Abstract
Thymosin alpha 1 (Tα1) has immunomodulatory and anti-tumor effects in patients and has been commercialized in worldwide. An innovative technique is therefore impending to achieve high-yield expression and purification of Tα1 to meet the increasing requirements for clinical applications. Tα1 can enhance T cells, dendritic cells and antibody responses, and also augment an anti-tumor immune response. In the current study, we developed a novel technique to produce Tα1 concatemer and investigated its capability in anti-tumor immunotherapy. We expressed the recombinant 2×Tα1 concatemer protein (Tα1② protein) in Escherichia coli. The purity of Tα1② was higher than 95% as assessed by HPLC analysis. In vitro, Tα1② could stimulate the proliferation of mouse splenic lymphocyte, and increase the apoptosis of tumor cell lines. In vivo, Tα1② significantly inhibited the tumor growth in B16 tumor-bearing mice. Compared with Tα1, the Tα1② is of more effective bioactivity than Tα1. The purified Tα1② is a promising substitute for synthetic Tα1 because of its potent anti-tumor effects. We concluded that the expression system for Tα1 concatemer was constructed successfully, which could serves as a highly efficient tool for the production of large quantities of the highly active protein.
Keywords: Thymosin alpha 1, concatemer, protein expression, purification, tumor suppression
Introduction
Tα1 is a heat stable, acidic polypeptide consisting of 28 amino acid residues with a molecular weight of 3108 Da and an isoelectric point of pH4.2 [1]. As a biological response modifier, Tα1 enhances immune response and promotes specific lymphocyte functions, including lymphoproliferative responses to mitogens, maturation of T-cells [2], antibody production, and T-cell-mediated cytotoxicity [3-5]. It can antagonize apoptosis of T cells induced by dexamethasone or CD3 monoclonal antibody [6] or sera of tumor-bearing mice [7]. Tα1 also can stimulate lymphokine production of IFN-γ, IFN-α, IL-2, and macrophage migration inhibitory factor (MIF) [8]. It also is capable to activate infected dendritic cells through toll-like-receptor signaling. Furthermore, Tα1 can significantly inhibit proliferation and induce apoptosis in many tumor cell lines [9-10]. Previous studies showed that Tα1 could reduce tumor size in mice [11], and partly restore cellular immunity [12].
Thymosin α1 belongs to the thymus extract denominated by thymosin fraction5 (TF5) which shows immunoregulatory properties [13]. Tα1 is an immune modifier that can trigger maturational events in lymphocytes, augment T-cell function, and promote reconstitution of immune defects [14]. Because of the previously reported potential, Tα1 has been used worldwide for the treatment of a number of diseases including immunodeficiencies [15-16], malignancies [17], and infections such as HBV[18], HIV [19], either in stand-alone therapy or in conjunction with IFN-α [20]. Recently, Tα1 has also been used as an adjuvant for vaccines against influenza and hepatitis [21].
It is widely accepted that Tα1 affects tumor bearing hosts by activating infected dendritic cells through toll-like-receptor signaling, augmenting specific lymphocyte functions, increasing the expression of tumor and MHC-I antigens, and enhancing their immune responsiveness. Thus, Tα1 can influence the inflammation balance. In vivo, high doses of Tα1 can improve anti-tumor efficacy of chemo-immunotherapy against B16 melanoma and increase the average survival time of treated mice [22]. Moreover, clinical trials using Tα1 in the treatment of patients with cancer indicate that this agent is nontoxic [23-24]. Tα1 can cause few side effects and has the benefit of arresting neurotoxicity induced by chemotherapy [25]. These reports all indicate the safe applications of Tα1 in future clinical practice for malignant tumor patients.
Although the Tα1 has been successfully applied in clinical trials, but the high costs greatly limited its clinical use. Increasing therapeutic applications for Tα1 has attracted rising interests in optimizing methods for its production and purification. Recombinant DNA techniques has been applied in many laboratories to produce Tα1 including the expression of Tα1 as fusion proteins [26]. The shortcoming of this method is that the fusion protein must be removed because the peptide might be inactive [27] and the bioactivities of the fusion proteins were lower than that of Tα1 [28]. Tα1 in concatemer form expressed as a His6 tagged fusion protein was also studied [29-30]. Data showed this kind of fusion protein exhibited a weeker stimulation on mice lymphocytes proliferation than synthetic Tα1 [30]. Furthermore, His6 tag sequence may not be safe and may lead to some problems in clinical research. On the other hand, the affinity purification of the protein by His6 tag is costly. The Tα1 concatemer protein was also expressed in inclusion body form and had to be cleaved by hydroxylamine to release the active Tα1 monomer [27]. Obviously, the current techniques available are not suitable for large-scale manufacture of Tα1 and the activity of this protein in vivo are also questioned.
In this study, a recombinant gene encoding recombinant 2×Thymosin alpha 1 tandem repeats (Tα1②) was synthesized, inserted into expression vector pET-22b (pET-Tα1②) and expressed in E.coli. We have developed an economic and scalable purification protocol to produce the recombinant Tα1② protein with the final yielding ratio of 4.5mg purified protein out of per gram of cell paste.
Remarkably, the bioactivity of the protein was more effective than that of synthetic Tα1. MTT assay shows high dose Tα1 can inhibit B16 cell proliferation and induce cell apoptosis, which prompted us to investigate the anti-tumor efficacy of Tα1 in vivo. We administered high dose of Tα1 monomer or Tα1② to B16 bearing melanoma C57 mice, and found that Tα1② treatments (8mg/kg and 16mg/kg) caused the conspicuous regression of subcutaneous tumor. Tα1② had an inhibitory effect on the growth of several tumor cell lines which is even more significant than Tα1 from synthetic. All the results suggested that this strategy for production of Tα1 was effective and simple.
Materials and Methods
Materials
Expression vectors pET-22b and E. coli BL21 were kept in our laboratory. Thymosin 2 concatemer gene was synthesized by Beijing AuGCT Company. The synthetic Tα1 was purchased from BACHEM Company (catalogue no. 62304-98-7). The anti- Tα1 antibody (catalogue no. ab55635) was purchased from Abcam Company. The secondary goat anti-mice IgG antibody conjugated with HRP (catalogue no. Zb-2305) was purchased from Beijing Zhongshan Company. The TUNEL detection kit was purchased from KeyGEN Company (catalogue no. KGA702).
Construction of Recombinant plasmid pET- Tα1②
Tα1② gene was designed and synthesized. Gly and Ser as a linker were inserted between the two monomer sequences of Tα1. The synthesized sequences is as follows: Sense strand, 5'-CATATGTCTGATGCAGCGGTTGACACCAGCTCGGAAATCACCACCAAAGACCTGAAAGAGAAGAAAGAGGTGGTGGAAGAAGCGGAGAACGGATCCAGCGATGCGGCGGTTGACACCAGCTCGGAAATCACCACCAAAGACCTGAAAGAGAAGAAAGAGGTGGTGGAAGAAGCGGAGAACTAGAAGCTT-3' (NdeⅠand Sal I restriction site was underlined). The synthesized gene was cloned into pET-22b (+) vector. The recombinant plasmid was identified by DNA sequencing. E. coli strain BL21 (DE3) was transformed with the correct recombinant plasmid containing the Tα1② gene. The expression of the Tα1② gene was driven by a T7 promoter, which can be regulated by inducing the culture with isopropyl β-D-thiogalactopyranoside (IPTG) thereby allowing high level of expression of the Tα1② gene.
Expression of Tα1②
The recombinant plasmid containing the Tα1② gene was transformed into E. coli. BL21(DE3) and plated on Luria Broth (LB)-Agar, containing 100μg/ml ampicillin. After an overnight incubation at 37℃, a single colony was used to inoculate 10 ml of LB media containing 100μg/ml ampicillin and incubated overnight at 37℃ with shaking at 200 rpm. Then 3 milliliters of culture was transferred to 300ml fresh LB medium with 100μg/ml ampicillin in a 500ml shake Task. The culture was then induced after reaching an optical density of 0.6 at 600 nm by the addition of IPTG to a final concentration of 0.5 mM and cultured at 37℃, 200 rpm for 4h. Samples were collected at 1, 2, 3 and 4h respectively. Electrophoresis of the expression samples and the molecular marker were carried out on SDS-PAGE gels, stained with coomassie brilliant blue (CBB) G-250. The optimal induction time was confirmed by analysing the protein expression level at different times.
The culture was centrifuged at 10,000 rpm for 15 min at 4℃. The supernatant was carefully removed, and the cell pellet was washed by gently suspending in lysis buffer (20mM Tris-HCl, 150mM NaCl, and 1mM EDTA, pH 8.5). The washed cell mass (10 g) was collected by centrifuging at 10,000 rpm, 4℃ for 15 min and stored at -20℃.
Purification of Tα1②
Ten grams (wet weight) of pellet was suspended in 100 ml of lysis buffer in a beaker maintained on ice. The cells were disrupted with a sonicator programmed for 25 min of 5 sec on and 10 sec off. The sonicate was then centrifuged at 14,000 rpm at 4℃ for 15 min. The supernatant was transferred to a clean tube without disturbing the pellet. Ammonium sulfate was added to the cleared lysate to 60% saturation and kept for 60min at 4℃. The supernatant collected by centrifugation at 10,000 rpm for 20 min at 4℃ was loaded onto a hydrophobic column at room temperature. The column was equilibrated with ammonium sulfate of 60% saturation and eluted with a linear gradient of ammonium sulfate from 60% to 0%. Then the eluted fractions were collected and dialyzed against buffer A (20mM citric acid- citrate, 1mM EDTA, pH 5.0) thoroughly. The resulting solution was applied to Q Sepharose fast flow column pre-equilibrated with buffer A and eluted with a linear gradient of NaCl from 0 to 1M in buffer B (20mM citric acid- citrate, 1mM EDTA, 1 mol/L NaCl, pH 5.0). The protein was monitored by measuring the UV absorbency at 215 nm. The eluted fractions were pooled and dialyzed against 20mM phosphate buffer(pH 7.2) at 4℃ with frequent buffer changes and then stored at -20℃.
Western-blot analysis
For western blots, after electrophoresis the samples and molecular weight standards were electrophoretically transferred to an NC membrane. The membrane was probed with a mouse anti-human Tα1 monoclonal antibody (Abcam, catalogue no. ab55635) followed by incubation with specific HRP-conjugated goat anti-mice IgG (Beijing Zhongshan Company, catalogue no. Zb-2305). The immunoreactive protein was visualized with an enhanced chemiluminescence, (Nanjing keygen Company, catalogue no. KGP1123).
Proliferation assay of Tα1② protein on mouse splenic lymphocytes
The proliferation response of splenocytes was determined by MTT assay. Spleens were removed from 6~8-week-old BALB/c mice and dispersed through nylon mesh to generate a single-cell suspension. The lymphocytes were separated by lymphocyte separating medium and collected by centrifugation at 1000 rpm for 10 min. The cell pellet was re-suspended in RPMI 1640 medium. One hundred microliter of the suspension was seeded in each well of 96 well plates at a concentration of 4×106 cells /ml and incubated in RPMI 1640 medium with 2.5 μg/ml concanavalin A (ConA) for 6 hours. After incubation, 90 μl of Tα1② protein that had been previously diluted with RPMI 1640 culture medium was added to all but the control wells. The synthetic Tα1 and RPMI 1640 culture media were used as positive and negative controls (3 parallel wells). After 66h incubation at 37℃, 20 μl of MTT (0.5 mg/ml) solution was added to each well, and the microplates were incubated for 4 hours at 37℃. After incubation, the plates were centrifuged (2,000 rpm, 25℃, 10 minutes). The supernatants were removed, and 100 μl of dimethyl sulfoxide (DMSO) was added to each well and incubated for 10 minutes at room temperature. After incubation, the solubilized reduced MTT was measured colorimetrically at 570 nm using a Benchmark microculture plate reader (Bio-Rad, USA). The optical densities results were used for calculate growth rate.
| Growth rate (%) = OD sample / OD control ×100% |
Inhibitory effect of Tα1② on tumor cell lines
HL60, M1, S180, B16 and Hep-G2 cells were cultured in RPMI1640 medium containing 10% fetal bovine serum at 37℃ in a humidified 5% CO2 incubator and regularly passaged. We harvested the cells when they are in the exponential phase of growth. Cells were re-suspended and diluted to 1 × 104 cells per ml in RPMI 1640 culture medium. Then 100μl (5×103cells) of diluted cells was seeded into each well. After incubation at 37℃ for 6h, 100 μl of Tα1② protein of different dilutions (37.5μg/ml, 75μg/ml, 150μg/ml, 300μg/ml and 600μg/ml) was added to the plate. The synthetic Tα1 and PBS were also used as positive and negative controls, respectively. After incubation for 24 h, 20μl of MTT (0.5 mg/ml) solution was added to each well, and the microplate was incubated for 4 hours at 37℃. After incubation, the plates were centrifuged (2,000 rpm, 25℃, 10 minutes). The supernatants were discarded and 100μl of DMSO was added to each well. Then the plate was swirled gently for 10 min at room temperature. The absorbance was read at 570 nm. The results were used for calculate Inhibition rate.
| Inhibition ratio (%) =(OD control-OD sample) / (OD control)×100% |
The IC50 (the drug concentration that causes a 50% reduction in cell proliferation) values of Tα1 and Tα1② were estimated from the inhibition ratios.
Cell cycle analysis and apoptosis assays
The effects of Tα1② on both cell cycle and apoptosis in B16 cells were analyzed using flow cytometry. For cell cycle analysis, cells were plated into 12-well plates. Tα1②, Tα1 (150μg/ml) and PBS (used as negative control) were added and kept for 16 h. Then the cells were collected and washed with PBS without Ca2+ and Mg2+ and fixed with 70% ethanol. Cells were re-suspended in 1× binding buffer (10 mM HEPES-NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2) and 5 μl of propidium iodide/105 cells, and incubated at room temperature for 15 min. Cells were acquired and the percentage of cells in each stage of the cell cycle was analyzed by flow cytometry.
Cells were plated into 12-well plates. Tα1②, Tα1 (150μg/ml) and PBS (used as negative control) were added and kept for 16 h. Cells floating in the medium combined with adherent layer were trypsinized and incubated with annexin V-FITC/propidium iodide. Samples were immediately analyzed by flow cytometry for cell cycle and apoptosis assays. Detection of apoptosis through annexin V and PI staining was done as following: Cells were washed three times in PBS and re-suspended in binding buffer at 1 × 106 cells/ml. An aliquot of 1 × 105 cells was stained with annexin V-FITC and PI for 15 minutes at room temperature. Analysis was performed on a BD FacsCalibur flow cytometer. Cells were considered to be early apoptotic if they exhibited staining for annexin V. The double positive population was considered to be in the late stage of apoptosis
Detection of apoptotic cells by TUNEL Sections were performed with the Cell Death Detection kit (KeyGEN, China), according to the manufacturer's instructions. Apoptotic cells were examined with fluorescence microscope. At 400× magnifications, 20 visual fields were randomly selected to count the number of apoptotic cells and the total number of cells, respectively. The apoptosis index (AI) = (number of apoptotic cells / number of total cells) × 100%.
In vivo anti-tumor effect
C57BL/6 mice were challenged subcutaneously with B16 melanoma cells rearward, 5×105 cells / mouse. On the tenth day, after tumors were larger than 0.01cm3 in the majority of the mice, we discarded the mice without tumors or with oversized tumors and divided the others stochastically into 5 groups at random. The administration of drugs began at the same day. The 5 groups were separately injected intraperitoneally with 150μl of saline, synthesized Tα1 (8mg/Kg/day), high dose Tα1② (16mg/Kg/day), middle dose Tα1②(8mg/Kg/day) and low dose Tα1② (4mg/Kg/day). Tumor size was measured every 2 days or every day and calculated according to the following formula: Tumor size (cm3) =π×length×wide2/6. The administration lasted for twelve days. Then the mice were sacrificed and the tumor tissue was dissected for body mass. The results were used for statistic analysis.
Statistical analysis
All the data were analyzed with SPSS 13.0 software and student t-test was used. A P-value of less than 0.05 indicated a statistically significant difference.
Results
Plasmid construction and identification
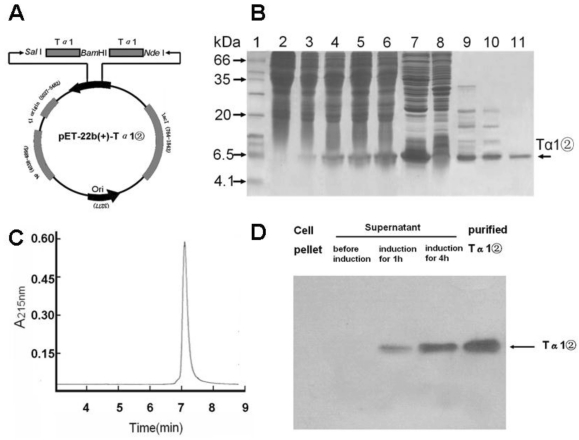
The tandem Tα1 gene of 2 repeats was synthesized and cloned into pET-22b (+) plasmid (pET-22b -Tα1②) (Fig.1A). DNA sequence analysis confirmed that recombinant Tα1② gene was correct.
Figure 1.
Plasmid construction ,Tα1② expression and purification and western blot analysis. (A) Schematic map of pET-22b(+)-Tα1② expression vector. (B) SDS-PAGE analysis of Tα1② expression and purification. Lane 1, molecular weight standards (kDa); lane 2, total cell lysate before induction; lane 3~6, total cell lysate after induction for 1, 2, 3 and 4h; lane 7, supernatant of total cell lysate after induction; lane 8, pellet of total cell lysate after induction; Lane 9, supernatant after 60% (NH4)2SO4 salt fractionation; Lane 10, Phenyl column wash; Lane 11, pooled Tα1② eluted from Q-Sepharose FF column. (C) HPLC analysis of purifiedTα1②. HPLC analyses were performed on a Beckman's HPLC system. The sample (5μg) in PBS was injected onto a 7.5mm×300mm G2000SW column (TOSOH Corporation) at a flow rate of 0.5mL/min. Peaks were detected by monitoring at a wavelength of 215 nm. (D) Detection of Tα1② protein by Western blot in cell pellet and supernatant, as well as Tα1② eluted from Q-Sepharose FF column.
Expression, Purification and Western-blot analysis of Tα1②
The expression plasmid pET22b-Ta1② was transformed into E.coli BL21(DE3). Tα1②(6.3 kD) was expressed in soluble form with the presence of 0.5mM IPTG for 4 hours(Fig.1B). Tα1② was precipitated by ammonium sulfate, purified by hydrophobic chromatography and anion exchange chromatography and harvested with purity over 95% by HPLC (Fig.1C). Western blot analysis confirmed that Tα1② could be recognized by a polyclonal antibody against the human Tα1 (Fig.1D). This purification process resulted in final yielding ratio as 4.5mg purified Tα1②/g cell paste (Table 1).
Table 1.
Purification of Tα1② (10 g of wet cell paste)
| Purification step | Protein concentration (mg/ml) | Volume (ml) | Total protein (mg) | Purity (%) | Yield (%) |
|---|---|---|---|---|---|
| E. coli cell lysate | 9.8 | 105 | 1029 | 21.6 | — |
| Salt fractionation | 2.1 | 95 | 199.5 | 54.2 | 48.6 |
| Phenyl Sepharose | 1.2 | 86 | 103.2 | 65.7 | 30.5 |
| Q Sepharose | 1.8 | 26 | 47 | 95.5 | 20.2 |
Tα1② stimulated the proliferation of mouse splenic lymphocytes in vitro
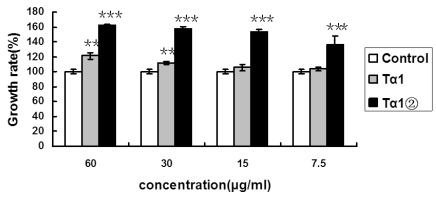
To examine whether Tα1② could enhance the proliferation of splenic lymphocytes, we compared the proliferation ratio of mice lymphocytes using purified Tα1② and synthetic Tα1. The concentrations were set as following: 60μg/ml, 30μg/ml, 15μg/ml and 7.5μg/ml. The MTT assay result showed that 30μg/ml synthetic Tα1 could induce significant proliferation of mouse spleen lymphocytes compared to the control(P<0.005), whereas 7.5μg/ml Tα1②could induce significant proliferation (Fig.2).
Figure 2.
Tα1② protein promotes the proliferation of mouse spleen lymphocytes. Control group: Cultured cells were exposed to 2.5μg/ml ConA only; Tα1②group: Cultured cells were exposed to 2.5μg/ml ConA and various concentrations (7.5μg/ml, 15μg/ml, 30μg/ml and 60μg/ml) ofTα1②;Tα1 group: Cultured cells were exposed to 2.5μg/ml ConA and various concentrations (7.5μg/ml, 15μg/ml, 30μg/ml and 60μg/ml) of Tα1. Cell proliferation was determined by the MTT viability assay. The assays were repeated in triplicate. The data are expressed as the mean±S.E.M. of groups consisting of three observations. (**P<0.005, ***P<0.001)
Tα1② inhibited the growth of the tumor cell lines in vitro
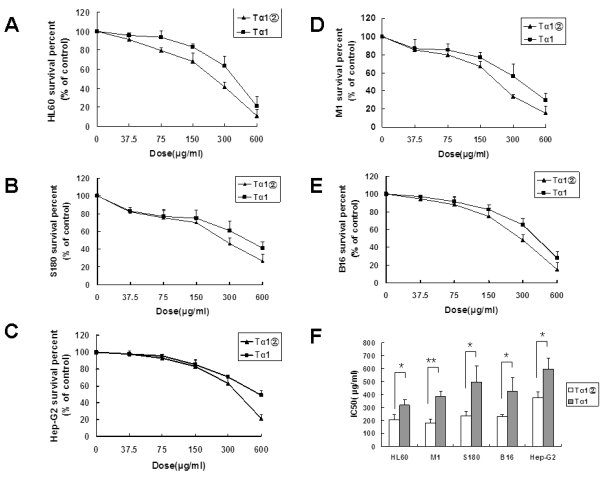
Previous studies showed that Tα1 could inhibit the growth of the tumor cell lines. In this study, the inhibition effect of Tα1② on 5 tumor cell lines was validated. After 24 h of treatment with Tα1② and synthetic Tα1, the proliferation of all tumor cells was significantly inhibited, and showed the dose-dependent effect. As shown in Fig.3, HL60 [IC50(Tα1) =318.75 μg/ml, IC50(Tα1②) =204.9μg/ml ], M1 [IC50(Tα1) =385.05 μg/ml, IC50(Tα1②) =182.7μg/ml], S180 [IC50(Tα1) =495.9μg/ml, IC50(Tα1②) =239.4μg/ml], and B16 [IC50(Tα1) =426.3μg/ml, IC50(Tα1②) =230.4μg/ml ] displayed relatively low IC50 values compared with HepG-2 [IC50(Tα1) =596μg/ml, IC50(Tα1②) =376.5μg/ml ].
Figure 3.
Inhibitory effect of Tα1② on tumor cell lines. Cultured cells (5×103 cells/well) were exposed to various concentrations ofTα1 andTα1② (37.5μg/ml, 75μg/ml, 150μg/ml, 300μg/ml and 600μg/ml) for 24 h. Cell proliferation was determined by the MTT viability assay. The assays were repeated in triplicate. The data were expressed as the mean± (SD) of groups consisting of four observations. (A) Inhibition curve of HL60; (B) Inhibition curve of S180; (C) Inhibition curve of Hep-G2; (D) Inhibition curve of M1; (E) Inhibition curve of B16; (F) IC50 of Tα1 and Tα1② for different cell lines. (**P<0.01, *P<0.05)
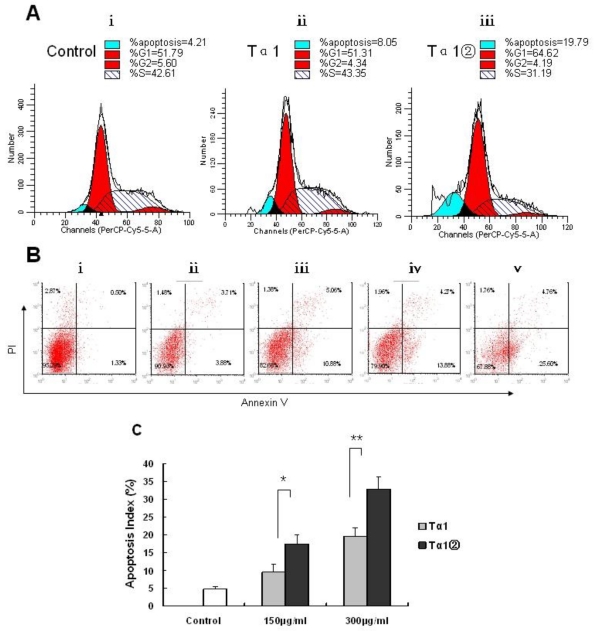
Effects of Tα1② treatment on cell cycle progression and apoptosis in B16 cells
To elucidate potential cellular mechanisms of Tα1②-induced growth inhibition, we examined the cell cycle progress and cell apoptosis by flow cytometry. Tα1② at a concentration of 150μg/ml resulted in the decreasing the proportion of cells in the S phase after 16h of exposure (Fig.4A). To assess the effects of Tα1② on induction of B16 cell apoptosis, we performed flow cytometry after 16 h of treatment with150 μg/ml, 300μg/ml Tα1② and Tα1. Flow cytometric analysis showed both Tα1② and Tα1② can induce apoptosis compared with the control group in a dose-dependent manner (Fig.4B). Evaluation of apoptosis by TUNEL assay showed that Tα1② induced a higher apoptotic rate in B16 cells compared with Tα1 at both doses (17.45±2.56% vs. 9.42±2.27% for 150μg/ml and 32.72±3.38 % vs. 19.58±2.31% for 300μg/ml). The altered cell cycle and increased apoptosis may be the mechanisms against the proliferative action of Tα1② in B16 cells in vitro.
Figure 4.
Effect of Tα1② on cell cycle progression and apoptosis in B16 cells. (A)Cultured B16 cells were exposed to 150μg/ml Tα1 andTα1② for 16 h then acquired and the percentage of cells in each stage of the cell cycle was analyzed. ⅰ.control group, ⅱ. 150μg/ml Tα1 group, ⅲ. 150μg/ml Tα1② group. Proportion of cells in the G1, S, and G2 phases and apoptosis was calculated using the Cell Quest software. (B) Detection of apoptosis through annexin V and PI staining. ⅰ.control group, ⅱ. 150μg/ml Tα1 group, ⅲ. 150μg/ml Tα1② group, ⅳ.390μg/ml Tα1 group,ⅴ.300μg/ml Tα1② group. (C) TUNEL assay comparison-The apoptosis index (AI) detected. Data are represented as mean ± SD. * and** showed p<0.05 and p<0.01 for n=3 triplicate after 16 hours of treatment with different concentrations PBS, Tα1 and Tα1② using TUNEL assay.
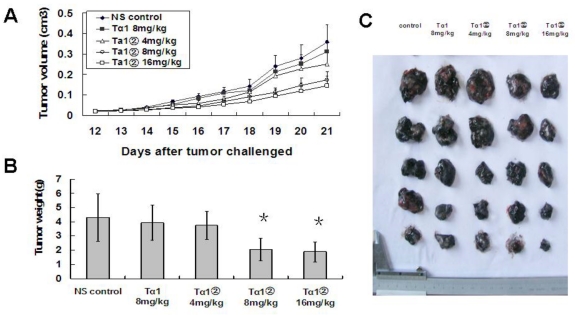
Ta1② inhibited the growth of tumor in the B16 bearing mice
To evaluate the bioactivity of Tα1② further, we observed the anti-tumor ability of Tα1② in the tumor-bearing mice in vivo. In a randomized, controlled study, high dose of Tα1② and synthesized Tα1 were given to B16 bearing mice. Our data showed that the treatment of Tα1② (high dose=16mg/Kg/day, middle dose =8mg/Kg/day) for 12 days led to retarded tumor growth (Fig.5A) and reduced tumor weight (Fig.5B) compared to control group(P<0.05). This anti-tumor effect is more evident in initial stages of tumor growth. Tα1 at the dose of 8mg/Kg/day did not show any statistical significance (P=0.755) compared to the control group. These results show the activity of Tα1② in vivo is also more effective than the synthesized Tα1.
Figure 5.
Tα1② inhibited the growth of tumor in the B16 bearing mice. C57BL/6 mice were challenged subcutaneously with B16 melanoma cells rearward for 10 days, followed by administration with synthesizedTα1 and different doses of Tα1② for another twelve days. Then the mice were executed and the tumor tissue was dissected and weighed. The data were expressed as the mean± (SD) of groups consisting of four observations. (A) Tumor volume measurement. B) Tumor weight of different groups, (*P<0.05). (C) Picture of isolated tumors.
Discussion
Previous studies have confirmed the immuno-modulating effects of Tα1 when it was used in combination with cytokines and chemotherapy, which was effective in inhibiting tumor growth and controlling infective diseases, particularly in an immunocompromised host [31].
However, the Tα1 used in clinics is mainly produced by chemical synthesis and animal thymus extraction [32-35]. The amount of Tα1 extracted from calf or porcine thymus is limited. In addition, the impurity and quality variation of extracted Tα1 also restricts its application. Tα1 from this source is often impure so that it may cause heterogeneous allergies. Although chemically synthesized Tα1 can reach a high level of purity, it is necessary to get rid of by-products at each step. Thus, its cost is very expensive [27]. Prokaryotic expression systems allow for high expression of foreign genes and are widely used for producing large amounts of proteins or peptides. However, it is difficult to extract the micromolecular recombinant peptide from fermentation broth when Tα1 (3.1kDa) is expressed alone. In this study, we designed a protein sequence of two repeats of Tα1, Tα1②, whose molecular weight was doubled (about 6.3kDa). A linker GS (Gly-Ser) was inserted between two repeats of Tα1. The flexibility and simple structure of GS linker may help the two monomer Tα1 peptides to keep their conformation and function and also adds flexibility to the expressed protein.
Active Tα1② was successfully expressed in E.coli suggesting that our design was reasonable. In addition, unlike regular high expression of other proteins, no inclusion bodies were found during the E.coil expression period, making it easier to purify the recombinant protein. Some of the known biological effects of Tα1 include enhanced T-cell, dendritic and antibody responses, chemo-protection properties, modulation of cytokine and chemokine production, and the ability to block steroid-induced apoptosis of thymocytes [36]. In this study, we discarded the widely used heat treatment [29], though it is of great help to remove most miscellaneous proteins. We used ammonium sulfate precipitation instead of heat treatment to avoid possible adverse effects on the bioactivity of Tα1② protein caused by heat denaturation.
Natural Tα1 can stimulate the proliferation of splenic lymphocytes of mice. Consequently, we evaluated the capability of Tα1② to stimulate the proliferation of mouse spleen lymphocytes after Tα1②was purified. The result showed that the Tα1② was of much stronger ability to promote proliferation than synthetic Tα1. A possible explanation for this phenomenon is that the conformation of the former might have stronger affinity with the Tα1 receptor compared with the latter. Tα1② may promote receptor dimerization better than the monomer.
Tα1 is believed to augment an anti-tumor immune response by activation of specific signaling pathways in lymphoid cells, Firstly, Tα1 has been shown to increase lymphocytic infiltration to sites of disease, an effect which may be facilitated by the release of certain chemokines stimulated by Tα1. Second, Tα1 mediates maturation and differentiation of dendritic cells which synthesizes specific cytokines leading to an increase in anti-tumor T-cells. Anti-melanoma T-cells have been shown to correlate with tumor regression and improve survival of patients with melanoma [36]. Evidence showed that Tα1 can significantly suppress proliferation and induce apoptosis in many tumor cell lines, suggesting that Tα1 was a potent therapeutic agent for some kinds of malignant tumors [9, 11]. Thus, we compared the effect of Tα1② and synthetic Tα1on tumor cell lines. The data showed that the 5 tumor cell lines (HL60, M1, S180, B16 and Hep-G2) were more sensitive to Tα1② and the effect of Tα1② on the tumor cell lines was much higher than Tα1. More significant cycle arrest and increase in apoptosis was induced after B16 cells exposed to Tα1② compared to Tα1. Ta1② inhibited the growth of tumor in the B16 bearing mice. The activity of Tα1② in vivo is also more effective than the synthesized Tα1. These effects were attributed to be mediated by stimulation of the host immune response and direct suppression of proliferation and induction of apoptosis in some cancer cell lines. Therefore, the anti-tumor mechanism and signal pathway remains to be explained further. We are working on further discussion of its in vivo efficacy and interaction. Above all, our data indicated that the Tα1② we engineered with stronger bioactivity may be a good candidate for tumor therapy.
Conclusion
We developed a strategy for expression and purification of Tα1②. The final yields were 4.5mg purified protein per gram of cell paste. The purity of the protein was higher than 95%. This is the first report on successful expression of recombinant Tα1 with higher bioactivity than the commercial Tα1. Our study suggests that this new purification protocol provides a cost effective and an efficient way to produce large quantities of high purity recombinant Tα1② which will greatly enhance the development and therapeutic application of this biologically potent molecule.
Acknowledgments
This work was supported by grant from Natural Science Foundation of China (NSFC, No. 30900537;81072050).
References
- 1.Goldstein Al, Low TL, McAdoo M. et al. Thymosin alpha l: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc. Natl. Acad. Sci. 1977;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancell CD, Phipps J, Young L. Thymosin alpha-1. Am. J. Health Syst. Pharm. 2001;58(10):879–885. doi: 10.1093/ajhp/58.10.886. [DOI] [PubMed] [Google Scholar]
- 3.Sztein MB, Goldstein AL. Thymic hormones--a clinical update. Springer Semin Immunopathol. 1986;9:1–18. doi: 10.1007/BF00201901. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein AL, Badamchian M. Thymosins: chemistry and biological properties in health and disease. Expert Opin Biol Ther. 2004;4(4):559. doi: 10.1517/14712598.4.4.559. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Li M, Yang H. et al. Roles of thymosins in cancers and other organ systems. World J. Surg. 2005;29(3):264–270. doi: 10.1007/s00268-004-7817-2. [DOI] [PubMed] [Google Scholar]
- 6.Baumann CA, Badamchian M, Golstein AL. Thymosin α1 antagonizes dexamethasone and CD3-induced apoptosis of CD4+CD8+ thymocytes through the activation of cAMP and protein kinase C dependent second messenger pathways. Mechanism of Ageing and Development. 1997;94:85–101. doi: 10.1016/s0047-6374(96)01860-x. [DOI] [PubMed] [Google Scholar]
- 7.Roy R, Singh SM, Shanker A. Mechanism of thymocyte apoptosis induced by serum of tumor-bearing host: The molecular events involved and their inhibition by thymosin α-1. Int. J. Immunopharmacol. 2000;22:309–321. doi: 10.1016/s0192-0561(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 8.Wolf GT, Hudson J, Peterson KA. et al. Interleukin 2 receptor expression in patients with head and neck squamous carcinoma.Effects of thymosin alpha 1 in vitro. Arch. Otolaryngol Head Neck Surg. 1989;115:1345–9. doi: 10.1001/archotol.1989.01860350079019. [DOI] [PubMed] [Google Scholar]
- 9.Ying-zhe Fan, Hui Chang, Ye Yu. et al. Thymosin alpha1 suppresses proliferation and induces apoptosis in human leukemia cell lines. Peptides. 2006;27(9):2165–2173. doi: 10.1016/j.peptides.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Spangelo BL. et al. Presence of a peptide component of thymosin fraction-5 manifesting discrete cytostatic properties in HL-60 human promyelocytic leukemia cells. International Immunopharmacology. 2005;5(7-8):1317–1329. doi: 10.1016/j.intimp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Moody TW, Fagarasan M, Zia F. et al. Thymosin alpha 1 down-regulates the growth of human non-small cell lung cancer cells in vitro and in vivo. Cancer Res. 1993;53:5214–8. [PubMed] [Google Scholar]
- 12.Hadden JW. Thymic Endocrinology. Annals of the New York Academy of Sciences. 1998;840:352–358. doi: 10.1111/j.1749-6632.1998.tb09574.x. [DOI] [PubMed] [Google Scholar]
- 13.Low TL. et al. The chemistry and biology of thymosin. J. Biol. Chem. 1979;254:981–986. [PubMed] [Google Scholar]
- 14.Low TL, Goldstein AL. Thymosins: structure, function and therapeutic applications. Thymus. 1984;6:27–42. [PubMed] [Google Scholar]
- 15.Li CL, Zhang T, Saibara T. et al. Thymosin alpha 1 accelerates restoration of T-cell-mediated neutralizing antibody response in immunocompromised hosts. Int. Immunopharmacol. 2002;2:39–46. doi: 10.1016/s1567-5769(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 16.Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol. 2003;3:1061–71. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 17.Garaci E, Pica F, Rasi G. et al. Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application. Int. J. Immunopharmacol. 2000;22:1067–76. doi: 10.1016/s0192-0561(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 18.Leung N. Treatment of chronic hepatitis B: case selection and duration of therapy. Gastroenterol Hepatol. 2002;17:409–14. doi: 10.1046/j.1440-1746.2002.02767.x. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick D, Pido-Lopez J, Pires A. et al. A pilot study of the safety and efficacy of thymosin alpha 1 in augmentingimmune reconstitution in HIV-infected patients with low CD4 counts taking highly active antiretroviral therapy. Clin Exp Immunol. 2003;134:477–81. doi: 10.1111/j.1365-2249.2003.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garaci E, Pica F, Sinibaldi-Vallebona P. et al. Thymosin alpha(1) in combination with cytokines and chemotherapy for the treatment of cancer. Int Immunopharmacol. 2003;3:1145–50. doi: 10.1016/S1567-5769(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 21.Billich A. Thymosin α1(SciClone Pharmaceuticals) Current Opioion in Investigational. Drugs. 2002;3:698–707. [PubMed] [Google Scholar]
- 22.Pica F, Fraschetti M, Matteucci C. et al. High doses of thymosin alpha 1 enhance the anti-tumor efficacy of combination chemo-immunotherapy for murine B16 melanoma. Anticancer Res. 1998;18:3571–3578. [PubMed] [Google Scholar]
- 23.Moody TW, Tuthill C, Badamchian M. et al. Thymosin alpha1 inhibits mammary carcinogenesis in Fisher rats. Peptides. 2002;23:1011–1014. doi: 10.1016/s0196-9781(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 24.Rasi G, Pierimarchi P, Sinibaldi Vallebona P. et al. Combination therapy in the treatment of chronic viral hepatitis and prevention of hepatocellular carcinoma. Int. Immunopharmacol. 2003;3:1169–1176. doi: 10.1016/S1567-5769(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.An TT, Liu XY, Fang J. Primary assessment of treatment effect of thymosin alpha1 on chemotherapy-induced neurotoxicity. Ai Zheng. 2004;23(11):1428–30. [PubMed] [Google Scholar]
- 26.Qiong Shen, Ruiyang Tian, Wenzhe Ma. Construction and expression of a new fusion protein, thymosin a1-cBLyS in E.coli. Biotechnology Letters. 2005;27:143–148. doi: 10.1007/s10529-004-7333-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Lai ZT, Lu MK. et al. Expression and Hydroxylamine Cleavage of Thymosin Alpha 1 Concatemer. Journal of Biomedicine and Biotechnology. 2008;2008:736060. doi: 10.1155/2008/736060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You-Feng Yang, Han-Ying Yuan, Nan-Song Liu. Construction, expression and characterization of human interferon α2b-(G4S)n-thymosin α1 fusion proteins in Pichia pastoris. World J. Gastroenterol. 2005;11:2597–2602. doi: 10.3748/wjg.v11.i17.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei-Fu CHEN, Hong-Ying ZHANG, Geng-Feng FU. Overexpression of Soluble Human Thymosin Alpha 1 in Escherichia coli. Acta. Biochim. Biophys. Sin. (Shanghai) 2005;37:147–151. [PubMed] [Google Scholar]
- 30.Chen Y, Zhao L, Shen G. et al. Expression and analysis of thymosin α1 concatemer in Escherichia coli. Biotechnol. Appl. Biochem. 2008;49:51–56. doi: 10.1042/BA20070055. [DOI] [PubMed] [Google Scholar]
- 31.Yong Qin, Fu-Ding Chen, Liang Zhou. et al. Proliferative and anti-proliferative effects of thymosin α1 on cells are associated with manipulation of cellular ROS levels. J. Chemico-biological Interactions. 2009;180:383–8. doi: 10.1016/j.cbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Low TL, McClure JE, Naylor PH. et al. Isolation of thymosin alpha 1 from thymosin fraction 5 of different species by high-performance liquid chromatography. J. Chromatogr. 1983;26:533–44. doi: 10.1016/s0021-9673(01)90924-0. [DOI] [PubMed] [Google Scholar]
- 33.Danho W, Gabriel TF, Makofske RC. Isolation and identification of thymosin alpha 1 from calf spleen using high performance liquid chromatography. Int. J. Pept. Protein Res. 1984;23:630–6. doi: 10.1111/j.1399-3011.1984.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang SS, Makofske R, Bach A. et al. Automated solid phase synthesis of thymosin alpha 1. Int. J. Pept. Protein Res. 1980;15:1–4. doi: 10.1111/j.1399-3011.1980.tb02543.x. [DOI] [PubMed] [Google Scholar]
- 35.Wong TW, Merrifield RB. Solid-phase synthesis of thymosin alpha 1 using tert-butyloxycarbonylaminoacyl-4-(oxymethyl) phenylacetamidomethyl-resin. Biochemistry. 1980;19:3233–8. doi: 10.1021/bi00555a021. [DOI] [PubMed] [Google Scholar]
- 36.Allan L Goldstein, Adsm L Goldstein. From lab to bedside: emerging clinical applications of thymosin α1. Expert. Opin. Biol. Ther. 2009;9:593–608. doi: 10.1517/14712590902911412. [DOI] [PubMed] [Google Scholar]