV-unifected N = 696 (%) | Hazard ratio (HR) (95% CI) | |
|---|---|---|---|---|---|---|---|
| Incidence rate (95% CI) per 1000 child-daysb | – | 0.56 (0.28–1.12) | 0.95 (0.72–1.26) | 2.33 (1.52–3.57) | 0.64 (0.46–0.88) | ||
| Total | 52 (42–80) | 8 (4.5) | 49 (7.7) | 21 (17.9) | 36 (5.2) | ||
| Respiratory (n = 23) | 62 (42–91) | 4 (2.2) | 19 (3.0) | 1.37 (0.47–4.03) | 9 (7.7) | 14 (2.0) | 4.02 (1.74–9.29) |
| Gastrointestinal (n = 24) | 50 (41–71) | 3 (1.7) | 21 (3.3) | 1.99 (0.59–6.68) | 9 (7.7) | 15 (2.2) | 3.79 (1.66–8.66) |
| Neurological (n = 3) | 46 (29–81) | 0 | 3 (0.5) | – | 1 (0.9) | 2 (2.9) | 3.21 (0.29–35.49) |
| Infections (n = 4) | 45 (36–61) | 0 | 4 (0.6) | – | 1 (1.9) | 3 (0.4) | 2.11 (0.22–20.33) |
| Malnutrition (n = 1) | 63 (–) | 0 | 1 (0.2) | – | 0 | 1 (0.1) | – |
| Other/unknown (n = 2) | 42 (–) | 1 (0.6) | 1 (0.2) | 0.28 (0.02–4.41) | 1 (0.9) | 1 (0.1) | 5.98 (0.37–95.62) |
aBirth related: birth asphyxia, neonatal jaundice, congenital pneumonia, acute respiratory distress syndrome; Respiratory: pulmonary tuberculosis, PCP, pneumococcal pneumonia, other chest infection; Gastrointestinal: acute infection, chronic infection; Neurological: meningitis, encephalitis; Other infections: infection unspecified, HIV, viral infection, septicemia, urinary tract infection; Malnutrition: kwashiorkor, marasmus; and Other: immunization/adverse event, anemia requiring transfusion, unknown.
bOverall incidence rate excludes birth-related diagnoses and events within the first 24 hours of life.
Fig. 1.
Kaplan–Meier: Probability (95% CI) of remaining free from hospitalization within the first 100 days of life according to infant HIV status and mode of infant feeding.The overall log-rank p-value was p < 0.001. The overall log-rank p-value between breast- and formula-fed infants was p = 0.1406. The overall log-rank p-value between HIV-infected and HIV-uninfected infants was p < 0.0001. Among breast-fed infants, the log-rank p-value between HIV-infected and HIV-uninfected infants was p = 0.1730. Among formula-fed infants, the log-rank p-value between HIV-infected and HIV-uninfected infants was p < 0.0001.
Infant mortality
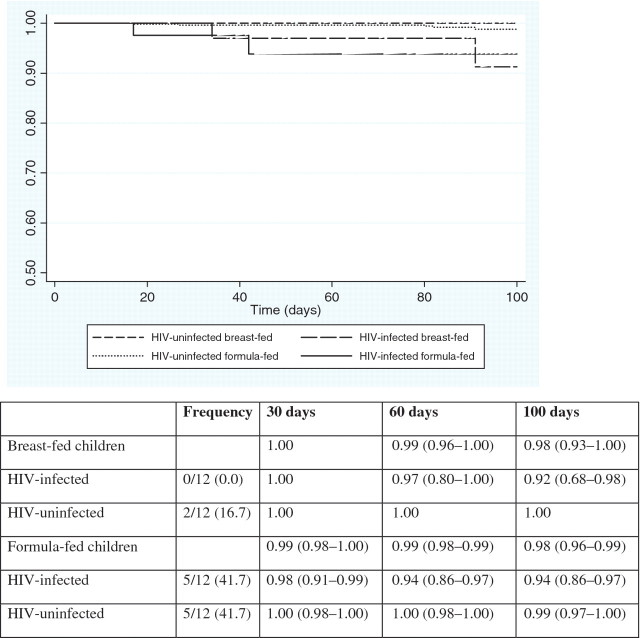
Among the 12 infants who died, four deaths occurred during the neonatal period. The main causes of death were gastrointestinal infections (25%), pneumonia (20%) and other infections (25%). The overall mortality rate was 0.18 per 1000 child-days. The mortality rate among formula-fed infants (0.19 per 1000 child-days) was higher than among breast-fed infants (0.14 per 1000 child-days) (p = 0.1958), but this was not statistically significant. The mortality rate among HIV-infected infants (0.72 per 1000 child-days) was eight times higher than among HIV-uninfected infants (0.09 per 1000 child-days) (p < 0.0001). As shown in Fig. 2, among breast-fed infants, 100% of HIV-infected infants and 92% of HIV-uninfected infants remained alive (p = 0.0047), and among formula-fed infants, 94% of HIV-infected infants and 99% of HIV-uninfected infants remained alive (p = 0.0005). The difference in overall probability of remaining alive between HIV-infected and HIV-uninfected infants was also significant (p < 0.0001).
Fig. 2.
Kaplan–Meier: probability (95% CI) of remaining alive within the first 100 days of live according to infant HIV status and mode of infant feeding. The overall log-rank p-value was p = 0.0003. The overall log-rank p-value between breast- and formula-fed infants was p = 0.6689. The overall log-rank p-value between HIV-infected and HIV-uninfected infants was p < 0.0001. Among breast-fed infants, the log-rank p-value between HIV-infected and HIV-uninfected infants was p = 0.0047. Among formula-fed infants, the log-rank p-value between HIV-infected and HIV-uninfected infants was p = 0.0005.
Multivariable analysis of infant morbidity and mortality
Tables 3 and 4 present the Cox proportional hazards regression models for predictors of infant death and hospitalization. The occurrence of hospitalization was significantly higher with infant HIV infection (HR: 2.61; 95% CI: 1.40–4.85; p = 0.002) and maternal PVL >100 000 copies ml−1 (HR: 1.87; 95% CI: 1.01–3.48; p = 0.048), and lower with maternal age <20 years (HR: 0.25; 95% CI: 0.07–0.88; p = 0.031), but not with infant-feeding method, infant birth weight, infant ARV prophylaxis, maternal death and maternal CD4 cell count. The occurrence of death was significantly higher with infant HIV infection (HR: 4.10; 95% CI: 1.18–14.31; p = 0.027) and maternal PVL >100 000 copies ml−1 (HR: 6.93; 95% CI: 1.64–29.26; p = 0.008), but not with infant-feeding status, infant birth weight, infant ARV prophylaxis, maternal CD4 cell count and maternal age.
Table 3.
Predictors of hospitalization among infants born to HIV-infected mothers within the first 100 days of life (N = 813)
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Category | Hazard ratio (HR) (95% CI) | p-value | Hazard ratio (HR) (95% CI) | p-value |
| Maternal correlates | ||||
| Maternal age | ||||
| ≤20 | 0.34 (0.13–0.92) | 0.034 | 0.25 (0.07–0.88) | 0.031 |
| 20–25 | 0.47 (0.26–0.95) | 0.034 | 0.51 (0.24–1.07) | 0.075 |
| 25–30 | 0.79 (0.41–1.51) | 0.473 | 0.79 (0.40–1.57) | 0.502 |
| >30 | 1.00 | – | 1.00 | – |
| Maternal CD4 cell count | ||||
| ≤350 cells µl−1 | 1.82 (1.06–3.15) | 0.031 | 1.27 (0.70–2.28) | 0.427 |
| >350 cells µl−1 | 1.00 | – | 1.00 | – |
| Maternal PVL | ||||
| >100 000 copies ml−1 | 2.39 (1.38–4.13) | 0.002 | 1.87 (1.01–3.48) | 0.048 |
| ≤100 000 copies ml−1 | 1.00 | – | 1.00 | – |
| Maternal death | ||||
| Yes | 1.63 (0.40–6.69) | 0.50 | 0.40 (0.05–3.07) | 0.380 |
| No | 1.00 | – | 1.00 | – |
| Infant correlates | ||||
| Infant prophylaxis status | ||||
| Zidovudine | 1.15 (0.68–1.93) | 0.604 | 0.92 (0.53–1.62) | 0.782 |
| Nevirapine | 1.00 | – | 1.00 | – |
| Infant HIV status | ||||
| HIV infected | 3.43 (2.00–5.88) | <0.0001 | 2.61 (1.40–4.85) | 0.002 |
| HIV uninfected | 1.00 | 1.00 | – | |
| Infant birth weight | ||||
| ≤2500 g | 1.24 (0.67–2.30) | 0.496 | 1.05 (0.55–2.02) | 0.879 |
| >2500 g | 1.00 | – | 1.00 | – |
| Infant feeding methoda | ||||
| Formula feeding | 1.02 (0.98–1.06) | 0.33 | 1.03 (0.99–1.07) | 0.102 |
| Breast milk | 1.00 | – | 1.00 | – |
aInfant-feeding method was analyzed as a time-dependent covariate in the multivariable analysis.
Table 4.
Predictors of mortality among infants born to HIV-infected mothers within the first 100 days of life (N = 813)
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Category | Hazard ratio (HR) (95% CI) | p-value | Hazard ratio (HR) (95% CI) | p-value |
| Maternal correlates | ||||
| Maternal age | ||||
| ≤20 | 1.66 (0.28–9.99) | 0.579 | 1.78 (0.23–13.57) | 0.577 |
| 20–25 | 1.02 (0.19–5.59) | 0.981 | 1.24 (0.22–6.92) | 0.809 |
| 25–30 | 1.00 (0.17–5.97) | 0.998 | 1.23 (0.20–7.46) | 0.824 |
| >30 | 1.00 | – | 1.00 | – |
| Maternal CD4 cell count | ||||
| ≤350 cells µl−1 | 3.80 (1.11–12.98) | 0.033 | 1.02 (0.52–2.02) | 0.945 |
| >350 cells µl−1 | 1.00 | – | 1.00 | – |
| Maternal PVL | ||||
| >100 000 copies ml−1 | 10.26 (2.72–38.71) | 0.001 | 6.93 (1.64–29.26) | 0.008 |
| ≤100 000 copies ml−1 | 1.00 | – | 1.00 | – |
| Infant correlates | ||||
| Infant prophylaxis status | ||||
| Zidovudine | 0.97 (0.31–3.02) | 0.964 | 1.25 (0.36–4.31) | 0.725 |
| Nevirapine | 1.00 | – | 1.00 | – |
| Infant HIV status | ||||
| HIV infected | 7.99 (2.53–25.17) | <0.0001 | 4.10 (1.18–14.31) | 0.027 |
| HIV uninfected | 1.00 | – | 1.00 | – |
| Infant birth weight | ||||
| ≤ 2500 g | 2.99 (0.95–9.42) | 0.062 | 1.48 (0.42–5.25) | 0.547 |
| >2500 g | 1.00 | – | 1.00 | – |
| Infant feeding methoda | ||||
| Formula feeding | 1.08 (0.91–1.29) | 0.369 | 1.04 (0.95–1.14) | 0.361 |
| Breast milk | 1.00 | – | 1.00 | – |
aInfant-feeding method was analyzed as a time-dependent covariate in the multivariable analysis.
Discussion
The current study among infants born to HIV-infected South African women provides further evidence of the potent detrimental effect of HIV infection on outcomes in early infancy, contributes to the ongoing debate about the most beneficial approach to infant feeding, and emphasizes the importance of increasing timely access to HAART among pregnant women [17, 19]. Our findings indicate that infant HIV status is the primary predictor of morbidity and mortality soon after birth. HIV-infected infants were at over twice the risk of hospitalization and at over four times the risk of death compared to HIV-uninfected infants. Early detection of infant HIV infection should be a priority in order to intervene as soon as possible. Elevated maternal PVL also predicted infant morbidity and mortality, highlighting the importance of early diagnosis of maternal HIV as well as the need to promptly initiate HIV-infected mothers on HAART.
Infant feeding method did not influence the risk of infant morbidity and mortality in this urban setting where women had ready access to clean water and infant formula [23, 24]. The findings of the current study are concordant with a multi-site African study that found that mortality among infants born to HIV-infected mothers was associated with maternal clinical factors as well as infant HIV status, but was not associated with infant-feeding practice [27]. A recent 2-year observational study from Cote d’Ivoire also found that 2-year adverse health outcomes and mortality were similar among breast-fed and formula-fed infants [20]. Similar findings have been documented in Kenya and South Africa [28, 29]. However, other recent studies from African settings have suggested that breast-feeding is strongly protective against infant mortality among HIV-infected infants [30, 31]. Comparing risk of hospitalization and mortality attributable to feeding method between studies is not straightforward and can be misleading due to differing outcome definitions, varying periods of follow-up and study entry after birth, differing regional settings with variable access to safe formula and water, and varying ART strategies.
In the current study, infants of HIV-infected mothers with PVL >100 000 copies/ml were at almost 2-fold greater risk of hospitalization and at >6-fold greater risk of mortality, which were independent of the effect of infant HIV infection status. This finding of the impact of maternal immunodeficiency on childhood morbidity and mortality is in accordance with earlier studies from multiple African settings [21, 32, 33]. Maternal PVL is not only a major predictor of the risk of perinatal HIV transmission [34], but may also closely predict viral levels in maternal breast milk [35] and the health of both HIV-infected and HIV-uninfected infants [33, 36]. Women with higher PVL may also be at higher risk of co-infection with other pathogens that could be transmitted to their offspring [37]. HIV-infected mothers with unsuppressed viremia may be less likely to be able to provide appropriate child care that could harm their infants compared to healthy mothers. Infants of younger women were at significantly lower hazard of hospitalization, which was independent of maternal PVL and CD4 cell count. This finding suggests that rather than maternal HIV disease, other social and cultural factors associated with younger maternal age may be protective against infant morbidity. Our results highlight the importance of prompt initiation of HAART among HIV-infected pregnant women, but not necessarily all women of childbearing age. Decreasing breast milk viral load through HAART throughout lactation is likely to reduce the risk of infection [38, 39], and also provide the infant with the beneficial aspects of breast-feeding if the mother chooses to breast-feed.
In the current study, the incidence of hospitalization for diarrhea and pneumonia, which constituted over two-thirds of all hospitalizations, was similar among formula-fed when compared to breast-fed infants, but the risk of hospitalization due to diarrhea and pneumonia was close to four times greater among HIV-infected infants than HIV-uninfected infants. Among non-HIV infected mothers, sustained breast-feeding has been shown to be associated with a lower risk of gastrointestinal and respiratory illness in infants from multiple regional settings [40, 41]. The most common cause of infant pneumonia requiring hospitalization in our study population has been shown to be PCP [42]. Many infectious complications can be prevented through primary prophylaxis with co-trimoxazole in this infant population with a substantial risk of HIV infection [12], and further efforts are needed to assure timely prophylaxis initiation.
We are unable to make conclusive comments on an association between infant-feeding method and morbidity and mortality among infants born to HIV-infected mothers [43]. It is possible that the benefits accrued through breast milk exposure require a longer duration of lactation, but the protective effects of breast-feeding likely decrease over time [13]. Despite possible gains in avoiding HIV transmission through avoiding breast-feeding, healthcare workers, policy makers and mothers must also consider the extra morbidity, primarily from diarrhea and acute respiratory infections soon after birth, that could be associated with not breast-feeding [13, 44]. In the absence of timely access to HAART in settings where there is available clean water and a safe supply of breast-feeding substitutes, alternatives to breast-feeding with appropriate nutritional counseling and care could be considered as a safe intervention [45]. Our results may not be generalizable to all resource-limited settings where the provision of safe drinking water is not available and where the supply of non-breast milk substitutes may be intermittent. It is advisable that feeding decisions be context specific [46].
Due to the exclusion criteria of the original clinical trial, these findings may be generalizable only to full-term infants. Data on maternal co-infections was not available in the current study, however all women with CD4 cell counts <200 cells µl−1 were started on co-trimoxazole prophylaxis per study protocol. At the time of the study, antiretroviral therapy was not freely available in the public sector. The current study utilized hospital admission data to assess infant morbidity and mortality and it is possible that these outcomes could have been under ascertained or misclassified. However, we reviewed infant clinical records over time to confirm hospital admissions and participants who did not follow-up for visits were contacted by study staff. Additionally, we were able to examine clinical outcomes only during the first 3 months of life, and hence we were unable to assess feeding method in the postweaning (>6 months) period.
Despite the data being from 2000 to 2002, these findings are still relevant in light of high HIV-associated infant morbidity and mortality as well as due to a lack of adequate HAART coverage of mothers and their infants in this region. These findings, showing the excessive morbidity and mortality in HIV-infected infants, highlight the need for rapid infant HIV diagnosis, prompt initiation of HAART in HIV-infected infants and their mothers, and interventions that will enhance child health. Unlike earlier studies from African settings, the current study included a sizeable number of women who did not breast-feed, but a larger sample of women may be necessary to assess the impact of feeding method on infant morbidity and mortality. Our detailed collection of feeding methods over time allowed us to assess feeding as a time-dependent measure, while many earlier studies have assessed feeding at a single time point. A strength of the current study was detailed outcome data obtained on a large cohort of infants through physician diagnosis and patient records, which allowed for exclusion of outcomes that were unrelated to feeding method and infant HIV status.
The findings of the current study can assist in the development of further HIV diagnostic, monitoring and care interventions that can be integrated into current HAART treatment programs for HIV-infected women and their infants [47]. New evidence supports the concept that ART given to the infant during breast-feeding can reduce postnatal transmission [38], and that ART can substantially reduce early infant mortality and HIV progression by >75% among South African infants [48]. Despite increasing access to HAART along with PMTCT in South Africa, major gaps in coverage remain. Optimizing the health of HIV-infected women and their infants involves a careful consideration of competing clinical risks as well as local circumstances. Given the overwhelming impact HIV infection has on infant health outcome, the increased provision of HAART could have the impact of not only decreasing MTCT, but substantially improving both maternal and child health in hyperendemic HIV settings.
Funding
Funding was made available from Bristol Myers Squibb “Secure the Future” fund. KKV was supported by a National Institutes of Mental Health (NIMH) Ruth Kirchstein National Research Service Award (NRSA) (grant no. F30 MH079738-01A2). KO received support from the Fogarty International Center (grant no. TW007370/3).
Acknowledgements
The authors thank Dr L. Kuhn, Sergievsky Center, Columbia University, New York, NY, USA for her helpful comments, especially on statistical analysis and Dr K.H. Mayer, Brown University, Providence, RI, USA for his comments.
References
- 1.Human Sciences Research Council. South African National HIV Prevalence, Incidence, Behavior, and Communication Survey, 2008: A Turning Tide Among Teenagers? Cape Town, South Africa, HSRC Press; 2008. [Google Scholar]
- 2.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. J Am Med Ass. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 3.Newell M, Brahmbhatt H, Ghys PD. Child mortality and HIV infection in Africa: a review. AIDS. 2004;18(Suppl 2):S27–34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 4.Zaba B, Whitworth J, Marston M, et al. HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology. 2005;16:275–80. doi: 10.1097/01.ede.0000155507.47884.43. [DOI] [PubMed] [Google Scholar]
- 5.John-Stewart G, Mbori-Ngacha D, Ekpini R, et al. Ghent IAS Working Group on HIV in Women Children. Breast-feeding and Transmission of HIV-1. JAIDS. 2004;35:196–202. doi: 10.1097/00126334-200402010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray G, Saloojee H. Breast-feeding, antiretroviral prophylaxis, and HIV. N Eng J Med. 2008;359:189–91. doi: 10.1056/NEJMe0803991. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. HIV and infant feeding: Guidelines for decision-makers. 2004 http://whqlibdoc.who.int/hq/2003/9241591223.pdf (27 August 2009, date last accessed) [Google Scholar]
- 8.Coutsoudis A, Dabis F, Fawzi W, et al. Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infectious Disease. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 9.Coovadia H, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. The Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 10.Coutsoudis A, Pillay K, Kuhn L, et al. South African Vitamin A Study Group. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–87. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 11.Iliff P, Piwoz EG, Tavengwa NV, et al. ZVITAMBO study group. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 12.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. The Lancet. 2002;359:2097–104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 13.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. The Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 14.Kuhn L, Stein Z. Infant survival, HIV infection, and feeding alternatives in less-developed countries. Am J Pub Health. 1997;87:926–31. doi: 10.2105/ajph.87.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Cock K, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. J Am Med Ass. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 16.Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews. 2007;24:CD003510. doi: 10.1002/14651858.CD003510.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Meyers T, Moultrie H, Naidoo K, et al. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis. 2007;196(Suppl 3):S474–81. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett J, Shao JF. Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. Lancet Infect Dis. 2009;9:637–49. doi: 10.1016/S1473-3099(09)70227-0. [DOI] [PubMed] [Google Scholar]
- 19.Becquet R, Ekouevi DK, Arrive E, et al. Universal antiretroviral therapy for pregnant and breast-feeding HIV-1-infected women: towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis. 2009;49:1936–45. doi: 10.1086/648446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becquet R, Bequet L, Ekouevi DK, et al. ANRS 1201/1202 Ditrame Plus Study Group. Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d'Ivoire. PLoS Med. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilongozi D, Wang L, Brown L, et al. HIVNET 024 Study Team. Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. J Pediatr Infect Dis. 2008;27:808–14. doi: 10.1097/INF.0b013e31817109a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray G, Urban M, Chersich MF, et al. for the PEP Study Group. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS. 2005;19:1289–97. doi: 10.1097/01.aids.0000180100.42770.a7. [DOI] [PubMed] [Google Scholar]
- 23.Statistics South Africa. General Household Survey. Statistics South Africa, Pretoria. 2008 [Google Scholar]
- 24.National Department of Health. Protocol for providing a comprehensive package of care for the prevention of mother to child transmission of HIV (PMTCT) in South Africa. National Department of Health Pretoria. 2001 [Google Scholar]
- 25.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2007. http://apps.who.int/classifications/apps/icd/icd10online/ (24 June 2009, date last accessed) [Google Scholar]
- 26.Alioum A, Cortina-Borja M, Dabis F, et al. Ghent Group. Estimating the efficacy of interventions to prevent mother-to-child transmission of human immunodeficiency virus in breastfeeding populations: comparing statistical methods. Ame J Epidemiol. 2003;158:596–605. doi: 10.1093/aje/kwg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newell M, Coovadia H, Cortina-Borja M, et al. Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. The Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 28.Mbori-Ngacha D, Nduati R, John G, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. J Am Med Ass. 2000;286:2413–20. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobat R, Moodley D, Coutsoudis A, Coovadia H. Breastfeeding by HIV-1-infected women and outcome in their infants: a cohort study from Durban, South Africa. AIDS. 1997;11:1627–33. doi: 10.1097/00002030-199713000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Taha T, Kumwenda NI, Hoover DR, et al. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull WHO. 2006;84:546–54. doi: 10.2471/blt.05.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox M, Brooks D, Kuhn L, et al. Reduced mortality associated with breast-feeding-acquired HIV infection and breast-feeding among HIV-infected children in Zambia. JAIDS. 2008;48:90–6. doi: 10.1097/QAI.0b013e31816e39a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee A, Bosch RJ, Hunter DJ, et al. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. JAIDS. 2007;46:599–606. doi: 10.1097/QAI.0b013e31815a5703. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–61. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mofenson L, Lambert JS, Stiehm ER, et al. Pediatric AIDS Clinical Trials Group Study 185 Team. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N Eng J Med. 1999;341:385–93. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 35.John G, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–12. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 36.Ioannidis J, Tatsioni A, Abrams EJ, et al. Maternal viral load and rate of disease progression among vertically HIV-1-infected children: an international meta-analysis. AIDS. 2004;18:99–108. doi: 10.1097/00002030-200401020-00012. [DOI] [PubMed] [Google Scholar]
- 37.Brayfield B, Phiri S, Kankasa C, et al. Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J Infect Dis. 2003;187:559–68. doi: 10.1086/367985. [DOI] [PubMed] [Google Scholar]
- 38.Kumwenda N, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Eng J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 39.Kilewo C, Karlsson K, Ngarina M, et al. Mitra Plus Study Team. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. JAIDS. 2009;52:406–16. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 40.Quigley M, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:837–42. doi: 10.1542/peds.2006-2256. [DOI] [PubMed] [Google Scholar]
- 41.Coutsoudis A, Pillay K, Spooner E, et al. Morbidity in children born to women infected with human immunodeficiency virus in South Africa: does mode of feeding matter? Acta Paediatrica. 2003;92:890–5. [PubMed] [Google Scholar]
- 42.Ruffini D, Madhi SA. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalized for severe pneumonia. AIDS. 2002;16:105–12. doi: 10.1097/00002030-200201040-00013. [DOI] [PubMed] [Google Scholar]
- 43.Bertolli J, Hu DJ, Nieburg P, et al. Decision analysis to guide choice of interventions to reduce mother-to-child transmission of HIV. AIDS. 2003;17:2089–98. doi: 10.1097/00002030-200309260-00010. [DOI] [PubMed] [Google Scholar]
- 44.Wilfert C, Fowler MG. Balancing maternal and infant benefits and the consequences of breast-feeding in the developing world during the era of HIV infection. J Infect Dis. 2004;195:165–7. doi: 10.1086/510255. [DOI] [PubMed] [Google Scholar]
- 45.Rollins N, Becquet R, Bland RM, et al. Infant feeding, HIV transmission and mortality at 18 months: the need for appropriate choices by mothers and prioritization within programmes. AIDS. 2008;22:2349–57. doi: 10.1097/QAD.0b013e328312c740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. HIV and infant feeding: Update. 2007 cited. http://whqlibdoc.who.int/publications/2007/9789241595964_eng.pdf (27 August 2009, date last accessed) [Google Scholar]
- 47.Abrams E, Myer L, Rosenfield A, et al. Prevention of mother-to-child transmission services as a gateway to family-based human immunodeficiency virus care and treatment in resource-limited settings: rationale and international experiences. Am J Obstet Gynecol. 2007;197(Suppl 3):S101–6. doi: 10.1016/j.ajog.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 48.Violari A, Cotton MF, Gibb DM, et al. CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]